Abstract
Placental tissues encompass all the tissues which support fetal development, including the placenta, placental membrane, umbilical cord, and amniotic fluid. Since the 1990s there has been renewed interest in the use of these tissues as a raw material for regenerative medicine applications. Placental tissues have been extensively studied for their potential contribution to tissue repair applications. Studies have attributed their efficacy in augmenting the healing process to the extracellular matrix scaffolds rich in collagens, glycosaminoglycans, and proteoglycans, as well as the presence of cytokines within the tissues that have been shown to stimulate re-epithelialization, promote angiogenesis, and aid in the reduction of inflammation and scarring. The compositions and properties of all birth tissues give them the potential to be valuable biomaterials for the development of new regenerative therapies. Herein, the development and compositions of each of these tissues are reviewed, with focus on the structural and signaling components that are relevant to medical applications. This review also explores current configurations and recent innovations in the use of placental tissues as biomaterials in regenerative medicine.
1. Introduction
Placental tissues, or extra-embryonic tissues, also commonly referred to as birth tissues, support and protect fetal development during gestation. They include the placental disc, the umbilical cord, placental membrane, and amniotic fluid (Figure 1A). These tissues are transient, as they only exist to support the fetus until birth. Placental tissues are of great interest in medicine, specifically as a biomaterial because of the exceptional properties of fetal tissues and the ease of access to them as raw materials [1, 2]. Unlike cadaveric tissue, placental tissues are of a consistent age, donated from a screened population of healthy donors, do not require extensive procedures to collect, and would otherwise be discarded as medical waste [3]. Common tissue collection practices involve packaging for transport following routine cesarian section deliveries, and vaginal deliveries under certain circumstances, with sterile materials and transport solutions. Sterile containers are commonly used to transport the tissue to avoid contamination, and storage time is kept as minimal as possible under refrigerated conditions to ensure material stability [3, 4].
Figure 1.
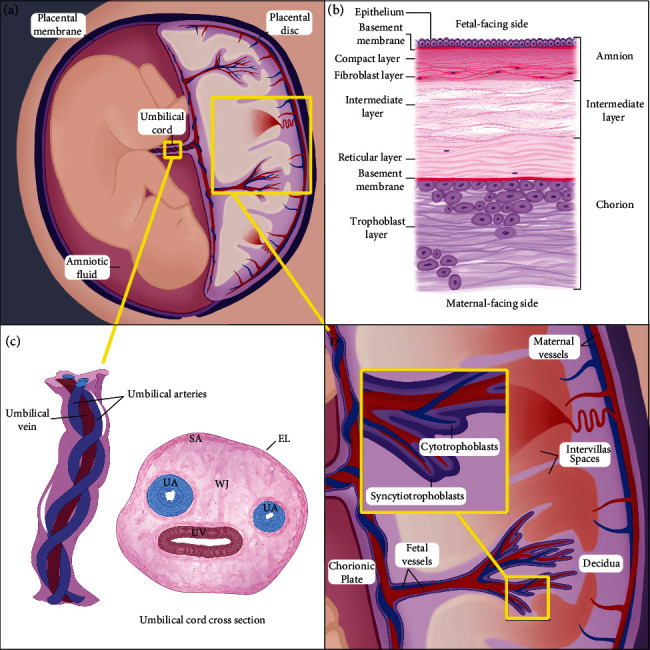
The anatomy of placental tissues. (a) The anatomy of the fetal compartment, (b) the anatomy of the placental membrane layers, (c) the anatomy and cross section of the umbilical cord, including the epithelial layer (EL), subamnion (SA), Wharton's Jelly (WJ), umbilical arteries (UA), and umbilical vein (UV), and (d) the anatomy of the placental disc.
In the 16th century, medical use of the human placenta was described in China [5]. The first documented use of placental tissues in modern medicine dates back to the beginning of the 20th century where application of placental membrane in skin grafting was first published showing superior efficacy over cadaveric tissue by Davis in 1910 [6]. The first studies showing therapeutic efficacy of placental tissues appear between the 1930s and 1980s [2]. Several reports were published in the 1930-1940s on the use of placental membrane in surgical and wound applications followed by clinical reports and trials through the 1970s and 1980s. As contemporary standards of cleanliness and sterility developed through the ‘80s and ‘90s, scientists began to experiment with dehydration and sterilization of the tissue. This led to the increased safety, usability, and access of today's shelf-stable products.
Given the clinical benefits of placental membrane grafts in wound and surgical applications, research has further focused on developing and testing new tissue- and cell-based therapies derived from placental tissues [4]. Since the turn of the century, applications of these tissues have included uses in cell therapy, bioengineering, and regenerative medicine [3]. The majority of clinical studies have utilized placental membrane, umbilical cord veins, and amniotic fluid [3]. However, extensive preclinical experimentation has been performed on the placental disc, Wharton's Jelly, and umbilical arteries [3]. The wide array of applications stems from the varied functions and forms of the placental tissues and their rich composition of extracellular matrix (ECM) components, signaling molecules, and growth factors [3].
Over the last 30 years there has been vast interest in the use of implanted, injected, or cultured stem cells as a therapy to help regenerate damaged tissues [7]. Placental tissues are a rich source of stem cells that have been extensively researched. The use of multipotent mesenchymal stromal cells (MSCs) from umbilical cord blood, Wharton's Jelly, and placenta, for instance, have been thoroughly described [8, 9] and are not a subject of this review. Recent research regarding the regenerative mechanism of action of MSCs has shown that they provide therapeutic benefit through the release of cytokines [10]. This, along with the clinical success of non-viable placental tissue allografts, further suggests a therapeutic potential for placental tissues as biomaterials [2]. This review will discuss the raw material potential of placental tissues as sources of ECM components, scaffolds, signaling molecules and growth factors for use in regenerative therapies. We will emphasize the structural and soluble biochemical composition of these tissues and discuss current innovations using these tissues as biomaterials in regenerative medicine.
2. Placental Membrane
2.1. Anatomy and Development
The placental membrane (PM) surrounds the developing fetus and creates the amniotic fluid-filled fetal compartment during gestation (Figure 1A). The PM is structured in three major layers: the fetal-facing amnion, the maternal-facing chorion, and the intermediate layer in between, as shown in Figure 1B [11, 12]. The amnion, which is composed of epithelium, basement membrane, compact layer, and fibroblast layer, encloses the fetus and the amniotic fluid, which cushions and protects the fetus during gestation [13]. The chorion, the maternal-facing layer, in contact with the maternal decidua, is comprised of three layers: the reticular layer, a basement membrane and a trophoblast layer [11, 14, 15]. The chorion forms a barrier between the fetal environment and the maternal immune system [16, 17]. The intermediate layer is a spongey network of collagens and proteoglycans that functions as a physical barrier between the amnion and chorion. The structure of this layer aids in the movement of the amnion along the chorion, supported by the high concentration of hyaluronic acid (HA) which hydrates and lubricates the tissue [11, 18–20].
During gestation, the amnion and chorion form separately after blastocyst implantation around day 14 of gestation, alongside the development of the placental disc [21, 22]. The wall of the blastocyst becomes the chorion. By day 10 post-conception, the amnion is visible, forming from the amniogenic cells that make up the inner trophoblast layer of the cellular mass of the embryo [21]. The PM continuously grows throughout gestation to accommodate the growing fetus. Following the end of month 3 of gestation, the amnion is visibly separated from the chorion leave, the precursor to the chorion, by the chorionic cavity. As the amniotic sac fills with fluid and gradually expands, the amnion adheres to the innermost surface of the chorion and the chorionic cavity disappears. Although now closely adhered, amniotic and chorionic layers of the PM do not fuse and remain histologically separate [21]. At term, the literature reports native PM thickness can range from 0.02 mm to 0.6 mm and the average surface area of the PM is 1600 cm2 [1, 23].
2.2. Physiology
The PM acts to provide a barrier and protect the fetus from the maternal environment and withstand the stretching and pressure created by the fetus as it develops and moves [14, 24]. In addition, the PM is metabolically active and provides an immune barrier to infection and a barrier to immune rejection by the mother [25]. Over the course of gestation, the PM constantly undergoes matrix remodeling to maintain its barrier integrity and accommodate a growing fetus [26]. The composition of each layer of the PM varies slightly to support the overall function of the membrane. The basement membrane of the amnion is a dense tissue layer that acts as a permeable barrier for the exchange of nutrients [11, 27]. The compact layer provides tensile strength to the membrane and is the most fibrous of the amnion layers due to the high content of collagens secreted from the mesenchymal cells in the fibroblast layer [11, 12, 28]. The fibroblast layer contributes to the tensile strength of the membrane and provides a scaffold for cell-cell interactions [29, 30]. The intermediate layer functions as a barrier between the amnion and the chorion, providing mechanical support in the form of a nonfibrillar network made up mostly of proteoglycans, glycosaminoglycans (GAGs) and type III collagen [11, 18, 20]. The reticular layer provides a scaffold for the chorion with a network of fibers and is embedded with mesenchymal cells similar to the fibroblast layer in the amnion [31]. The basement membrane between the reticular layer and the trophoblasts increases the structural integrity of the chorion by providing cell scaffolding for the trophoblast layer and contributes to the immune privilege of the tissue [16, 32, 33]. The trophoblast layer, the outermost layer of the chorion, consists of trophoblasts, myofibroblasts and macrophages [16].
The overall PM structure supports resident cells while maintaining tensile strength and elasticity during the regular turnover of the ECM that is facilitated by endogenous cytokines in the PM [34]. The PM is an immune privileged tissue contributing to its function as an immune barrier between the mother and fetus, preventing maternal rejection of the fetus. Studies in the early 1980s found no human leukocyte antigen (HLA) -A, -B, -C, or -DR molecules in human amnion, but more recent studies have detected these class I and II molecules in various cells of the tissue [35]. However, while almost all cells of the PM have been shown to express class I HLA molecules, the tissue has been found to be immunologically inert with almost no reports of immune rejection [35, 36] and there is little to no major histocompatibility complex (MHC) reactivity in unmatched adult recipients [36]. This limited reactivity is reportedly due to the active suppression of B cells, T cells, and dendritic cells, from interactions such as the binding of CD8+ T cells to HLA-G molecules [35, 36]. Further Fas ligand expression on amnion epithelial cells is suggested to signal apoptosis of host lymphocytes, helping to prevent maternal rejection of the fetus, along with the immunosuppressive actions of cell surface and soluble HLA-G [35].
2.3. Structural Composition
The PM is composed of a network of collagens (types I, III, IV, V and VI), laminin, fibronectin, HA, vitronectin, elastin, and proteoglycans, which provide mechanical strength, elasticity, flexibility, and appropriate stiffness to support the barrier integrity in the uterine environment (Table 1) [12, 18, 34, 37, 38]. Each layer of the PM has a different composition to serve its individual function [20]. The ECM of the amnion contains collagens types I, III, IV, V and VI secreted by the mesenchymal cells in the fibroblast layer, as well as elastin, fibronectin, laminin, HA, and sulfated proteoglycans [12, 19, 34]. The chorion ECM consists of collagens types I, III, IV, V, and VI, elastin, fibronectin, laminin and HA [34]. The intermediate layer is composed of collagen types I, III, and IV, sulfated proteoglycans and glycoproteins. Due to the high concentration of proteoglycans and glycoproteins, the intermediate layer allows the amnion and chorion to glide against each other [14, 18, 28].
Table 1.
Extracellular matrix components of placental membrane, amniotic fluid, umbilical cord Wharton's Jelly and placental disc.
| Extracellular Matrix Components of Placental Tissues | ||||
|---|---|---|---|---|
| ECM Component | Placental Membrane | Amniotic Fluid | Umbilical Cord | Placental Disc |
| Collagen I | [26, 28] | [109, 127]∗∗ | [188] | [232, 233, 235, 254] |
| Collagen II | [28] | NR | [205] | [235] |
| Collagen III | [28] | [109, 127]∗∗ | [188] | [232, 233, 254] |
| Collagen IV | [28] | [108, 109]∗∗ | [188] | [3, 232, 254] |
| Collagen V | [28] | NR | [188] | [232, 235] |
| Collagen VI | [28] | NR | [205] | [235] |
| Collagen XII | [28] | NR | [205] | [254] |
| Collagen XIII | [28] | NR | NR | [254] |
| Collagen XIV | [28] | NR | [258] | [235, 254] |
| Collagen XX | NR | NR | NR | [254] |
| Collagen XXVIII | NR | NR | NR | [254] |
| Aggrecan | [28] | NR | NR | [254] |
| Agrin | [259] | NR | NR | [254] |
| Chondroitin sulfate proteoglycans | [260] | [135]∗∗ | [191] | [254] |
| Elastin | [28] | NR | NR | [254] |
| Fibrinogen | NR | NR | NR | [254] |
| Fibrillin | [28] | NR | [261] | [3] |
| Fibronectin | [28] | [127]∗∗ | [262] | [3, 232, 235, 236, 238, 240, 254] |
| Ficolin-2 | NR | NR | NR | [254] |
| Heparin sulfate proteoglycan | [12] | [135]∗∗ | [188] | [3, 254] |
| Hyaluronic acid | [12] | [135]∗∗ | [194] | [241] |
| Dermatan sulfate | [260] | [135]∗∗ | [263] | [241] |
| Chondroitin 6-sulfate | [264] | [135]∗∗ | [188] | [241] |
| Chondroitin 4-sulfate | [264] | [135]∗∗ | [188] | [241] |
| Laminins alpha 1, 2, 3, 4; beta 1, 2; gamma 1, 3 | [43] Laminin a2, a3, a5 [43] a1, a4: ND[43] b1-3, gamma 1-2 [43] gamma 3: ND |
NR | NR | [3, 218, 232, 235, 254] |
| Nidogen | [265] | NR | ND | [254] |
| Vitronectin | [28] | NR | NR | [254] |
| Osteopontin | NR | NR | NR | [254] |
∗∗ Levels are from second trimester AF collection and measurements; NR = not reported; ND = not detected.
The PM contains different types of collagen (types I, III, IV, V, and VI) secreted by fibroblasts which serve different purposes depending on which layer they are found in [28]. Collagen types I and III form parallel bundles throughout all PM layers, except the trophoblast layer, to support the mechanical integrity of the tissue during gestation [20, 28]. Collagens type IV and V form connections to the basement membranes of both the amnion and the chorion [39]. The fibrous structure of the amnion is attributed to the collagens type I, III, V and VI present [20]. The chorion, the thickest of the layers, contains large amounts of collagens type I and IV, which form scaffolding for resident cells in the PM [20, 28]. Additionally, the presence of collagen in the trophoblast layer of the chorion assists in the anchoring of the PM to the maternal decidua [14, 20]. The intermediate layer is comprised of collagen type I, III and IV and contains a higher density of proteoglycans and mucopolysaccharides [20]. PM is attractive to researchers interested in collagen-rich sources for wound healing applications because of the role of collagen in reducing inflammation in wound healing [40].
Glycoproteins, such as fibronectin and laminin, are ECM components that contribute to the structural integrity of the PM. Fibronectin interacts with collagens and proteoglycans, and influences cell morphology, adhesion, migration, proliferation and differentiation by binding to the cells in both the amnion and chorion [28, 41] Laminins provide a connection between neighboring membrane layers by binding to other proteins, like collagens [28, 42], [43]. Proteoglycans contribute to the tensile strength and integrity of the PM, as well as to cell proliferation and differentiation, and assist in binding of growth factors in the other layers of the PM [14, 18]. When utilized as a biomaterial in wound management, this naturally occurring ECM scaffold can provide structural support and facilitate intercellular and intracellular signaling between cells, cell attachment and cellular migration essential to proper wound remodeling [44, 45]. PM ECM components have been shown to contribute to decreased inflammation, prevention of infection, and reduction in scarring and pain at the site of injury [44, 46].
The ECM also contains components that give the PM flexibility and cushioning from the pressures of gestation. Elastin fibers contribute to the integrity and tensile strength of the PM as it stretches during gestation [15, 20]. GAGs, such as HA, are another component that provides the ECM with elasticity and lubrication. Studies on the distribution of HA in PM have observed its presence in collagen-rich (specifically types IV and V) areas of the tissue, for example, the basement membrane or reticular layer of PM, as well as intermediate layer, but not in the epithelium of the amnion. The chorion and intermediate layer have a greater presence of HA than the amnion [34, 47, 48]. The GAG content of the intermediate layer, specifically the HA, contributes to the hydrophilic nature of the layer [20]. HA has also been shown to play a role in the reduction of inflammation and readily accessible biomaterials that provide HA are of growing interest in the regenerative medicine field [20, 38].
2.4. Cytokines & Antimicrobial Components
Extensive research into the cytokine content of both native PM and processed PM allografts has been done to help understand the role of PM in gestation and potential clinical applications. The layers of the PM are a reservoir of cytokines and growth factors which regulate the pathways that maintain the ECM structural integrity during gestation (Table 2). Evaluating the cytokine and growth factor content in the amnion, the intermediate layer, and chorion is of interest for varying applications of the tissue [20, 49–51]. Specific focus has been given to growth factors such as epithelial growth factor (EGF), fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), transforming growth factor alpha (TGFα), transforming growth factor beta (TGFβ), keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and platelet-derived growth factors (PDGFs), interleukins (ILs) such as IL-4, IL-6, IL-8, and IL-10, interferons (IFNs), and protease inhibitors including tissue inhibitors of metalloproteinases (TIMPs) TIMP-1, TIMP-2, and TIMP-4 because of their applicability to therapeutic applications [49, 50, 52–54]. These signaling components help regulate multiple aspects of the healing cascade, including angiogenesis, inflammation, and tissue remodeling [20, 51]. Basic FGF (bFGF), endocrine-derived vascular endothelial growth factor (EG-VEGF), VEGF and PDGF-AA, and PDGF-BB stimulate the activity and migration of matrix metalloproteinases (MMPs), which are responsible for the breakdown of existing ECM in preparation for remodeling of the PM [34, 55]. TIMPs are important inhibitors that regulate the activity of MMPs to prevent excessive breakdown of the ECM and deposit ECM components [56, 57]. In addition to the active role MMPs play in the remodeling of the placental membrane during gestation, they are an active component in the onset of labor, where high levels of MMPs and reduced levels of TIMPs induce the degradation of the PM ECM resulting in membrane rupture [55]. Specifically, at the onset of labor, MMP-9 concentrations are mainly localized near the cervix and the concentrations rise throughout the PM as labor progresses [55].The tensile strength of healthy term PM has been correlated to the distribution of MMP-9 with the weakest areas having the highest concentration [31].
