Abstract
Cataplexy in the narcoleptic canine may be modulated by systemic administration of monoaminergic compounds. In the present study, we have investigated the effects of monoaminergic drugs on cataplexy in narcoleptic canines when perfused locally via microdialysis probes in the amygdala, globus pallidus/putamen, basal forebrain, pontine reticular formation and ventral tegmental area of narcoleptic and control Doberman pinchers. Cataplexy was quantified using the Food-Elicited Cataplexy Test and analyzed by electroencephalogram, electrooculogram and electromyogram. Local perfusion with the monoaminergic agonist quinpirole, 7-OH-DPAT and BHT-920, into the ventral tegmental area produced a dose-dependent increase in cataplexy without significantly reducing basal muscle tone. Perfusion with the antagonist raclopride in the same structure produced a moderate reduction in cataplexy. Local perfusion with quinpirole, 7-OH-DPAT and BHT-920 into the globus pallidus/putamen also produced an increase, while raclopride produced a decrease, in cataplexy in narcoleptic canines. In control animals, none of the above drugs produced cataplexy or muscle atonia when perfused into either the ventral tegmental area or the globus pallidus/putamen. Other monoaminergic drugs tested in these two brain areas; prazosin, yohimbine, amphetamine, SKF 38393 and SCH 23390 had no effects on cataplexy. Local perfusion with each of the above listed drugs had no effect on cataplexy in any of the other brain regions examined. These findings show that cataplexy may be regulated by D2/D3 dopaminergic receptors in the ventral tegmental area and perhaps the globus pallidus/putamen. It is suggested that neurons in the mesolimbic dopamine system of narcoleptics are hypersensitive to dopaminergic autoreceptor agonists.
Keywords: Narcolepsy, Cataplexy, Dopaminergic system, Ventral tegmental area
1. Introduction
Narcolepsy is a sleep disorder occurring in approximately 0.05% of the population [27]. It is characterized by excessive daytime sleepiness, fragmented night-time sleep, sleep onset periods of rapid eye movement (REM) and sudden periods of muscle atonia called cataplexy [1,21]. Canine narcolepsy is an animal model of the disorder with many clinical features paralleling those found in humans, including excessive daytime sleepiness, fragmented night-time sleep and symptoms of abnormal REM sleep such as cataplexy [6,28,42,64]. Cataplexy in the narcoleptic canine can be elicited by the emotional stimulation produced by food, and consequently this symptom can be reliably and easily quantified using a food-elicited cataplexy test (FECT) [6].
The current treatment of human narcolepsy involves a combination of tricyclic antidepressant drugs, which are active on cataplexy and REM related symptoms and stimulant compounds, such as modafinil and methamphetamine, which are active on somnolence [1,36,50]. The same compounds are also active in narcoleptic canines, on which a thorough analysis of the pharmacological control of cataplexy and somnolence has recently been carried out [42,43,60]. These studies have demonstrated that the therapeutic effects of antidepressant compounds on canine cataplexy are mediated primarily through the blockade of adrenergic uptake. Specific adrenergic uptake inhibitors are much more effective than dopaminergic or serotonergic uptake inhibitors in reducing cataplexy in the canine model [43,57]. Studies in progress also suggest that activation of dopaminergic transmission via uptake blockade or stimulation of dopamine release is critical for the alerting effect of most stimulant compounds on narcolepsy [60]. All the stimulant compounds available to date share this pharmacological property and some newly developed monoamine uptake blockers specific for the dopamine transporter have EEG arousal properties [20,26,44,49,60].
Using the narcoleptic canine model, we have attempted to further investigate the receptors that are involved in the postsynaptic mediation of the therapeutic effects of the monoamine uptake inhibitors and release enhancers, with special emphasis on the adrenergic control of cataplexy [42,58]. The results demonstrated that the most likely candidate receptor for the mediation of the anticataplectic effects of adrenergic uptake inhibitor is the α-lb receptor [41,57]. α-I specific receptor agonists are potent suppressants of cataplexy whereas α-I antagonists enhance canine cataplexy, a phenomenon that has been confirmed in human patients taking prasozin as an antihypertensive therapy [41,57]. We have also shown that adrenergic α-2 modulation, most probably presynaptically mediated, has significant effects on cataplexy, with some atypical α-2 agonists (such as BHT-920 and xylazine) increasing while several (1–2 antagonists decrease canine cataplexy [55]. Consistent with these findings, significant changes in tissue levels of noradrenaline in the pontine reticular formation, amygdala and preoptic hypothalamus [15,46] as well as an increase in α-2 receptor numbers in the locus coeruleus (LC) have been found in the narcoleptic canine brain [17]. In human postmortem studies a decrease in α-I adrenergic receptor binding in the cortex and an increase in α-2 receptor binding in the LC has been reported [2,32,37] These studies indicate that the central noradrenergic projections, originating in the locus coeruleus, might be altered in the narcoleptic canine brain but to date no studies have examined the effects of local pharmacological manipulations in the locus coeruleus on the symptoms of narcolepsy.
Studies on the ability of dopaminergic drugs to modulate cataplexy in narcoleptic canines have shown that low doses of D2 dopamine receptor agonists such as quinpirole and BHT-920 (mixed D2 and α-2 agonist) stimulate cataplexy potently, while D2 and DI dopamine receptor antagonists reduce cataplexy and the DI agonist SKF 38393 have no effect [56]. This surprising result contrasts with the finding that dopaminergic uptake inhibitors have absolutely no effects on canine cataplexy [43]. Studies on the levels of biogenic amines in narcoleptic canines, demonstrating several neurochemical abnormalities in the cerebrospinal fluid (CSF) and in specific brain regions, suggest a role for dopaminergic systems in the regulation of sleepiness and abnormal REM sleep in narcolepsy. Thus, reduced levels of dihydroxyphenylacetic acid (DOPAC) have been reported in CSF [7] and elevated DOPAC levels and an increase in the DOPAC/dopamine ratio has been shown in the amygdala and rostral caudate nucleus [15,39,46]. Furthermore, an increase in D2 dopamine receptors in the rostral caudate and the amygdala of narcoleptic canines has also been reported [10]. Changes in dopaminergic function have also been demonstrated in studies on postmortem human narcoleptic brains which reported decreased DOPAC levels in the rostral caudate and amygdala [32] and an increase in DI and D2 dopamine receptor binding in the caudate and possibly (though not significant) an increase in DI dopamine receptor binding in the lateral globus pallidus [2,30,32,37]. However, while the studies on postmortem human brain tissue have consistently shown an increase in D2 receptor binding in the caudate it should be noted that in vivo imaging studies on patients failed to observe this difference [25,30]. Nevertheless, these studies indicate the possibility of a pathophysiological change in the nigrostriatal and mesolimbic dopamine systems of narcoleptic subjects [38].
Previous work in our laboratory has shown that FECT induced cataplexy in the narcoleptic canine can be modulated by local administration of cholinergic drugs in several brain areas [59,61–63] Local perfusion of low doses of cholinergic compounds into the pontine reticular formation of narcoleptic canines produced a strong increase in cataplexy [61,62] which appeared to be mediated by M2 muscarinic receptors [63]. These results parallel previous work on the cholinergic regulation of cataplexy [8,13,31], as well as what is known about the pharmacological control of REM sleep by cholinergic drugs (for review and references, see [68,69]). We also recently found that cholinergic stimulation in the basal forebrain elicited cataplexy in narcoleptic animals [59] whereas very large doses of carbacol were needed to induce muscle atonia in control animals.
In contrast with the data described above for cholinergic systems, very little is known regarding the neuroanatomical substrates which mediate the effects of monoaminergic drugs on cataplexy and REM sleep. In the present study, we have studied the effects of local perfusion of monoaminergic compounds via microdialysis probes implanted in various brain regions on cataplexy in the narcoleptic canine. Both dopaminergic and adrenergic drugs which have been previously shown to modulate cataplexy when given systemically were tested. Brain regions studied included areas which have been previously shown to have altered monoaminergic levels (amygdala, preotic hypothalamus) and/or increased monoaminergic receptor binding (IOC, amygdala, lateral globus pallidus/putamen and PRF at the level of the LC) in either human or canine narcoleptic postmortem brain. We also tested monoaminergic drugs in the ventral tegmental area (VTA) because it is the primary nucleus of all mesolimbic and mesocortical dopaminergic projections. These studies should reveal whether, if at all, monoaminergic drug modulation of cataplexy can be mediated via specific brain areas and if so, should indicate which specific dopaminergic and/or noradrenergic neuronal systems are critical for the pathophysiology of narcolepsy. Furthermore, since many of the monoaminergic drugs which reduce the symptoms of narcolepsy are also known to reduce REM sleep, it is possible that such brain regions might also be involved in the monoaminergic regulation of REM sleep.
2. Materials and methods
All studies were performed on adult Doberman pinchers, which included eight narcoleptic (five males and three females), four control (three males and one female) and one heterozygous (male) canine. Heterozygous canines have only one copy of the narcolepsy transmitting gene, canarc-l, and do not express cataplexy spontaneously [42]. All animals were between I and 9 years of age, weighed 24–34 kg, and were bred at the Stanford University narcoleptic dog colony. Because of the great value of all implanted narcoleptic canines, most (10 of the 13 used in this study) have been used in previous experiments (see [61–64]). If so, each animal was given a ‘wash-out’ period of at least 10 days before further use. The animals were kept under a 12:12 h light/ dark schedule with food and water available ad libidum.
2.1. Surgical procedures
Guide cannulae and recording electrodes were surgically implanted in all canines using sterile procedures according to the protocol of Reid and colleagues (for complete description see [61]). Briefly, the animals were anesthetized using isofluorane (2% mixture in air administered via a trachial tube) and placed in a Kopf stereotaxic device. For cataplexy recording, they were implanted with screw electrodes in the skull over lateral parietal and the midfrontal cortex for recording of electroencephalogram (EEG) and in the orbit of the frontal bone for the recording of electrooculogram (EOG). Stranded stainless steel wires were inserted into the dorsal neck musculature for recording the electromyogram (EMG). The guide cannulae consisted of two 20-mm stainless steel cannulae separate by 2 mm and soldered to a nut stack, and were lowered into position so that the ventral tips touched the surface of the cortical dura. The guide cannulae bundles were placed bilaterally, one bundle over each hemisphere, so that a single guide cannula (caudal of each pair of stainless steel cannulae) was positioned over the PRF at the level of the LC (L: 3.2, A: 3.0 from stereotaxic zero according to Lim and colleagues [34], and V: 39.0 from the surface of the brain), the VTA (L: 1.5, A: 13.0, V: 36.0), the central nucleus of the amygdala (L: 13.0, A: 23.0, V: 36.0) and the BF adjacent to the preoptic hypothalamus (L: 5.0, A: 29.0, V: 33.0). Taking advantage of the more rostral guides from the guide cannulae bundles over the amygdala, bilateral cannulae over the lateral globus pallidus/putamen (GP/putamen) (L: 13.0 A: 25.0, V: 27.0) were also obtained. The recording electrodes, electrical plug and guide cannulae were cemented to the skull using dental acrylic and the skin was sutured. During recovery from surgery all animals were under 24 h surveillance at the Stanford University Department of Comparative Medicine Intensive Care Unit and post-surgical treatment included analgesics for the first 12–48 h and a regimen of daily antibiotic treatment for up to 5 days. The animals were allowed at least 4 weeks to recover from surgery.
One day prior to experimentation, the microdialysis probes (70 mm shaft with 5 mm membrane, CMA/ 10, CMA/Microdialysis, Stockholm, Sweden) were lowered bilaterally into the brain region of interest and anchored in place while the animals were under isofluorane anesthesia. The left and right probes were always implanted at the same coordinate on the anterior-posterior (AP) axis. Each probe was tested for in vitro recovery of 10−7 M dopamine before implantation and the relative recovery found was: 26 ± 3%, n = 26 (mean ± S.E.M.). After completing the experiment the probes were removed and the animals returned to their cages.
