Abstract
Ingestion of thalidomide was associated with a reduction in the upregulation of the granulocyte activation marker CD11b and a reduced capacity to release elastase and lactoferrin after stimulation with lipopolysaccharide or lipoteichoic acid. A single oral dose of thalidomide attenuates neutrophil activation upon ex vivo stimulation with bacterial antigens.
Thalidomide has been rediscovered as an anti-inflammatory drug in the treatment of a wide range of diseases, including mycobacterial infection, sarcoidosis, Crohn's disease, and AIDS-related ulcers (reviewed in reference 8). Most studies on the mode of action of thalidomide have focused on mononuclear cells. However, granulocytes constitute a first line of defense to an invading microorganism. Furthermore, in auto-immune diseases, inflammation is also characterized by the influx of granulocytes (4). Activation of granulocytes is associated with an upregulation of CD11b and a concurrent downregulation of l-selectin (5). Activation is further characterized by granulocyte degranulation, resulting in the release of the granule products elastase and lactoferrin (10, 14). Knowledge of the effects of thalidomide on granulocyte functions is limited. Treatment of human umbilical vein endothelial cells with thalidomide resulted in stimulation or inhibition of transmigration of neutrophils, depending on the stimulus used (3). Thalidomide attenuated neutrophil chemotaxis in vitro but did not influence respiratory burst activity (3). In the present study we determined whether an oral dose of thalidomide influenced the ability of bacterial antigens to induce granulocyte activation in healthy humans.
Six healthy male subjects with a median age of 38 years (range, 33 to 44 years) ingested 400 mg of thalidomide orally (racemic mixture, purchased from Grünenthal, GmbH, Stolberg, Germany). Venous blood was collected directly before ingestion of thalidomide and 3, 6, and 24 h thereafter, using a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson, Mountain View, Calif.). The present study was performed simultaneously with investigations that determined the effect of thalidomide on human immunodeficiency virus coreceptor expression on CD4+ T cells and on the production of Th1 and Th2 cytokines by peripheral blood lymphocytes (7, 13). The study was approved by the institutional research and ethics committees of the Academic Medical Center, Amsterdam, The Netherlands, and written informed consent was obtained from all volunteers. Anticoagulation was obtained using heparin (final concentration, 10 U/ml of blood; Leo Pharmaceutical Products, Weesp, The Netherlands). For determination of CD11b and l-selectin expression on granulocytes, whole blood was added to sterile polypropylene tubes and diluted 1:2 with RPMI 1640 (BioWhittaker, Verviers, Belgium), to which either lipopolysaccharide (LPS) from Escherichia coli (10 ng/ml; serotype 0111:B4; Sigma, St. Louis, Mo.) lipoteichoic acid (LTA) from Staphylococcus aureus (1 μg/ml; Sigma), or no stimulus was added. After incubation for 24 h at 37°C, blood was prepared for fluorescence-activated cell sorter analysis as follows. Erythrocytes were lysed with bicarbonate-buffered ammonium chloride solution (pH 7.4). Leukocytes were recovered after centrifugation at 600 × g for 5 min and counted. A total of 106 cells was resuspended in phosphate-buffered saline containing EDTA (100 mM), sodium azide (0.1%) and bovine serum albumin 5% (cPBS); placed on ice; and incubated for 1 h with either CD11b monoclonal antibody (clone M1/70) or a rat anti-human l-selectin monoclonal antibody (Pharmingen, San Diego, Calif.). Nonspecific staining was controlled for by incubation of cells with isotypic FITC-labeled rat IgG2 (Coulter Immunotech). Cells were then washed twice in ice-cold cPBS and resuspended for flow cytofluorometric analysis (Calibrite; Becton Dickinson Immunocytometry Systems, San Jose, Calif.). For each test, at least 106 granulocytes were counted. Mean cell fluorescence (MCF) at >570 nm of forward and side angle scatter-gated granulocytes was assessed. Data are represented as the difference between MCF intensities of specific and nonspecifically stained cells. Neutrophil degranulation was investigated exactly as described previously (2, 11, 12). In brief, whole blood diluted 1:5 with RPMI 1640 was incubated for 2 h at 37°C with LPS, LTA, or no stimulus. Thereafter, plasma was prepared by centrifugation and stored at −20°C until assays were performed. Elastase and lactoferrin were measured as described previously (12). All values are given as means ± standard errors of the means (SEM). Comparisons between LPS or LTA effects and control (RPMI) incubation before thalidomide ingestion were done with the paired Wilcoxon test. Changes in time relevant to t = 0 (time of thalidomide ingestion) were analyzed by one-way analysis of variance. A P of <0.05 was considered statistically significant.