Table 2.
Signaling components of placental membrane, amniotic fluid, umbilical cord Wharton's jelly and placenta.
| Signaling Components of Placental Tissues | |||||
|---|---|---|---|---|---|
| Functional role in regenerative medicine | Cytokine | Placental membrane | Amniotic fluid | Umbilical cord | Placental disc |
| Angiogenic signal | TGF -β2 | [266] | [267]∗∗; [268]∗∗(TGF- β1 and - β2) | [269] | [3, 217, 243, 245, 252, 254, 270] |
| TGF-α | [266] | [271] | [272] | [273] | |
| Angiogenin | [51] | [274]∗∗ | NR | [252] | |
| VEGF | [26] VEGF-A; [26, 275–277] ND (VEGF-D) |
[142]∗∗; [278] | [194](VEGF-A); NR (VEGF-B); NR (VEGF-C); NR (VEGF-D) | [3, 244, 245, 252, 254, 270] | |
| PlGF | [51, 58] | [279]∗∗ | NR | [245, 246, 252] | |
| FGF-1 (aFGF) | [51] | NR | [198] | [252, 254] | |
| FGF-6 | NR | NR | NR | [252, 254] | |
| FGF-2 (bFGF) | [266] | [271] | [198] | [252, 254] | |
| HGF | [51] | [271] | [194] | [252, 254] | |
| PDGF-AA, -BB | [26] PDGF-AA; [51] PDGF-BB | [280]∗∗ (PDGF-BB) | [194] PDGF-AA; NR (PDGF-BB) | [244, 252, 254] | |
| ANG | [51] | [281] | NR | [3] | |
| Anti inflammatory signal | IL-4 | [26] | [142]∗∗; [280]∗∗ | NR | [243, 245, 282, 283] |
| IL-10 | [26] | [142]∗∗; [267]∗∗ | NR | [243, 245, 282, 283] | |
| IL-13 | NR | [142]∗∗ | NR | [243] | |
| IL-1ra | [51] | [142]∗∗ | [194] | [243] | |
| IL-6 | [58] | [142]∗∗; [267]∗∗;[284] | [194] | [217, 243, 244, 252, 283] | |
| IL-2 | [51] | [285] [142]∗∗; [280]∗∗; [284] | NR | [243, 283] | |
| Adiponectin | [286] | [287] | NR | [244, 288] | |
| Protease inhibitor | TIMP-1 | [26] | [289], [290] | [194] | [252] |
| TIMP-2 | [26] | [289–291] | [194] | [252] | |
| TIMP-4 | [26] | [290] | [292] | [293] | |
| Antimicrobial | IFN γ | NR | [142]∗∗; [280]∗∗ | [194] | [243, 245, 283] |
| Lactoferrin | [20] | [144] | NR | [294] | |
| HBD-1 | [20, 60] | [153] | NR | [60] | |
| HBD-2 | [295] | [145] | [296] | [60, 248] | |
| HBD-3 | [295] | NR | NR | [60] | |
| HNP-3 | [297] | [298] | NR | [297] | |
| Elafin/Trappin-2 | [297] | [297] | NR | [60, 248] | |
| SLPI | [297] | [200] | NR | ND [60] | |
| Tissue remodeling | TGF-β1, 2, 3 | [26] | [268]∗∗ (TGF- β 1 and β 2) | [186] | [3, 17, 243, 245, 252, 254, 270] |
| TGF-α | [51] | [271] | [194] | [273] | |
| Activin | [38] | [299] | NR | [245] | |
| VEGF | [26] VEGF-A; [26, 275–277] ND (VEGF-D) |
[142]∗∗, [278] | [194](VEGF-A); NR (VEGF-B); NR (VEGF-C); NR (VEGF-D) | [3, 244, 245, 252, 254, 270] | |
| PlGF | [33, 51] | [279]∗∗ | NR | [245, 246, 252] | |
| FGF-1, -2, -3, -4, -5, -6, -7 | FGF-1: [51]; FGF-2: [266]; FGF-3: NR; FGF-4: [33]; FGF-5: [20]; FGF-6: [20]; FGF-7: [33] | NR | FGF-1: [198]; FGF-2: [198]; FGF-3: NR; FGF-4: [300]; FGF-5: NR; FGF-6: NR; FGF-7: [194] | [252, 254] | |
| EGF | [33] | [271, 301] | [186] | [252, 254, 270] | |
| HGF | [51] | [271] | [194] | [3, 252, 254] | |
| IGF-1 | [51] | [271, 302] | NR | [3, 252, 254] | |
| PDGF-AA, -BB | [26, 51] | [280]∗∗ (PDGF-BB) | [194] PDGF-AA; NR (PDGF-BB) | [244, 252, 254] | |
| Adiponectin | [286] | [279]∗∗ | NR | [244, 288] | |
| IGFBP-1, -2, -3, -4 | [303] ND (IGFBP-1), ND (IGFBP-2), IGFBP-3, ND (IGFBP-4) |
[304], [305] | NR | [252] | |
| MMP-2 | [55] | [55], [306] | [197] | [56] | |
| MMP-9 | [31], [55] | [55], [125], [306] | [197] | [56] | |
∗∗ Levels are from second trimester AF collection and measurements; NR = not reported; ND = not detected.
The amnion and chorion are comparable in terms of the types of cytokines present but differ in their distribution and concentration [51]. The concentrations range from less than 1 pg/mg to over 6,000 pg/mg, normalized by dry weight, depending on the thickness and location [33, 38, 50, 58]. Multiple cytokines have been found in significantly higher concentrations in fresh chorion compared to other layers, including adiponectin (APN), angiogenin (ANG), ANG-2, bFGF, EG-VEGF, HGF, insulin-like growth factor 1 (IGF-1), PDGF-AA, PDGF-BB, TIMP-2 and TIMP-4 [51]. The greater concentrations per surface area found in the chorion are attributed to the chorion being thicker than the amnion [51]. The amnion layer, however, does contain higher levels of several components, including galectin-7, IL-1F5 and TGF-β1 [51]. The proteomic profile of the intermediate layer has been recently investigated, demonstrating that the layer not only contributes to the overall structural integrity of the membrane but also functions as a source of signaling components important to wound healing applications, such as PDGF-AA, PDGF-BB, TIMP-1, TIMP-2, TIMP-4, TGF-β1, bFGF, EGF, and VEGF [20].
The PM also serves as a barrier to bacterial infection during gestation, containing layers of antimicrobial peptides, such as human defensins, elafin, secretory leukocyte protease inhibitor (SLPI), and histones H2A and H2B [59, 60]. Human β-defensin (HBD) is produced by epithelial cells in the PM [61–63]. Defensins are antimicrobial peptides that prevent the growth of bacteria [61]. The PM also contains cystatin E which has anti-viral properties [64]. There are greater levels of bacterial inhibition from the maternal-facing chorion than the fetal-facing amnion, which points to the role of the chorion as a key barrier layer in preventing infection and rejection [63].
2.5. Clinical Applications and Uses in Regenerative Medicine
The PM has the longest history of use in modern medicine of all placental tissues with the first documented case in 1910 where it was used in skin grafting [6]. This membrane has been extensively researched for use in wound applications, where the sheet configuration easily translates to a wound covering. Apart from its regenerative properties, the tissue is attractive in wound healing applications because it is a sustainable material, typically classified as medical waste, that is immune privileged [36, 65]. While acute and chronic wounds and burns are still the most prevalent application for PM, there is a wide variety of surgical and sports medicine applications where PM is being investigated as a treatment, including ocular reconstruction, spinal surgery, nerve reconstruction, dermatological cases, and as a barrier membrane for adhesion or scarring prevention [4, 11, 12, 36, 66].
The wide array of applications and advances in tissue processing technology has led to the emergence and investigation of numerous PM configurations, including as single-layer, multi-layer, full-thickness and composite grafts, as a micronized powder, and as an injectable matrix [11, 34, 67–70]. Processing and preservation techniques also vary. Depending upon the application, the PM may be left untreated or decellularized to remove donor cells and DNA from the tissue, for example using dispase, EDTA, etc.[71, 72]. Additional processing techniques can include dehydration methods such as oven dehydration and lyophilization, cryopreservation, and cryomilling [26, 67, 69, 70]. Studies have shown that lyophilization prevents deterioration at room temperature for a more stable product that is comparable to fresh placental tissue [66, 69, 73]. Characterization of placental membrane configurations has demonstrated retention of their regenerative potential after processing. However, some studies have reported reduced concentrations of cytokines, as well as altered tissue structures and appearance compared to fresh PM [20, 37, 51, 58].
Over the last two decades, there has been an increased use of PM allografts in the treatment of wounds and burns as more evidence continues to emerge regarding the ECM, therapeutic components, and proven efficacy [20, 74, 75]. Processed PM has been a successful treatment for venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), and chronic wounds, demonstrating decreased time to wound closure [74], On a cellular level, the PM ECM has scaffold characteristics that are beneficial for anchorage-dependent cell attachment and endogenous cytokines that promote proliferation, angiogenesis, cellular migration, tissue remodeling, and create an environment for the resolution of the inflammatory stage of healing and improved remodeling [12, 20, 21, 34, 37, 38]. Clinical applications of dehydrated PM as burn dressings have shown that the PM encourages re-epithelialization of damaged tissue, stimulated by EGF, KGF and HGF, reducing inflammation, decreasing healing time and decreasing scar tissue development [76]. Implanted acellular PM graft in a subcutaneous mouse model encouraged migration and infiltration of host cells during the healing process, specifically of fibroblast-like cells, macrophages, endothelial cells, and fewer T-cells than the control graft [77]. PM has been successfully used in ophthalmology for almost 30 years for the treatment of corneal lesions, retinal detachment, and limbal cell regeneration [78, 79]. Both intact and denuded PM used in ocular reconstruction in a rabbit model achieved re-epithelialization and integration into the rabbit tissue by 3-weeks post-surgery, with lower inflammatory responses noted in denuded PM treated eyes [80]. Extract from digested amnion used in eye drops for corneal treatment has been shown to successfully cultivate limbal stem cells clinically [81].
Placental membrane-derived materials have been increasingly used surgically in reconstructive, vascular, OB-GYN, abdominal, spinal, and neurological cases. Dehydrated PM usage has been shown to result in superior recovery and lower rates of reherniation following microdiscectomy [82]. Retrospective case studies have shown the potential benefits of PM to prevent retethering of the spinal cord following microsurgical intradural lysis adhesion [83]. The preventative use of PM against failed back surgery syndrome (FBSS) caused by epidural adhesions resulted in a significant decrease in the incidence of epidural fibrosis formation compared to standard closure in canine and rat models [84, 85]. There is evidence of PM use in a variety of reconstructive applications including vaginoplasty and vestibuloplasty [86, 87]. The clinical uses of PM in the field of urology are numerous, including penile reconstruction, microsurgical cord denervations, posterior urethroplasty, hypospadias repairs, and urogenital indications, such as vesicovaginal fistulas, as reviewed by Ootamasathien et al. (2017).
A review of the literature will show dozens of animal study investigations of PM as an intraabdominal adhesion barrier, resulting in improved outcomes in various animal models. A retrospective cohort study of 120 patients with 40 patients receiving dehydrated PM barriers used following intrauterine adhesiolysis and 80 patients in the control group found PM to reduce adhesion reformation and improve subsequent menstruation compared to the control group [89]. A study investigating the use of PM as an adhesion barrier following laparoscopic resection of endometriosis with adhesiolysis found that after 1-2weeks no adhesions were observed at point of PM placement in 14 out of 15 cases [90] In a rat model, PM used as a nerve wrapping resulted in a decrease in scar tissue development and an increase in sciatic function, suggesting that in addition to functioning as a barrier, the PM assisted in regenerating the nerve [91]. Recently, a reconstituted, injectable PM powder was investigated in a post-myocardial infarction tissue repair procedure in a rat model, resulting in a decrease of myocardial infarction scar formation and improved left ventricular function [68].
More recently, use of PM in the field of dermatology has been explored, including applications of PM in Mohs surgery, in the management of chronic radiation necrosis, and in the treatment of pyoderma gangrenosum cases [92–94], A case series of the use of dehydrated PM Mohs micrographic surgery for large full-thickness (to the bone) scalp wounds found treatment was well tolerated and resulted in the development of granulation tissue [95]. A case of a 77-year old female with pyoderma gangrenosum treated with dehydrated PM as an adjunct to immunosuppressives reported pain reduction from 10/10 to 5/10 within hours of application that decreased to 0/10 after 1 week and a 56% reduction in wound size after 3 applications.[93].
PM products have also been investigated and used in the past decade to treat various musculoskeletal and orthopedic conditions, including ligament and tendon repair and osteoarthritis (OA) [79]. In a case study focused on flexor tendon injuries, a blunt dissected amnion wrapped tendon resulted in a higher range of active motion, a higher rank on the Strickland and Glogovac scale, compared to the control group [96]. Similarly, a combination injectable matrix composed of PM and umbilical cord has been used to treat knee OA in humans. Results from the pilot study showed an increase in cartilage thickness/volume and a decrease in lesions compared to the control group [97]. In a rat model for Achilles tendon repair utilizing type II diabetic rats to impair healing, dehydrated dual layer amnion/chorion showed improved cell migration, lower failure rates, and more favorable mechanical strength of the tendon compared to untreated controls [98]. Micronized PM has been utilized as an injectable treatment for OA, and in rats with induced OA has shown to result in joints with higher proteoglycan content than controls [99]. Intra-articular injection of lyophilized PM in rabbits for OA showed similar improvements compared to a saline control [79]. A controlled, multi-center trial for PM-derived barriers to encase flexor tendons following repair resulted in significantly increased range of motion and functional scores compared to control [79].
3. Amniotic Fluid
3.1. Anatomy and Development
Amniotic fluid (AF) begins to form around week two of gestation and is the product of water accumulated from maternal circulation that has filled the extracelomic cavity (Figure 1A). In addition to water accumulation via maternal vessels of the decidua and chorion laeve, AF constituents are derived from filtration from the fetal vessels of the umbilical cord and chorionic plate, secretory processes of the amniotic epithelium, and filtration from intracorporeal fetal vessels via the fetal skin [100]. As pregnancy progresses to week 8, prior to the keratinization of fetal skin, water, proteins and other metabolites diffuse freely and bidirectionally between the fetus and AF [101–103]. During this time, the composition of AF resembles maternal and fetal serum. To meet the requirements of development, towards the second half of gestation, the composition of AF begins to change due to fluid regulation and exchange pathways, such as fetal swallowing, lung fluid excretion, fetal urination, and intramembranous absorption [104]. AF volume is primarily regulated through fetal swallowing and intramembranous exchange across the PM. This intramembranous flow transfers solutes and fluid between the maternal circulation and the amniotic cavity. Meanwhile, fetal swallowing allows these substances to be transferred from the amniotic cavity into fetal circulation [104].
A low density population of heterogeneous AF cells called amniocytes is present in AF that are derived from the fetal tissue [105]. Reported cell concentrations in freshly collected AF samples are on the order of magnitude of 105 cells/mL [106, 107]. Amniocytes collected from routine amniocentesis performed during 14-16 weeks gestation have been differentiated and subclassified into one of 3 main groups based on their morphological and growth characteristics in culture: 60.8% amniotic fluid specific type (AF-type) cells, 33.7% epithelioid (E-type), and 5.5% fibroblastic type (F-type) [108]. To identify subclassification of amniocytes in AF, cell culture must be performed to observe the morphological characteristics of the matured colonies [108]. Morphologically, mature E-type cells are large polygonal cells with smooth margins that grow in close contact with each other in culture. They resemble AF-type cells but can be distinguished by their broader growth margin [108]. Mature AF-type cell colonies have pleomorphic morphology and can be differentiated after 12-14 days. They are distinguished by their characteristic “bulls-eye” growth pattern that is not found in E- or F-type AF cell colonies [108, 109]. It has been suggested that AF-type cells in AF fluid are derived from extraembryonic, fetal trophoblastic tissues, yet their function during gestation is not well understood [110, 111]. F-type cells originate from fetal fibrous connective tissue and dermal fibroblasts and generate a ribbed and streaming pattern as they mature in culture. They are spindle-shaped and overlap, forming multi-layered sheets in areas of greater density when cultured [108, 109]. A very small subpopulation, approximately 0.1-0.5%, of AF cells are CD117+ and have differentiation potential. AF mesenchymal stem cells (AF-MSCs) have been an area of focus for regenerative medicine in recent years [112–115]. As the vast majority of studies focused on amniocytes have been performed in vitro, our understanding of their proliferative potential and heterogenous phenotype in vivo is still evolving, especially given the processing of AF-derived products that would likely render MSCs non-viable [112, 116, 117]. A 2019 study found that while 3 AF-derived injectable products all contained signaling components that modulate healing, there were no cells with known characteristics of MSCs in the product [117]
3.2. Physiology
One of the most important functions of AF is its role in fetal support and protection throughout gestation [103]. While AF provides a unique environment that supports fetal growth and movement, it plays a large role in cushioning and protecting the fetus against thermal and mechanical shock [103]. AF also acts to help protect the integrity of the umbilical cord by providing a mechanical cushion between the fetus and the uterine wall, preventing the umbilical cord from being compressed during gestation [101]. Separate from mechanical protection, AF contributes to the innate immunity of the fetal system. A host of antimicrobial and bacteriostatic peptides have been identified in AF (Table 2), which help protect the fetal environment from infection or common bacterial or fungal pathogens [103].
Additionally, AF acts as a reservoir for nutrients, such as essential free amino acids, proteins, lipids, electrolytes, and carbohydrates, as observed in mid-trimester AF [118]. These nutrients originate from maternal plasma and the placenta and are transported to the fetal cavity through placental circulation or, less commonly, across the PM and into the AF through carrier-mediated transporters and channels located in the PM [104, 119, 120]. The composition of these nutrients constantly changes to meet the needs of the developing fetus [118]. At full-term, AF composition is about 98% water and electrolytes. Peptides, carbohydrates, lipids, hormones, and signaling molecules comprise the other 2% [101]. These non-water constituents are soluble components that aid in the structure, protection, nutrition, and overall well-being of fetal development. Glucose is the primary energy source for the fetus during gestation and is transported to the fetus via the placenta as well as from swallowing AF [121, 122]. In an animal model, essential amino acids in AF, such as glutamine, are required for gastrointestinal development, growth, and function, as well as fetal nitrogen and carbon metabolism. Amino acid concentrations naturally decrease towards the end of gestation but could be further reduced by alterations in maternal diet [120].