2.2. Experimental protocols
All canines were given at least 3 days of habituation to the experimental chamber before testing. A complete description of the experimental chamber has been made previously [61]. The probes were perfused via a 3-m inlet line at 2.0 ml/ min with artificial CSF (125 mM NaCl, 0.5 mM NaH2PO4, 2.5 Na2HPO4, 1.2 caC12, 2.5 mM KCI, 1 mM MgC12, pH 7.4) using a Harvard Pump. Cataplexy was measured using the FECT (see [61]) combined with recordings of EEG, EOG and EMG. In each FECT, the subject ate ten small bites of wet dog food which were lined up on the floor in a semi-circle over a distance of approximately 3 m and cataplectic attacks were scored when the animal stopped forward motion and the hind quarters were lowered towards the floor, thus initiating either a partial or complete attack. A simultaneous loss of muscle tone was usually noted by the neck EMG recordings. Time required to eat all ten bites of food and successfully move away was also recorded.
Experiments were performed on days 1 –5, beginning approximately 18 h after microdialysis probe implantation, between the hours of 10.00 h and 16.00 h. Each animal was connected to the dialysis inlet lines and perfused for 90 min with CSF before testing for cataplexy. Al the end of this 90-min control period, baseline levels of cataplexy were measured using four consecutive FECT trials performed over a period of 20 min. After completion of the 4 FECT trials CSF was perfused for another 20 min and then the perfusion medium was switched from CSF to CSF plus drug using a manual liquid switch (CMA/110, CMA Microdialysis). All drugs were tested bilaterally at various concentrations in the perfusion medium (pH 7.0); BHT-920 (10–5–102 M), quinpirole (10−5–10−2 M), 7-OHDPAT (10−5 10−2 M), SKF 38393 (10−5-10−2 M), raclopride (10−3 10−2 M), SCH 23390 (10−3-10−2 M), yohimbine (10−3 M), prazosin (10−3 M) and amphetamine (10−3 M). Drugs which were tested at more than one concentration were done so by increasing the concentration of the drug over the course of an experiment, e.g., 10−5 M was perfused for the first hour, 10−4 M for the second hour and 10−3 M for the third hour, e.g., cataplexy was measured by two consecutive FECT trials during 20–30 min and 50–60 min of each I h drug concentration period. Control levels of cataplexy were tested under the same schedule in narcoleptic canines receiving no drug infusion. Animals were awake at the start of all FECT trials.
In brain regions where a modulation of cataplexy was elicited by local drug perfusion, perfusate samples were also collected during the first 2 days of experimentation. Briefly, 10-min samples were collected during the 90-min baseline period, the 20-min FECT stimulation period and the 20-min post-FECT perfusion period (all samples collected while perfusing with CSF). Samples were collected bilaterally, on a short outlet feeding into collection tubes attached to the side of the headstage, from both narcoleptic and control canines. To control for motor effects of the FECT procedure, samples were also collected from narcoleptic canines when stimulated by motor activity similar to that during an FECT, without causing any cataplexy, during a 20-min stimulation period. All samples were analyzed for dopamine and DOPAC content by reverse phase HPLC coupled to an electrochemical detector (LC4B, BAS, West Lafayette, IN). The reverse phase ion pairing column (100 × 3.2) was prepacked with Phase Il ODS 3-μm particulate, the mobile phase contained M Na2HPO4, 0.09 M EDTA and 1.3 I-octanesulfonic acid in an 18% methanol solution adjusted to pH 2.95 with phosphoric acid. Electrochemical detection was done using a dual glassy carbon electrode (BAS-LC4B) set at 0.65 V. The limit of detection for dopamine was approximately 0.02 pmol.
2.3. Sleep stage scoring and spectral analysis
EEG, EMG and EOG were recorded at all times during the experiments. For sleep scoring a 30-s epoch was used. Wake included all episodes with low voltage mixed frequency cortical tracing in which EMG was not inhibited. Sleep onset (SI), or drowsiness, was scored when the animal laid quietly with eyes partially open or closed and cortical EEG showed trains of relatively slow wave (5–7 Hz) without the development of spindles and EMG amplitude showed a moderate decrease from wakefulness. Synchronous waves at 4–7 Hz, 50–100 μV occasionally appeared on a background of low voltage fast activity. Light sleep (LS), indicative of unequivocal sleep, was scored when EEG patterns were more synchronous and higher in amplitude than in SI, and sleep spindles (10–14 Hz) and/or K complexes (in fronto-parietal recordings) were present. Deep sleep (DS), was scored when high amplitude slow waves (less than 4 Hz) constituted more than 20% of a 30-s epoch. REM sleep was scored when a low-voltage mixed-frequency EEG tracing was observed together with rapid eye movements on the EOG tracing and a significant drop in EMG activity. Cataplexy (spontaneous or FECT induced) was scored when an abrupt drop in EMG during wakefulness was observed. The EEG pattern during cataplexy was low voltage, mixed frequency, and rapid eye movements and muscle twitching could sometimes, though not often, be seen. For this scoring stage the previous two epochs could be wake, SI or cataplexy.
Power spectral analysis was performed on 10-s epochs of EEG recorded during cataplexy. Briefly, EEG was filtered at 35 Hz low pass and 0.3 Hz high pass settings and digitized at 100 Hz onto an IBM PC computer using SCORE software (courtesy of Dr. Dale Edgar). The EEG was then Fourier analyzed in 10-s epochs using POWER-SPECTRUM software (courtesy of Dr. Dale Edgar). 10-s epochs from each cataplexy condition (baseline or drug induced), within the same animal, were selected according to specific criteria (see below) and means and standard deviation of the EEG power density (V2 per Hz) for each 0.2 Hz frequency band (0–20 Hz) were calculated. The 10-s epochs from each condition were chosen based on the following criteria: (l) sufficient ( > 0.0001 V2 ) spectral power from the EEG; (2) clean of noise and recording artifact; (3) entire epoch within a cataplectic attack (no transition phases included); and (4) both baseline and drug induced cataplexy recordings must fulfill the above criteria and be made on the same day, from the same animal.
2.4. Histology
Histological verification of the probe placements was performed on all animals. Four control, one heterozygous, and eight narcoleptic Doberman pinchers, weighing 28–35 kg, were deeply anesthetized with pentobarbital (50 mg/kg), implanted with injection cannulae (same gauge as CMA 10 probes) into the area of interest at the same coordinates as the microdialysis probes (in some animals a 1.0 μl injection of 2% cresyl violet was made), perfused transcardially with 4 1 of saline containing heparin and then 4 1 of 4% paraformaldehyde, 0.1% glutaraldehyde, 0.2% picric acid in 0.1 M phosphate buffer saline (PBS) at 40C. The brains and the first 4–5 cm of the spinal cords were removed and post-fixed in the same fixative without glutaraldehyde for 6–16 h. The tissue was then dissected into 1 −1.5 cm blocks and cryoprotected in 30% sucrose, 0.1 M PBS, 0.l% sodium azide at 4°C for 3–5 days. Selected blocks were frozen in liquid C02 and cut at 30 mm in a freezing cryostat at −24°C. Twelve serial sections were prepared in 0.l M PBS and rinsed for 30 min with three changes. Free-floating sections were then stored in the same solution with 0.1% sodium azide added as a preservative at 4°C until used. Adjacent sections were stained with thionin and for tyrosine hydroxylase immunoreactivity (TH-IR). Probe placement for all of the animals was determined using the thionin stained sections. Probe location in these cases was estimated by the presence of lesion/ scar tissue in the region of interest.
In two of the narcoleptic canines studied in the VTA the sections stained for TH-IR were used to further analyze probe location in relation to the mesencephalic dopamine cell body regions. The sections were rinsed in 0.1 M PBS, 0.3% Triton X-100 and treated with 10% normal goat TH serum in the same solution for 90 min followed by incubation in the primary antibody for 60 h at 4°C. Anti-TH antibody (Eugen Tech) was used at 1:100. After rinsing 3 × 10 min in PBS with 0.3% Triton X-I00 the sections were incubated in biotinylated anti-rabbit (TH) IgG (Vector, for 1:400–800) for 90 min at room temperature, rinsed 3 X 10 min in PBS with 0.3% Triton X-100 and treated with ABC (Vector, 1:400) for 90 min. The product was visualized after incubation in 0.02% diaminobenzidine (DAB) in 0.05 M Tris-HCI (pH 7.6) together with 0.003% H2O2 for 30 min. When the primary antibody was omitted or the secondary antibody was replaced with an irrelevant IgG no staining was observed. After being mounted onto gelatin coated slides, the sections were dehydrated, cleaned with xylene and coverslipped with Permount.
2.5. Drugs
Methamphetamine (monaminergic neurotransmitter releaser), yohimbine HCI (α-2 antagonist) (Sigma, St. Louis, MO), prazosin HCI (α-I antagonist), quinpirole (D2 dopamine agonist), 7-OH-DPAT (D3 dopamine agonist), SCH 23390 (m dopamine antagonist), SKF 38393 (DI dopamine agonist) (Research Biochemicals Incorporated, Natick, MA), raclopride (D2 dopamine antagonist) (Astra Pharmaceuticals, Södertälje, Sweden) and BHT-920 (α-2 and D2 dopaminergic agonist) (Forsch, FRG) were dissolved directly into CSF and tested for pH (all were approximately 7) before local administration.
2.6. Statistics
All results are presented as mean ± S.E.M. Dopamine levels are presented using percent of control values (the control value was obtained from the final 10-min sample prior to behavioral stimulation). FECT scores for cataplectic attacks and elapsed time during control and drug treatments were analyzed with one-way repeated ANOVA, followed by post-hoc Fisher PLSD tests (Statview 512, Abacus Concepts, Berkeley, CA). FECT scores were measured in duplicates at each time point. Dopamine levels during FECT and motor stimulation and were also analyzed with one-way repeated ANOVA. Dopamine levels were measured bilaterally and repeated testing was employed (each animal tested up to 2 consecutive days). The results from the control animals studied in the lateral GP/putamen should be considered as exploratory (due to the small animal number). Comparison of separate treatments/animal groups was done using two-way repeated ANOVA.
3. Results
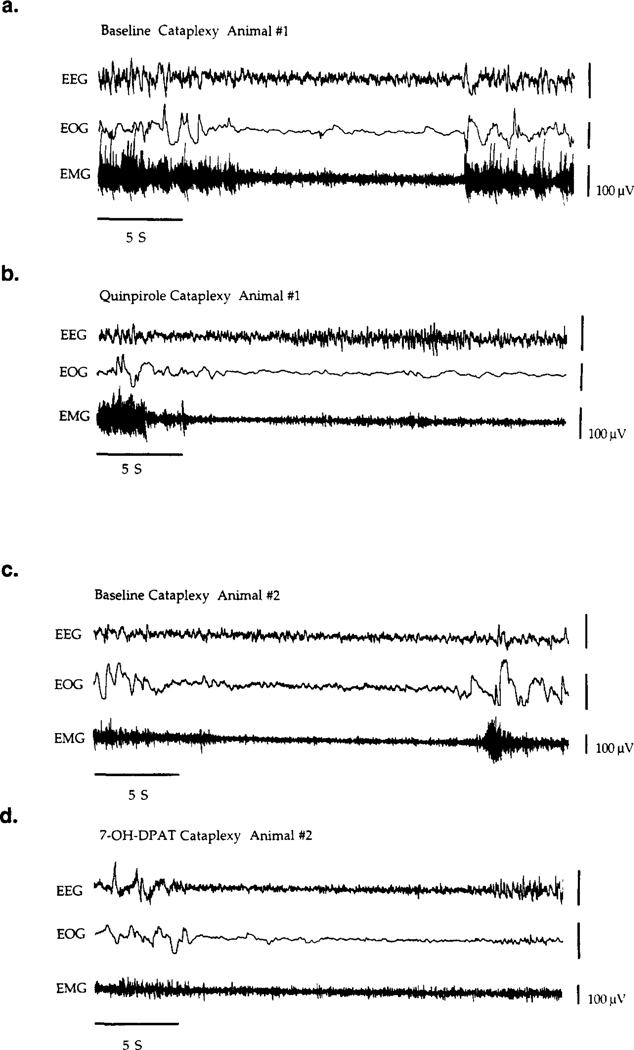
Basal FECT induced cataplectic attacks ranged from partial, in which the animal could remain upright though a clear loss of muscle tone was indicated by the drop in EMG signal, to complete, in which the animal would go down on all four limbs and remain atonic for up to I min. During cataplexy, the EEG signal remained desynchronized and the EOG was mostly quiet. Rapid eye movement was not usually observed. Typical baseline cataplectic attacks (complete attacks) are shown in Fig. 3a,c. While some variability in the levels of cataplexy existed, between different animals and within individuals between different experiment days (particularly when separated by one month or greater), the FECT scores were relatively stable on any given day over a 4 h control period with no drug treatment (Table 1), averaging approximately 2–3 cataplectic attacks per FECT (F7,100 = 0.866, P = 0.678) and elapsing over an approximate time of 40–60 s (F7,100 = 0.554, P=0.878). The control canines and the heterozygous canine did not exhibit cataplexy and normally completed a FECT within 15–25 s.
