Abstract
Background: Osteoarthritis (OA) is a degenerative disease which serious affects patients. Ligusticum chuanxiong (CX) has been shown to have a certain curative effect on osteoarthritis in traditional Chinese medicine therapy. This study is based on network pharmacology and molecular docking technology to explore the potential mechanism of CX.
Methods: Components of CX to treat osteoarthritis were screened in the TCMSP database and targets were predicted by the PharmMapper database, the osteoarthritis targets were collected from the GeneCards database, and intersection genes were found to be the possible targets of CX anti-OA. The STRING database and Cytoscape software were utilized for protein-protein interaction analysis and further screening of core targets. The Metascape database was used for KEGG and GO enrichment analyses. Then, the top 10 pathways were selected to construct “drug-compound-target-pathway-disease” network analysis. Finally, molecular docking was used to analyze the binding affinity of seven compounds with core targets and TNF-α.
Results: Seven compounds with 253 non-repetitive targets of CX were screened from the TCMSP database and 60 potential intersection targets of CX anti-OA were found. PPI network analysis showed that the core targets were ALB, AKT1, IGF1, CASP3, MAPK1, ANXA5, and MAPK14, while GO and KEGG pathway enrichment analyses showed that the relevant biological processes involved in the treatment of osteoarthritis by CX might include the MAPK cascade and reactive oxygen species metabolic process. The KEGG pathway analysis result was mainly associated with the MAPK signaling pathway and PI3K-AKT signaling pathway. We further docked seven ingredients with MAPK1 and MAPK14 enriched in the MAPK pathway, and TNF-α as the typical inflammatory cytokine. The results also showed good binding affinity, especially FA, which may be the most important component of CX anti-OA.
Conclusion: Our research revealed the potential mechanism of CX in the treatment of OA, and our findings can also pave the way for subsequent basic experimental verification and a new research direction.
Keywords: osteoarthritis, network pharmacology, molecular docking, MAPK pathway, ligusticum chuanxiong
Introduction
Osteoarthritis (OA) is a degenerative disease of the joints, it mainly occurs in knee joints and the clinical manifestations are pain, swelling, and limited movement (Katz et al., 2021). The pathological changes are cartilage degeneration, subchondral bone sclerosis, and synovitis (Abramoff and Caldera, 2020). It was reported that there are more than 250 million people around the world suffering from osteoarthritis, and half of the world’s population aged 65 and older suffer from OA (Litwic et al., 2013). Without radical treatment, osteoarthritis has become the main reason for the worldwide public problem of limb disability. Currently, non-steroidal anti-inflammatory drugs and intra-articular local injection of glucocorticoids were used as the first-line therapy for OA, mainly leading to reduced symptoms and restored joint function (Sharma, 2021). These drugs with short term efficacy not only cause serious damage to digestive tract, liver, and kidney function, but also accelerate the progress of arthritis in the long run (Dragos et al., 2017).
The research of traditional Chinese medicine (TCM) on osteoarthritis can be traced back to thousands of years ago (Yuan et al., 2016). It was first mentioned in “Huangdi Neijing”. Osteoarthritis is called bone arthralgia in TCM. According to the principle of diagnosis and treatment based on an overall analysis of the illness and the patient’s condition, the treatment for OA in TCM mainly focused on tonifying the liver and kidney, expelling wind and dampness, promoting blood circulation, and dispersing cold. Rich theoretical knowledge and therapeutic drugs have been accumulated for thousands of years (Wang C. et al., 2020).
The medicinal value of Ligusticum chuanxiong (CX) is in its roots and stems. It has been used in traditional Chinese medicine for thousands of years which was first mentioned in “Shennong’s herbal classic” (Chen Z. et al., 2018). It is one of the most important and commonly used drugs for promoting blood circulation and removing blood stasis. It is known as an “important medicine for headaches in various meridians” (Ran et al., 2011). It also plays a significant role in ischemic diseases, dizziness, and irregular menstruation (Wang M. et al., 2020). In addition, it also has a certain effect on rheumatism and joint pain, including osteoarthritis (Zhou et al., 2019a). Many researchers have focused on CX in recent years. In 1977, Chinese researchers first isolated tetramethylpyrazine (TMP), which is the most important alkaloid in CX (Sue et al., 2009). And 189 active components have currently been found, with some basic studies showing that the active components of CX can improve the vitality of chondrocytes, inhibit the apoptosis of chondrocytes, and upregulate the expression of type II collagen which is a major part of cartilage tissue (Ju et al., 2010). Undoubtedly, CX or its isolate compounds are potential candidates of the treatment for osteoarthritis in the view of modern drug development. However, only a few studies about CX have focused on the treatment of osteoarthritis, and the scope of these studies is not adequate (Ye et al., 2011; Luo Y. et al., 2020). Therefore, a new way or concept may be needed to help us sufficiently explore the mechanism of CX anti-OA.