3.3. Structural Composition
AF analyzed mid-trimester contains ECM components that are secreted by fetal cells suspended in the AF or shed from placental tissues and deposited into AF by fetal urine (Table 1) [123, 124]. Collagen types I, III, and IV are major components of the PM [125]. As the PM is weakened in preparation for spontaneous rupture of the fetal membranes, enzymatic constituents of AF, such as MMP-9, degrade and metabolize the collagen present in the PM, likely leading to collagen deposition into AF at term [125, 126].
It has also been proposed that cultured fetal F-type cells in AF are a dictator of collagen deposition, the main component of the fetal dermal ECM [108], and are responsible for the production of type I and type III collagen and fibronectin in fetal ECM development [109, 111, 127]. In culture, F-type and AF-type amniocytes produce a spectrum of collagenous proteins. In vitro F-type cells predominantly synthesize type I and type III collagen, but AF-type cells synthesize considerably less collagen [128]. Although the importance of these amniocytes during gestation is not well studied, these findings suggest a role of amniocytes in collagen synthesis [127], which may contribute to signaling fetal wound repair and dermal growth [109, 129]. The function of E-type cells in AF is not widely understood but they have been shown to be involved in secretion of type IV collagen and cell-to-cell organization of both keratin and vimentin fibers in culture, further suggesting their role in fetal development [108, 130].
AF contains multiple GAGs and nutrients that may play an important role in providing protection, cushioning, and lubrication to the developing fetus [131–133]. The cushioning and lubrication properties of ECM components present in AF, such as HA, have been a topic of interest in regenerative medicine for their potential therapeutic effects on treating synovial joint pain and prevention of adhesion formation [47, 134]. HA (34%), chondroitin-6-sulfate (20%), non-sulfated chondroitin (14%), chondroitin-4-sulfate (13%), heparan sulfate (6%), and dermatan sulfate (5%) make up most of the GAGs found in AF [135].
The relatively low concentrations of hyaluronidase in AF allow for an environment rich in HA [136]. HA serves to increase AF viscosity, provides support and lubrication in the uterine environment, and may also play an important role in fetal wound healing [137]. Studies show that HA provides a mesenchymal signal for regenerative healing in fetal wounds, contributing to the scarless healing observed in fetuses, and potentially providing intercommunication between the fetus and surrounding tissues through the AF [132, 138, 139].
Fibronectin is a glycoprotein found in high concentrations in AF compared to plasma [140]. It is secreted by both F-type and AF-type cells and is associated with the pericellular and PM ECM [127]. The physiological functions of fibronectin in AF are relatively unknown, but fibronectin present in AF carries double the carbohydrates when compared to plasma fibronectin, allowing for better protection against proteolytic digestion [140]. Fibronectin may be responsible for the rapid, scarless wound healing observed in fetal wounds as deposition of fibronectin occurs earlier in fetal wounds than in adult wounds [131, 141].
3.4. Cytokines and Antimicrobial Components
AF is rich in cytokines that are secreted rapidly from fetal cells, including fetal macrophages, suspended in AF as they are signaled to aid in tissue remodeling, protease inhibition, antimicrobial defense, and angiogenic and anti-inflammatory signaling during gestation (Table 2) [103, 114, 142]. Many are investigated for their role in therapeutic applications, such as EGF, bFGF, IL-1ra, PDGF-BB, VEGF, and TIMPs. It is believed that many of these cytokines do not readily cross the placenta. Interleukins present in AF are important for cellular communication and the regulation of inflammation [143–145]. Most research on the proteomic make up of AF comes from pre-term analysis via amniocentesis in the study of biomarkers indicative of spontaneous pre-term delivery and other conditions, such as pre-eclampsia [16]. Further studies have investigated the AF-MSC secretory profile and their impacts on wound healing, revealing that AF-MSC conditioned media has higher profiles of IL-6, IL-8, VEGF, EGF, and TGF-β that successfully promoted healing pathways in dermal fibroblasts [146]. In second trimester AF, the two most abundant cytokines are anti-inflammatory IL-1ra and pro-inflammatory interferon gamma-induced protein 10 (IP-10) [142]. IL-1ra in AF helps protect the fetus against inflammatory damage caused by overactivation of IL-1β [142]. IGF-1 is an important factor in tendon healing, as it has a positive effect on tenocyte proliferation in vitro [147]. In AF, growth factors such as IGF-1 are primarily involved in intrauterine growth via enterocyte proliferation and has been shown to increase fetal intestinal growth and maturity [148, 149]. The IGF system is the dominant endocrine determinant of fetal growth because of its role in regulating insulin and growth hormone [150]. IGF-1 plays an important role in tissue regeneration and wound healing, and has been especially studied in the repair of tendon and joints [151, 152]. For instance, IGF-1 has been shown to have a positive effect on tenocyte proliferation in vitro, and to play a role in preventing cartilage metabolism in the development of OA [147, 151].
AF is also a key regulator of the innate immune system and contains enzymes, immunomodulatory mediators, and antimicrobial polypeptides and lipids that aid in antimicrobial defense and fetal skin immunity [136]. Human β-defensin-1 (HBD-1) concentrations increase in the presence of microorganism infection in the amniotic cavity, demonstrating the participation in host-defense mechanisms [153]. Other critical antimicrobial components of AF include HBD-2, lysozyme, cystatin C, and lactoferrin, which prevent infection via iron binding function that inhibits bacterial, viral, fungal, and parasitic infection.
3.5. Clinical Applications and Uses in Regenerative Medicine
AF has become increasingly used in regenerative medicine applications because of the rich mixture of growth factors, antimicrobial factors, amniocytes, and GAGs [154]. The material translates well as a raw material for medical use because of its availability and the immune privileged state of placental-derived tissues. The antimicrobial constituents of AF contribute to its favorable potential as a biomaterial in regenerative medicine, especially in the treatment of chronic wound infections [143, 154]. Many configurations and processing techniques for AF have been investigated for therapeutic applications. Minimally filtered or centrifuged AF solutions have been used or investigated in near-native states for the treatment of OA, tendonitis, plantar fasciitis, wound healing, and adhesion prevention [155–159]. Unlike most allograft or biological tissues currently used for biomedical purposes, AF is advantageous for the supplementation of fluid environments. Following filtration or centrifugation to remove various constituents, different preservation techniques have been investigated, including cryopreservation and lyophilization to a powder; additionally, combinations of AF-derived allografts and other materials, such as alginate-based hydrogels have been investigated [137, 154, 157, 160, 161]. AF has also been used to make amniotic suspension injectable products, containing a combination of micronized human PM and AF components, such as cells [147, 156, 158].
AF-derived materials have been investigated for use in treating OA, rheumatoid arthritis (RA), various tendon injuries, bone defects, corneal defects, abdominal adhesions, and in novel wound healing therapies [133, 154]. As an injectable therapy, AF has been administered in the form of an amniotic fluid suspension or as a fresh medium [147, 156, 158, 162]. It has been suggested that the high HA content in AF makes it a promising candidate for treatment of RA and OA when injected into the synovial fluid of diseased joints [162]. This is likely due to the cushioning and protection properties of HA and the anti-inflammatory components of AF [47, 156, 162]. Vines et al. found that a single intra-articular injection of an amniotic suspension allograft (ASA) containing cryopreserved amniotic fluid cells statistically increased IgG and IgE levels in moderate to severe OA patients, demonstrating the feasibility for the treatment of knee OA [158]. In an animal model with induced OA, treatment with cryopreserved ASA containing AF cells resulted in significant decreases in pain thresholds and swelling [156]. There were also increased levels of IL-10 in the synovial fluid surrounding the joint induced with OA, suggesting the promotion of an anti-inflammatory environment when treated with the ASA-containing AF cells [156]. When studying the effect of ASAs containing AF cells on tendon healing, Kimmerling et al. found that ASA injections greatly increased density and migration of tenocytes as well as deposition of ECM proteins, including collagen and elastin, in vitro [147]. In the presence of the ASA, less TGF-β1 was produced and IL-8 was downregulated, suggesting an anti-inflammatory effect of the AF-containing allograft. These findings allow for a better understanding of the potential use of ASAs containing AF cells in tendon repair and healing [147]. AF injections have also been investigated for stimulating neochondrogenesis when injected under free perichondral grafts in rabbits. A single 0.3mL dose of freshly collected AF was effective in promoting proliferation and differentiation of chondrocytes as well as stimulating new cartilage formation in the treatment of certain cartilage defects [162]. It was suggested that these results were likely due to the high concentrations of HA and other growth factors in AF [162]. In an animal model, freshly collected AF was applied topically to tendon sheaths to evaluate the effect on peritendinous adhesion formation and differences in tensile strength. Significantly fewer adhesions were observed in tendons treated with AF and they had significantly higher load values compared to the untreated tendon controls. Their results suggest that human AF is effective in facilitating tendon healing and preventing adhesion formation [134].
Amniotic fluid for the treatment of chronic soft tissue defects and wounds has been increasingly utilized over the last decade [154]. In chronic diabetic wounds, AF is a relatively new innovation in the treatment of chronic soft tissue defects and wounds [154]. In chronic diabetic wounds treated with processed, lyophilized AF, Bazrafshan et al. found significant increases in the rate of wound closure compared to control wounds not treated with AF [154]. A significant increase in the number of large vessels in the wounds injected with AF was also observed. Recently, alginate hydrogel-electrospun silk fibroin fibers combined with AF resulted in increased cellular proliferation, spreading, and higher collagen secretion in vitro [161].
Longaker et al. found that in incisional wounds of fetal lambs, cultured fetal fibroblasts in AF produced more collagen than adult fibroblasts, leading to a faster rate of collagen diffusion into the AF from the ECM of placental tissues, and therefore reduced scar formation [163]. In an in vitro wound model, Nyman et al. found that the rate of re-epithelialization was significantly increased and hyaluronidase activity was inhibited when treated with, centrifuged, filtered mid-trimester AF collected from amniocentesis [137]. Fresh AF has been used to treat various corneal disorders because of its reported positive effects on corneal sensitivity and decreased scar formation in the cornea [160]. In a study evaluating the effect of fresh AF on corneal epithelial defects in rabbits, topical application of AF to the cornea resulted in accelerated re-epithelialization and corneal wound healing and protection of corneal keratinocytes [160]. EGF is important in AF for promoting the growth of various developing organ systems, importantly the gastrointestinal tract and the lungs [164]. Investigation of EGF stimulation of hyaluronan synthesis in vitro resulted in abundant hyaluronan levels during keratinocyte migration into an open wound [165]. Signaling components from AF have been shown to retain their bioactivity after processing and lyophilization, promoting increased wound closure rates, cellular proliferation, and stimulating angiogenesis in induced chronic diabetic wounds [154].
4. Umbilical Cord
4.1. Anatomy and Development
The umbilical cord (UC) is a tubular connection between the fetus and placenta and acts as a conduit for vessels that provide an exchange of oxygen and nutrient-rich blood between the developing fetus and the placenta [166]. The UC protects and facilitates blood flow despite various forces within the fetal compartment that could compress, kink, or extend the tissue, such as fetal grasping and external pressures [167]. The UC consists of three blood vessels (a vein and two arteries), a thick supporting extracellular matrix (Wharton's Jelly), and a simple amniotic epithelium that encases the tissue. The UC vein takes nutrient-rich blood from the placenta to the fetus, and the two arteries return nutrient-depleted blood to the placenta (Figure 1C) [168, 169]. The arteries do not have an elastic membrane or an adventitia to protect the arterial integrity and blood flow, rather they are protected by smooth muscle cells and a mucoid connective tissue called the Wharton's Jelly [168–172]. This thick connective tissue constitutes the majority of the umbilical cord and has become an interesting point of discussion in regenerative medicine, and as such is the focus of this section. At term, the Wharton's Jelly is divided into three zones: the perivascular Wharton's Jelly, the intermediate Wharton's Jelly, and the subamnion [167]. These zones are characterized by the differentiation stages of the cellular populations and structure of the ECM [167]. The high concentration of ECM components in Wharton's Jelly, like water-soluble proteoglycans and HA, mixed with collagen structures, interact with water resulting in unique biomechanical properties that protect the blood vessels from compression [173]. Surrounding the entirety of the UC is an epithelial layer lining, or the “amnion” of the UC, which is contiguous with the epithelial layer of the PM and contributes to the stability of the UC by protecting it from mechanical forces in the womb [174].
The development of the UC occurs alongside that of the amnion. The precursor UC, called the body stalk, connects the embryo to the developing placenta. Around day 18 of gestation, the amniotic cavity expands and surrounds and covers the body stalk. During the first trimester, the UC lengthens, pushing the embryo further into the amniotic sac [168]. Blood flow has been documented in the UC around the end of the 5th week of gestation [175, 176]. At term, the UC length can vary between 35 – 70 cm long and 1 – 2 cm in diameter [167, 174, 177].
4.2. Physiology
The UC connective tissue is structured to protect the integrity of the blood vessels so as to maintain constant blood flow to the fetus [174]. The smooth muscle cells located in the arterial media facilitate contraction and blood flow in place of elastin, and the Wharton's Jelly provides a rigid structure to encase the arteries and protect vessel integrity [178]. The Wharton's Jelly also physically assists in the UC arterial contractions, regulating the pulsations that drive blood flow to the fetus [179, 180]. The contractibility of the smooth muscle cells in the vessel walls is provided by endocrine mediators like serotonin, angiotensin, and oxytocin, as well as paracrine mediators such as prostaglandins [100].
The structure of the Wharton's Jelly is honeycomb in nature, composed of a network of interconnected collagen pores that contain water-binding GAGs [169]. This porous structure allows for the movement of the mucoid ground substance as the UC contracts and expands during blood vessel contractions. These compartments also allow for the diffusion of signaling components from the Wharton's Jelly to other areas such as the amniotic cavity [181]. The composition of the Wharton's Jelly is not homogeneous and, therefore, the mechanical properties across the tissue vary. The porosity at the fetal end of the UC is higher than the maternal side [182]. Young's modulus, measuring elasticity, and the Aggregate modulus, measuring stiffness, of Wharton's Jelly is higher on the fetal side of the UC, indicating that this area of the tissue resists deformation, likely due to the smaller pore sizes at the fetal end [183]. The behavior of these smaller pores resisting flow is called poroelastic behavior and is aided by hydrophilic molecules, such as HA and proteoglycans [169].
The Wharton's Jelly is sparsely populated with cells and investigation of the distribution of cells in the Wharton's Jelly by electron microscopy and immunohistochemical staining shows that Wharton's Jelly contains cells at various levels of differentiation from fibroblasts towards myofibroblasts [184]. The density of cells is higher around the UC vessels and lower at the epithelial lining [172, 178]. Several cell types have been identified in the UC tissue, including myofibroblasts, stromal cells, mast cells, and MSCs, which are responsible for maintaining the Wharton's Jelly ECM [167, 179, 185, 186].
4.3. Structural Composition
Wharton's Jelly ECM is abundant and consists mainly of collagens (over 500mg/g tissue), proteoglycans, and GAGs, like HA and heparan sulfate, immobilized and imbedded in the collagen network, which provides structural support and maintains the mucoid ground substance (Table 1) [187, 188]. The proportional relationship of collagen type I (47%), III (40%), V (12%), and other collagens in Wharton's Jelly shows that collagen type I and III are the most abundant in this matrix and their proportional concentration is consistent with that of other soft tissues which typically report a higher amount of collagen type I than collagen type III [188]. Collagen is recruited to increase the stiffness of the tissue [173]. Proteoglycans, such as chondroitin sulfate, dermatan sulfate, heparan sulfate and keratin sulfate, also help provide structural support to Wharton's Jelly by regulating cellular interactions, and modulating the organization of the ECM matrix as it undergoes remodeling during gestation [189–191]. Wharton's Jelly does not contain elastin fibers, so under low strains the GAGs contribute to the elasticity of the material and the ability to retain shape under high strain [186, 189]. Up to 70% of the GAG content in UCs is HA and these high concentrations (approximately 4mg/ml) give the tissue a hydrophilic nature [188, 192, 193].
4.4. Cytokines and Antimicrobial Components
The ECM of Wharton's Jelly is embedded with a variety of growth factors (Table 2), including aFGF, bFGF, IGF-1, PDGFs, EGFs TGF-β1, IL-1, MMPs, and TIMPs [186, 194]. These signaling factors are vital to cell migration, proliferation, differentiation and remodeling of the UC ECM to maintain mechanical stability, elasticity and resistance to compression necessary to protect the UC vessels [186]. The low number of cells, especially myofibroblasts, present in Wharton's Jelly are especially stimulated to synthesize and regenerate the ECM of the Wharton's Jelly [195]. Reservoirs of signaling molecules are distributed throughout the ECM to be easily accessible to cells that regulate the tissue remodeling process of the ECM and vessels [172, 186]. For instance, high levels of MMP-2 drive the UC vessel reconstruction during gestation [196, 197]. Sulphated GAGs present in the Wharton's Jelly, such as heparin sulphate and heparin found in proteoglycans, bind cytokines and growth factors to the ECM [195]. Binding to proteoglycans protects these signaling molecules from degradation via proteolysis, creating a stable reservoir available for remodeling [195]. Studies have reported that growth factors, such as bFGF, TGFβ, and IGF, are embedded close to these cells and their release from the ECM during matrix remodeling stimulates the surrounding cells [195, 197, 198].
Proteomic analysis of the Wharton's Jelly has identified cytokines of interest to researchers for regenerative medicine applications, such as anti-inflammatory cytokines and antimicrobial components [194]. Pro-inflammatory cytokines and anti-inflammatory cytokines work in a cascade to respond to tissue damage by removing foreign bodies through the inflammatory response before anti-inflammatory cytokines rapidly reduce inflammation and potential scarring [194, 199]. The anti-inflammatory cytokine IL-1ra is present in Wharton's Jelly, competing with IL-1 to prevent cell changes and prevent inflammation [194, 200]. Other immunomodulatory cytokines found in Wharton's Jelly, such as RANTES, IL-6R, and IFN-γ, play a vital role in the regulation of T cells, the immune response, macrophage activation and contribute to bacterial immunity in fetal tissues [194, 201]. Although further investigation is needed for a complete proteomic profile of UC tissue, initial studies suggest there is a large concentration of cytokines of interest for applications such as cartilage regeneration and tendon repair [186, 194, 195, 202].