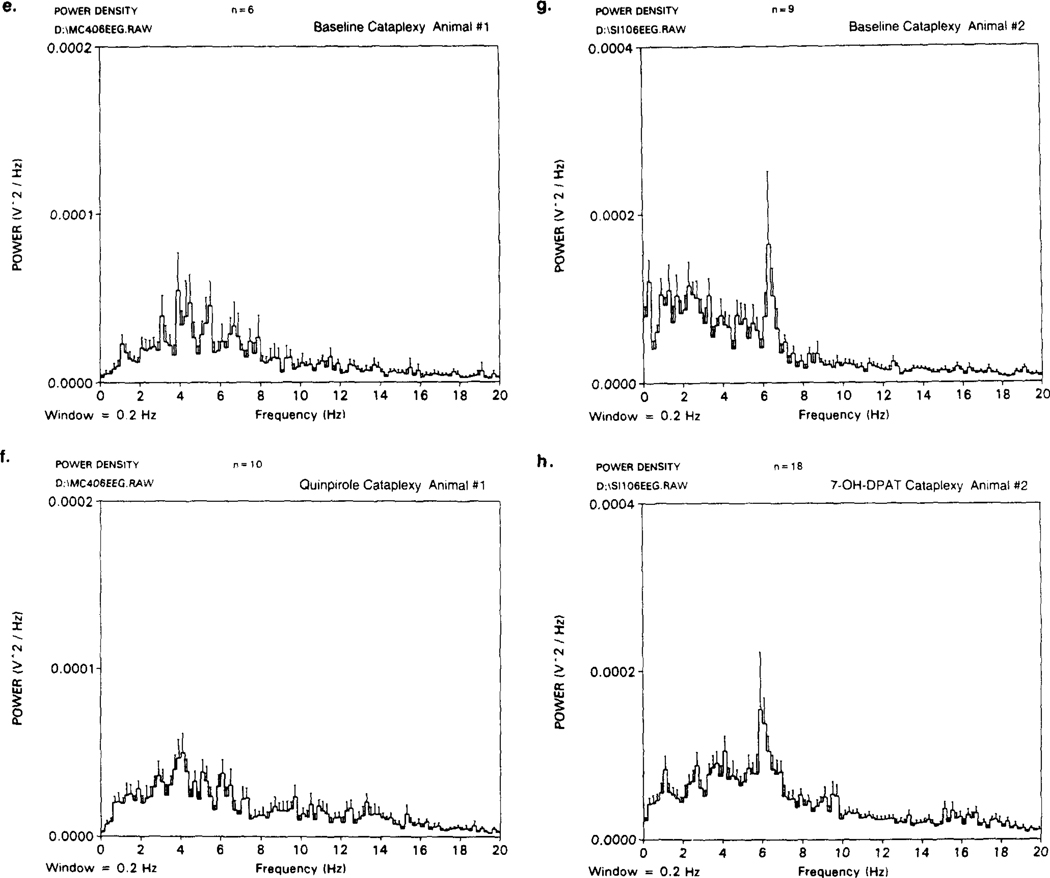
Fig. 3.
Polygraph recordings and spectral analysis of cataplexy in two narcoleptic canines receiving either quinpirole (10−3 M) (a,c,e,f) or 7-OH-DPAT (10−3 M) (b,d,g,h) in the ventral tegmental area (VTA). Polygraph recording of (a,c) baseline cataplexy, (b) bilateral quinpirole perfusion and (d) bilateral 7-OH-DPAT perfusion in the VTA are shown. Recordings in (a) and (c) were obtained from the same animal (animal no. 1). Recordings in (b) and (d) were obtained from the same animal (animal no. 2). All EEG recordings were bipolar, taken from fronto-parietal cortex leads, and were taken on the same day from each animal. Means and standard deviation of the EEG power density (V2 per Hz) for each 0.2 Hz frequency band (0–20 Hz) were calculated by averaging all 10-s epochs which were free of artifact for each condition; baseline (e,g), quinpirole (10−3 M) (f) and 7-OH-DPAT (10−3 M) (h). Power spectra in (e,f) and (g,h) were taken from the same animal, respectively.
Table 1.
Basal levels of FECT induced cataplexy in narcoleptic canines
| Time (min) | −20 | −10 | 20 | 50 | 80 | 110 | 140 | 170 |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Attacks (n = 8): | 2.9 ± 0.3 | 3.4 ± 0.4 | 2.6 ± 0.4 | 3.1 ± 0.4 | 2.4 ± 0.5 | 2.7 ± 0.4 | 2.4 ± 0.3 | 2.4 ± 0.9 |
| Elapsed time (s): | 61 ± 10 | 49 ± 7 | 55 ± 11 | 53 ± 5 | 49 ± 7 | 52 ± 7 | 56 ± 5 | 44 ± 10 |
Baseline cataplexy in eight narcoleptic canines perfused bilaterally with cerebrospinal fluid in the pontine reticular formation. Control levels of cataplexy were measured using the same schedule as drug perfused animals, once for each animal. The mean number of cataplectic attacks and elapsed time for two food-elicited cataplexy tests (FECT) per test period is shown. During each FECT the subject ate 10 bites of wet dog food lined up along the floor (3 m) and cataplectic attacks were scored when the animal stopped forward motion and the hind limbs were lowered towards the floor. The total time required to eat all ten bites of food and return to (or remain in) an upright position was also recorded.
Cataplexy in narcoleptic canines was enhanced by local drug perfusion in the lateral GP/putamen and VTA. No other brain regions mediated any effects. For an overall summary of the cataplexy modulatory effects of locally perfused monoaminergic drugs, see Table 2.
Table 2.
Modulation of cataplexy by local perfusion with monoaminergic drugs
| Perfused drug (bilateral) | VTA (n = 4) | PRF (n = 4) | GP/putamen (n = 4) | BF (n = 6) | Amygdala (n = 4) |
|---|---|---|---|---|---|
|
| |||||
| Dopaminergic: | |||||
| Agonists: (10−4 – 10−2 M) | |||||
| Quinpirole | + + | 0 | + | 0 | 0 |
| SKF38393 | 0 | 0 | 0 | 0 | 0 |
| 7-OH-DPAT | + + | 0 | + | 0 | 0 |
| Amphetamine | 0 | 0 | 0 | 0 | 0 |
| Antagonists: (10−3 – 10−2 M) | |||||
| Raclopride | - | 0 | - | 0 | 0 |
| SCH 23390 | 0 | 0 | 0 | 0 | 0 |
| Adrenergic: | |||||
| agonists: (10−4 – 10−2 M) | |||||
| BHT-920 | + + | 0 | + | 0 | 0 |
| Antagonists: (10−3 M) | |||||
| Yohimbine | 0 | 0 | 0 | 0 | 0 |
| Prazosin | 0 | 0 | 0 | 0 | 0 |
Effects of several monoaminergic compounds perfused locally in various brain regions on cataplexy in narcoleptic canines. All drugs are classified according to primary neurotransmitter receptor activity, although BHT-920 is also a strong agonist at D2 dopamine receptors. Cataplexy in narcoleptic canines was measured using the repeated Food-Elicited Cataplexy Tests (FECT) during the middle (20–30 min) and end (50–60 min) of a 60-min perfusion with each drug. 0 indicates no significant effect on cataplexy versus four baselines FECT’s, – indicates a significant reduction (P < 0.05) (but not a complete inhibition) in cataplexy versus four baseline FECT’s, + indicates a significant increase (P < 0.05) in cataplexy versus four baseline FECT’s and + + indicates a significant increase (P < 0.0l) in cataplexy versus four baseline FECT’s followed by the development of status cataplecticus in at least 50% of the animals. Significance was determined using a one-factor repeated ANOVA. The number of animals tested for each brain region studied, and each drug studied in that brain region, is shown in parenthesis under the each brain region heading.
3.1. Local drug perfusion in the amygdala, BF and pontine reticular formation
None of the monoaminergic drugs tested in the amygdala, BF and PRF had any effects on FECT induced cataplexy in the narcoleptic canines. Drugs were tested bilaterally in (l) the amygdala (n = 4): BHT-920 (10−510−3 M), quinpirole (10−5–10−3 M), SKF 38393 (10−510−3 M), raclopride (10−3 M), yohimbine (10−3 M), prazosin (10−3 M) and amphetamine (10−3 M), (2) the BF (n = 6): same drugs as in amygdala, and (3) the PRF (n 4): BHT-920 (10−5–10−3 M), yohimbine (10−3 M), methoxamine (10−3 M), prazosin (10−3 M) and amphetamine (10−3 M) (see Table 2).
3.2. Local drug perfusion in the lateral globus pallidus / putamen
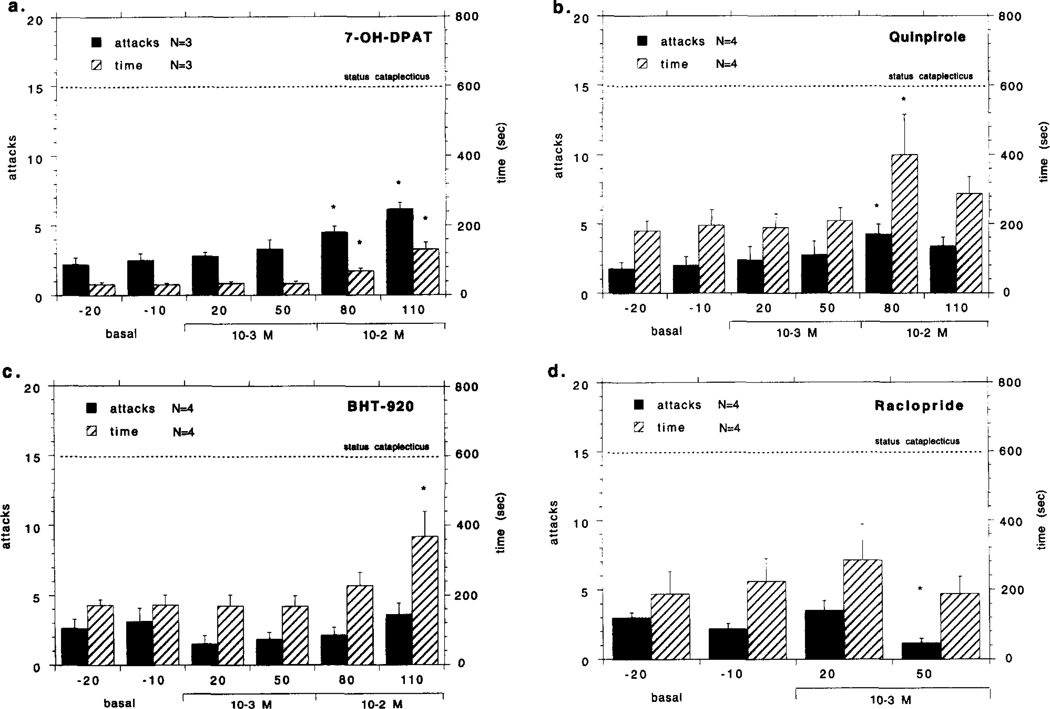
The effects of 7-OH-DPAT, quinpirole, BHT-920 and raclopride perfusion in the lateral GP/putamen on cataplexy in the narcoleptic canines are shown in Fig. 1a–d. Bilateral quinpirole or 7-OH-DPAT perfusion (both 10−3 − 10−2 M) in the lateral GP/putamen produced a moderate increase in FECT induced cataplexy that was significant at the highest dose, evident in both the number of attacks and elapsed FECT time. Bilateral perfusion with BHT-920 (10−3 − 10−2 M) in the lateral GP/putamen had no effect on the number of FECT induced cataplectic attacks; however, the highest concentration did cause an increase in elapsed FECT time (for F-values, see Fig. 1a–d legend). In general, complete cataplectic attacks became more prevalent and would last longer during quinpirole and 7-OH-DPAT perfusion, while the effects of BHT-920 were similar but weaker. There was no significant decrease in basal muscle tone as noted by the EMG during perfusion with any of the above drugs. During quinpirole, 7–0HDPAT and BHT-920 stimulated cataplectic attacks the EEG was desynchronized, the EMG was greatly reduced and the EOG signal was mostly quiet, similar to baseline cataplexy. Bilateral perfusion with raclopride (10−3 M) in the GP/putamen of narcoleptic canines produced a moderate reduction in the number of FECT induced cataplectic attacks; however, no change in elapsed FECT time was observed (for F-values, see Fig. 1a–d legend). All other drugs tested in the lateral GP/putamen did not have any significant effects on FECT induced cataplexy: SKF 38393 (n= 4), yohimbine (n = 4), prazosin (n= 4) and amphetamine (n= 4) (all drugs perfused bilaterally at 10−3 M) (see Table 2).