CX, as with other traditional Chinese medicines, has complex action mechanisms and multi-channel interaction (Tao et al., 2013). In the past, traditional Chinese medicine was regarded as empirical medicine because of the lack of modern research methods and technologies (Luo E. et al., 2020). Network pharmacology is a new method of combining computer science and medicine which was first proposed by Hopkins in 2008 (Hopkins, 2008). Its interactive network based on “multi-gene”, “multi-target”, and “multi-channel” interactions coincides with the thinking and methods of TCM. Network pharmacology has become an emerging discipline to help us deeply study traditional Chinese medicine.
Active components of CX were screened and predicted by network pharmacology. By constructing the “drug-component-target-pathway-disease” interactive network and utilizing GO and KEGG pathway enrichment analysis and molecular docking technology, the potential molecular mechanism of CX in the treatment of osteoarthritis was explored. Our research may provide guidance for subsequent basic experimental research.
Methods
Pharmacokinetics and Component Screening of CX
The TCMSP database (http://lsp.nwu.edu.cn/tcmsp.php) (Ru et al., 2014) was utilized which can predict absorption, distribution metabolism, and excretion (ADME) characteristic information, such as oral bioavailability (OB), drug similarity (DL), Caco-2 permeability (Caco-2), and blood-brain barrier (BBB) issues. OB value is the most important feature of oral drugs, which can evaluate the efficacy of drugs distributed to the whole-body circulation after absorption. It is difficult to reach the effective concentration of most TCM components to exert their efficacy in specific tissues and organs, probably due to their small OB value. According to the recommendations of the TCMSP database and previous studies, OB value ≥30% is considered to have relatively reliable pharmacological activity. DL is a concept of physicochemical properties and molecular structure. It is based on the chemical structure of existing drugs to evaluate whether new compounds meet the characteristics of drugs, so as to become a new drug. The threshold DL value is 0.18 according to previous research (Zhang et al., 2020). We used the chemical name “Ligusticum chuanxiong” to search for its pharmacokinetic characteristics, and the active ingredients were further screened by taking oral bioavailability (OB) ≥ 30% and drug likeness (DL) ≥ 0.18 as the screening conditions (Ai et al., 2020).
Target Prediction of CX Against OA
The reverse pharmacophore localization database PharmMapper (http://www.lilab-ecust.cn/pharmmapper/) (Liu et al., 2010) was used to predict the potential targets of CX. After screening the active components from the TCMSP database, the SDF structure formats of these small molecular compounds were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and uploaded to the PharmMapper server. Species “Homo sapiens” was selected in uniport (https://www.uniprot.org/) (Uszkoreit et al., 2021) to standardize the Uniprot ID to the gene symbol. At the same time, the targets of OA were predicted in the Gene Cards database (https://www.genecards.org/). The keyword “osteoarthritis” was used to collect potential genes. We selected a correlation score >1 of OA targets, norm fit >0.6 of CX component targets for further analysis, and the overlapping area of the Venn diagram represented the potential targets of CX anti-OA.
PPI Network Construction
The STRING (http://string-db.org; Version 11.5) (Feng et al., 2019) database was used to construct a protein-protein interaction (PPI) network for CX anti-OA to analyze the functional interaction between proteins. The network confidence score ≥0.4 was set to obtain targets with “Homo sapiens” being selected in Cytoscape software (version 3.8.1) further. The MCODE (molecular complex detection) and Cytohubba plug-ins were used to collect the core targets of CX anti-OA to construct the regulatory network.