4.5. Clinical Applications and Uses in Regenerative Medicine
UC tissue has found applications in regenerative medicine as a tissue graft and scaffold [203, 204]. The structure of the UC tissue provides an ECM scaffold for successful cell attachment and a reservoir of growth factors that play a role in tissue regeneration and cellular proliferation, as demonstrated by work with Wharton's Jelly and bone marrow MSCs and osteocytes [186, 205]. The composition of native Wharton's Jelly is attractive for wound healing applications because its natural ECM scaffold can overcome the shortcomings of other synthetically designed scaffolds that do not provide an ideal microenvironment [206]. UC tissue has been investigated for use in multiple applications, including wound healing, cardiovascular repair, cartilage repair, and treatment of diseased joints [3, 99, 204, 207–209]. Chemically stabilized umbilical cord vessels are popular for vascular reconstruction because of their length and they are often chosen over synthetic alternatives [3]. The configurations of UC tissue being researched are varied, including Wharton's Jelly sheet configurations of varying thicknesses, UC electrospun scaffolds, powderized configurations mixed with amnion material, and hydrogel compositions [205, 206, 209–211]. The processing methods that have been investigated are equally varied, including cryopreserved, lyophilized, cryomilled, electrospun, and combinations with pharmaceutical vehicles [206, 212, 213].
Clinical applications of cryopreserved, devascularized UC have been used for the treatment of DFU, with complete wound closure and re-epithelialization in the majority of patients and decreased development of scar tissue, linked to the downregulation of TGF-β [211]. In vitro, the extracted soluble components of a lyophilized UC sheet promoted human dermal fibroblast wound closure and the promotion of angiogenesis, and in an in vivo rat model the lyophilized, devascularized UC grafts were shown to resorb into the wound beds within 3 months [207]. A study evaluating the potential of UC tissue use in cartilage repair applications characterized Wharton's Jelly and drew parallels between its composition and that of native cartilage [204]. Decellularized and electrospun Wharton's Jelly scaffolds were investigated in cartilage tissue engineering, demonstrating desirable mechanical properties, biocompatibility, and support for cell attachment [206]. Wharton's Jelly has also been combined with other fetal tissues such as amnion in micronized configurations that can be resuspended for injection [209]. These matrix injections have been targeted for use in joint spaces where the disease state involves a decrease in the concentration of proteoglycans and damage to the ECM [99, 208]. In a rat model, knee joints injected with umbilical cord-amniotic membrane matrix saw a reduction of disease progress and reduced inflammation with no immune response, attributed to the presence of interleukins, such as IL-4, which inhibit the breakdown of proteoglycans, as well as IL-10 signaling the biosynthesis of new proteoglycans and collagen type II [209].
5. Placental Disc
5.1. Anatomy and Development
The placental disc is the transient organ that exists to support fetal development through the exchange of nutrients between maternal and fetal bloodstreams (Figure 1D). To maintain an immunological barrier, the placental disc is structured to keep the blood streams separate while maximizing surface area for the transport of nutrients between them. The placental disc begins to develop from the embryonic blastocyst when it implants into the maternal endometrium after fertilization [214–217]. The outer cells of the blastocyst develop into the placental trophoblasts, which invade the maternal tissue [214–216]. These fetal trophoblasts eventually form the fetal portion of the placental organ [214, 215]. At term, this is comprised of the chorionic plate with vascular protrusions, called chorionic villi, that extend into uterine tissue [214–217]. The UC vessels insert into the chorionic plate where they branch out into a network of vessels and capillaries in the villous structures [2]. Mature chorionic villi consist of vessels supported by a mesenchymal core, basement membranes, and a tissue layer encapsulating each villus made up of a syncytiotrophoblast layer, a cytotrophoblast layer, and an ECM [215, 218]. Trophoblast cells, mesenchymal cells, and endothelial cells of vessels are the main cell types of the placental disc [2, 215]. The syncytiotrophoblast cell layer results from the differentiation of cytotrophoblast cells creating a barrier between the maternal and fetal compartments [214–216, 218].
The maternal side of the placental disc consists of the basal plate, formed from decidual endometrial tissue [2]. Maternal blood flow is supplied by radial arteries of the myometrium that are supplied from ovarian and uterine vasculature [219]. This is pumped into the intervillous space between the basal and chorionic plates, washing the chorionic villi in maternal blood for the active and passive diffusion of gases, proteins, and nutrients
As pregnancy progresses, the placental disc grows to increase surface area to accommodate the growing demands of the fetus, allowing for additional blood flow. This is also marked by a reduction in the cytotrophoblast layer, resulting in a single syncytiotrophoblast layer between fetal and maternal blood flow [219]. At term, the placental disc is 15 to 20cm in diameter, 2 to 3cm thick and weighs approximately 500g. This gives term placental discs almost 15m2 of surface area for exchange between mother and fetus [219].
5.2. Physiology
The placental disc is the site of exchange for gases and metabolites between maternal and fetal blood, and the secretion of hormones for the support of healthy pregnancy. Substances in the maternal blood pass from the intervillous space, through the syncytiotrophoblast layer, the fetal connective tissue, and finally the fetal capillary endothelium to enter the fetal blood stream [219, 220]. Fetal lungs are not utilized for gas exchange until after birth, so all exchange of oxygen and carbon dioxide goes through the placental organ [219, 221]. Oxygen and carbon dioxide molecules are small enough to pass through the chorionic villi and fetal capillaries via passive diffusion, relying on their partial pressure gradients between the intervillous space and umbilical arteries [219, 221]. Key metabolites required for fetal development pass through the placental disc, including fatty acids, amino acids, water, electrolytes, and glucose [220, 222]. While water and electrolytes can mainly pass the placental barrier by passive diffusion or the aid of simple water channels in the trophoblasts, other metabolites require carriers, enzymatic cleavage before transfer, or facilitated transfer [219, 222–225]. For example, as fetal gluconeogenesis is limited, a high demand for maternal supplied glucose is needed, which requires the active use of glucose transporters [219, 224, 226].
The placental disc also serves an endocrine and immunological function, producing hormones and passing maternal immunity to the fetus. The syncytiotrophoblast layer acts as the endocrine tissue of the placental disc, which secretes human chorionic gonadotropin (HCG), human chorionic somatomammotropic hormone, human growth hormone variant, progesterone, and estrogens [219, 227–229]. These support pregnancy by stimulating metabolite production and enabling uterine and placental growth [219, 229]. To provide initial passive immunity to the fetus, the placental disc serves an important immunological role, allowing for the transport of maternal IgG class antibodies to the fetus [230]. These IgG antibodies are transported into the fetal blood stream via pinocytosis. IgG antibodies bind to the syncytiotrophoblasts, where they are then enclosed in vesicles and transported to the fetal blood stream to provide initial immunity for the fetus [231]
A major function of the placental disc is to maintain immune privilege and prevent rejection of the fetal tissue by the maternal immune system. One way this is achieved is by the low number of MHC molecules present in the tissue. No classical MHC class I or II antigens are found on the syncytiotrophoblast cells, in direct contact with maternal tissue [217]. Other cells of the placental disc express very low amounts of classical MHC molecules (HLA-A, HLA-B, and HLA-C), preventing the maternal immune system from recognizing the cells as not “self” [2]. The invasive cytotrophoblasts do have nonclassical class I HLA-G molecules, which have been hypothesized to prevent rejection from the mother by acting as the “universal “self” transplantation antigen” [217].
5.3. Structural Composition
The placental disc ECM is heavily structured in collagens, fibronectin, laminin, proteoglycans and GAGs (Table 1). The structure has a strong, organized collagenous skeleton to mechanically support the chorionic vessels and the trophoblast cell layer [232]. The basement membrane of the chorionic vessels and the stroma of the chorionic villi have a dense ECM that provides mechanical support and structure for the growth and differentiation of chorionic cells [232–234]. The villi structure is a continuous matrix of collagen fibrils and bundles woven together in different layers [232]. The structure of the network is dependent upon the stem size, thickness, and cell population [232]. The villi basement membranes contain the characteristic collagen type IV as well as collagens type I and III [233]. Additionally, collagens types I, II, III, IV, V, VI, and XIV have been extracted from human placental disc (Table 1) [232, 235]. Collagen XIV binds strongly to basement membrane-derived heparan sulfate and may be relevant in situ [235]. The trophoblasts are in contact with collagen throughout the life of the placental disc where it helps regulate trophoblast function [232]. The chorionic plate ECM plays a role in mediating development where collagen type IV has been shown to function in the differentiation of mesodermal cell lineages [232].
The placental disc contains high levels of insoluble fibronectin in the ECM [236]. Fibronectin promotes cell adhesion and has been shown to promote cell migration in culture and plays a role in the organization of the placental disc [236, 237]. Fibronectin has been particularly studied for its interaction with proteoglycans, for instance, binding to heparan sulfate in the placental disc [238]. This is structurally distinct fibronectin from the forms found in AF and maternal plasma [239]. Placental-derived fibronectin can bind twice as much carbohydrate as the plasma type, increasing its resistance to protease degradation and regulating cell interactions with tissue and other cells [240]. The fibronectin composition of chorionic villi changes through gestation and fibronectin is thought to play a role in the development of the placental tissue [239]. Higher fibronectin concentrations in early pregnancy correspond with the rapid proliferation rates of the cytotrophoblast cell column and shell in early villi development [239]. It's postulated that the trophoblasts produce less fibronectin as they mature, with more fibronectin being produced by the fibroblast and endothelial cells of the villi core [239].
Laminin is present in placental disc basement membranes of the villous trophoblast, fetal capillaries, and maternal portion of the placental disc [218, 234]. As villi develop, laminin becomes more concentrated at the villi mesenchymal cores where it contacts the trophoblast cell layer [235]. Additionally, an array of GAGs has been detected throughout placental disc tissue, including HA, dermatan sulfate, chondroitin 6-sulfate, small amounts of heparan sulfate, and large amounts of chondroitin 4-sulfate [241]. GAGs are bound or associated with the collagens of the basement membranes and fibronectin [241]. The large concentration of heparan sulfate is likely due to the extensive vascularity of the placental disc, as it is found in vessel walls [241]. Many roles have been ascribed to these molecules in utero, including providing electrochemical barriers to immunocompetent cells at the maternal sinuses and mesenchymal core, preventing structural compression of the placental disc due to the high water content held by these molecules, and acting as endogenous inhibitors of thrombin, preventing coagulation of blood and the loss of nutrient exchange [241, 242].
5.4. Cytokines and Antimicrobial Components
The majority of known cytokines have been detected in the placental disc and associated membranes (Table 2); however, the concentrations in the placental disc are known to vary with the progression of pregnancy and are not well characterized [243, 244]. The host of cytokines produced by the placental disc help modulate the immunological processes required for implantation, placental growth, and pregnancy [243]. Resident placental disc cells, including trophoblasts, endothelial cells, and stromal cells, secrete hormones and cytokines to support tissue metabolism, endocrine function, placental proliferation, angiogenesis, apoptosis, trophoblast invasion of the maternal tissue, and to help prevent rejection of the fetal tissue [244, 245]. For instance, a major role of the placental disc is to continually develop vasculature and villi to support fetal development. Placental growth factor (PlGF) is produced by trophoblast cells specifically and functions with VEGF, PDGFs, and leptin to promote vascular development [244, 246]. MMPs are also drivers of remodeling of the spiral arteries of the placental disc during gestation;, they are responsible for the migration of cytotrophoblasts, and the implantation into the maternal wall [56]. The placental disc also acts as a microbial barrier between fetal and maternal tissues, expressing an array of antimicrobial peptides that interplay with those expressed by the fetus and maternal cells to provide broad-spectrum microbial protection [247]. These include elafin/trappin-2, bactericidal/permeability-increasing protein (BPI), SLPI, HBD2, acyloxyacyl hydrolase (AOAH), and cathelicidin (CAP18) [247, 248].
5.5. Clinical Applications and Uses in Regenerative Medicine
Recent interest in the placental disc as a biomaterial has increased due to its unique function, physiology, biochemical composition and immune privilege [1, 2, 234]. This material translates well into the regenerative medicine space because of its low immunogenicity and structural and signaling components which are advantageous to the wound healing process [3]. Additionally, placental discs yield a large amount of material that can be manipulated into different tissue product configurations, processed to extract ECM components, and combined with other materials to yield gels and devices [234]. Researchers have investigated potential therapeutic applications of different components of the placental disc for several decades [2]. These include applications in the treatment of wounds, RA intervertebral disc degeneration, as well as in surgery, neurology, gynecology, and dermatology [2]. Processing of placental disc to obtain useable, clean extract involves decellularization and additional processing to obtain an extract of desired proteins, enzymes, fatty acids, and other metabolites useful in the wound healing process [249]. These processes have been developed to retain the majority of ECM and cytokine content of the cells [234]. Pharmaceutically prepared placental disc extracts have been shown to enhance the proliferation of fibroblasts and cord blood cells in vitro [2]. Animal studies have shown these placental disc extracts reduce concentrations of free radicals and inflammatory cytokines, as well as increase the proliferation of progenitor cells in vitro [2]. Use of a human placental disc hydrolysate in ligament healing of a rodent model showed marked anti-inflammatory effects, though did not impact structural characteristics of the ligament [250]. Similarly, a pharmaceutical hydrolyzed extract had antioxidative and anti-apoptotic effects, having a positive impact on skin flap survival in a rat model [251]. The presence of TGFβ, VEGF, and FGF in placental disc extracts are associated with the increased rate of epithelialization, amplification of angiogenesis, and reduction in pain when treating non-healing wounds and burns [2]. The use of placental disc-derived ECM as a dermal substitute has also been investigated because of the similarities in cellular arrangement to that of skin [252]. Resulting studies showed that the homogenized, decellularized substitute modulated dermal healing and promoted the growth of new cells [252]. The ECM composition of the placental disc has been shown to provide the ground material for the adhesion and proliferation of cells [253].
Hydrogels prepared from isolated placental disc ECM have been shown to retain the high collagen, laminin, glycoprotein, and growth factor content of the native placental disc and to support cardiomyocytes in culture and blood vessel formation by endothelial cells, which is associated with the regenerative, pro-angiogenic, and mitogen characteristics of the ECM components [254]. Placental disc extracts combined with other materials, like silk fibroin, have shown success in a rat model for full-thickness wounds, demonstrating enhanced angiogenesis, granulation tissue formation, and re-epithelialization, resulting in improved epidermal-dermal junctions compared to a collagen-incorporated silk fibroin [255]. There has also been interest in using the placental disc ECM as a scaffold to generate organs and tissues in vitro and in vivo due to the matrix and vasculature structure of the placental disc [256, 257]. Initial results show that the placental disc framework successfully supported transplanted liver fragments and the structure is able to re-endothelialize with adjacent cells [256, 257].
6. Conclusion
Placental tissues are a rich, varied, and sustainable source of healthy ECM and soluble biochemical components. These tissues are available from a healthy, screened population and are otherwise treated as medical waste. Research and clinical usage of these tissues since the turn of the 20th century demonstrate the current and future potential of these materials within regenerative medicine applications.
Evaluation of the composition of these native placental tissues reveals many similarities in the variety of signaling components that regulate inflammation, angiogenesis, tissue remodeling, and immune function. The varied structural compositions of these tissues have led to a wide array of configurations, suited to many regenerative applications. This in-depth comparison of placental tissue types can offer a roadmap to the selection of a placental biomaterial most suited to new applications. The placental membrane is a thin sheet with varying layers of collagens, laminins, elastins, proteoglycans, and GAGs. Amniotic fluid is another distinctive choice as a biomaterial that has the advantage of being an immune privileged fluid with soluble regulatory components and cellular constituents. The available material from the UC structure provides a scaffold rich in HA and collagens with unique biomechanical properties that can be advantageous in tissue engineering. The placental disc provides a high volume of collagens, fibronectin, laminin, and a high concentration of heparin sulfate that can be configured in varied formats.
While the use of placental membrane in wound applications is well established, novel configurations of placental tissue for a wide array of clinical applications are increasingly being investigated and implemented in recent years. Consistently, results from placental tissue use in animal models and clinical studies have shown therapeutic impacts, attributed to the combination of collagens, fibronectins, proteoglycans, GAGs, cytokines, and growth factors contained within these tissues. Increasingly, these tissues represent a unique raw material source for the development of innovative configurations for use in regenerative medicine.
Acknowledgments
We thank Avery Edelman for design support in figure creation. This work was supported by StimLabs, LLC.
Data Availability
This article is a review article. All data can be found in the cited references.
Conflicts of Interest
All authors declare that they are employees of StimLabs, LLC.