Fig. 1.
Effects of bilateral perfusion with various dopaminergic compounds in the GP/putamen (GP: globus pallidus) on cataplexy in narcoleptic canines. Local perfusion 7-OH-DPAT (10 M) (a), quinpirole (10−4–10−2 M) BHT-920 (10−4–10−2 M) (c) and raclopride M) in the narcoleptic canines is shown. Drugs were mixed into artificial cerebrospinal fluid and perfused through microdialysis probes at the indicated concentrations over the course of a 4-h experiment: none during the first hour,10−4 M during the second hour, 10−3 M during the third hour and 10−2 M during the fourth hour. Each drug was tested with four narcoleptic canines, though the same animals were not always used. The mean number of cataplectic attacks and elapsed time for two food-elicited cataplexy tests (FECT) per test period is shown. For the purpose of figure presentation, status cataplecticus (complete muscle atonia) was arbitrarily designated as 15 attacks elapsed over 600 s. Each drug perfusion time point was compared with the basal time points using a Fisher PLSD post-hoc test; * indicates P < 0.05 satisfactory for comparison with either basal time point. Univariate analyses of number of cataplectic attacks over the tested dose range for each drug were; 7-OH-DPAT:F4,20 = 9.851, P=0.001 Quinpirole: F4,28=3.244, P = 0.048; BHT-920: F4,28 = 1.322, P = 0.279; Raciopride: F2,14=4.235, P = 0.028. Univariate analyses of elapsed FECT time over the tested dose range for each drug were; 7-OH-DPAT: F 4,20= 19.366, P = Quinpirole: F4,28=3.975, P = 0.026; BHT-920: F4,28=4.257, P = 0.006; Raciopride: F2,14=1.112, P = 0.655.
Two control canines were studied in the lateral GP/putamen, and none of the drugs which increased cataplexy in the lateral GP/putamen of narcoleptic canines (7-OH-DPAT, quinpirole and BHT-920, all bilaterally perfused at 10−3 − 10−2 M) had any effect on FECT scores in the control animals, as no cataplectic attacks were observed (all scores were zero) and elapsed FECT time did not change (F4.12 = 1.566, P = .489) (data not shown). Furthermore there was no noticeable effect on muscle tone, as detected by behavioral observation and EMG analysis, produced by any of these monoaminergic agonists.
3.3. Local drug perfusion in the ventral tegmental area
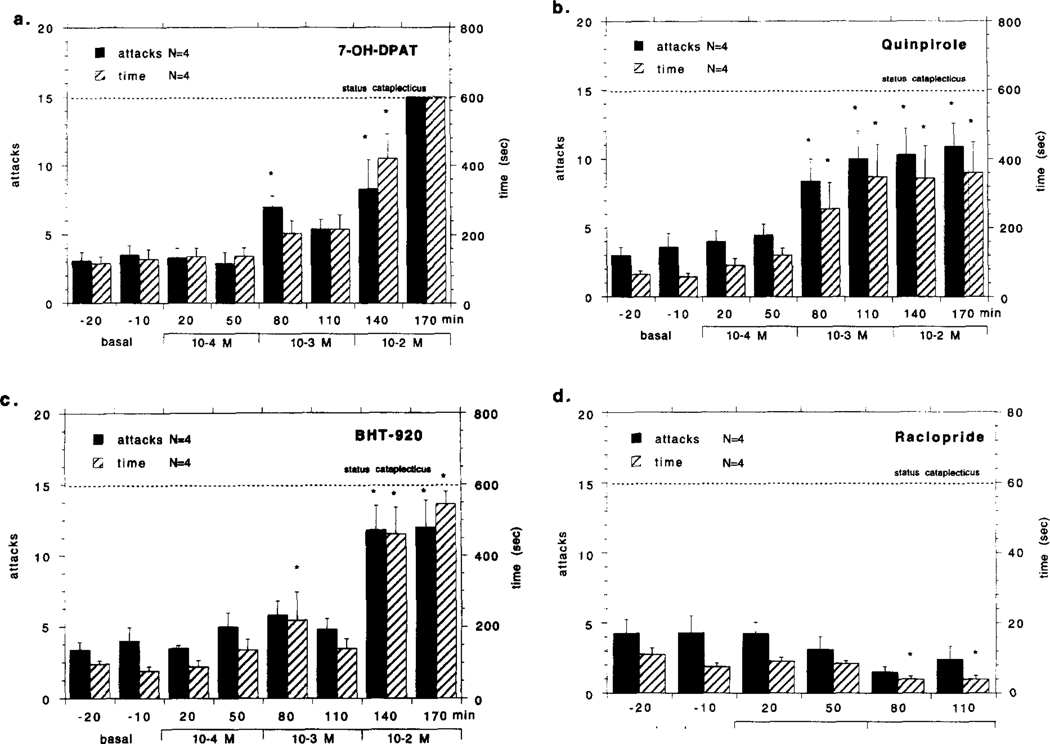
The effects of bilateral perfusion with 7-OH-DPAT, quinpirole, BHT-920 and raclopride (all at 10−4−10−2 M) in the VTA on cataplexy in the narcoleptic canines are shown in Fig. 2a–d. All three monoaminergic agonists (7-OH-DPAT, quinpirole, BHT-920) produced a dose-dependent increase in cataplexy that was evident in number of cataplectic attacks and elapsed time (for F-values, see Fig. 2a–d legend). During agonist perfusion complete cataplectic attacks became more prevalent and would last longer than during the baseline period, though there was no significant decrease in basal muscle tone during the time between FECT’s as noted by the EMG. During 7-OH-DPAT perfusion complete muscle atonia (status cataplecticus) was observed in all four animals at the highest concentration (10−2 M) (Fig. 2a). During quinpirole perfusion status cataplecticus was observed in two of the four animals tested (Fig. 2b). During BHT-920 perfusion status cataplecticus was observed in 3 of the 4 animals tested (Fig. 2c). Bilateral perfusion with raclopride (10−3- 10−2 M) in the VTA of narcoleptic canines did not significantly reduce the number of FECT induced cataplectic attacks; however, a significant decrease in elapsed FECT time was observed at the highest dose (for F-values, see Fig. 2a–d legend). All other monoaminergic drugs tested in the VTA did not have any significant effects on FECT induced cataplexy: SKF 38393 (n = 4), yohimbine (n = 4), amphetamine (n=4) and prazosin (n = 4) (all drugs perfused bilaterally at 10−3 M) (see Table 2).
Fig. 2.
Effects of bilateral perfusion with various dopaminergic compounds in the ventral tegmental area (VTA) on cataplexy in narcoleptic canines. Local perfusion 7-OH-DPAT (10−4–10−2 M) (a), quinpirole (10−4–10−2 M) (b), BHT-920 (10−4–10−2 M) (c) and raclopride (10−3 M) in the narcoleptic canines is shown. The mean number of cataplectic attacks and elapsed time for two food-elicited cataplexy tests (FECT) per test period is shown. For the purpose of figure presentation, status cataplecticus (complete muscle atonia) was arbitrarily designated as 15 attacks elapsed over 600 s, thus, for these levels of cataplexy there was no statistical variability in the score. The same four narcoleptic canines were tested for each drug. Each drug perfusion time point before status cataplecticus was compared with the basal time points using a Fisher PLSD post-hoc test; * indicates P < 0.05 satisfactory for comparison with either basal time point. Univariate analyses of number of cataplectic attacks over the tested dose range for each drug were; 7-OH-DPAT: F5,35 = 11 .923, P = 0.001; Quinpirole: 5.378, P = 0.001 ; BHT-920: 8.146, P = 0.001 ; Raciopride: F4.28 = 1.264, P = 0.303. Univariate analyses of elapsed FECT time over the tested dose range for each drug were: 7-OH-DPAT: F5.35 = 11.511, P = 0.001; Quinpirole: F6.42 = 4.226, P = 0.002; BHT-920: F6.42 = 11.715, P = 0.001 ; Raciopride: F4.28 = 2.826, P = 0.039.
The control and heterozygous canines all responded similarly to 7-OH-DPAT, quinpirole and BHT-920 perfusion in the VTA, and are therefore presented together as the control group. None of these monoaminergic agonists produced cataplexy in the control group. At all concentrations tested there were no cataplectic attacks observed (all scores were zero) and elapsed FECT time did not change (7-OH-DPAT: = 0.435, 0.851; Quinpirole: F6,42 = 1.194, P = 0.327; BHT 920: 1.365, P- 0.270) (data not shown). Furthermore, there was no noticeable effect on basal muscle tone produced by any of these monoaminergic agonists, as detected by behavioral observation and EMG analysis.
The EEG, EOG and EMG polygraph recordings taken from narcoleptic canines during quinpirole (10 M) or 7-OH-DPAT (10−3 M) stimulated cataplexy (VTA perfusion) are shown in Fig. 3a–d. Spectral analysis comparing EEG frequency during cataplexy between baseline and quinpirole M) (Fig. 3e,f) or baseline and 7–01-1DPAT (10−3 M) (Fig. 3g,h) (different animal for each drug) is also shown. During quinpirole and 7-OH-DPAT perfusion, complete cataplectic attacks became more prevalent and would last longer. As with basal cataplexy (Fig. 3a,c), the cataplectic attacks were associated with a desynchronized EEG and a decrease in EMG activity at the onset of the attack; however, visual analysis of the polygraph recordings revealed that with long lasting attacks ( > 15 s) SI frequently occurred and was evident during 10−3 M and 10−2 M drug perfusion (see Fig. 3b,d). This was observed in three out of four animals tested with either 7-OH-DPAT (10−3 M) or quinpirole (10−3 M). In one animal, these “sleep attacks” even occurred spontaneously (without FECT stimulation) with either drug. However, spectral analysis comparing baseline and drug stimulated periods of cataplexy (averaging all 10-s epochs which were free of artifact for each condition) did not reveal a significant effect on spectra of drug treatment in any of the animals showing this tendency, for either quinpirole (e,f) or 7-OH-DPAT (g,h). The recordings in Fig. 3a–d resemble typical baseline and drug induced cataplectic attacks and were chosen for representation primarily because these animals had the best baseline cataplexy recordings (long and clearly defined), good spectral power from the EEG and were clean of noise and recording artifact. All recordings and spectral analysis of quinpirole (a,c,e,f) or 7-OH-DPAT (b,d,g,h) were made on the same day and from the same animal, respectively.