GO and KEGG Pathway Enrichment Analysis
GO function and KEGG pathway enrichment analyses were conducted to explore the core mechanism and pathway of CX anti-OA in the Metascape (http://www.metascape.org/) (Zhou et al., 2019b) database. We searched the gene symbols of common targets in Metascape by limiting the species to “Homo sapiens”, and setting the cut-off p value as 0.01 and min overlap as three for enrichment analysis, including biological process (BP), cell composition (CC), molecular function (MF), and KEGG pathways. The results were imported into bioinformatics software (http://www.bioinformatics.com.cn/). Sequentially, the highest pathway in the KEGG mapper (http://www.kegg.jp.org/) (Kanehisa and Sato, 2020) was highlighted to show its specific molecular mechanism in this pathway.
Construction of the Drug–Compound-Target–Pathway-Disease (D-C-T-P-D) Network
Cytoscape (version 3.8.2) (Piñero et al., 2021) software was used to establish the D-C-T-P-D network model, and drugs, active compounds, cross genes. and diseases were introduced respectively. The top core pathway in the previous KEGG pathway enrichment analysis was inputted to establish the relationship between these elements and build a complete regulatory network by using the degree value for internal ranking.
Molecular Docking
The Swiss dock (http://www.swissdock.ch/) (Grosdidier et al., 2011) database was used to analyze the molecular binding affinity of the molecular docking between the active components of CX and the core target for the treatment of OA. Target protein was obtained by comprehensively evaluating the resolution and release time in the Protein Data Bank (PDB) (www.rcsb.org) website (Carugo, 2021), and chemical structures of active components were downloaded from the PubChem database. Openbabel software was used to convert the SDF format into the mol2 format (O'Boyle et al., 2011). The specific binding sites and atomic distances between active components and proteins were determined by UCSF chimera software (Pettersen et al., 2004).