References
- 1.Gupta S., Gupta R. Placental Tissues- From Reproductive to Regenerative Biology. Int. J. Sci. Res. IJSR . 2014;3:607–612. [Google Scholar]
- 2.Pogozhykh O., Prokopyuk V., Figueiredo C., Pogozhykh D. Placenta and Placental Derivatives in Regenerative Therapies: Experimental Studies, History, and Prospects. Stem Cells International . 2018;2018 doi: 10.1155/2018/4837930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore M. C., Van De Walle A., Chang J., Juran C., McFetridge P. S. Human Perinatal-Derived Biomaterials. Advanced Healthcare Materials . 2017;6(18):p. 1700345. doi: 10.1002/adhm.201700345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silini A. R., Cargnoni A., Magatti M., Pianta S., Parolini O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Frontiers in Bioengineering and Biotechnology . 2015;3 doi: 10.3389/fbioe.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ober W. B. Notes on placentophagy. Bulletin of the New York Academy of Medicine . 1979;55(6):591–599. [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J. S. Skin Transplantation with a review of 550 cases at the Johns Hopkins Hospital. The Johns Hopkins Medical Journal . 1910;15(307):307–398. [Google Scholar]
- 7.Brunt K. R., Weisel R. D., Li R.-K. Stem cells and regenerative medicine - future perspectives. Canadian Journal of Physiology and Pharmacology . 2012;90(3):327–335. doi: 10.1139/y2012-007. [DOI] [PubMed] [Google Scholar]
- 8.Lindenmair A., Hatlapatka T., Kollwig G., et al. Mesenchymal Stem or Stromal Cells from Amnion and Umbilical Cord Tissue and Their Potential for Clinical Applications. Cell . 2012;1(4):1061–1088. doi: 10.3390/cells1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava M., Ahlawat N., Srivastava A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. Journal of Obstetrics and Gynecology of India . 2018;68(1):15–19. doi: 10.1007/s13224-017-1034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan A. I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Translational Medicine . 2017;6(6):1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamede A. C., Carvalho M. J., Abrantes A. M., Laranjo M., Maia C. J., Botelho M. F. Amniotic membrane: from structure and functions to clinical applications. Cell and Tissue Research . 2012;349(2):447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 12.Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., Seifalian A. M. Properties of the amniotic membrane for potential use in tissue engineering. European Cells & Materials . 2008;7:88–99. doi: 10.22203/eCM.v015a07. [DOI] [PubMed] [Google Scholar]
- 13.Verbruggen S. W., Oyen M. L., Phillips A. T. M., Nowlan N. C. Function and failure of the fetal membrane: Modelling the mechanics of the chorion and amnion. PLoS One . 2017;12(3) doi: 10.1371/journal.pone.0171588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant-Greenwood G. D. The extracellular matrix of the human fetal membranes: structure and function. Placenta . 1998;19(1):1–11. doi: 10.1016/S0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 15.Hieber A. D., Corcino D., Motosue J., et al. Detection of elastin in the human fetal membranes: proposed molecular basis for elasticity. Placenta . 1997;18(4):301–312. doi: 10.1016/S0143-4004(97)80065-3. [DOI] [PubMed] [Google Scholar]
- 16.Hong J.-S., Romero R., Kusanovic J. P., et al. Trophoblast islands of the chorionic connective tissue’ (TICCT): a novel placental histologic feature. Placenta . 2013;34(4):360–368. doi: 10.1016/j.placenta.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.-S., Romero R., Kim J. S., et al. Coexpression of myofibroblast and macrophage markers: novel evidence for an in vivo plasticity of chorioamniotic mesodermal cells of the human placenta. Laboratory Investigation . 2008;88(4):365–374. doi: 10.1038/labinvest.3700749. [DOI] [PubMed] [Google Scholar]
- 18.Meinert M., Eriksen G. V., Petersen A. C., et al. Proteoglycans and hyaluronan in human fetal membranes. American Journal of Obstetrics and Gynecology . 2001;184(4):679–685. doi: 10.1067/mob.2001.110294. [DOI] [PubMed] [Google Scholar]
- 19.Parry S., Strauss J. F. Premature rupture of the fetal membranes. New England Journal of Medicine . 1998;338(10):663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 20.Roy A., Griffiths S. Intermediate layer contribution in placental membrane allografts. Journal of Tissue Engineering and Regenerative Medicine . 2020;14(8):1126–1135. doi: 10.1002/term.3086. [DOI] [PubMed] [Google Scholar]
- 21.Baradaran-Rafii A., Aghayan H.-R., Arjmand B., Javadi M.-A. Amniotic Membrane Transplantation. British Journal of Ophthalmology . 2020;2(1) [Google Scholar]
- 22.Sadler T. W., Langman J. Langman’s medical embryology . 12th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2020, https://www.ncbi.nlm.nih.gov/nlmcatalog/101562744. [Google Scholar]
- 23.Rao T. V., Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. Archives of Surgery . 1981;116(7):891–896. doi: 10.1001/archsurg.1981.01380190029007. [DOI] [PubMed] [Google Scholar]
- 24.Favaron P. O., Carvalho R. C., Borghesi J., Anunciação A. R. A., Miglino M. A. The Amniotic Membrane: Development and Potential Applications - A Review. Reproduction in Domestic Animals . 2015;50(6):881–892. doi: 10.1111/rda.12633. [DOI] [PubMed] [Google Scholar]
- 25.Togarrati P. P., Dinglasan N., Yee E., et al. Potential of Membranes Surrounding the Fetus as Immunoprotective Cell-Carriers for Allogeneic Transplantations. Transplantation direct . 2019;5(6):p. e460. doi: 10.1097/TXD.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei J., Priddy L. B., Lim J. J., Massee M., Koob T. J. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Advances in Wound Care . 2016;6(2):43–53. doi: 10.1089/wound.2016.0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keene D. R., Sakai L. Y., Lunstrum G. P., Morris N. P., Burgeson R. E. Type VII collagen forms an extended network of anchoring fibrils. The Journal of Cell Biology . 1987;104(3):611–621. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss J. F. Extracellular matrix dynamics and fetal membrane rupture. Reproductive Sciences . 2013;20(2):140–153. doi: 10.1177/1933719111424454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rousselle P., Keene D. R., Ruggiero F., Champliaud M. F., Rest M., Burgeson R. E. Laminin 5 binds the NC-1 domain of type VII collagen. The Journal of Cell Biology . 1997;138(3):719–728. doi: 10.1083/jcb.138.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J., Ockleford C. D. Laser scanning confocal examination and comparison of nidogen (entactin) with laminin in term human amniochorion. Placenta . 1994;15(1):95–106. doi: 10.1016/S0143-4004(05)80240-1. [DOI] [PubMed] [Google Scholar]
- 31.Uchide N., Ohyama K., Bessho T., Takeichi M., Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators of Inflammation . 2012;2012 doi: 10.1155/2012/270670.270670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourne G. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgraduate Medical Journal . 1962;38:193–201. doi: 10.1136/pgmj.38.438.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koob T. J., Lim J. J., Massee M., Zabek N., Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. Journal of Biomedical Materials Research Part B: Applied Biomaterials . 2014;102(6):1353–1362. doi: 10.1002/jbm.b.33141. [DOI] [PubMed] [Google Scholar]
- 34.Lei J., Priddy L. B., Lim J. J., Koob T. J. Dehydrated Human Amnion/Chorion Membrane (dHACM) Allografts as a Therapy for Orthopedic Tissue Repair. Techniques in Orthopaedics . 2017;32(3):149–157. doi: 10.1097/BTO.0000000000000229. [DOI] [Google Scholar]
- 35.Kubo M., Sonoda Y., Muramatsu R., Usui M. Immunogenicity of Human Amniotic Membrane in Experimental Xenotransplantation. Investigative Ophthalmology & Visual Science . 2001;42(7):1539–1546. [PubMed] [Google Scholar]
- 36.Chopra A., Thomas B. Venation-Inspired Growth Technique for Stiffener Layout Design of Plate and Shell Structures. Journal of Biomimetics Biomaterials and Tissue Engineering . 2013;18(1):1–12. doi: 10.4028/www.scientific.net/JBBTE.18.1. [DOI] [Google Scholar]
- 37.Cooper L. J., Kinoshita S., German M., Koizumi N., Nakamura T., Fullwood N. J. An investigation into the composition of amniotic membrane used for ocular surface reconstruction. Cornea . 2005;24(6):722–729. doi: 10.1097/01.ico.0000154237.49112.29. [DOI] [PubMed] [Google Scholar]
- 38.Mohan R., Bajaj A., Gundappa M. Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. Journal of International Society of Preventive & Community Dentistry . 2017;7(1):15–21. doi: 10.4103/jispcd.JISPCD_359_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malak T. M., Ockleford C. D., Bell S. C., Dalgleish R., Bright N., Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta . 1993;14(4):385–406. doi: 10.1016/S0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- 40.Rangaraj A., Harding K., Leaper D. Role of collagen in wound management. Wounds uk . 2011;7(2):54–63. [Google Scholar]
- 41.Pankov R., Yamada K. M. Fibronectin at a glance. Journal of Cell Science . 2002;115(20):3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 42.Aumailley M., El Khal A., Knöss N., Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biology . 2003;22(1):49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- 43.Takashima S., Yasuo M., Sanzen N., et al. Characterization of laminin isoforms in human amnion. Tissue & Cell . 2008;40(2):75–81. doi: 10.1016/j.tice.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Ghatak S., Maytin E. V., Mack J. A., et al. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. International Journal of Cell Biology . 2015;2015:20. doi: 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kannaiyan J., SuriyaNarayanan S., Palaniyandi M., Rajangam B., Chhabra H., Pandey A. Amniotic membrane as a scaffold in wound healing and diabetic foot ulcer: an experimental technique and recommendations. International Journal of Research in Medical Sciences . 2016;4(8):p. 8. doi: 10.18203/2320-6012.ijrms20162206. [DOI] [Google Scholar]
- 46.ElHeneidy H., Omran E., Halwagy A., Al-Inany H., Al-Ansary M., Gad A. Amniotic membrane can be a valid source for wound healing. International Journal of Women's Health . 2016;8:225–231. doi: 10.2147/IJWH.S96636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goa K. L., Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs . 1994;47(3):536–566. doi: 10.1016/j.ijbiomac.2019.11.066. [DOI] [PubMed] [Google Scholar]
- 48.Higa K., Shimmura S., Shimazaki J., Tsubota K. Hyaluronic acid-CD44 interaction mediates the adhesion of lymphocytes by amniotic membrane stroma. Cornea . 2005;24(2):206–212. doi: 10.1097/01.ico.0000133999.45262.83. [DOI] [PubMed] [Google Scholar]
- 49.Keelan J. A., Blumenstein M., Helliwell R. J. A., Sato T. A., Marvin K. W., Mitchell M. D. Cytokines, prostaglandins and parturition--a review. Placenta . 2003;24(Supplement A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 50.Koob T. J., Rennert R., Zabek N., et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. International Wound Journal . 2013;10(5):493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McQuilling J. P., Vines J. B., Kimmerling K. A., Mowry K. C. Proteomic Comparison of Amnion and Chorion and Evaluation of the Effects of Processing on Placental Membranes. Wounds: A compendium of Clinical Research and Practice . 2017;29(6):E38–E42. [PMC free article] [PubMed] [Google Scholar]
- 52.Bomfim Pereira M. G., Pereira Gomes J. A., Rizzo L. V., Cristovam P. C., Silveira L. C. Cytokine Dosage in Fresh and Preserved Human Amniotic Membrane. Cornea . 2016;35(1):89–94. doi: 10.1097/ICO.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 53.Paolin A., Trojan D., Leonardi A., et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell and Tissue Banking . 2016;17(3):399–406. doi: 10.1007/s10561-016-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paradowska E., Blach-Olszewska Z., Gejdel E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta . 1997;18(5–6):441–446. doi: 10.1016/s0143-4004(97)80045-8. [DOI] [PubMed] [Google Scholar]
- 55.Weiss A., Goldman S., Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front. Biosci. J . 2007;12:649–659. doi: 10.2741/2089. [DOI] [PubMed] [Google Scholar]
- 56.Majali-Martinez A., Hiden U., Ghaffari-Tabrizi-Wizsy N., Lang U., Desoye G., Dieber-Rotheneder M. Placental membrane-type metalloproteinases (MT-MMPs): Key players in pregnancy. Cell Adhesion & Migration . 2016;10(1–2):136–146. doi: 10.1080/19336918.2015.1110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley S., Leask R., Denison F., Wisely K., Calder A., Howe D. Secretion of tissue inhibitors of matrix metalloproteinases by human fetal membranes, decidua and placenta at parturition. The Journal of Endocrinology . 1999;162(3):351–359. doi: 10.1677/joe.0.1620351. [DOI] [PubMed] [Google Scholar]
- 58.Koob T. J., Lim J. J., Zabek N., Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. Journal of Biomedical Materials Research. Part B, Applied Biomaterials . 2015;103(5):1133–1140. doi: 10.1002/jbm.b.33265. [DOI] [PubMed] [Google Scholar]
- 59.Kim H. S., Cho J. H., Park H. W., Yoon H., Kim M. S., Kim S. C. Endotoxin-Neutralizing Antimicrobial Proteins of the Human Placenta. The Journal of Immunology . 2002;168(5):2356–2364. doi: 10.4049/jimmunol.168.5.2356. [DOI] [PubMed] [Google Scholar]
- 60.King A. E., Paltoo A., Kelly R. W., Sallenave J.-M., Bocking A. D., Challis J. R. G. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta . 2007;28(2–3):161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Reviews Immunology . 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A., Chandra R. V., Reddy A. A., Reddy B. H., Reddy C., Naveen A. Evaluation of clinical, antiinflammatory and antiinfective properties of amniotic membrane used for guided tissue regeneration: A randomized controlled trial. Dental Research Journal . 2015;12(2):127–135. [PMC free article] [PubMed] [Google Scholar]
- 63.Zare-Bidaki M., Sadrinia S., Erfani S., Afkar E., Ghanbarzade N. Antimicrobial Properties of Amniotic and Chorionic Membranes: A Comparative Study of Two Human Fetal Sacs. Journal of Reproduction & Infertility . 2017;18(2):218–224. [PMC free article] [PubMed] [Google Scholar]
- 64.Ni J., Abrahamson M., Zhang M., et al. Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. Journal of Biological Chemistry . 1997;272(16):10853–10858. doi: 10.1074/jbc.272.16.10853. [DOI] [PubMed] [Google Scholar]
- 65.Vo A., Diller R., Kellar R. Characterization and Clinical Applications of Amniotic Membranes. J. Pharmacol. Clin. Res. . 2017;4(4, article 555645) [Google Scholar]
- 66.Ilic D., Vicovac L., Nikolic M., Lazic Ilic E. Human amniotic membrane grafts in therapy of chronic non-healing wounds. British Medical Bulletin . 2016;117(1):59–67. doi: 10.1093/bmb/ldv053. [DOI] [PubMed] [Google Scholar]
- 67.Fenelon M., B Maurel D., Siadous R., et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Materials Science & Engineering. C, Materials for Biological Applications . 2019;104, article 109903 doi: 10.1016/j.msec.2019.109903. [DOI] [PubMed] [Google Scholar]
- 68.Henry J. J. D., Delrosario L., Fang J., et al. Development of Injectable Amniotic Membrane Matrix for Postmyocardial Infarction Tissue Repair. Advanced Healthcare Materials . 2020;9(2, article ???) doi: 10.1002/adhm.201900544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson A., Gyurdieva A., Dhall S., Danilkovitch A., Duan-Arnold Y. Understanding the Impact of Preservation Methods on the Integrity and Functionality of Placental Allografts. Annals of Plastic Surgery . 2017;79(2):203–213. doi: 10.1097/SAP.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 70.Thomasen H., Pauklin M., Steuhl K.-P., Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. . 2009;247(12):1691–1700. doi: 10.1007/s00417-009-1162-y. [DOI] [PubMed] [Google Scholar]
- 71.Ferenczy P. A. V. H., Souza L. B. D. Comparison of the preparation and preservation techniques of amniotic membrane used in the treatment of ocular surface diseases. Revista Brasileira de Oftalmologia . 2020;79(1):71–80. doi: 10.5935/0034-7280.20200016. [DOI] [Google Scholar]
- 72.Gilpin A., Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Research International . 2017;2017 doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dhall S., Sathyamoorthy M., Kuang J. Q., et al. Properties of viable lyopreserved amnion are equivalent to viable cryopreserved amnion with the convenience of ambient storage. PLoS One . 2018;13(10, article e0204060) doi: 10.1371/journal.pone.0204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castellanos G., Bernabé-García Á., Moraleda J. M., Nicolás F. J. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta . 2017;59:146–153. doi: 10.1016/j.placenta.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Parolini O., Solomon A., Evangelista M., Soncini M. Human Placenta: Structure and Development, Circulation and Functions . Nova Science Publishers, Inc; 2010. Human Term Placenta as a Therapeutic Agent: From the First Clinical Applications to Future Perspectives; pp. 1–49. [Google Scholar]
- 76.Milan P. B., Amini N., Joghataei M. T., et al. Decellularized human amniotic membrane: From animal models to clinical trials. Methods . 2020;171:11–19. doi: 10.1016/j.ymeth.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 77.Wilshaw S.-P., Kearney J., Fisher J., Ingham E. Biocompatibility and Potential of Acellular Human Amniotic Membrane to Support the Attachment and Proliferation of Allogeneic Cells. Tissue Engineering. Part A . 2008;14(4):463–472. doi: 10.1089/tea.2007.0145. [DOI] [PubMed] [Google Scholar]
- 78.Kim J. C., Tseng S. C. G. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean Journal of Ophthalmology . 1995;9(1):32–46. doi: 10.3341/kjo.1995.9.1.32. [DOI] [PubMed] [Google Scholar]
- 79.Huddleston H. P., Cohn M. R., Haunschild E. D., Wong S. E., Farr J., Yanke A. B. Amniotic Product Treatments: Clinical and Basic Science Evidence. Current Reviews in Musculoskeletal Medicine . 2020;13(2):148–154. doi: 10.1007/s12178-020-09614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jie J., Yang J., He H., et al. Tissue remodeling after ocular surface reconstruction with denuded amniotic membrane. Scientific Reports . 2018;8(1):p. 6400. doi: 10.1038/s41598-018-24694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baradaran-Rafii A., Asl N. S., Ebrahimi M., et al. The role of amniotic membrane extract eye drop (AMEED) in _in vivo_ cultivation of limbal stem cells. The Ocular Surface . 2018;16(1):146–153. doi: 10.1016/j.jtos.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Anderson D. G., Popov V., Raines A. L., O’Connell J. Cryopreserved Amniotic Membrane Improves Clinical Outcomes Following Microdiscectomy. Clin. Spine Surg. . 2017;30(9):413–418. doi: 10.1097/BSD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 83.Walker C. T., Godzik J., Kakarla U. K., Turner J. D., Whiting A. C., Nakaji P. Human Amniotic Membrane for the Prevention of Intradural Spinal Cord Adhesions: Retrospective Review of its Novel Use in a Case Series of 14 Patients. Neurosurgery . 2018;83(5):989–996. doi: 10.1093/neuros/nyx608. [DOI] [PubMed] [Google Scholar]