3.4. Dopamine levels’ in the lateral globus pallidus / putamen and ventral tegmental area during cataplexy
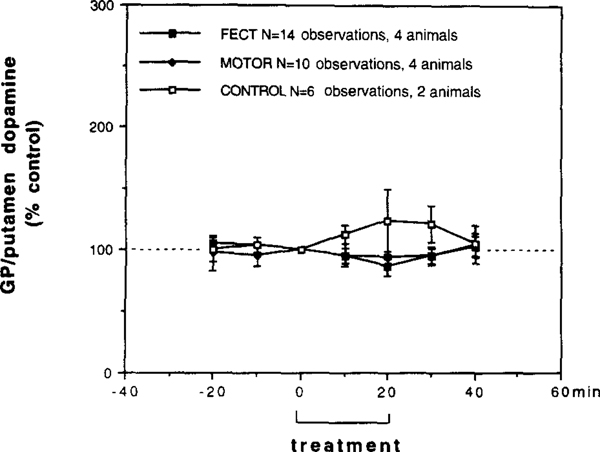
From the four narcoleptic canines studied in the lateral GP/putamen, perfusion samples were collected from a total of eight bilateral in vivo microdialysis sessions (4 animals tested on 2 consecutive days, 16 total observations). From the two control canines studied in the lateral GP/putamen (no heterozygous canines were used in this study) perfusion samples were collected from a total of four bilateral in vivo microdialysis sessions (2 animals tested on 2 consecutive days, 8 total observations). Dopamine and DOPAC levels in both narcoleptic and control animals were essentially stable after 60 min of perfusion and baseline levels were obtained from the 80–90-min sample (the last 10-min perfusion period prior to FECT stimulation). Baseline dopamine levels in the narcoleptic and control lateral GP/putamen (narcoleptic: 0.076 ± 0.010 pmol/10 min, n = 16; control: 0.053 ± 0.019 pmol/10 min, n = 8) were not siginicantly different (t(22) = 1.135, P = 0.276). Baseline levels of DOPAC in the narcoleptic and control lateral GP/putamen (narcoleptic: 6.4 ± 3.1 pmol/10 min, n = 16; control: 9.8 ± 3.4 pmol/10 min, n=8) were also not significantly different (t(22) 1.672, P = 0.212). Because of the lack of any effects on lateral GP/putamen DOPAC levels seen in our study, we have focused on the dopamine data and therefore do not show any DOPAC figures. The effects of FECT stimulation in narcoleptic and control canines and locomotor stimulation without cataplexy in narcoleptic canines on lateral GP/putamen dopamine levels are shown in Fig. 4. During FECT induced cataplexy in narcoleptics dopamine levels were not significantly effected (F6,78 = 1.196,P = 0.316), though a trend towards a decrease was observed. During FECT stimulation in controls dopamine levels were also not significantly effected (F6,30 = 0.474, P = 0.809), though a trend towards an increase was observed. A repeated measures comparison between narcoleptics and controls, however, did not reveal a significant difference in FECT stimulated dopamine levels (F6,108 = 0.129, P= 0.988). During locomotor stimulation with cataplexy in narcoleptics, the levels of dopamine were unchanged (F6,54 = 0.329, P = 0.919).
Fig. 4.
Behavioral tests on the extracellular levels of dopamine in the lateral GP/putamen (GP: globus pallidus) of narcoleptic (n=4) and control canines (n=2) measured by in vivo microdialysis. FECT treav ment represents data from seven bilateral microdialysis sessions in four narcoleptic canines (each animal tested 2 times = 14 total obervations), MOTOR treatment represents data from five bilateral microdialysis sessions in four narcoleptic canines (three animals tested I time, one animal tested 2 times =10 total observations) and CONTROL treatment represents data from three bilateral microdialysis sessions in two control canines (one animal tested onc time, one animal tested 2 times =6 total observations). During FECT treatment each animal performed two FECT trials per min, during MOTOR treatment each animal performed two MOTOR activity trails without having cataplexy per 10 min and during CONTROL treatment each animal performed two FECT trials per 10 min (no cataplexy occurred). Within each group the treatment and post-treatment time points were compared with pretreatment time points (Fishers PLSD post-hoc test). No significant differences were found.
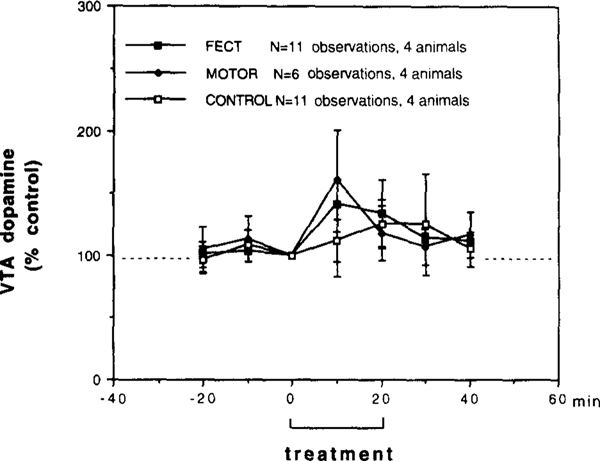
From the four narcoleptic canines studied in the VTA, perfusion samples were collected from a total of six bilateral in vivo microdialysis sessions (two animals tested on 2 consecutive days, two animals tested on I day, twelve total observations). From the four control canines studied in the VTA, perfusion samples were obtained from six separate bilateral in vivo microdialysis sessions (two animals tested on 2 consecutive days, two animals tested on I day, twelve total observations). Dopamine and DOPAC levels in both narcoleptic and control animals were essentially stable after 60 min of perfusion and baseline levels were obtained from the 80–90-min sample (the last 10-min perfusion period prior to FECT stimulation). Baseline dopamine levels in the narcoleptic and control VTA (narcoleptic: 0.052 ± 0.015 pmol/10 min, n = 12; control: 0.046 ± 0.017 pmol/l0 min, n = 12) were not sigificantly different (t(22) = 0.317, P = 0.757). Baseline levels of DOPAC in the narcoleptic and control lateral VTA (narcoleptic: 0.564 ± 0.102 pmol/l0 min, n = 12; control: 0.448 ± 0.201 pmol/10 min, n = 12) were also not significantly different (t(22) = 0.502, P = 0.678). Because of the lack of any effects on lateral VTA DOPAC levels seen in our study, we have focused on the dopamine data and therefore do not show any DOPAC figures. The effects of FECT stimulation in narcoleptic and control canines and locomotor stimulation without cataplexy in narcoleptic canines on lateral VTA dopamine levels are shown in Fig. 5. During FECT induced cataplexy in narcoleptics dopamine levels increased approximately 50%; however, because of considerable variation between animals this effect was not significant (F6,60 = 1.920, P = 0.09). During FECT stimulation in controls dopamine levels increased approximately 25%; however, this effect was also not significant (F6,60 = 0.911 P =0.493). A repeated measures comparison between narcoleptics and controls did not reveal a significant difference in FECT stimulated dopamine levels (F6.120 = 0.846, P = 0.539). During locomotor stimulation without cataplexy in narcoleptics dopamine levels showed a similar increase which, again, was not statistically significant due to variability (F6,30 = 1.449, P = 0.227).
Fig. 5.
Behavioral tests on the extracellular levels of dopamine in the ventral tegmental area (VTA) of narcoleptic (n = 4) and control canine group (3 controls, 1 heterozygous) (n = 4) measured by in vivo microdialysis. FECT treatment represents data from six bilateral microdialysis sessions in four narcoleptic canines (two animals tested 2 times, two animals tested 1 time = 11 total observations (one observation run lost due to sample contamination)). MOTOR treatment represents data from four bilateral microdialysis sessions in four narcoleptic canines (each animal tested 1 time = 6 total observations (2 observation runs lost due to sample contamination)). CONTROL treatment represents data from six bilateral microdialysis sessions in four control canines (two animals tested 2 times, two animals tested 1 time = 11 total observations (one observation run lost due to sample contamination)). During FECT treatment each animal performed two FECT trials per 10 min, during MOTOR treatment each animal performed two MOTOR activity trails without having cataplexy per 10 min and during CONTROL treatment each animal performed two FECT trials per 10 min (no cataplexy occurred). Within each group the treatment and post-treatment time points were compared with pretreatment time points (Fishers PLSD post-hoc test). No significant differences were found.
3.5. Histology
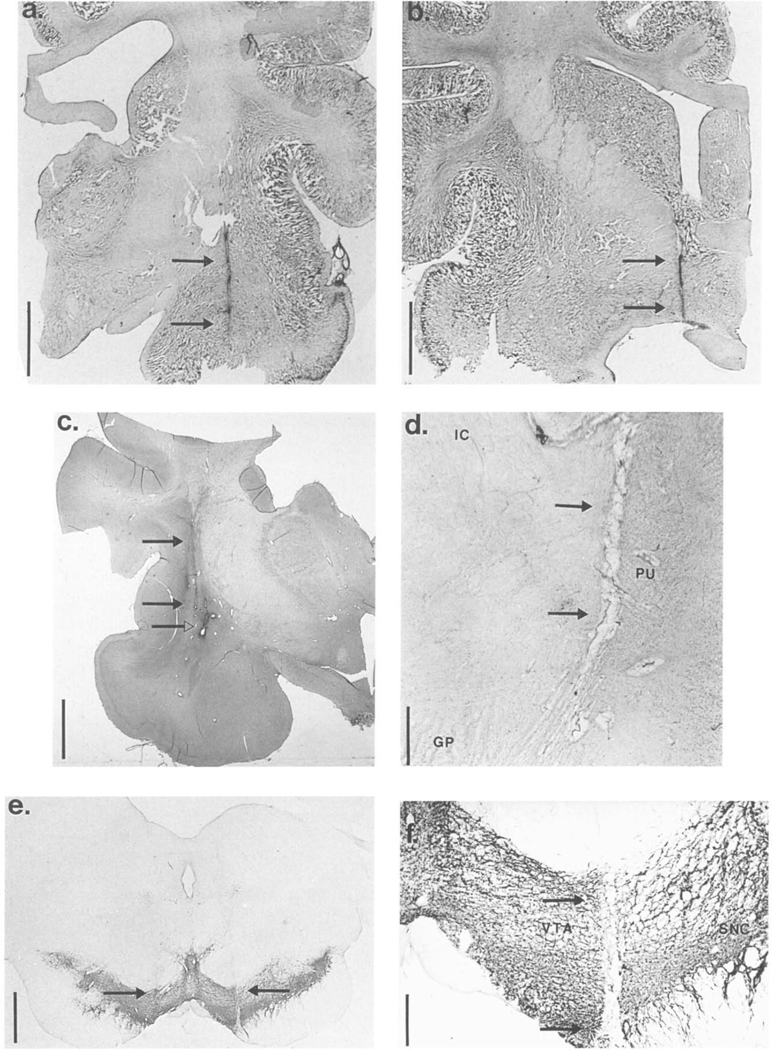
Histological analysis of the microdialysis probe locations in the amygdala, lateral GP/putamen, BF, PRF and VTA of the narcoleptic and control canines showed that all animals were properly cannulated as the injection tracts were accurately implanted into each brain region of interest. Bilateral implantation was well oriented across the rostrocaudal plane (both tracts within 0.5–1.0 mm of the same coronal plane). In addition, the laterality of the tracts within each brain region were approximately the same on each side (both tracts were within 0.5–1.0 mm of the same position along the medio-lateral axis). Microphotographs showing typical probe locations in the amygdala, BF, lateral GP/putamen and VTA are shown in Fig. 6a–f. Each photomicrograph shows a tract(s) made by lowering an injection cannula (same gauge as the probes) into the brain at the same coordinates as the microdialysis probes. The coronal section with the ventral most tip of the tract is shown and the tracts are indicated by arrows. Analysis of probe tracts in the PRF can be seen in previous papers [61,63] showing microdialysis probe locations in the PRF at, or just ventromedial of, the LC. Probe locations in the amygdala were near to the central nucleus of the amygdaloid complex (Fig. 6a). Probe locations in the BF were in the magnocellular regions of the BF near to the lateral preoptic region and the diagonal band of Broca (Fig. 6b) (see also [60]). Probe locations in the lateral GP/putamen were within the putamen, adjacent to the claustrum and extended down into the dorsal regions of the lateral GP (Fig. 6c,d). The lateral GP/putamen location was always 2 mm rostral and 5 mm dorsal of the amygdala location and thus, can also be estimated from the photomicrograph of the amygdala probe tract (Fig. 6a). Probe locations in the VTA were placed in the central portion of the VTA, near the substantia nigra pars compacta, and terminated within the dopamine cell body region (Fig. 6e,f). The sections in Fig. 6e,f were stained for TH-IR. In Fig. 6e tracts in both hemispheres penetrating into the TH-IR cell bodies of the VTA are evident, In Fig. 6f a clean tract extending through the entire dorso-ventral dimension of the. VTA can be seen, demonstrating that implantation of the VTA caused no damage to the dopamine cell bodies along the cannula and that the 5 mm dialyzing membrane of the microdialysis probe was almost entirely within the area of the dopamine cell bodies.
Fig. 6.