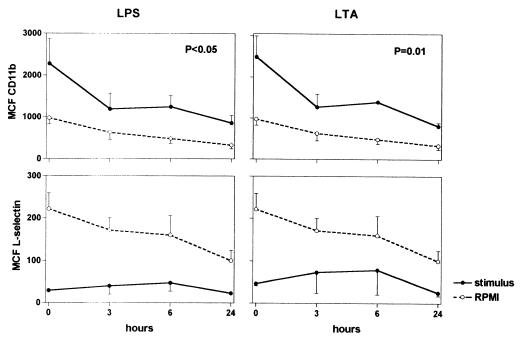
An oral dose of 400 mg of thalidomide did not result in a change in leukocyte counts or differentiation in six healthy volunteers (data not shown). Besides drowsiness, volunteers did not experience side effects. Activation of neutrophils is associated with enhanced expression of CD11b and a concurrently reduced expression of l-selectin (5). Previously, it was shown that CD11b on granulocytes of a volunteer who had ingested thalidomide without further stimulation showed a reduction of CD11b expression (9). In our study, in blood that was immediately processed for fluorescence-activated cell sorter analysis (i.e., not incubated), granulocyte CD11b and l-selectin did not change over time after ingestion of thalidomide (data not shown). Ex vivo stimulation with LPS or LTA induced an increase in the expression of CD11b and a decrease in the expression of l-selectin, indicative of an activated state of the granulocytes (P < 0.05 for all versus incubation with RPMI [see t = 0 in Fig. 1]). Ingestion of thalidomide inhibited LPS- and LTA-induced upregulation of CD11b (P < 0.05 versus value obtained at t = 0), an effect that was already evident after 3 h and which lasted at least 24 h (thereby ruling out a circadian effect) (Fig. 1). Thalidomide did not influence LPS- or LTA-induced downregulation of l-selectin. Both CD11b and l-selectin expression tended to decrease after ingestion of thalidomide in unstimulated blood (P = 0.59 and 0.19, respectively).
FIG. 1.
(MCF) of granulocytes expressing CD11b and l-selectin in blood from 6 volunteers before and after ingestion of thalidomide at t = 0. Blood was obtained before and at various time points after ingestion of thalidomide (400 mg) and was subsequently incubated for 24 h with either LPS (10 ng/ml) (solid lines, left panels), LTA (1 μg/ml) (solid lines, right panels), or RPMI (dotted lines). Data are means ± SEM (error bars). P values reflect significance for change after ingestion of thalidomide at t = 0.
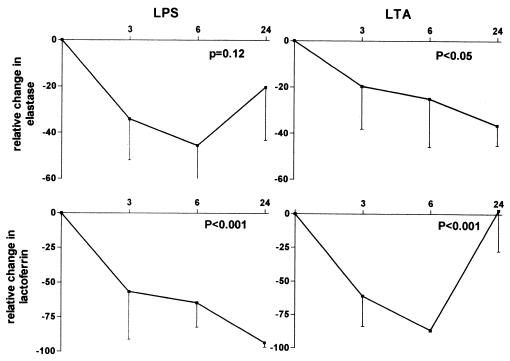
Granulocytes are equipped to attack ingested organisms or immune complexes by release of their granules. Neutrophils contain at least two types of granules: primary and secondary, or azurophilic and specific granules. Azurophilic granules are the main storage of proteases, such as elastase, which can degrade almost all components of the extracellular matrix and cleaves a variety of plasma proteins (6). Lactoferrin is found in specific granules and has a high affinity for iron, thereby eliminating iron from the cell environment (14). Since iron is an important microbial growth factor, lactoferrin may enhance antibacterial host defense. Having established that thalidomide reduces activation of cellular markers on granulocytes, we next determined whether thalidomide influences granulocyte degranulation. Since granulocytes are the only producers of elastase and lactoferrin (1, 6), levels of these products measure granulocyte degranulation. The concentrations of elastase and lactoferrin in plasma obtained from blood that was not incubated did not change after thalidomide ingestion (data not shown). Incubation of whole blood with LPS and LTA resulted in profound degranulation of neutrophils, although the interindividual variation was relatively large; i.e., elastase concentrations in supernatants were 19.7 ± 5.1 ng/ml (RPMI), 127.7 ± 82.3 ng/ml (LPS), and 150.5 ± 75.9 ng/ml (LTA), and lactoferrin concentrations were 10.8 ± 3.0 ng/ml (RPMI), 567.3 ± 163.5 ng/ml (LPS), and 60.8 ± 29.3 ng/ml (LTA) (for all LPS and LTA values, P < 0.05 versus RPMI). Levels of elastase and lactoferrin in unstimulated blood (i.e., incubated for 2 h without stimulus) did not change over time (data not shown). Thalidomide ingestion resulted in a decreased release of elastase and lactoferrin (Fig. 2).