Results
Screened CX Compounds Targets and OA Disease Targets
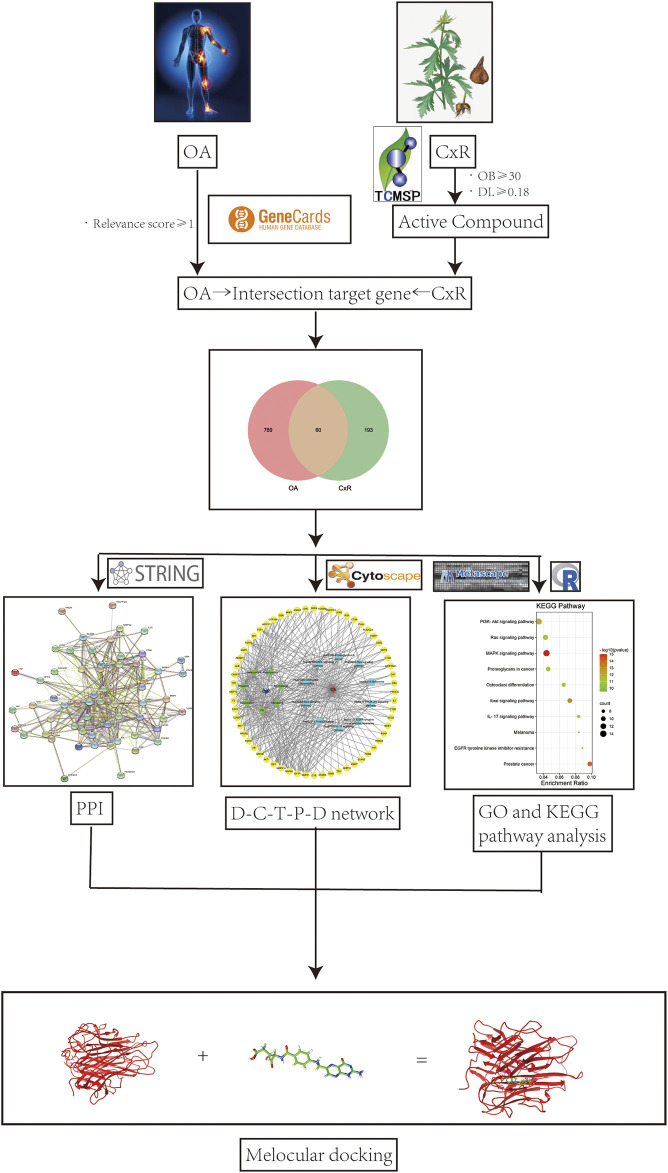
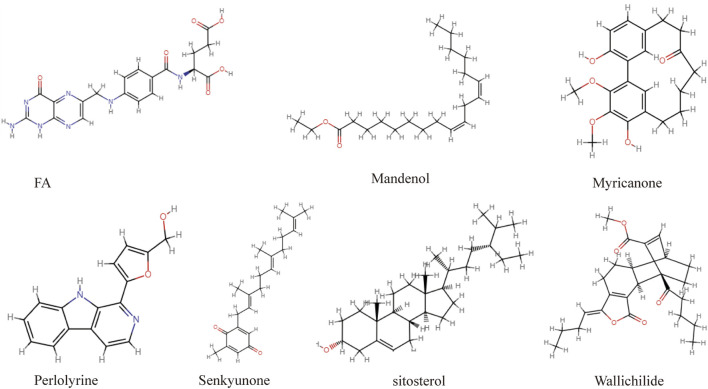
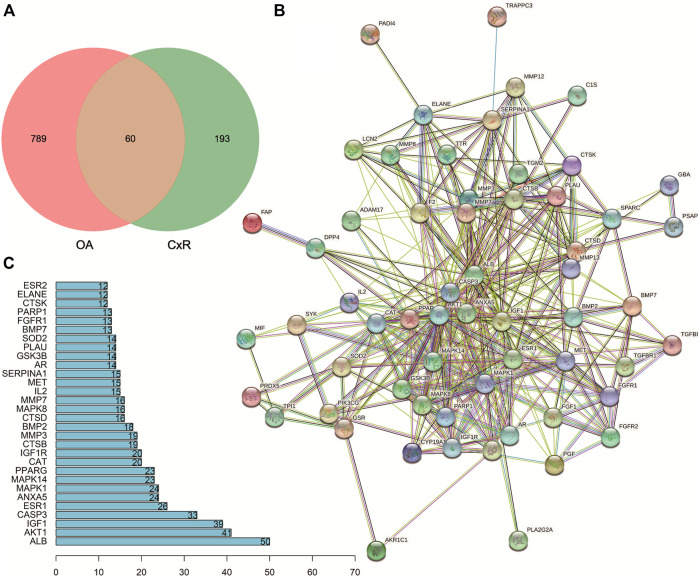
The flaw chart of this study as displayed in Figure 1. A total of 189 ingredients isolated from CX were collected from the TCMSP database. According to the screening criteria of OB ≥ 30% and DL ≥ 0.18, seven compounds were identified, including mandenol, myricanone, perlolyrine, senkyunone, wallichilide, sitosterol, and FA (structure in Figure 2, detail in Table 1). PharmMapper was used for target prediction, and the results were selected with norm fit >0.6 for subsequent analysis. We obtained 100 targets of mandenol, 56 of myricanone, 68 of perlolyrine, 116 of senkyunone, 152 of wallichilide, 98 of sitosterol, and 177 of FA. With repeat targets excluded, 253 drug targets were obtained finally. And a total 3,107 targets of osteoarthritis were identified from the GeneCards database. We selected the 849 results with reference score >1 for subsequent analysis. Both targets of CX and OA were imported into a Venn diagram and the 60 overlapped genes were the potential targets for CX anti-OA (entrez IDs shown in Table 2).
FIGURE 1.
Flow chart of network pharmacology to study the potential molecular mechanism of CX in the treatment of OA.
FIGURE 2.
The chemical structure formula of active components of CX screened from the TCMSP database.
TABLE 1.
The ADME characteristics of CX active compounds according to the TCMSP database.
| NO. | Molecule ID | Molecule name | Molecular weight | OB(%) | DL |
|---|---|---|---|---|---|
| 1 | MOL001494 | Mandenol (Ethyl linolate) | 308.56 | 42 | 0.19 |
| 2 | MOL002135 | Myricanone | 356.45 | 40.6 | 0.51 |
| 3 | MOL002140 | Perlolyrine | 264.3 | 65.95 | 0.27 |
| 4 | MOL002151 | senkyunone | 326.52 | 47.66 | 0.24 |
| 5 | MOL002157 | wallichilide | 412.57 | 42.31 | 0.71 |
| 6 | MOL000359 | sitosterol | 414.79 | 36.91 | 0.75 |
| 7 | MOL000433 | FA | 441.45 | 68.96 | 0.71 |
OB, oral bioavailability; DL, drug-likeness.