Histological analysis of microdialysis probe locations in the narcoleptic and control canines. Microphotographs showing typical probe locations in the (a) amygdala, (b) basal forebrain, (c,d) lateral globus pallidus/putamen (e,f) ventral tegmental area (VTA) are shown. Each photomicrograph in (a-d,f) shows a tract in one hemisphere made by lowering an injection cannula (same gauge as the microdialysis probes) into the brain at the same coordinates as the microdialsyis probes (done before sacrificing each animal). The photomicrograph in (e) shows tracts in both hemispheres. The coronal section with the ventral most tip of the tract is shown and the tracts are indicated by arrows. The injection tract shown in the amygdala is located within the central nucleus of the amygdaloid complex (a). The injection tract in the basal forebrain is located in the magnocellular regions of the BF near to the lateral preoptic region and the diagonal band of Broca (b). The injection tracts in the lateral globus pallidus/putamen were within the putamen, adjacent to the claustrum, and extended partially down to the lateral GP (abbreviations: GP-globus pallidus, IC-internal capsule, PI-Lputamen) (c,d). In (c) the arrows with filled triangles indicate a tract from a previously implanted microdialysis probe while the arrow with an open triangle shows the site of injection of 1.0 μl 2% cresyl violet made by the injection cannula used for the histological verification procedure. (c) and (d) were taken from different animals, narcoleptic and control, respectively. The injection tracts in the VTA were within the central portion of the VTA, just medial of the substantia nigra pars compacta (abbreviations: SNC-substantia nigra pars compacta, VTA-ventral tegmental area) (e,f). The sections in (e,f) were stained for tyrosine hydroxylase-immunoreactivity (TH-IR); (e) and (f) were taken from the same animal. The vertical bars in (a–c,e) indicate 5 mm and the vertical bars in (d,f) indicate 1 mm.
4. Discussion
In the present study, local perfusion with D2 and D3 dopaminergic agonists in the VT A and lateral GP/ putamen produced a robust increase in cataplexy in narcoleptic canines. In addition, local perfusion with a D2 dopaminergic antagonist in the same regions produced a mild decrease in cataplexy in narcoleptic canines. All other brain regions studied, including the PRF, amygdala and BF, did not mediate any such dopaminergic effects. Local perfusion with selective adrenergic drugs known to modulate cataplexy in narcoleptic canines when given peripherally, prazosin and yohimbine, failed to produce any change in cataplexy when perfused in any of the brain regions studied: PRF, VTA, amygdala, BF and lateral GP/putamen. Furthermore, the mixed α-2/D2 dopamine agonist BHT 920 was unable to modulate cataplexy when perfused in the PRF, near to the LC. These findings are the first to show specific brain regions where dopaminergic drugs may effectively modulate cataplexy in narcoleptic canines, and are indicative of a neuronal population which mediates the cataplexy modulatory effects of peripherally administered dopaminergic drugs. The inability of any of the locally perfused adrenergic drugs to modify cataplexy, however, leaves the question about the site of action for peripherally administered adrenergic drugs unresolved.
These results indicate that localized pharmacological modulation of central dopaminergic neurotransmission has strong effects on cataplexy, a pathological equivalent of REM sleep atonia. This was especially obvious after dopamine autoreceptor modulation in the VTA where quinpirole, BHT-920 or 7-OH-DPAT stimulation dramatically exacerbated cataplexy, an effect also associated with indications of behavioral sedation. A similar but quantitatively much weaker effect could also be observed after dopaminergic stimulation in the lateral GP/putamen area. 7-OH-DPAT is a recently developed D3 receptor agonists which has high potency at both the D3 and D2 receptors (D3 affinity nearly 100 × greater than D2 atTinity) [35] Quinpirole has potent activity at the D2 receptor, and recent studies characterizing the D3 receptor have demonstrated significant affinity also at this site (nearly X greater than D2 affinity) [19,35,70,71]. BHT-920, originally developed as a dopamine autoreceptor agonist, is active at both D2 receptors and α-2 adrenergic receptors [3,24,55]. Therefore, in both the VTA and the lateral GP/putamen, the effects are likely to be mediated via D2 or D3 but not DI receptors, as quinpirole, BHT-920 and 7-OH-DPAT were very active whereas SKF 38393 (DI agonist) was inactive in modulating cataplexy. These results are consistent with previously published data obtained on cataplexy after systemic administration of dopaminergic compounds [56]. In this study, D2 antagonists and very small doses (pre-emetic) of dopamine autoreceptor D2/D3 receptor agonists, but not DI agonists, had very potent effects on cataplexy [56]. Interestingly, D3 receptors have been suggested to act as autoreceptors [35,71], possibly mediating dopamine synthesis inhibition [40]. Furthermore, BHT-920 applied locally onto VTA neurons produces a potent reduction in neuronal firing relative to its dopamine receptor affinity [66]. Thus, it may be suggested that autoreceptor mediated inhibition of dopaminergic VTA projection neurons produces cataplexy in narcoleptic canines.
Dopaminergic D2 and D3 receptor specific compounds have biphasic effects on locomotor activity, a phenomenon that presumably involves dopaminergic autoreceptor and postsynaptic receptors at low and high doses, respectively [12,14,16,23]. Similar results have been generated regarding the effects of dopaminergic compounds on sleep, though the data is more fragmentary. Low doses of D2 and D3 dopaminergic agonists have been shown to enhance sleep (both REM and slow wave sleep) in various animal species whereas opposite effects are obtained at high doses, the converse being true with dopamine antagonists [16,23,33,51,52,76,77]. The sedative effects of dopaminergic agonism have been suggested to reflect activity at dopamine autoreceptors [33]. Most stimulant compounds used in the treatment of narcolepsy have presynaptic effects on dopamine uptake or release which are likely critical for their ability to reduce excessive daytime sleepiness [44,50,60]. Consistent with this, experimental treatments for narcolepsy aimed at upregulating the level of endogenous dopamine neurotransmission, such as treatment with L-DOPA or I–tyrosine, have also been shown to reduce the drowsiness symptoms of narcolepsy [9,65].
Whereas there is little doubt that dopaminergic drugs may effect sleep, the role of central dopaminergic systems in the regulation of natural sleep has been debated for many years. Lesions of the dopamine containing perikarya of the VTA and substantia nigra have been shown to produce a state of behavioral unresponsiveness and immobility in cats which is not associated with a significant decrease in wakefulness and desynchronyzed cortical EEG activity [29]. This, together with the fact that the firing rate of dopaminergic neurons in the VTA and substantia nigra does not change across the sleep cycle [45,72,74,75], suggests that dopaminergic systems might be involved in reactive behavioral arousal rather than natural sleep regulation. Nevertheless, others have found that bilateral injections of D2 and autoreceptor dopamine agonists in the VTA, but not the substantia nigra, nucleus accumbens, prefrontal cortex or caudate nucleus, evoked a behavioral and electrocortical equivalent of sleep [4,5]. Though this study failed to analyzed possible differential effects on REM sleep versus slow wave sleep, it was noted that intraVTA injections produced a form of sleep which was similar to that following peripheral injections of the same dopaminergic drugs. These studies are consistent with our observation of an increase in sleep episodes during quinpirole and 7-OH-DPAT induced cataplexy in VTA perfused narcoleptic canines. However, though a trend suggesting increased sleep onset was observed by objective scoring of the polygraphs, spectral analysis of the EEG recordings during drug induced cataplexy did not reveal a significant increase in sleep related spectral power. It must be recognized that this study did not directly investigate the effects of local dopaminergic drugs on sleep in narcoleptic canines (due to the necessity to perform multiple cataplexy tests and to collect perfusate samples, the animals were disturbed frequently). Future studies on this question need to be performed. Nevertheless, our findings indicate that mesolimbic dopamine neurons might be involved in the pathophysiology of both increased somnolence and cataplexy in narcolepsy.
The enhancement of cataplexy observed after perfusion with dopaminergic agonists in the VTA suggest that changes in mesolimbic dopamine neuronal activity may have critical impact on the regulation of REM sleep. This result agrees well with the fact that dopamine autoreceptor and D2 compounds have been shown to modulate not only slow wave sleep but also REM sleep [51,76,77]. Thus, it has been reported that low doses of D2 agonists and antagonists enhance and inhibit REM sleep, respectively, whereas high doses have the opposite effect [51,52,77]. However, others have suggested that the dopaminergic system does not have a regulatory effect on REM sleep [54]. Interestingly, REM sleep deprivation has been shown to reduce the number of D2 dopamine receptors relative to DI receptors (increase in DI/D2 ratio) in the rat caudate [22], whereas narcoleptic canines and humans (‘REM enhanced’) have been shown to have increased numbers of D’2 dopamine receptors in the same brain structure.
Our findings that autoreceptor stimulation in the VTA enhanced cataplexy, which likely coincides with decreased dopamine neurotransmission in the nucleus accumbens and other mesolimbic structures, do not fit well with previous studies indicating that the monoaminergic regulation of cataplexy is primarily mediated via adrenergic systems [41,43,55,57]. Stimulant compounds with preferential impact on dopaminergic systems, such as dopamine uptake blockers, have absolutely no effect on cataplexy [43] and relatively little effect on REM sleep [54] but dramatically reduce slow wave sleep [60]. Likewise, in the present study locally perfused amphetamine had no effects on cataplexy in any of the brain regions studied. This discrepency might suggest that dopaminergic uptake inhibitors preferentially effect neuron projections that are involved in the control of slow wave sleep, whereas REM sleep and cataplexy might be regulated by a different population of dopamine neurons. This could be due to differential anatomical distribution of uptake site or autoreceptor proteins in dopaminergic neurons (VTA, substantia nigra) or terminal structures (limbic cortex, caudate, putamen, nucleus accumbens) [11,48]. Though we did not study all of the potential mesolimbic and extrapyramidal target structures that could be involved, our finding that dopaminergic drug infusion into the lateral GP/putamen area, but not the amygdala or BF, could exacerbate cataplexy is consistent with the suggestion that select dopamine pathways are involved in the regulation of cataplexy.
A surprising result of this study was the finding that dopaminergic stimulation in the lateral GP/putamen area also modulated cataplexy, even if the effect was much weaker than after perfusion in the VTA. This finding was made rather serendipidously, as the first observation was made when attempting to study the amygdala using the rostral amygdala guide cannulae and more dorsally oriented implantation coordinates (which actually hit the lateral GP/putamen as described in the Materials and Methods section). The finding that the lateral GP/putamen area could mediate dopaminergic stimulation of cataplexy might suggest the involvement of the nigrostriatal system in the regulation of REM sleep and narcolepsy, as proposed by Mamelak [38]. Previous work has indicated that D2 receptor binding is enhanced in the putamen of postmortem narcoleptic brains [32] which might account for the sensitivity of dopaminergic drugs in this area to modulate cataplexy. In contrast, no effects were observed after perfusion of the same compounds in the BF or amygdala, the latter which has been shown to have increased D2 receptor binding in narcoleptic canines [10]. While the BF is anatomically located very close to the nucleus accumbens, the main site of projection of the dopaminergic mesolimbic system, our microdialysis probes were not actually targeted at this structure (the BF in these animals was also studied for cholinergic effects [59]. Thus, it will be necessary to study dopaminergic compounds injected in the substantia nigra pars compacta, caudate and the core of the nucleus accumbens in order to more thoroughly assess the respective importance of the nigrostriatal and mesolimbic systems in the regulation of cataplexy and abnormal REM sleep in narcolepsy. Indeed, consistent findings of increased D2 receptor binding in the caudate [2,10,32,37] make it imperative that this site be studied. Alternatively, the proximity of the substantia nigra pars compacta to our probes in the VTA suggests the possibility that cataplexy could be elicited by simultaneous modulation of both nigrostriatal and mesolimbic dopamine projections.
Dopamine release in both the VTA and lateral GP/putamen areas was also analyzed and it was found that there were no significant differences between control and narcoleptic canines during FECT stimulation and cataplexy. No significant effects on the dopamine metabolite DOPAC were seen in each region either. The mild increase in dopamine levels seen in the VTA seemed to be better correlated with motor activity than with cataplexy. However, while an increase in dendritic dopamine release in the VTA might indicate that dopamine autoreceptors could be stimulated during cataplexy (consistent with our local drug perfusion data) it should be noted that the regulation of dendritic dopamine release is poorly understood. There was a small, but insignificant, decrease in dopamine release in the lateral GP/putamen during FECT induced cataplexy in the narcoleptic canines. In contrast, the control animals showed a small, but insignificant, increase in dopamine release during FECT stimulation, though this reflects data from only two animals and as such should be considered exploratory. While we did not find statistical evidence for a difference between narcoleptic and control canines the trends in these findings might suggest a reduction in the firing of nigrostriatal dopamine neurons, which terminate in the putamen, during cataplexy. This would be consistent with the suggestion that nigrostriatal dopamine neurons may also be involved in the regulation of cataplexy.