FIG. 2.
Effect of thalidomide ingestion on degranulation of granulocytes in six healthy humans. Blood was obtained before and at various time points after ingestion of thalidomide (400 mg) and was subsequently incubated for 2 h with either LPS (10 ng/ml) (left panels) or LTA (1 μg/ml) (right panels). Elastase and lactoferrin concentrations were measured in supernatants. Data are expressed as mean percentage inhibition ± SEM (error bars) relative to levels of elastase or lactoferrin after incubation of whole blood before ingestion of thalidomide (t = 0) with LPS or LTA (these concentrations at t = 0 are given in the text). Elastase and lactoferrin levels measured after incubation with RPMI only did not change after ingestion of thalidomide (see text). P values reflect significance for change after ingestion of thalidomide at t = 0. NS, nonsignificant.
We found that in vivo exposure of healthy humans to thalidomide resulted in reduced granulocyte CD11b expression and inhibition of the release of elastase and lactoferrin from neutrophilic granules, induced by ex vivo stimulation with gram-positive and gram-negative bacterial antigens. Some caution is warranted with respect to the interpretation of these results, considering that commercially available bacterial antigen preparations such as LPS and LTA may not be completely pure. These findings shed new light on the anti-inflammatory properties of thalidomide and suggest that this compound may inhibit granulocyte-mediated tissue injury.
Acknowledgments
This work was supported by grants from the Mr. Willem Bakhuys Roozeboom Foundation to N. P. Juffermans and the Royal Dutch Academy of Arts and Sciences to T. van der Poll.
REFERENCES
- 1.Bennett R M, Kokocinski T. Lactoferrin turnover in man. Clin Sci. 1979;979:453–460. doi: 10.1042/cs0570453. [DOI] [PubMed] [Google Scholar]
- 2.Bouma M G, Jeunhomme T M, Boyle D L, Dentener M A, Voitenok N N, van den Wildenberg F A, Buurman W A. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol. 1997;158:5400–5408. [PubMed] [Google Scholar]
- 3.Dunzendorfer S, Schratzberger P, Reinisch N, Kähler C M, Wiedermann C J. Effects of thalidomide on neutrophil respiratory burst, chemotaxis, and transmigration of cytokine- and endotoxin-activated endothelium. Naunyn-Schmiederberg's Arch Pharmacol. 1997;356:529–535. doi: 10.1007/pl00005087. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan F M, Maini R N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 5.Hogg J C, Doerschuk C M. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 6.Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- 7.Juffermans N P, Verbon A, Olszyna D P, van Deventer S J H, Speelman P, van der Poll T. Thalidomide suppresses up-regulation of human immunodeficiency virus coreceptors CXCR4 and CCR5 on CD4+ T cells in humans. J Infect Dis. 2000;181:1813–1816. doi: 10.1086/315478. [DOI] [PubMed] [Google Scholar]
- 8.Marriott J B, Muller G, Dalgleish A G. Thalidomide as an emerging immunotherapeutic agent. Immunol Today. 1999;20:538–540. doi: 10.1016/s0167-5699(99)01531-5. [DOI] [PubMed] [Google Scholar]
- 9.Neubert R, Nogueira A C, Neubert D. Thalidomide and the immune system. 2. Changes in receptors on blood cells of a healthy volunteer. Life Sci. 1992;51:2107–2116. doi: 10.1016/0024-3205(92)90162-i. [DOI] [PubMed] [Google Scholar]
- 10.Nuijens J H, Abbink J J, Wachtfogel Y T, Colman R W, Eerenberg A J, Dors D, Kamp A J, Strack van Schijndel R J, Thijs L G, Hack C E. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–168. [PubMed] [Google Scholar]
- 11.Ogilvie A C, Hack C E, Wagstaff J, van Mierlo G J, Eerenberg A J, Thomsen L L, Hoekman K, Rankin E M. IL-1 beta does not cause neutrophil degranulation but does lead to IL-6, IL-8, and nitrite/nitrate release when used in patients with cancer. J Immunol. 1996;156:389–394. [PubMed] [Google Scholar]
- 12.Schultz M J, Speelman P, Hack C E, Buurman W A, van Deventer S J H, van der Poll T. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J Antimicrob Chemother. 2000;46:235–240. doi: 10.1093/jac/46.2.235. [DOI] [PubMed] [Google Scholar]
- 13.Verbon A, Juffermans N P, Speelman P, van Deventer S J H, ten Berge I J M, Guchelaar H-J, van der Poll T. A single oral dose of thalidomide enhances the capacity of lymphocytes to secrete gamma interferon in healthy humans. Antimicrob Agents Chemother. 2000;44:2286–2290. doi: 10.1128/aac.44.9.2286-2290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]