TABLE 2.
The detailed entrez IDs of 60 potential targets of CX anti-OA.
| NO. | Target | Symbol | Entrez ID | NO. | Target | Symbol | Entrez ID |
|---|---|---|---|---|---|---|---|
| 1 | Disintegrin and metalloproteinase domain-containing protein 17 | ADAM17 | P78536 | 31 | Neutrophil gelatinase-associated lipocalin | LCN2 | P80188 |
| 2 | Aldo-keto reductase family 1 member C1 | AKR1C1 | Q04828 | 32 | Mitogen-activated protein kinase 1 | MAPK1 | P28482 |
| 3 | RAC-alpha serine/threonine-protein kinase | AKT1 | P31749 | 33 | Mitogen-activated protein kinase 14 | MAPK14 | Q16539 |
| 4 | Serum albumin | ALB | P02768 | 34 | Mitogen-activated protein kinase 8 | MAPK8 | P45983 |
| 5 | Annexin A5 | ANXA5 | P08758 | 35 | Hepatocyte growth factor receptor | MET | P08581 |
| 6 | Androgen receptor | AR | P10275 | 36 | Macrophage migration inhibitory factor | MIF | P14174 |
| 7 | Bone morphogenetic protein 2 | BMP2 | P12643 | 37 | Macrophage metalloelastase | MMP12 | P39900 |
| 8 | Bone morphogenetic protein 7 | BMP7 | P18075 | 38 | Collagenase 3 | MMP13 | P45452 |
| 9 | Complement C1s subcomponent | C1S | P09871 | 39 | Stromelysin-1 | MMP3 | P08254 |
| 10 | Caspase-3 | CASP3 | P42574 | 40 | Matrilysin | MMP7 | P09237 |
| 11 | Catalase | CAT | P04040 | 41 | Neutrophil collagenase | MMP8 | P22894 |
| 12 | Cathepsin B | CTSB | P07858 | 42 | Protein-arginine deiminase type-4 | PADI4 | Q9UM07 |
| 13 | Cathepsin D | CTSD | P07339 | 43 | Poly [ADP-ribose] polymerase 1 | PARP1 | P09874 |
| 14 | Cathepsin K | CTSK | P43235 | 44 | Placenta growth factor | PGF | P49763 |
| 15 | Aromatase | CYP19A1 | P11511 | 45 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | PIK3CG | P48736 |
| 16 | Dipeptidyl peptidase 4 | DPP4 | P27487 | 46 | Phospholipase A2, membrane associated | PLA2G2A | P14555 |
| 17 | Neutrophil elastase | ELANE | P08246 | 47 | Urokinase-type plasminogen activator | PLAU | P00749 |
| 18 | Estrogen receptor | ESR1 | P03372 | 48 | Peroxisome proliferator-activated receptor gamma | PPARG | P37231 |