Locally perfused adrenergic compounds in the narcoleptic canines were unable to affect cataplexy in any of the brain regions investigated in this study. This was somewhat discouraging considering the great amount of pharmacological data showing that adrenergic drugs are potent modulators of canine narcolepsy [41–43,55,57]. Furthermore, the reported changes in tissue levels of noradrenaline in the preoptic hypothalamus (near the BF), amygdala and nucleus pontis oralis of the PRF [15,39], as well as the increase in α-2 receptor binding in the LC [17] and putamen of narcoleptics [2], would suggest that a localized modulation of the adrenergic system, particularly in the LC, would be able to modulate cataplexy. However, it is possible that our probes were not able to adequately stimulate the LC, since it is such a small structure relative to the microdialysis probes and in some animals was just above the dorsal edge of the dailyzing membrane (see [61] for detailed illustration). The same argument could also be made for the BF and lateral GP/putamen probes, which were not in exactly the same brain region that showed an increase in noradrenaline levels (preoptic hypothalamus) [15] and α-2 receptor binding [2]. Alternatively, it is possible that adrenergic drugs must act in concert at several neuronal sites in order to produce any significant modulation of cataplexy. Nevertheless, the site of action of adrenergic drugs, and the possibility that cataplexy in canine narcolepsy is regulated by a select noradrenergic nucleus or pathway, remains unresolved.
Our findings have significance for the understanding of the neuroanatomical pathophysiology of narcolepsy. Previous experiments using the narcoleptic canine model have shown that narcoleptic animals are hypersensitive to cholinergic stimulation in the PRF [61,63] and the BF [59] and that acetylcholine release in the PRF is enhanced during cataplexy [62]. These findings suggest an abnormal cholinoceptive-cholinergic interaction in narcolepsy which we hypothesized to be central to the occurrence of abnormal REM sleep and cataplexy in narcolepsy [59]. In the present study, we have similar evidence indicating that narcoleptic animals are hypersensitive to dopaminergic autoreceptor stimulation in the VTA. In this context, the suggestion that inhibition of mesolimbic dopamine neurons could also mediate cataplexy opens up the possibility of abnormal dopaminergic-cholinergic interactions in the pathology of narcolepsy. Thus, the activity of cholinergic systems could be upregulated while the activity of dopaminergic neurons could be downregulated. Consistent with such an hypothesis, neuroanatomical connections between the VTA and pontine cholinergic systems and between the globus pallidus and both the midbrain dopamine and pontine cholinergic systems have been demonstrated [18,53,67,73,78]. The present results might be critical in explaining the existence of excessive daytime sleepiness in narcolepsy since the mesolimbic dopamine system is critical to the alerting effects of stimulant compounds [50]. Additionally, our finding might also indicate the neuronal system which integrates the ability of emotions to trigger cataplexy. Indeed, mesolimbic dopamine arising from the VTA has long been suggested to mediate motivation and reward (see [47,78]) and a recent study has shown a selective increase in the firing of midbrain dopaminergic neurons upon the presentation of appetitive stimuli to monkeys [47], the most potent trigger of cataplexy in our narcoleptic animals. Further studies on the pharmacological interaction of dopaminergic and cholinergic drugs, investigating how they modulate sleep, cataplexy and neurotransmitter release in cataplexy relevant areas of the narcoleptic canine brain, are needed to address these questions and are presently underway.
Acknowledgements
We would like to thank Ms. Molly Hazelhorst for her clerical assistance. Research supported by grants from the National Institute of Neurological Disorders and Stroke at NIH to E. Mignot, W.C. Dement and J. Siegel: NS27710, NS23724 and NS14610.
Abbreviations:
- BF
basal forebrain
- CSF
cerebrospinal fluid
- DOPAC
dihydroxyphenylacetic acid
- DS
deep sleep
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- FECT
food-elicited cataplexy test
- GP
globus pallidus
- LC
locus coerulcus
- LS
light sleep
- PBS
phosphate buffer saline
- PRF
pontine reticular formation
- REM
rapid eye movement
- SI
sleep onset
- TH-IR
tyrosine hydroxylase-immunoreactivily
- VTA
ventral tegmental area
References
- [1].Aldrich MS, The neurobiology of narcolepsy-cataplexy, Prog. Neurobiol, 41 (1993) 533–541. [DOI] [PubMed] [Google Scholar]
- [2].Aldrich MS, Hollingsworth Z. and Penney JB, Autoradiographic studies of postmortem narcoleptic brain, Neurophysiol. Clin, 23 (1993) 35–45. [DOI] [PubMed] [Google Scholar]
- [3].Anden NE, Nilsson H, Ros E. and Thornstrom U, Effects of BHT-920 and BHT-933 on dopamine and noradrenaline receptors in the rat brain, Acta Pharmacol. Toxicol, 52 (1983) 51–56. [DOI] [PubMed] [Google Scholar]
- [4].Baggeta G, De Sarro GB, Priolo E. and Nistico G, Ventral tegmental area: site through Wich dopamine D2-receptor agonists evoke behavioural and electrocortical sleep in rats, Br. J, Pharmacol, 95 (1988) 860–866,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baggeta G, De Sarro GB, Priolo E, Marra R. and Nistico G, Inhibition by pertusis toxin of the soporific effects induced by stimulation of dopamine D2 autoreceptors in the ventral tegmental area in rats, Neuropharmacology, 28 (1989) 941–947. [DOI] [PubMed] [Google Scholar]
- [6].Baker TL and Dement WC, Canine narcolepsy-cataplexy syndrome: evidence for an inherited monoaminergic-cholinergic imbalance. In McGinty DJ, Drucker-Colfn R, Morrison A. and Parmengiani P. (Eds), Brain Mechanisms and Sleep, Raven, New York, 1985, pp. 199–233. [Google Scholar]
- [7].Barchas JD, Dement WC, Faull K. Foutz AS and Holman RB, The concentrations of amine metabolites in cerebrospinal fluid from normal and narcoleptic dogs, J. Physiol, 296 (1979) 94P–95P. [PubMed] [Google Scholar]
- [8].Boehme RE, Baker TL, Mefford LN, Barchas JD, Dement WC and Ciaranello RD, Narcolepsy: cholinergic receptor changes in an animal model, Life Sci., 34 (1984) 1825–1828. [DOI] [PubMed] [Google Scholar]
- [9].Boivin DB and Montplaisir J, The effects of L-DOPA on excessive daytime sleepiness in narcolepsy, Neurology, 41 (1991) 1267–1269. [DOI] [PubMed] [Google Scholar]
- [10].Bowersox SS, Kilduff T.s., Faull KF, Zeller-DeAmicis L, Dement WC and Ciaranello RD, Brain dopamine receptor levels elevated in canine narcolepsy, Brain Res., 402 (1987) 44–48. [DOI] [PubMed] [Google Scholar]
- [11].Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB and Levey AI, The dopamine transporter: immunochemical characterization and localization in brain, J. Neurosci, 15 (1995) 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Costall B, Lim SK and Naylor RJ, Characterization of the mechanisms by which purported dopamine agonists reduce spontaneous locomotor activity of mice, Eur. J. Pharmacol, 73 (1981) 175–188. [DOI] [PubMed] [Google Scholar]
- [13].Delashaw JB, Foutz AS, Guilleminault C. and Dement WC, Cholinergic mechanisms and cataplexy in dogs, Exp. Neurol, 66 (1979) 745–757. [DOI] [PubMed] [Google Scholar]
- [14].DiChiara G, Porceddu ML, Vargiu L, Argiolas A. and Gessa GL, Evidence for dopamine receptors mediating sedation in the mouse brain, Nature, 264 (1976) 564–567. [DOI] [PubMed] [Google Scholar]
- [15].Faull KF, Zeller-DeAminis L.c., Raddle L, Bowersox SS, Baker TL, Kilduff TS and Dement WC, Biogenic amines concentrations in the brains of normal narcoleptic canines: current status, Sleep, 9 (1986) 107–110. [DOI] [PubMed] [Google Scholar]
- [16].Ferrari F. and Giuliani D, Behavioral effects induced in rats and chicks by D2 dopamine agonist. Physiol- Behav., 54 (1993)695–700 [DOI] [PubMed] [Google Scholar]
- [17].Fruhstorter B, Mignot E, Bowersox S, Nishino S, I)emenl WC and Guilleminault C, Canine narcolepsy is associated with an elevated number of α-2 receptors in the locus coeruleus, Brain Res., 500 (1989) 209–214. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Rill E, The peduncuiopontine nucleus, Prog. Neurobiol, 36 (1991) 363–389. [DOI] [PubMed] [Google Scholar]
- [19].Gehlert DR, Gackenheimer SL, Seeman P. and Schaus J, Autoradiographic localization of [3 H]quinpirole binding to dopamine D2 and D3 receptors in rat brain, Eur. J. Pharmacol, 21 1 (1992) 189–194. [DOI] [PubMed] [Google Scholar]
- [20].Gouzoulis E, Steiger A, Ensslin M, Kovar A. and Hcrmmle L,Sleep EEG effects of 3,4-methylencdioxyethamphetamine (MDE: ‘eve’) in healthy volunteers, Biol. Psychiatry, 32 (1992) 1 108–1 1 17. [DOI] [PubMed] [Google Scholar]
- [21].Guilleminault C, Passuoant P. and Dement WC, Narcolepsy, Spectrum, New York, 1976. [Google Scholar]
- [22].Hamdi A, Brock J, Ross K. and Prasad C, Effects of rapid eye movement sleep deprivation on the properties of striatal dopaminergic system, Pharm. Biochem. Behau, 46 (1993) 863–866. [DOI] [PubMed] [Google Scholar]
- [23].Helton DR, Modlin DL and Williams PD, Behavioral characterization of the new potent nonselective dopamine agonist pergolide, Arzneimitttel-Forschung, 42 (1992) 885–890. [PubMed] [Google Scholar]
- [24].Hinzen D, Hornykiewics O, Kobinger, Pichler L, Pifi C. and Schingnitz G, The dopamine autoreceptor agonist BHT-920 stimulates denervated postsynaptic brain dopamine receptors in rodent and primate models of Parkinson’s Disease, Eur. J, Pharmacol, 131 (1986) 75–86. [DOI] [PubMed] [Google Scholar]
- [25].Hublin C, Launes J, Nikkinen P. and Partinen M, Dopamine D&receptors in human narcolepsy: a SPECT study with 1231–1BZM, Acta Neurol. Scand, 90 (1994a) 186–9. [DOI] [PubMed] [Google Scholar]
- [26].Hublin C, Partinen M, Heinonen E H„ Puuka P, and Salmi T, Selegiline in the treatment of narcolepsy, Neurology, 44 (1994b) 2095–2101. [DOI] [PubMed] [Google Scholar]
- [27].Hublin C, Partinen M, Kaprio J, Koskenvuo M. and Guilleminault C, Epidemiology of narcolepsy, Sleep, 17 (1994) S7–S12. [DOI] [PubMed] [Google Scholar]
- [28].Kaitin K.l., Kilduff TSS and Dement WC, Sleep fragmentation in canine narcolepsy, Sleep, 9 (1986) 1 16–1 19. [DOI] [PubMed] [Google Scholar]
- [29].Jones BE, Bobillier P, Pin C. and Jouvet M, The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat, Brain Res., 58 (1973) 157–177. [DOI] [PubMed] [Google Scholar]