- 84.Tao H., Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. European Spine Journal . 2009;18(8):1202–1212. doi: 10.1007/s00586-009-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uçkun Ö. M., Divanlioglu D., Akdag R., Menekşe G., Dalgiç A., Belen A. D. Dehydrated human amnion/chorion membrane allograft for preventing epidural fibrosis after laminectomy: an experimental rat model. The European Respiratory Journal . 2020;6(6) doi: 10.18621/eurj.523854. [DOI] [Google Scholar]
- 86.Keerthi R., Vaibhav N., Raut R. Amniotic membrane as a biological scaffold after vestibuloplasty. J. Maxillofac. Oral Surg. . 2015;14(Suppl 1):383–387. doi: 10.1007/s12663-014-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rathod S., Samal S. K. Secondary Vaginal Atresia Treated with Vaginoplasty Using Amnion Graft: A Case Report. J. Clin. Diagn. Res. JCDR . 2014;8(11):OD05–OD06. doi: 10.7860/JCDR/2014/10674.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oottamasathien S., Hotaling J. M., Craig J. R., Myers J. B., Brant W. O. Amniotic therapeutic biomaterials in urology: current and future applications. Transl. Androl. Urol. . 2017;6(5):943–950. doi: 10.21037/tau.2017.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng X., Li T., Zhao Y., Guo Y., Xia E. Safety and Efficacy of Amnion Graft in Preventing Reformation of Intrauterine Adhesions. Journal of Minimally Invasive Gynecology . 2017;24(7):1204–1210. doi: 10.1016/j.jmig.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Dulemba J., Mirzakhani P., Istwan N. B. Evaluation of Dehydrated Human Amnion/Chorion Membrane as an Adhesion Barrier in Women Undergoing Robotic Laparoscopy. Gynécologie et Obstétrique . 2016;6(10):1–5. doi: 10.4172/2161-0932.1000405. [DOI] [Google Scholar]
- 91.Lemke A., Ferguson J., Gross K., et al. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomaterialia . 2018;66:335–349. doi: 10.1016/j.actbio.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 92.Regulski M. J., Danilkovitch A., Saunders M. C. Management of a chronic radiation necrosis wound with lyopreserved placental membrane containing viable cells. Clin. Case Rep. . 2019;7(3):456–460. doi: 10.1002/ccr3.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snyder R. J., Ead J., Glick B., Cuffy C. Dehydrated Human Amnion/Chorion Membrane as Adjunctive Therapy in the Multidisciplinary Treatment of Pyoderma Gangrenosum: A Case Report. Ostomy/Wound Management . 2015;61(9):40–49. [PubMed] [Google Scholar]
- 94.Wisco O. J. Case series: The use of a dehydrated human amnion/chorion membrane allograft to enhance healing in the repair of lower eyelid defects after Mohs micrographic surgery. JAAD Case Rep. . 2016;2(4):294–297. doi: 10.1016/j.jdcr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyons A. B., Chipps L. K., Moy R. L., Herrmann J. L. Dehydrated human amnion/chorion membrane allograft as an aid for wound healing in patients with full-thickness scalp defects after Mohs micrographic surgery. JAAD Case Rep. . 2018;4(7):688–691. doi: 10.1016/j.jdcr.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu C., Bai J., Yu K., Liu G., Tian S., Tian D. Biological Amnion Prevents Flexor Tendon Adhesion in Zone II: A Controlled, Multicentre Clinical Trial. BioMed Research International . 2019;3 doi: 10.1155/2019/2354325. https://www.hindawi.com/journals/bmri/2019/2354325/ () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castellanos R., Tighe S. Injectable Amniotic Membrane/Umbilical Cord Particulate for Knee Osteoarthritis: A Prospective, Single-Center Pilot Study. Pain Medicine . 2019;20(11):2283–2291. doi: 10.1093/pm/pnz143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McQuilling J. P., Sanders M., Poland L., et al. Dehydrated Amnion/Chorion Improves Achilles Tendon Repair in a Diabetic Animal Model. Wounds Compend. Clin. Res. Pract. . 2019;31(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 99.Willett N. J., Thote T., Lin A. S. P., et al. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Research & Therapy . 2014;16(1):p. R47. doi: 10.1186/ar4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benirschke K., Burton G. J., Baergen R. N. Pathology of the Human Placenta . 6th ed. Berlin Heidelberg: Springer-Verlag; 2012. [DOI] [Google Scholar]
- 101.Fitzsimmons E. D., Bajaj T. StatPearls . Treasure Island (FL): StatPearls Publishing; 2020. Embryology, Amniotic Fluid. Accessed: Apr. 29, 2020. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK541089/ [PubMed] [Google Scholar]
- 102.Tisi D. K., Emard J. J., Koski K. G. Total Protein Concentration in Human Amniotic Fluid Is Negatively Associated with Infant Birth Weight. The Journal of Nutrition . 2004;134(7):1754–1758. doi: 10.1093/jn/134.7.1754. [DOI] [PubMed] [Google Scholar]
- 103.Tong X.-L., Wang L., Gao T.-B., Qin Y.-G., Qi Y.-Q., Xu Y.-P. Potential function of amniotic fluid in fetal development---novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J. Chin. Med. Assoc. JCMA . 2009;72(7):368–373. doi: 10.1016/S1726-4901(09)70389-2. [DOI] [PubMed] [Google Scholar]
- 104.Beall M. H., van den Wijngaard J. P. H. M., van Gemert M. J. C., Ross M. G. Amniotic fluid water dynamics. Placenta . 2007;28(8–9):816–823. doi: 10.1016/j.placenta.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Davydova D. A., Vorotelyak E. A., Smirnova Y. A., et al. Cell Phenotypes in Human Amniotic Fluid. Acta Naturae . 2009;1(2):98–103. doi: 10.32607/20758251-2009-1-2-98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson M. M., Emery A. E. H. Amniotic Fluid Cells; Prenatal Sex Prediction and Culture. British Medical Journal . 1970;1(5695):523–526. doi: 10.1136/bmj.1.5695.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Votta R. A., de Gagneten C. B., Parada O., Giulietti M. Cytologic study of amniotic fluid in pregnancy. American Journal of Obstetrics and Gynecology . 1968;102(4):571–577. doi: 10.1016/0002-9378(68)90540-1. [DOI] [PubMed] [Google Scholar]
- 108.Hoehn H., Bryant E. M., Karp L. E., Martin G. M. Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. I. Clonal morphology and growth potential. Pediatric Research . 1974;8(8):746–754. doi: 10.1203/00006450-197408000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Priest R. E., Priest J. H., Moinuddin J. F., Keyser A. J. Differentiation in human amniotic fluid cell cultures: I: Collagen production. Journal of Medical Genetics . 1977;14(3):157–162. doi: 10.1136/jmg.14.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Megaw J. M., Priest J. H., Priest R. E., Johnson L. D. Differentiation in human amniotic fluid cell cultures: II: Secretion of an epithelial basement membrane glycoprotein. Journal of Medical Genetics . 1977;14(3):163–167. doi: 10.1136/jmg.14.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Priest R. E., Marimuthu K. M., Priest J. H. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab. Investig. J. Tech. Methods Pathol. . 1978;39(2):106–109. [PubMed] [Google Scholar]
- 112.Cananzi M., Atala A., De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reproductive Biomedicine Online . 2009;18(Suppl 1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- 113.Kim E. Y., Lee K.-B., Kim M. K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Reports . 2014;47(3):p. 135. doi: 10.5483/BMBRep.2014.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pierce J., Jacobson P., Benedetti E., et al. Collection and characterization of amniotic fluid from scheduled C-section deliveries. Cell and Tissue Banking . 2016;17(3):413–425. doi: 10.1007/s10561-016-9572-7. [DOI] [PubMed] [Google Scholar]
- 115.Skardal A., Mack D., Kapetanovic E., et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Translational Medicine . 2012;1(11):792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loukogeorgakis S. P., De Coppi P. Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications. Stem Cells Dayt. Ohio . 2017;35(7):1663–1673. doi: 10.1002/stem.2553. [DOI] [PubMed] [Google Scholar]
- 117.Panero A. J., Hirahara A. M., Andersen W. J., Rothenberg J., Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine . 2019;47(5):1230–1235. doi: 10.1177/0363546519829034. [DOI] [PubMed] [Google Scholar]
- 118.Cho C.-K. J., Shan S. J., Winsor E. J., Diamandis E. P. Proteomics Analysis of Human Amniotic Fluid. Molecular & Cellular Proteomics . 2007;6(8):1406–1415. doi: 10.1074/mcp.M700090-MCP200. [DOI] [PubMed] [Google Scholar]
- 119.Avagliano L., Garò C., Marconi A. M. Placental amino acids transport in intrauterine growth restriction. Journal of Pregnancy . 2012;2012 doi: 10.1155/2012/972562.972562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gurekian C. N., Koski K. G. Amniotic fluid amino acid concentrations are modified by maternal dietary glucose, gestational age, and fetal growth in rats. The Journal of Nutrition . 2005;135(9):2219–2224. doi: 10.1093/jn/135.9.2219. [DOI] [PubMed] [Google Scholar]
- 121.Battaglia F. C., Meschia G. Fetal nutrition. Annual Review of Nutrition . 1988;8:43–61. doi: 10.1146/annurev.nu.08.070188.000355. [DOI] [PubMed] [Google Scholar]
- 122.Tisi D. K., Burns D. H., Luskey G. W., Koski K. G. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care . 2011;34(1):139–144. doi: 10.2337/dc10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akella R. R. D., Kadali S. Amniotic fluid glycosaminoglycans in the prenatal diagnosis of mucopolysaccharidoses - A useful biomarker. Clin. Chim. Acta Int. J. Clin. Chem. . 2016;460:63–66. doi: 10.1016/j.cca.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 124.Sloan R., Archbold G. P., Lloyd F., Elliott R. J. Glycosaminoglycan accumulation in amniotic fluid during early pregnancy. Biochemical Society Transactions . 1996;24(1):p. 103S. doi: 10.1042/bst024103s. [DOI] [PubMed] [Google Scholar]
- 125.Athayde N., Edwin S. S., Romero R., et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. American Journal of Obstetrics and Gynecology . 1998;179(5):1248–1253. doi: 10.1016/S0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 126.Kumar D., Fung W., Moore R. M., et al. Proinflammatory Cytokines Found in Amniotic Fluid Induce Collagen Remodeling, Apoptosis, and Biophysical Weakening of Cultured Human Fetal Membranes. Biology of Reproduction . 2006;74(1):29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 127.Crouch E., Balian G., Holbrook K., Hoehn H., Bornstein P. Amniotic fluid fibronectin and the synthesis of fibronectin by amniotic fluid cells in culture. Annals of the New York Academy of Sciences . 1978;312:410–413. doi: 10.1111/j.1749-6632.1978.tb16821.x. [DOI] [PubMed] [Google Scholar]
- 128.Hoehn H., Salk D. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods in Cell Biology . 1982;26:11–34. doi: 10.1016/s0091-679x(08)61362-x. [DOI] [PubMed] [Google Scholar]
- 129.Parekh A., Hebda P. A. The Contractile Phenotype of Dermal Fetal Fibroblasts in Scarless Wound Healing. Curr. Pathobiol. Rep. . 2017;5(3):271–277. doi: 10.1007/s40139-017-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Virtanen I., von Koskull H., Lehto V. P., Vartio T., Aula P. Cultured human amniotic fluid cells characterized with antibodies against intermediate filaments in indirect immunofluorescence microscopy. The Journal of Clinical Investigation . 1981;68(5):1348–1355. doi: 10.1172/jci110382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larson B. J., Longaker M. T., Lorenz H. P. Scarless Fetal Wound Healing: A Basic Science Review. Plastic and Reconstructive Surgery . 2010;126(4):p. 1172. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lim J. J., Koob T. J. Placental Cells and Tissues: The Transformative Rise in Advanced Wound Care. Worldwide Wound Healing - Innovation in Natural and Conventional Methods, Cesar Joao Vicente da Fonseca - IntechOpen . 2016. Accessed: Apr. 29, 2020. [Online]. Available: https://www.intechopen.com/books/worldwide-wound-healing-innovation-in-natural-and-conventional-methods/placental-cells-and-tissues-the-transformative-rise-in-advanced-wound-care.
- 133.Werber B., Martin E. A prospective study of 20 foot and ankle wounds treated with cryopreserved amniotic membrane and fluid allograft. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. . 2013;52(5):615–621. doi: 10.1053/j.jfas.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 134.Özgenel G. Y., Şamli B., Özcan M. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J. Hand Surg. . 2001;26(2):332–339. doi: 10.1053/jhsu.2001.22524. [DOI] [PubMed] [Google Scholar]
- 135.Lee T. Y., Schafer I. A. Glycosaminoglycan composition of human amniotic fluid. Biochimica et Biophysica Acta . 1974;354(2):264–274. doi: 10.1016/0304-4165(74)90012-9. [DOI] [PubMed] [Google Scholar]
- 136.Underwood M. A., Gilbert W. M., Sherman M. P. Amniotic Fluid: Not just fetal urine anymore. Journal of the California Perinatal Association . 2005;25(5):341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 137.Nyman E., Huss F., Nyman T., Junker J., Kratz G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: in vitro studies of re-epithelialisation in human skin wounds. Journal of Plastic Surgery and Hand Surgery . 2013;47(2):89–92. doi: 10.3109/2000656X.2012.733169. [DOI] [PubMed] [Google Scholar]
- 138.Longaker M. T., Scott Adzick N., Hall J. L., et al. Studies in fetal wound healing, VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. Journal of Pediatric Surgery . 1990;25(4):430–433. doi: 10.1016/0022-3468(90)90387-o. [DOI] [PubMed] [Google Scholar]
- 139.Matsuo I., Kimura-Yoshida C. Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences . 2014;369(1657) doi: 10.1098/rstb.2013.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruoslahti E., Engvall E., Hayman E. G., Spiro R. G. Comparative studies on amniotic fluid and plasma fibronectins. Biochemical Journal . 1981;193(1):295–299. doi: 10.1042/bj1930295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Longaker M. T., Whitby D. J., Ferguson M. W. J., et al. Studies in fetal wound healing: III. Early deposition of fibronectin distinguishes fetal from adult wound healing. Journal of Pediatric Surgery . 1989;24(8):799–805. doi: 10.1016/S0022-3468(89)80540-8. [DOI] [PubMed] [Google Scholar]
- 142.Chow S. S. W., Craig M. E., Jones C. A., et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine . 2008;44(1):78–84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 143.Mao Y., Pierce J., Singh-Varma A., Boyer M., Kohn J., Reems J.-A. Processed human amniotic fluid retains its antibacterial activity. Journal of Translational Medicine . 2019;17(1):p. 68. doi: 10.1186/s12967-019-1812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pacora P., Maymon E., Gervasi M. T., et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. American Journal of Obstetrics and Gynecology . 2000;183(4):904–910. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 145.Soto E., Espinoza J., Nien J. K., et al. Human beta-defensin-2: A natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. Journal of Maternal-Fetal and Neonatal Medicine . 2007;20(1):15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoon B. S., Moon J. H., Jun E. K., et al. Secretory Profiles and Wound Healing Effects of Human Amniotic Fluid–Derived Mesenchymal Stem Cells. Stem Cells and Development . 2010;19(6):887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 147.Kimmerling K. A., McQuilling J. P., Staples M. C., Mowry K. C. Tenocyte cell density, migration, and extracellular matrix deposition with amniotic suspension allograft. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. . 2019;37(2):412–420. doi: 10.1002/jor.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Merimee T. J., Grant M., Tyson J. E. Insulin-like growth factors in amniotic fluid. The Journal of Clinical Endocrinology and Metabolism . 1984;59(4):752–755. doi: 10.1210/jcem-59-4-752. [DOI] [PubMed] [Google Scholar]
- 149.Underwood M., Sherman M. Nutritional Characteristics of Amniotic Fluid. NeoReviews . 2006;7(6):e310–e316. doi: 10.1542/neo.7-6-e310. [DOI] [Google Scholar]
- 150.Dawood M. Y. Hormones in amniotic fluid. American Journal of Obstetrics and Gynecology . 1977;128(5):576–583. doi: 10.1016/0002-9378(77)90046-1. [DOI] [PubMed] [Google Scholar]
- 151.Garoufalia Z., Papadopetraki A., Karatza E., et al. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed. Rep. . 2021;15(2):65–66. doi: 10.3892/br.2021.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei F. Y., Lee J. K., Wei L., Qu F., Zhang J. Z. Correlation of insulin-like growth factor 1 and osteoarthritic cartilage degradation: a spontaneous osteoarthritis in guinea-pig. European Review for Medical and Pharmacological Sciences . 2017;21(20):4493–4500. [PMC free article] [PubMed] [Google Scholar]
- 153.Varrey A., Romero R., Panaitescu B., et al. Human β-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. American Journal of Reproductive Immunology . 2018;80(4):p. e13031. doi: 10.1111/aji.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bazrafshan A., Owji M., Yazdani M., Varedi M. Activation of mitosis and angiogenesis in diabetes-impaired wound healing by processed human amniotic fluid. The Journal of Surgical Research . 2014;188(2):545–552. doi: 10.1016/j.jss.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 155.Gomoll A. H., Farr J., Cole B. J., et al. Safety and Efficacy of an Amniotic Suspension Allograft Injection Over 12 Months in a Single-Blinded, Randomized Controlled Trial for Symptomatic Osteoarthritis of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. . 2021;37(7):2246–2257. doi: 10.1016/j.arthro.2021.02.044. [DOI] [PubMed] [Google Scholar]
- 156.Kimmerling K. A., Gomoll A. H., Farr J., Mowry K. C. Amniotic Suspension Allograft Modulates Inflammation in a Rat Pain Model of Osteoarthritis. Journal of Orthopaedic Research . 2020;38(5):1141–1149. doi: 10.1002/jor.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ozgenel G. Y., Samli B., Ozcan M. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J. Hand Surg. . 2001;26(2):332–339. doi: 10.1053/jhsu.2001.22524. [DOI] [PubMed] [Google Scholar]