| 19 | Estrogen receptor beta | Esr2 | Q92731 | 49 | Peroxiredoxin-5, mitochondrial | PRDX5 | P30044 |
| 20 | Prothrombin | F2 | P00734 | 50 | Prosaposin | PSAP | P07602 |
| 21 | Prolyl endopeptidase FAP | FAP | Q12884 | 51 | Alpha-1-antitrypsin | SERPINA1 | P01009 |
| 22 | Fibroblast growth factor 1 | FGF1 | P05230 | 52 | Superoxide dismutase [Mn], mitochondrial | SOD2 | P04179 |
| 23 | Fibroblast growth factor receptor 1 | FGFR1 | P11362 | 53 | SPARC | SPARC | P09486 |
| 24 | Fibroblast growth factor receptor 2 | FGFR2 | P21802 | 54 | Tyrosine-protein kinase SYK | SYK | P43405 |
| 25 | Lysosomal acid glucosylceramidase | GBA | P04062 | 55 | TGF-beta receptor type-1 | TGFBR1 | P36897 |
| 26 | Glycogen synthase kinase-3 beta | GSK3B | P49841 | 56 | TGF-beta receptor type-2 | TGFBR2 | P37173 |
| 27 | Glutathione reductase, mitochondrial | GSR | P00390 | 57 | Protein-glutamine gamma-glutamyltransferase 2 | TGM2 | P21980 |
| 28 | Insulin-like growth factor I | IGF1 | P05019 | 58 | Triosephosphate isomerase | TPI1 | P60174 |
| 29 | Insulin-like growth factor 1 receptor | IGF1R | P08069 | 59 | Trafficking protein particle complex subunit 3 | TRAPPC3 | O43617 |
| 30 | Interleukin-2 | IL-2 | P60568 | 60 | Transthyretin | TTR | P02766 |
PPI Network Analysis
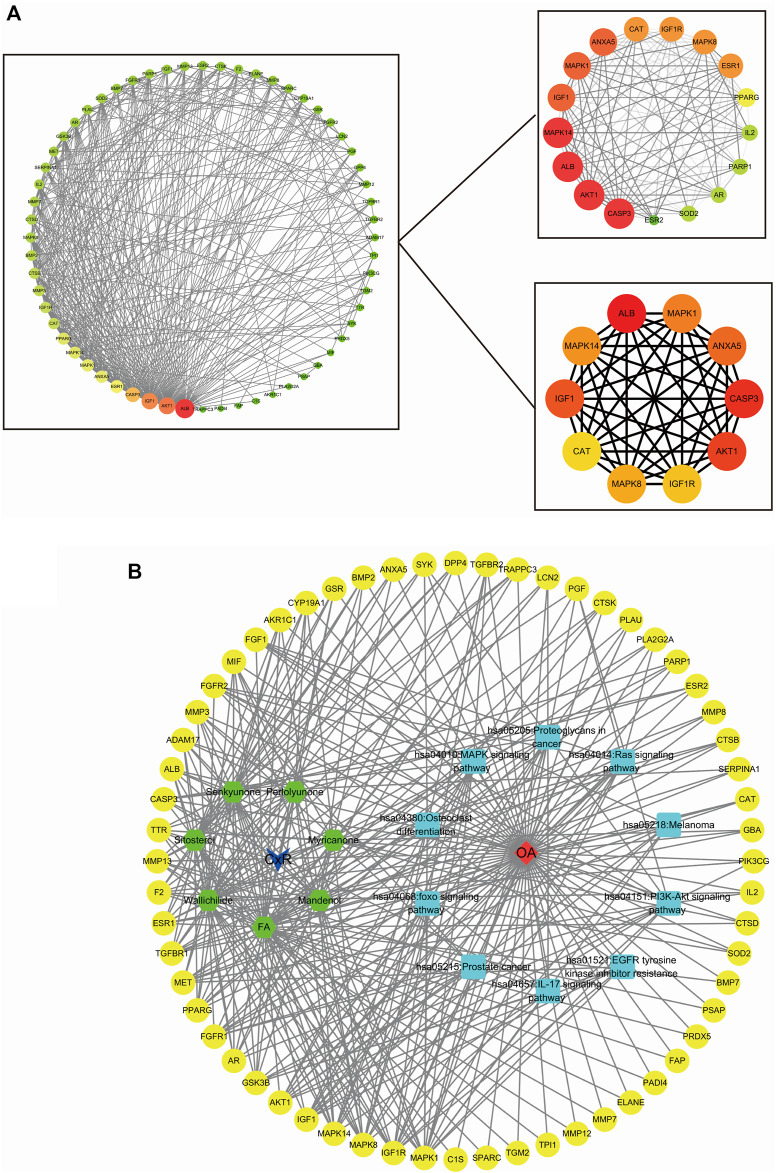
A total of 60 predicted targets were imported into STRING for PPI network analysis (Figure 3). The network complex included 60 nodes and 394 edges. The Cytoscape software was used to visualize and analyze the network by calculating centrality and other parameters. All the targets were arranged into circles according to these parameters. The high centrality value represented the important role in the network. Then the plug-ins MCODE and CytoHubba selected the core targets (Figure 4A). The top 10 core targets were ALB, AKT1, IGF1, CASP3, MAPK1, ANXA5, MAPK14, CAT, and IGF1R, respectively.
FIGURE 3.