- [30].Khan N, Antonini A, Parkes D, Dahlitz MJ, Meier-Ewert K,Weindl A. and Leenders KL, Striatal dopamine D2 receptors in patients with narcolepsy measured with PET and C-raclopride, Neurology, 44 (1994) 2102–4. [DOI] [PubMed] [Google Scholar]
- [31].Kilduff T.s., Bowersox S, Kaitin K.l., Baker TL, Ciaranello RD and Dement WC, Muscarinic cholinergic receptors and the canine model of narcolepsy, Sleep, 9 (1986) 102–106. [DOI] [PubMed] [Google Scholar]
- [32].Kish SJ, Mamelak M, Slimovitch C, Dixon LM, Lewis A, Shannak K, DiStephano L, Chang LJ and Hornykiewics O, Brain neurotransmitter changes in human narcolepsy, Neurology, 42 (1992) 229–234. [DOI] [PubMed] [Google Scholar]
- [33].Kropf W. and Kuschinsky K, Electroencephalographic correlates of the sedative effects of dopaminergic agonists presumabely acting on autoreceptors, Neurophrmacology, 30 (1991) 953–960. [DOI] [PubMed] [Google Scholar]
- [34].Lim RKS, Lui c.-N. and Moffitt RL, A Stereotaxic Atlas of the Dogs Brain, Thomas Books, Springfield, 1960. [Google Scholar]
- [35].Levesque D, Diaz J, Pilon C, Martres M-P, Giros B, Souil E, Schott D, Morgat J-M, Schwartz JC and Sokoloff P, Identification, characterization and localization of the dopamine D3 receptor in rat brain using 7 –[3H]hydroxyl- N-di-n-propyl-2-aminotetralin, Proc. Natl. Acad. sci. USA, 89 (1992) 8155–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lyons TJ and French J, Modafinil: the unique properties of a new stimulant, Aviation Space Environmental Medicine, 62 (1991) 432–5. [PubMed] [Google Scholar]
- [37].Malnelak M, A model for narcolepsy, Can. L Psychol, 45 (1991) 194–220. [DOI] [PubMed] [Google Scholar]
- [38].Mamelak M. A perspective on narcolepsy, Encephale, XVIII (1992) 347–351. [PubMed] [Google Scholar]
- [39].Mefford LN, Baker TL, Boehme R, Foutz A.s., Ciarranello RD, Barchas JD and Dement WC, Narcolepsy: biogenic amine deficits in an animal model, Science, 220 (1983) 629–632. [DOI] [PubMed] [Google Scholar]
- [40].Meller E, Bohmaker K. Goldstein M. and Basham DA, Evidence that striatal synthesis-inhibiting autoreceptors are dopamine D3 receptors, Eur. J. Pharmacol, 249 (1993) R5–R6. [DOI] [PubMed] [Google Scholar]
- [41].Mignot Fl., Guilleminault C. Bowersox S, Rappaport A. and [Dement WC, Effects of a-I adrenoreceptor blockade with prazosin in canine narcolepsy, Brain Res., 444 (1988) 184–186. [DOI] [PubMed] [Google Scholar]
- [42].Mignot E, Guilleminault C, Dement WC and Grumet FC, Genetically determined animal models of narcolepsy, a disorder or REM sleep. In Driscol P. (Ed.), Genetically l)etermined Animal Models Q/’ Neurobehanioral Dys/iwn.ction, Birkhauser Boston, Cambridge, MA, 1992, pp. 90–1 10. [Google Scholar]
- [43].Mignot E, Renaud A, Nishino S, Arrigoni J, Guilleminault C. and Dement WC, Canine cataplexy is preferentially controlled by adrenergic mechanisms: evidence using monoamme selective uptake inhibitors and release enhancers, Psychopharmacology, 1 13 (1993) 76–82, [DOI] [PubMed] [Google Scholar]
- [44].Mignot E, Nishino S, Guilleminault C. and Dement WC, Modafinil binds the dopamine uptake carrier site with low affinity, Sleep, 17 (1994) 436–437. [DOI] [PubMed] [Google Scholar]
- [45].Miller JD, Farber J, Gatz P, Roffwarg H. and German DC, Activity of mesencephalic dopamine and non dopamine neurons across stages of sleep and waking in the rat, Brain Res., 273 (1983) 133–141. [DOI] [PubMed] [Google Scholar]
- [46].Miller JD, Faull KF, Bowersox SS and Dement WC, CNS monoamines and their metabolites in canine narcoelpsy: a replication study, Brain Res., 509 (1990) 169–171. [DOI] [PubMed] [Google Scholar]
- [47].Mironowicz J. and Schultz W, Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli, Nature, 379 (1996) 449–451. [DOI] [PubMed] [Google Scholar]
- [48].Missaie C, Castalletti L, Govoni S, Spano PF, Trabucchi M. and Hanbauer 1, Dopamine uptake is differentially regulated in rat striatum and nucleus accumbens, J. Neurochem, 45 (1985) 51–56. [DOI] [PubMed] [Google Scholar]
- [49].Mitler MM, Erman M. and Hajdukovic R, The treatment of excessive daytime somnolence with stimulant drugs, Sleep, 16 (1993) 203–206. [DOI] [PubMed] [Google Scholar]
- [50].Mitier MM, Alrich MS, Koob GF and Zarcone VP, Narcolepsy and its treatment with stimulants. ASDSA Standards of Practice, Sleep, 17 (1994) 352–371. [PubMed] [Google Scholar]
- [51].Monti JM, Jantos H. and Fernandez M, Effects of selective dopamine D2 receptor agonist, quinpirole on sleep and wakefulness in the rat, Eur. J. Pharmacol, 169 (1989) 61–66. [DOI] [PubMed] [Google Scholar]
- [52].Monti JM, Fernandez M. and Jantos H, Sleep during acute dopamine DI agonist SKF 38393 or DI antagonist SCH 23390 administration in rats, Neuropsychopharmacology, 3 (1990) 153–162. [PubMed] [Google Scholar]
- [53].Morizumi T. and Hattori T, Separate neuronal populations of the rat globus pallidus projecting to the subthalamic nucleus, auditory cortex and pedunculopontine tegmental area, Neuroscience, 46 (1992) 701–710. [DOI] [PubMed] [Google Scholar]
- [54].Nicholson AN and Pacsoe PA, Dopaminergic transmission and the sleep-wakefulness continuum in man, Neuropharmacology, 29 (1990) 41 1–417. [DOI] [PubMed] [Google Scholar]
- [55].Nishino S, Haak L, Shepherd H, Guilleminault C, Sakai T, Dement WC and Mignot E, Effects of central α-2 adrenergic compounds on canine narcolepsy, a disorder of rapid eye movement sleep, J. Pharmacol. Exp. Then, 253 (1990) 1 145–1 152. [PubMed] [Google Scholar]
- [56].Nishino S, Arragoni J, Valtier D, Miller J, Guilleminault C, Dement WC and Mignot E, Involvement of dopamine D2-typc receptors in the regulation ot’ cataplexy in canine narcolepsy, J. Neurosci, I l (1991) 2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nishino S, Frushtorfer B, Arrigoni J. Guilleminault C, Dement WC and Mignot E, Further characterization of an a-I receptor subtype involved in the control of cataplexy in canine narcolepsy, J. Pharmacol- Exp. Ther, 264 (1993) 1079–1084 [PubMed] [Google Scholar]
- [58].Nishino S, Reid MS, Dement WC and Mignot E, Neuropharmacology and neurochemistry of canine narcolepsy, Sleep, 17 (1994) s84–s92. [DOI] [PubMed] [Google Scholar]
- [59].Nishino S, Tafti M, Reid MS, Shelton J, Siegel JM, Dement WC and Mignot E, Cholinergic stimulation in the basal forebrain ellicils cataplexy in narcoleptic canines, J. Neurosci, 15 (1995) 4806–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nishino S. Sampathkumaran R, Tafti M. Kambayashi T. Lister E, Dement WC and Mignot E, Is presynaptic activation of dopaminergic transmission important for the EEG arousal efTect of stimulant compounds? Sleep Res., 24 (1995) 310. [Google Scholar]
- [61].Reid MS, Geary LN, Nishino S. Siegel JM, Dement WC and Mignot E, Cholinergic mechanisms in canine narcolepsy: I.IOO modulation of cataplexy via local drug administration into the pontine reticular formation, Neuroscience, 59 (1994) 51 1–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Reid MS, Siegel JM, Dement WC and Mignot E, Cholinergic mechanisms in canine narcolepsy: Il. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy, Neuro- science, 59 (1994) 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reid MS, Tafti M, Nishino S, Siegel JM, Dement WC and Mignot E, Cholinergic regulation of cataplexy in canine narcolepsy in the pontine reticular formation is mediated by M2 muscarinic receptors, Sleep, 17 (1994) 424–435. [PMC free article] [PubMed] [Google Scholar]
- [64].Reid MS, Nishino S, Shelton J, Arrigoni L, Geary J, Dement WC and Mignot E, Evidence for excessive daytime slepiness in narcoleptic Doberman pinchers, Sleep Res., 23 (1994) 309. [Google Scholar]
- [65].Roufs JB, L-Tyrosine in the treatment of narcolepsy, Medical Hypothesis, 33 (1990) 269–273. [DOI] [PubMed] [Google Scholar]
- [66].Seutin V, scuvee-moreau J. Giesbers 1, Massotte L. and Dresse As, Effect of BHT-920 on monoaminergic neurons of the rat brain: an electrophysiological in vivo and in vitro study, Naunyn-Schmiedeberg ‘s Arch. Pharmacol, 242 (1990) 502–507. [DOI] [PubMed] [Google Scholar]
- [67].Shinoga Y, Takada M, Ogawa-Meguro R, Ikai Y. and Mizuno N, Direct projections from the globus pallidus to the midbrain and pons in the cat, Neurosci. Lett, 135 (1992) 179–183. [DOI] [PubMed] [Google Scholar]
- [68].Shiromani PJ, Gillin JC and Henriksen SJ, Acetylcholine regulation of REM sleep: basic mechanisms and clinical implica-tions for affective illness and narcolepsy, Anna. Rev. Pharmacol. Toxicol, 27 (1987) 137–156. [DOI] [PubMed] [Google Scholar]
- [69].Siegel JM, Brainstem mechanisms generating REM sleep. In Kryger MH, Roth T. and Dement WC (Eds.), Principles and Practice of Sleep Medicine, W.B. Saunders, New York, 1994, pp. 125–144. [Google Scholar]
- [70].Sokoloff P, Giros B, Manres M-P, Bouthenet M-L and dopamine receptor (D3) as a target for neuroleptics, Nature, 347 (1990) 146–151. [DOI] [PubMed] [Google Scholar]
- [71].Sokoloff Pa, Giros B, Martres M-P, Andrieux M, Besancon R, Pilon C, Bouthenet M-L, Souil E. and Schwartz J-C, Localization and function of the D3 dopamine receptor, Arzneim. -Forsch./Drug Res, 42 (1990) 224–230. [PubMed] [Google Scholar]
- [72].Steinfels GF, Heym J, Streckjer RE and Jacobs BL„ Behavioral correlates of dopmainergic activity in freely moving cats, Brain Res., 258 (1983) 217–228. [DOI] [PubMed] [Google Scholar]
- [73].Steininger TL, Rye DB and Wainer BH, Afferent projections to cholinergic peduncolopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. retrograde tracing studies, J. comp. Neurol, 321 (1992) 515–543. [DOI] [PubMed] [Google Scholar]
- [74].Trulson ME, Preusler DW and Howell GA, Activity of substantia nigra across the sleep-waking cycle in freely moving cats, Neurosci. Leti, 26 (1981) 183–188. [DOI] [PubMed] [Google Scholar]
- [75].Trulson ME and Preusler DW, Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep waking cycle and effects of stress, Exp. Neurol, 83 (1984)367–377. [DOI] [PubMed] [Google Scholar]
- [76].Wauquier A, Van den Broeck WAE and Janssen PAJ, Bipha-sic effects of pimozide on sleep-wakefulness in dogs, Life Sci., 27 (1978) 1469–1475. [DOI] [PubMed] [Google Scholar]
- [77].Wauquier A, Clincke GHC, Van den Broeck WAE and Prons DE, Active and permissive roles of dopamine in sleep-wakefulness regulation. In Wauqier A, Monti JM, Gaillard JM and Radsulavacki M. (Eds.), Sleep: Neurotranmitters and Neuromodulators, Raven Press, New York, 1985. [Google Scholar]
- [78].Yeomans JS, Mathur A. and Tampakeras M, Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopaminergic neurons, Behav. Neurosci, 107 (1993) 1077–1087. [DOI] [PubMed] [Google Scholar]