- 158.Vines J. B., Aliprantis A. O., Gomoll A. H., Farr J. Cryopreserved Amniotic Suspension for the Treatment of Knee Osteoarthritis. The Journal of Knee Surgery . 2016;29(6):443–450. doi: 10.1055/s-0035-1569481. [DOI] [PubMed] [Google Scholar]
- 159.Werber B. Amniotic Tissues for the Treatment of Chronic Plantar Fasciosis and Achilles Tendinosis. The Journal of Sports Medicine . 2015;2015, article e219896 doi: 10.1155/2015/219896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Castro-Combs J., Noguera G., Cano M., et al. Corneal wound healing is modulated by topical application of amniotic fluid in an ex vivo organ culture model. Experimental Eye Research . 2008;87(1):56–63. doi: 10.1016/j.exer.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 161.Ghalei S., Nourmohammadi J., Solouk A., Mirzadeh H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids and Surfaces. B, Biointerfaces . 2018;172:82–89. doi: 10.1016/j.colsurfb.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 162.Özgenel G. Y. The influence of human amniotic fluid on the potential of rabbit ear perichondrial flaps to form cartilage tissue. British Journal of Plastic Surgery . 2002;55(3):246–250. doi: 10.1054/bjps.2002.3811. [DOI] [PubMed] [Google Scholar]
- 163.Longaker M. T., Whitby D. J., Adzick N. S., et al. Studies in fetal wound healing VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. Journal of Pediatric Surgery . 1990;25(1):63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 164.Kelly E. J., Newell S. J., Brownlee K. G., et al. Role of epidermal growth factor and transforming growth factor alpha in the developing stomach. Archives of Disease in Childhood. Fetal and Neonatal Edition . 1997;76(3):F158–F162. doi: 10.1136/fn.76.3.F158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pienimaki J. P., Rilla K., Fulop C., et al. Epidermal Growth Factor Activates Hyaluronan Synthase 2 in Epidermal Keratinocytes and Increases Pericellular and Intracellular Hyaluronan. The Journal of Biological Chemistry . 2001;276(23):20428–20435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]
- 166.Wang Y., Zhao S. Placental Blood Circulation. Morgan & Claypool Life Sciences. 2010. Accessed: May 18, 2020. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK53254/ [PubMed]
- 167.Davies J. E., Walker J. T., Keating A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Translational Medicine . 2017;6(7):1620–1630. doi: 10.1002/sctm.16-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Benirschke K., Kaufmann P. Pathology of the Human Placenta . Springer; 2000. Anatomy and pathology of the umbilical cord and major fetal vessels; pp. 335–398. [Google Scholar]
- 169.Ferguson V. L., Dodson R. B. Bioengineering aspects of the umbilical cord. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 2009;144(Suppl 1):S108–S113. doi: 10.1016/j.ejogrb.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 170.Eyden B. P., Ponting J., Davies H., Bartley C., Torgersen E. Defining the myofibroblast: normal tissues, with special reference to the stromal cells of Wharton’s jelly in human umbilical cord. Journal of Submicroscopic Cytology and Pathology . 1994;26(3):347–355. [PubMed] [Google Scholar]
- 171.Mazza G., Roßmanith E., Lang-Olip I., Pfeiffer D. Marker profile for the evaluation of human umbilical artery smooth muscle cell quality obtained by different isolation and culture methods. Cytotechnology . 2016;68(4):701–711. doi: 10.1007/s10616-014-9822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stefańska K., Ożegowska K., Hutchings G., et al. Human Wharton’s Jelly—Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. Journal of Clinical Medicine . 2020;9(4):p. 1102. doi: 10.3390/jcm9041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pennati G. Biomechanical properties of the human umbilical cord. Biorheology . 2001;38(5–6):355–366. [PubMed] [Google Scholar]
- 174.Bosselmann S., Mielke G. Sonographic Assessment of the Umbilical Cord. Geburtshilfe und Frauenheilkunde . 2015;75(8):808–818. doi: 10.1055/s-0035-1557819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cochard L. R. Netter’s Atlas of Human Embryology . Teterboro, NJ: Icon Learning Systems; 2002. Accessed: Aug. 06, 2020. [Online]. Available: https://www.scholars.northwestern.edu/en/publications/netters-atlas-of-human-embryology-2. [Google Scholar]
- 176.Spurway J., Logan P., Pak S. The development, structure and blood flow within the umbilical cord with particular reference to the venous system. Australas. J. Ultrasound Med. . 2012;15(3):97–102. doi: 10.1002/j.2205-0140.2012.tb00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Balkawade N. U., Shinde M. A. Study of Length of Umbilical Cord and Fetal Outcome: A Study of 1,000 Deliveries. Journal of Obstetrics and Gynaecology of India . 2012;62(5):520–525. doi: 10.1007/s13224-012-0194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Takechi K., Kuwabara Y., Mizuno M. Ultrastructural and immunohistochemical studies of Wharton’s jelly umbilical cord cells. Placenta . 1993;14(2):235–245. doi: 10.1016/s0143-4004(05)80264-4. [DOI] [PubMed] [Google Scholar]
- 179.Corrao S., La Rocca G., Lo Iacono M., Corsello T., Farina F., Anzalone R. Umbilical cord revisited: from Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histology and Histopathology . 2013;28(10):1235–1244. doi: 10.14670/HH-28.1235. [DOI] [PubMed] [Google Scholar]
- 180.Debebe S. K., Cahill L. S., Kingdom J. C., et al. Wharton's jelly area and its association with placental morphometry and pathology. Placenta . 2020;94:34–38. doi: 10.1016/j.placenta.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vizza E., Correr S., Goranova V., Heyn R., Muglia U., Papagianni V. The collagen fibrils arrangement in the Wharton’s jelly of full-term human umbilical cord. Italian Journal of Anatomy and Embryology . 1995;100 Suppl 1:495–501. [PubMed] [Google Scholar]
- 182.Gervaso F., Pennati G., Boschetti F., Rigano S., Pigni A., Padoan A. Biomechanics Of The Human Umbilical Cord Under Compressive Loads . Florida: Sonesta Beach Resort in Key Biscayne; 2003. [Google Scholar]
- 183.Gervaso F., Boschetti F., Pennati G. Evaluation of the Wharton’s jelly poroelastic parameters through compressive tests on placental and foetal ends of human umbilical cords. Journal of the Mechanical Behavior of Biomedical Materials . 2014;35:51–58. doi: 10.1016/j.jmbbm.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 184.Nanaev A. K., Kohnen G., Milovanov A. P., Domogatsky S. P., Kaufmann P. Stromal differentiation and architecture of the human umbilical cord. Placenta . 1997;18(1):53–64. doi: 10.1016/s0143-4004(97)90071-0. [DOI] [PubMed] [Google Scholar]
- 185.Arno A., Smith A. H., Blit P. H., Shehab M. A., Gauglitz G. G., Jeschke M. G. Stem Cell Therapy: A New Treatment for Burns? Pharm. Basel Switz. . 2011;4(10):1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sobolewski K., Małkowski A., Bańkowski E., Jaworski S. Wharton’s jelly as a reservoir of peptide growth factors. Placenta . 2005;26(10):747–752. doi: 10.1016/j.placenta.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 187.Beiki B., Zeynali B., Seyedjafari E. Fabrication of a three dimensional spongy scaffold using human Wharton’s jelly derived extra cellular matrix for wound healing. Materials Science & Engineering. C, Materials for Biological Applications . 2017;78:627–638. doi: 10.1016/j.msec.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 188.Sobolewski K., Bańkowski E., Chyczewski L., Jaworski S. Collagen and glycosaminoglycans of Wharton’s jelly. Biology of the Neonate . 1997;71(1):11–21. doi: 10.1159/000244392. [DOI] [PubMed] [Google Scholar]
- 189.Bańkowski E., Sobolewski K., Romanowicz L., Chyczewski L., Jaworski S. Collagen and glycosaminoglycans of Wharton’s jelly and their alterations in EPH-gestosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 1996;66(2):109–117. doi: 10.1016/0301-2115(96)02390-1. [DOI] [PubMed] [Google Scholar]
- 190.Gogiel T., Bańkowski E., Jaworski S. Proteoglycans of Wharton’s jelly. The International Journal of Biochemistry & Cell Biology . 2003;35(10):1461–1469. doi: 10.1016/s1357-2725(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 191.Valiyaveettil M., Achur R. N., Muthusamy A., Gowda D. C. Characterization of chondroitin sulfate and dermatan sulfate proteoglycans of extracellular matrices of human umbilical cord blood vessels and Wharton’s jelly. Glycoconjugate Journal . 2004;21(6):361–375. doi: 10.1023/B:GLYC.0000046276.77147.b2. [DOI] [PubMed] [Google Scholar]
- 192.Kanayama N., Goto J., Terao T. The role of low molecular weight hyaluronic acid contained in Wharton’s jelly in necrotizing funisitis. Pediatric Research . 1999;45(4):510–514. doi: 10.1203/00006450-199904010-00009. [DOI] [PubMed] [Google Scholar]
- 193.Raio L., Cromi A., Ghezzi F., et al. Hyaluronan content of Wharton's jelly in healthy and Down syndrome fetuses. Matrix Biology . 2005;24(2):166–174. doi: 10.1016/j.matbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 194.Gupta A., El-Amin S. F., Levy H. J., Sze-Tu R., Ibim S. E., Maffulli N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. Journal of Orthopaedic Surgery . 2020;15 doi: 10.1186/s13018-020-1553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sobolewski K., Bańkowski E., Pałka J., Jaworski S. Binding of the basic fibroblast growth factor (bFGF) by soluble components of human umbilical cord. Acta Biochimica Polonica . 2002;49(4):999–1004. doi: 10.18388/abp.2002_3759. [DOI] [PubMed] [Google Scholar]
- 196.d’Ortho M. P., Will Horst, Atkinson Susan, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. European Journal of Biochemistry . 1997;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 197.Romanowicz L., Galewska Z. Extracellular matrix remodeling of the umbilical cord in pre-eclampsia as a risk factor for fetal hypertension. Journal of Pregnancy . 2011;2011 doi: 10.1155/2011/542695.542695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Malkowski A., Sobolewski K., Jaworski S., Bankowski E. FGF binding by extracellular matrix components of Wharton’s jelly. Acta Biochimica Polonica . 2007;54(2):357–363. doi: 10.18388/abp.2007_3257. [DOI] [PubMed] [Google Scholar]
- 199.Bielefeld K. A., Amini-Nik S., Alman B. A. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cellular and Molecular Life Sciences . 2013;70(12):2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang J.-M., An J. Cytokines, Inflammation and Pain. International Anesthesiology Clinics . 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tau G., Rothman P. Biologic functions of the IFN-γ receptors. Allergy . 1999;54(12):1233–1251. doi: 10.1034/j.1398-9995.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Connor D. E., Paulus J. A., Dabestani P. J., et al. Therapeutic potential of exosomes in rotator cuff tendon healing. Journal of Bone and Mineral Metabolism . 2019;37(5):759–767. doi: 10.1007/s00774-019-01013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hou X., Yuan Y., Yin J., Yang S., Xie L., Wang S. Fetal umbilical vein transplantation for the repair of middle cerebral artery injury. Neural Regeneration Research . 2013;8(34):3249–3254. doi: 10.3969/j.issn.1673-5374.2013.34.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Safari F., Fani N., Eglin D., Alini M., Stoddart M. J., Baghaban Eslaminejad M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. Journal of Biomedical Materials Research. Part A . 2019;107(8):1793–1802. doi: 10.1002/jbm.a.36698. [DOI] [PubMed] [Google Scholar]
- 205.Jadalannagari S., Converse G., McFall C., et al. Decellularized Wharton’s Jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One . 2017;12(2, article e0172098) doi: 10.1371/journal.pone.0172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lin L., Xu Y., Li Y., et al. Nanofibrous Wharton's jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Materials and Design . 2020;186, article 108216 doi: 10.1016/j.matdes.2019.108216. [DOI] [Google Scholar]
- 207.Bullard J. D., Lei J., Lim J. J., Massee M., Fallon A. M., Koob T. J. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. Journal of Biomedical Materials Research. Part B, Applied Biomaterials . 2019;107(4):1035–1046. doi: 10.1002/jbm.b.34196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gerwin N., Hops C., Lucke A. Intraarticular drug delivery in osteoarthritis. Advanced Drug Delivery Reviews . 2006;58(2):226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 209.Raines A. L., Shih M.-S., Chua L., Su C.-W., Tseng S. C. G., O’Connell J. Efficacy of Particulate Amniotic Membrane and Umbilical Cord Tissues in Attenuating Cartilage Destruction in an Osteoarthritis Model. Tissue Engineering. Part A . 2017;23(1–2):12–19. doi: 10.1089/ten.TEA.2016.0088. [DOI] [PubMed] [Google Scholar]
- 210.Basiri A., Farokhi M., Azami M., et al. A silk fibroin/decellularized extract of Wharton’s jelly hydrogel intended for cartilage tissue engineering. Progress in Biomaterials . 2019;8(1):31–42. doi: 10.1007/s40204-019-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marston W. A., Lantis JC 2nd, Wu S. C., et al. An open-label trial of cryopreserved human umbilical cord in the treatment of complex diabetic foot ulcers complicated by osteomyelitis. Wound Repair and Regeneration . 2019;27(6):680–686. doi: 10.1111/wrr.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bemenderfer T. B., Anderson R. B., Odum S. M., Davis W. H. Effects of Cryopreserved Amniotic Membrane-Umbilical Cord Allograft on Total Ankle Arthroplasty Wound Healing. The Journal of Foot and Ankle Surgery . 2019;58(1):97–102. doi: 10.1053/j.jfas.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 213.Brahm T. R. Human birth tissue laminate and methods of use. ” US9855301B1, Jan. 02, 2018 Accessed: Nov. 04, 2020. [Online]. Available: https://patents.google.com/patent/US9855301B1/en.
- 214.Boss A. L., Chamley L. W., James J. L. Placental formation in early pregnancy: how is the centre of the placenta made? Human Reproduction Update . 2018;24(6):750–760. doi: 10.1093/humupd/dmy030. [DOI] [PubMed] [Google Scholar]
- 215.Gude N. M., Roberts C. T., Kalionis B., King R. G. Growth and function of the normal human placenta. Thrombosis Research . 2004;114(5):397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 216.Huppertz B. The anatomy of the normal placenta. Journal of Clinical Pathology . 2008;61(12):1296–1302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 217.Roth I., Corry D. B., Locksley R. M., Abrams J. S., Litton M. J., Fisher S. J. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. The Journal of Experimental Medicine . 1996;184(2):539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Korhonen M., Virtanen I. Immunohistochemical localization of laminin and fibronectin isoforms in human placental villi. J. Histochem. Cytochem. Off. J. Histochem. Soc. . 2001;49(3):313–322. doi: 10.1177/002215540104900305. [DOI] [PubMed] [Google Scholar]
- 219.Griffiths S. K., Campbell J. P. Placental structure, function and drug transfer. Continuing Education in Anesthesia, Critical Care and Pain . 2014;15(2):84–89. doi: 10.1093/bjaceaccp/mku013. [DOI] [Google Scholar]
- 220.Brett K. E., Ferraro Z. M., Yockell-Lelievre J., Gruslin A., Adamo K. B. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. International Journal of Molecular Sciences . 2014;15(9):16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Desforges M., Sibley C. P. Placental nutrient supply and fetal growth. The International Journal of Developmental Biology . 2010;54(2–3):377–390. doi: 10.1387/ijdb.082765md. [DOI] [PubMed] [Google Scholar]
- 222.Smith C. H., Moe A. J., Ganapathy V. Nutrient transport pathways across the epithelium of the placenta. Annual Review of Nutrition . 1992;12:183–206. doi: 10.1146/annurev.nu.12.070192.001151. [DOI] [PubMed] [Google Scholar]
- 223.Knipp G. T., Audus K. L., Soares M. J. Nutrient transport across the placenta. Advanced Drug Delivery Reviews . 1999;38(1):41–58. doi: 10.1016/s0169-409x(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 224.Lager S., Powell T. L. Regulation of nutrient transport across the placenta. Journal of Pregnancy . 2012;2012 doi: 10.1155/2012/179827.179827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yudilevich D. L., Sweiry J. H. Transport of amino acids in the placenta. Biochimica et Biophysica Acta . 1985;822(2):169–201. doi: 10.1016/0304-4157(85)90007-3. [DOI] [PubMed] [Google Scholar]
- 226.Gluckman P. D. Endocrine and nutritional regulation of prenatal growth. Acta Paediatr. Oslo Nor . 1992;423:153–157. doi: 10.1111/j.1651-2227.1997.tb18399.x. [DOI] [PubMed] [Google Scholar]
- 227.Costa M. A. The endocrine function of human placenta: an overview. Reproductive Biomedicine Online . 2016;32(1):14–43. doi: 10.1016/j.rbmo.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 228.De Bonis M., Torricelli M., Severi F. M., Luisi S., De Leo V., Petraglia F. Neuroendocrine aspects of placenta and pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. . 2012;28(Suppl 1):22–26. doi: 10.3109/09513590.2012.651933. [DOI] [PubMed] [Google Scholar]
- 229.Evain-Brion D., Malassine A. Human placenta as an endocrine organ. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. . 2003;13:S34–S37. doi: 10.1016/s1096-6374(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 230.Palmeira P., Quinello C., Silveira-Lessa A. L., Zago C. A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clinical & Developmental Immunology . 2012;2012, article 985646 doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Malek A. Role of IgG antibodies in association with placental function and immunologic diseases in human pregnancy. Expert Review of Clinical Immunology . 2013;9(3):235–249. doi: 10.1586/eci.12.99. [DOI] [PubMed] [Google Scholar]
- 232.Sati L., Demir A. Y., Sarikcioglu L., Demir R. Arrangement of collagen fibers in human placental stem villi. Acta Histochemica . 2008;110(5):371–379. doi: 10.1016/j.acthis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 233.Bailey A. J., Sims T. J., Duance V. C., Light N. D. Partial characterization of a second basement membrane collagen in human placenta. Evidence for the existence of two type IV collagen molecules. FEBS Letters . 1979;99(2):361–366. doi: 10.1016/0014-5793(79)80992-8. [DOI] [PubMed] [Google Scholar]
- 234.Leonel L. C. P. C., Miranda C. M. F. C., Coelho T. M., et al. Decellularization of placentas: establishing a protocol. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. . 2018;51(1):p. e6382. doi: 10.1590/1414-431X20176382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brown J. C., Mann K., Wiedemann H., Timpl R. Structure and binding properties of collagen type XIV isolated from human placenta. The Journal of Cell Biology . 1993;120(2):557–567. doi: 10.1083/jcb.120.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nath S., Bhattacharyya D. Cell adhesion by aqueous extract of human placenta used as wound healer. Indian Journal of Experimental Biology . 2007;45(8):732–738. [PubMed] [Google Scholar]