Potential targets of CX anti-OA and the PPI network. (A) Venn diagram of potential targets. (B) The PPI network of 60 targets according to the STRING database. (C) Top 30 targets ranked by the degree value.
FIGURE 4.
Plug-in of Cytoscape was used to select the molecular complexes and core targets and the D-C-T-P-D network (A) MCODE and CytoHubba plug-ins of the Cytoscape software were used to choose the molecular complexes and core targets, respectively. (B) The drug-compound-target-pathway-disease network showed the potential mechanism of CX to treat OA.
GO Function and KEGG Pathway Enrichment Analysis
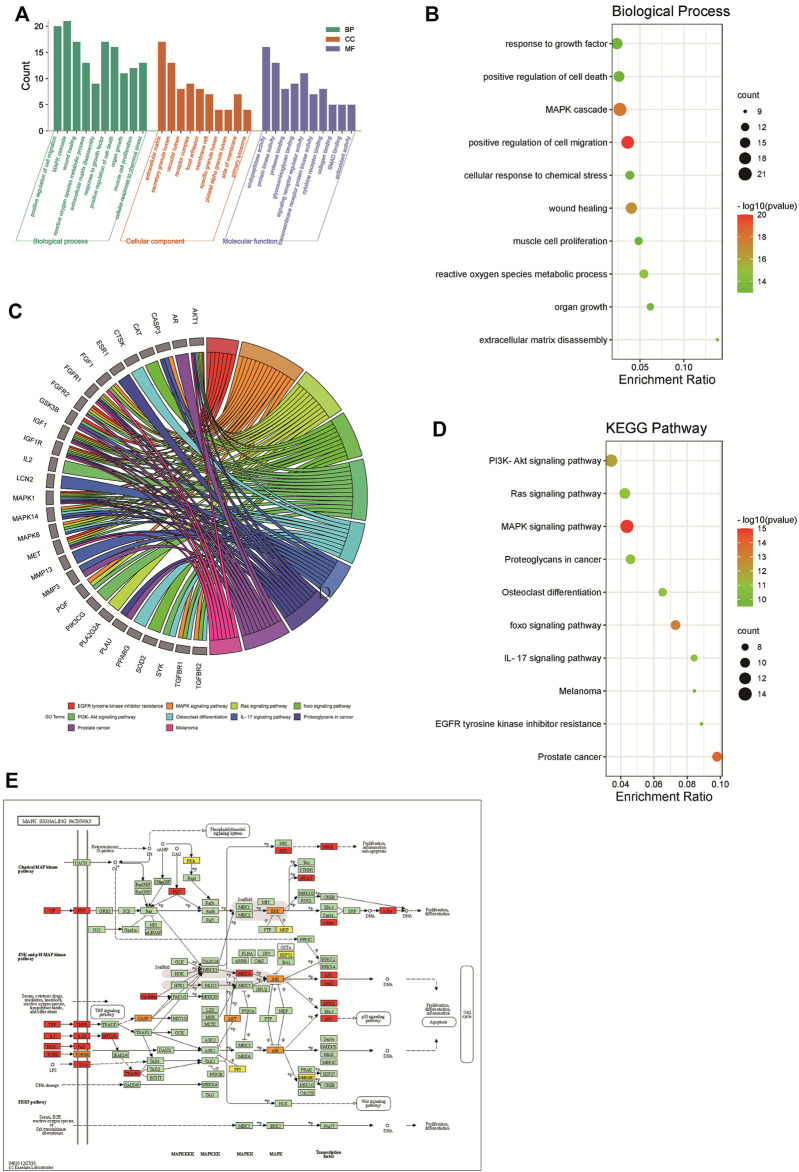
A total of 970 GO items were obtained from the Metascape database (p < 0.01), included 871 BP items, 40 CC items, and 59 MF items. We further selected the top 10 BP, CC, and MF catalogs for visualization (Figure 5A). In the histogram, the ordinary axis represents the degree of enrichment. According to our results of BP (Figure 5B), the function of active components of CX in osteoarthritis mainly focused on positive regulation of cell migration, MAPK cascade, wound healing, reactive oxygen species metabolic process, extracellular matrix disassembly, response to growth factor, positive regulation of cell death, organ growth, muscle cell proliferation, and cellular response to chemical stress. The MF items mainly included endopeptidase activity, protein kinase activity, protein binding, glycosaminoglycan binding, signaling receptor regulator activity, transmembrane receptor protein kinase activity, cytokine receptor binding, collagen binding, SMAD binding, and antioxidant activity. The abundant GO functions can also contribute to explaining to a certain extent that CX can be used to treat osteoarthritis and other diseases. KEGG pathway enrichment analysis showed that CX was mainly involved in 191 signaling pathways (p < 0.01) (Figures 5C,D), the top 10 enriched pathways were visualized by a bubble chart, the degree of gene enrichment was represented by abscissa, the amount of gene enrichment was represented by the bubble size, and p value was represented by the color depth. The main pathways of enrichment included EGFR tyrosine