- 237.Arai K. Y., Nishiyama T. Developmental Changes in Extracellular Matrix Messenger RNAs in the Mouse Placenta During the Second Half of Pregnancy: Possible Factors Involved in the Regulation of Placental Extracellular Matrix Expression. Biology of Reproduction . 2007;77(6):923–933. doi: 10.1095/biolreprod.107.061382. [DOI] [PubMed] [Google Scholar]
- 238.Isemura M., Sato N., Yamaguchi Y., et al. Isolation and characterization of fibronectin-binding proteoglycan carrying both heparan sulfate and dermatan sulfate chains from human placenta. The Journal of Biological Chemistry . 1987;262(18):8926–8933. doi: 10.1016/S0021-9258(18)47503-5. [DOI] [PubMed] [Google Scholar]
- 239.Yamaguchi Y., Isemura M., Yosizawa Z., et al. Changes in the distribution of fibronectin in the placenta during normal human pregnancy. American Journal of Obstetrics and Gynecology . 1985;152(6):715–718. doi: 10.1016/s0002-9378(85)80055-7. [DOI] [PubMed] [Google Scholar]
- 240.Takamoto M., Endo T., Isemura M., Kochibe N., Kobata A. Structures of asparagine-linked oligosaccharides of human placental fibronectin. J. Biochem. (Tokyo) . 1989;105(5):742–750. doi: 10.1093/oxfordjournals.jbchem.a122738. [DOI] [PubMed] [Google Scholar]
- 241.Calatroni A., di Ferrante N. The glycosaminoglycans of human term placenta. Carbohydrate Research . 1969;10(4):535–548. doi: 10.1016/S0008-6215(00)80122-6. [DOI] [Google Scholar]
- 242.de Oliveira G. B., Vale A. M., Santos A. C., Moura C. E. B., Rocha H. A. O., Oliveira M. F. Composition and significance of glycosaminoglycans in the uterus and placenta of mammals. Brazilian Archives of Biology and Technology . 2015;58(4):512–520. doi: 10.1590/S1516-8913201500281. [DOI] [Google Scholar]
- 243.Bowen J. M., Chamley L., Mitchell M. D., Keelan J. A. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta . 2002;23(4):239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 244.Hauguel-de Mouzon S., Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta . 2006;27(8):794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 245.Keelan J. A., Mitchell M. D. Placental cytokines and preeclampsia. Front. Biosci. J. Virtual Libr. . 2007;12:2706–2727. doi: 10.2741/2266. [DOI] [PubMed] [Google Scholar]
- 246.Dover N., Gulerman H. C., Celen S., Kahyaoglu S., Yenicesu O. Placental growth factor: as an early second trimester predictive marker for preeclampsia in normal and high-risk pregnancies in a Turkish population. Journal of Obstetrics and Gynaecology of India . 2013;63(3):158–163. doi: 10.1007/s13224-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Klaffenbach D., Friedrich D., Strick R., et al. Contribution of different placental cells to the expression and stimulation of antimicrobial proteins (AMPs) Placenta . 2011;32(11):830–837. doi: 10.1016/j.placenta.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 248.Frew L., Stock S. J. Antimicrobial peptides and pregnancy. Reprod. Camb. Engl. . 2011;141(6):725–735. doi: 10.1530/REP-10-0537. [DOI] [PubMed] [Google Scholar]
- 249.Park S. Y., Phark S., Lee M., Lim J. Y., Sul D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta . 2010;31(10):873–879. doi: 10.1016/j.placenta.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 250.Shin E. H., Kim M., Hada B., et al. Effects of Human Placenta Extract (Laennec) on Ligament Healing in a Rodent Model. Biological & Pharmaceutical Bulletin . 2019;42(12):1988–1995. doi: 10.1248/bpb.b19-00349. [DOI] [PubMed] [Google Scholar]
- 251.Kwon J. W., Hong S. E., Kang S. R., Park B. Y. Effect of Human Placental Extract Treatment on Random-Pattern Skin Flap Survival in Rats. Journal of Investigative Surgery . 2019;32(4):304–313. doi: 10.1080/08941939.2017.1417518. [DOI] [PubMed] [Google Scholar]
- 252.Choi J. S., Kim J. D., Yoon H. S., Cho Y. W. Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Engineering. Part A . 2013;19(3–4):329–339. doi: 10.1089/ten.TEA.2011.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Flynn L., Semple J. L., Woodhouse K. A. Decellularized placental matrices for adipose tissue engineering. Journal of Biomedical Materials Research. Part A . 2006;79(2):359–369. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 254.Francis M. P., Breathwaite E., Bulysheva A. A., et al. Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Biomaterialia . 52:92–104. doi: 10.1016/j.actbio.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 255.Rameshbabu A. P., Bankoti K., Datta S., et al. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Applied Materials & Interfaces . 2018;10(20):16977–16991. doi: 10.1021/acsami.7b19007. [DOI] [PubMed] [Google Scholar]
- 256.Kakabadze A., Kakabadze Z. Prospect of using decellularized human placenta and cow placentome for creation of new organs: targeting the liver (part I: anatomic study) Transplantation Proceedings . 2015;47(4):1222–1227. doi: 10.1016/j.transproceed.2014.09.181. [DOI] [PubMed] [Google Scholar]
- 257.Kakabadze Z., Kakabadze A., Chakhunashvili D., et al. Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology . 2018;67(5):1956–1969. doi: 10.1002/hep.29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kinzler W., Smulian J., Kistler C. A., Hahn R., Zhou P., Gordon M. Umbilical artery extracellular matrix remodeling in fetal growth restriction. American Journal of Obstetrics and Gynecology . 2004;191(6):p. S10. doi: 10.1016/j.ajog.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 259.Dietrich T., Schlotzer-Schrehardt U., Hofmann-Rummelt C., Seitz B., Kruse F. E. Comparative Analysis of Basement Membrane Composition of the Human Limbal and Amniotic Membrane Epithelia. Investigative Ophthalmology & Visual Science . 2005;46(13):2093–2093. [Google Scholar]
- 260.Brennan M., Oldberg A., Pierschbacher M., Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. The Journal of Biological Chemistry . 1984;259(22):13742–13750. doi: 10.1016/S0021-9258(18)89808-8. [DOI] [PubMed] [Google Scholar]
- 261.Franc S., Rousseau J. C., Garrone R., van der Rest M., Moradi-Améli M. Microfibrillar composition of umbilical cord matrix: characterization of fibrillin, collagen VI and intact collagen V. Placenta . 1998;19(1):95–104. doi: 10.1016/s0143-4004(98)90104-7. [DOI] [PubMed] [Google Scholar]
- 262.Pu L., et al. Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration. Stem Cell Research & Therapy . 2017;8(1):p. 72. doi: 10.1186/s13287-017-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Inoue S., Iwasaki M. Dermatan Sulfate-Chondroitin Sulfate Copolymers from Umbilical Cord. Isolation and Characterization. J. Biochem. (Tokyo) . 1976;80(3):513–524. doi: 10.1093/oxfordjournals.jbchem.a131306. [DOI] [PubMed] [Google Scholar]
- 264.Capehart A., Giamo A., Singhas C. DISTRIBUTION OF CHONDROITIN SULFATE GLYCOSAMINOGLYCANS IN THE HUMAN CHORIO-AMNIONIC MEMBRANE COMPLEX AT TERM. Journal of the North Carolina Academy of Science . 2003;119(1):1–12. [Google Scholar]
- 265.Bourne G. L. The microscopic anatomy of the human amnion and chorion. American Journal of Obstetrics and Gynecology . 1960;79(6):1070–1073. doi: 10.1016/0002-9378(60)90512-3. [DOI] [PubMed] [Google Scholar]
- 266.Koizumi N., Inatomi T., Sotozono C., Fullwood N. J., Quantock A. J., Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Current Eye Research . 2000;20(3):173–177. doi: 10.1076/0271-3683(200003)2031-9FT173. [DOI] [PubMed] [Google Scholar]
- 267.Heikkinen J., Möttönen M., Pulkki K., Lassila O., Alanen A. Cytokine levels in midtrimester amniotic fluid in normal pregnancy and in the prediction of pre-eclampsia. Scandinavian Journal of Immunology . 2001;53(3):310–314. doi: 10.1046/j.1365-3083.2001.00872.x. [DOI] [PubMed] [Google Scholar]
- 268.Lang A. K., Searle R. F. The immunomodulatory activity of human amniotic fluid can be correlated with transforming growth factor-beta 1 (TGF-beta 1) and beta 2 activity. Clinical and Experimental Immunology . 1994;97(1):158–163. doi: 10.1111/j.1365-2249.1994.tb06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Copland I. B., Adamson S. L., Post M., Lye S. J., Caniggia I. TGF-β3 Expression During Umbilical Cord Development and its Alteration in Pre-eclampsia. Placenta . 2002;23(4):311–321. doi: 10.1053/plac.2001.0778. [DOI] [PubMed] [Google Scholar]
- 270.Saito S. Cytokine network at the feto-maternal interface. Journal of Reproductive Immunology . 2000;47(2):87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 271.Hirai C., Ichiba H., Saito M., Shintaku H., Yamano T., Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. Journal of Pediatric Gastroenterology and Nutrition . 2002;34(5):524–528. doi: 10.1097/00005176-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 272.Rao C. V., Li X., Toth P., Lei Z. M. Expression of epidermal growth factor, transforming growth factor-alpha, and their common receptor genes in human umbilical cords. The Journal of Clinical Endocrinology and Metabolism . 1995;80(3):1012–1020. doi: 10.1210/jcem.80.3.7883816. [DOI] [PubMed] [Google Scholar]
- 273.Lysiak J., Han V., Lala P. Localization of Transforming Growth Factor in the Human Placenta and Decidua: Role in Trophoblast Growth1. Biology of Reproduction . 1993;49(5):885–894. doi: 10.1095/biolreprod49.5.885. [DOI] [PubMed] [Google Scholar]
- 274.Farina A., Carinci P., Di Luzio L. Angiogenin: a marker for preterm delivery in midtrimester amniotic fluid. American Journal of Obstetrics and Gynecology . 1997;177(2):483–484. doi: 10.1016/s0002-9378(97)70233-3. [DOI] [PubMed] [Google Scholar]
- 275.Irvin J., Danchik C., Rall J., et al. Bioactivity and composition of a preserved connective tissue matrix derived from human placental tissue. Journal of Biomedical Materials Research. Part B, Applied Biomaterials . 2018;106(8):2731–2740. doi: 10.1002/jbm.b.34054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McQuilling J. P., Burnette M., Kimmerling K. A., Kammer M., Mowry K. C. A mechanistic evaluation of the angiogenic properties of a dehydrated amnion chorion membrane in vitro and in vivo. Wound Repair and Regeneration . 2019;27(6):609–621. doi: 10.1111/wrr.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wolbank S., Hildner F., Redl H., van Griensven M., Gabriel C., Hennerbichler S. Impact of human amniotic membrane preparation on release of angiogenic factors. Journal of Tissue Engineering and Regenerative Medicine . 2009;3(8):651–654. doi: 10.1002/term.207. [DOI] [PubMed] [Google Scholar]
- 278.Vuorela-Vepsäläinen P., Alfthan H., Orpana A., Alitalo K., Stenman U. H., Halmesmäki E. Vascular endothelial growth factor is bound in amniotic fluid and maternal serum. Human Reproduction (Oxford, England) . 1999;14(5):1346–1351. doi: 10.1093/humrep/14.5.1346. [DOI] [PubMed] [Google Scholar]
- 279.Kalampokas E., Vrachnis N., Samoli E., et al. Association of adiponectin and placental growth factor in amniotic fluid with second trimester fetal growth. Vivo Athens Greece . 2012;26(2):327–333. [PubMed] [Google Scholar]
- 280.Maritati M., Comar M., Zanotta N., et al. Influence of vaginal lactoferrin administration on amniotic fluid cytokines and its role against inflammatory complications of pregnancy. Journal of Inflammation . 2017;14(1):p. 5. doi: 10.1186/s12950-017-0152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Buhimschi C. S., Bhandari V., Dulay A. T., et al. Amniotic Fluid Angiopoietin-1, Angiopoietin-2, and Soluble Receptor Tunica Interna Endothelial Cell Kinase-2 Levels and Regulation in Normal Pregnancy and Intraamniotic Inflammation-Induced Preterm Birth. The Journal of Clinical Endocrinology and Metabolism . 2010;95(7):3428–3436. doi: 10.1210/jc.2009-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Denison F. C., Kelly R. W., Calder A. A., Riley S. C. Cytokine secretion by human fetal membranes, decidua and placenta at term. Human Reproduction (Oxford, England) . 1998;13(12):3560–3565. doi: 10.1093/humrep/13.12.3560. [DOI] [PubMed] [Google Scholar]
- 283.Hara C. D., França E. L., Fagundes D. L., et al. Characterization of Natural Killer Cells and Cytokines in Maternal Placenta and Fetus of Diabetic Mothers. Journal of Immunology Research . 2016;2016 doi: 10.1155/2016/7154524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Halgunset J., Johnsen H., Kjøllesdal A. M., Qvigstad E., Espevik T., Austgulen R. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 1994;56(3):153–160. doi: 10.1016/0028-2243(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 285.Chaiworapongsa T., Romero R., Chaiworapongsa T., et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. The Journal of Maternal-Fetal & Neonatal Medicine . 2005;18(6):405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lappas M., Yee K., Permezel M., Rice G. E. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. The Journal of Endocrinology . 2005;186(3):457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 287.Mazaki-Tovi S., Romero R., Vaisbuch E., et al. ADIPONECTIN IN AMNIOTIC FLUID IN NORMAL PREGNANCY, SPONTANEOUS LABOR AT TERM, AND PRETERM LABOR: A NOVEL ASSOCIATION WITH intra-amniotic INFECTION/INFLAMMATION. The Journal of Maternal-Fetal & Neonatal Medicine . 2010;23(2):120–130. doi: 10.3109/14767050903026481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chen J., Tan B., Karteris E., et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia . 2006;49(6):1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 289.Korenovsky Y. V., Remneva O. V. Reference values of concentrations of matrix metalloproteinases-1, -2, -9 and the tissue inhibitor of matrix metalloproteinases (TIMP-1) in the amniotic fluid during physiological pregnancy and delivery. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. . 2016;10(3):240–242. doi: 10.1134/S1990750816030070. [DOI] [PubMed] [Google Scholar]
- 290.Tency I., Verstraelen H., Kroes I., et al. Imbalances between Matrix Metalloproteinases (MMPs) and Tissue Inhibitor of Metalloproteinases (TIMPs) in Maternal Serum during Preterm Labor. PLoS One . 2012;7(11, article e49042) doi: 10.1371/journal.pone.0049042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Maymon E., Romero R., Pacora P., et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. Journal of Perinatal Medicine . 2001;29(4):308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 292.Romanowicz L., Gogiel T., Galewska Z., et al. Divergent changes in the content and activity of MMP-26 and TIMP-4 in human umbilical cord tissues associated with preeclampsia. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 2018;231:48–53. doi: 10.1016/j.ejogrb.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 293.Zhang J., Cao Y.-J., Zhao Y.-G., Sang Q.-X. A., Duan E.-K. Expression of matrix metalloproteinase-26 and tissue inhibitor of metalloproteinase-4 in human normal cytotrophoblast cells and a choriocarcinoma cell line, JEG-3. Molecular Human Reproduction . 2002;8(7):659–666. doi: 10.1093/molehr/8.7.659. [DOI] [PubMed] [Google Scholar]
- 294.Thaler C. J., Labarrere C. A., Hunt J. S., McIntyre J. A., Faulk W. P. Immunological studies of lactoferrin in human placentae. Journal of Reproductive Immunology . 1993;23(1):21–39. doi: 10.1016/0165-0378(93)90024-c. [DOI] [PubMed] [Google Scholar]
- 295.Zaga-Clavellina V., Ruiz M., Flores-Espinosa P., et al. Tissue-specific human beta-defensins (HBD)-1, HBD-2 and HBD-3 secretion profile from human amniochorionic membranes stimulated with Candida albicans in a two-compartment tissue culture system. Reprod. Biol. Endocrinol. RBE . 2012;10(1):p. 70. doi: 10.1186/1477-7827-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sidhom E., Pilmane M., Kreicberga I. Molecular events in the Wharton’s jelly and blood vessels of human umbilical cord. Papers in Anthropology . 2017;26(2) doi: 10.12697/poa.2017.26.2.12. [DOI] [Google Scholar]
- 297.King A. E., Kelly R. W., Sallenave J.-M., Bocking A. D., Challis J. R. G. Innate immune defences in the human uterus during pregnancy. Placenta . 2007;28(11–12):1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 298.Espinoza J., Chaiworapongsa T., Romero R., et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. The Journal of Maternal-Fetal & Neonatal Medicine . 2003;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 299.Rosenberg V. A., Buhimschi I. A., Dulay A. T., et al. MODULATION OF AMNIOTIC FLUID ACTIVIN-A AND INHIBIN-A IN WOMEN WITH PRETERM PREMATURE RUPTURE OF THE MEMBRANES AND INFECTION-INDUCED PRETERM BIRTH. American Journal of Reproductive Immunology . 2012;67(2):122–131. doi: 10.1111/j.1600-0897.2011.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jeschke M. G., Gauglitz G. G., David T. T. P., Herndon N., Kita K. Umbilical Cord Lining Membrane and Wharton’s Jelly-Derived Mesenchymal Stem Cells: the Similarities and Differences. The Open Tissue Engineering and Regenerative Medicine Journal . 2011;4(1) Accessed: Nov. 04, 2020. [Online]. Available: https://benthamopen.com/ABSTRACT/TOTERMJ-4-21. [Google Scholar]
- 301.Hofmann G. E., Abramowicz J. S. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstetricia et Gynecologica Scandinavica . 1990;69(3):217–221. doi: 10.3109/00016349009028683. [DOI] [PubMed] [Google Scholar]
- 302.Bona G., Aquili C., Ravanini P., et al. Growth hormone, insulin-like growth factor-I and somatostatin in human fetus, newborn, mother plasma and amniotic fluid. Panminerva Medica . 1994;36(1):5–12. [PubMed] [Google Scholar]
- 303.Han V. K., Bassett N., Walton J., Challis J. R. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. The Journal of Clinical Endocrinology and Metabolism . 1996;81(7):2680–2693. doi: 10.1210/jcem.81.7.8675597. [DOI] [PubMed] [Google Scholar]
- 304.Darj E., Lyrenäs S. Insulin-like growth factor binding protein-1, a quick way to detect amniotic fluid. Acta Obstetricia et Gynecologica Scandinavica . 1998;77(3):295–297. doi: 10.1080/j.1600-0412.1998.770307.x. [DOI] [PubMed] [Google Scholar]
- 305.Martina N. A., Kim E., Chitkara U., Wathen N. C., Chard T., Giudice L. C. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. The Journal of Clinical Endocrinology and Metabolism . 1997;82(6):1894–1898. doi: 10.1210/jcem.82.6.3974. [DOI] [PubMed] [Google Scholar]
- 306.Goldman S., Weiss A., Eyali V., Shalev E. Differential activity of the gelatinases (matrix metalloproteinases 2 and 9) in the fetal membranes and decidua, associated with labour. Molecular Human Reproduction . 2003;9(6):367–373. doi: 10.1093/molehr/gag040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article is a review article. All data can be found in the cited references.