kinase inhibitor resistance, the MAPK signaling pathway, Ras signaling pathway, FoxO signaling pathway, PI3K-Akt signaling pathway, osteoclast differentiation, IL-17 signaling pathway, proteoglycans in cancer, prostate cancer, and melanoma. The KEGG mapper was used to color in targets in the MAPK pathway, the targets of CX anti-OA were colored in orange, other targets of CX but not involved in OA were colored in yellow, and other targets of OA in the MAPK pathway were colored in red (Figure 5E).
FIGURE 5.
GO function and KEGG pathway enrichment analyses of CX in the treatment of OA. (A) The GO function analysis, including biological process (BP), cellular component (CC), and molecular function (MF). (B) Bubble diagram of BP enrichment. (C) Gene ontology chord of the top 10 pathways in the CX anti-OA (D) Bubble diagram of KEGG pathway enrichment. (E) The MAPK pathway was colored by the KEGG mapper, the orange targets represent CX anti-OA, yellow represents other targets of CX not involved in OA treatment, and red represents other targets of OA in the MAPK pathway.
D-C-T-P-D Network Analysis
The D-C-T-P-D network of CX for the treatment of osteoarthritis is displayed in Figure 4B, showing the complex relationship between CX and osteoarthritis, including 79 total nodes (7 compound nodes, 60 target nodes, 10 core pathways, one CX node, and one OA node) and 359 edges. The targets, drug, and OA were represented by yellow circles, a blue arrow, and a red prism, respectively. While, the active compounds and core pathways were shown as green hexagons and blue rectangles, respectively. In the compound section, the degree of FA, perlolyunone, sitosterol, senkyunone K, wallichilide, mandenol, and myricanone was 42, 18, 23, 30, 37, 25 and 15, respectively, indicating that FA may be the most important active component in the treatment of osteoarthritis. Similarly, the degree of the MAPK pathway was 15, which was the most important signaling pathway, and that of MAPK1 was 16, which was the most important potential target predicted.
Molecular Docking Findings
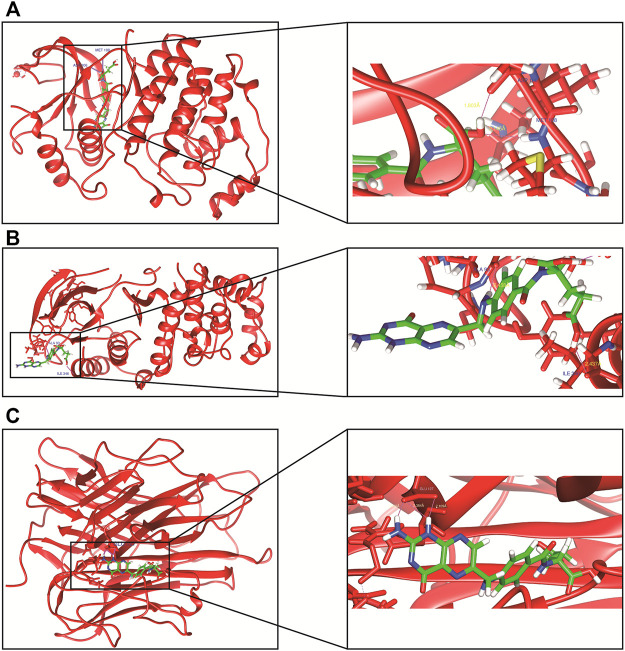
Finally, we employed molecular docking to determine the possibility of binding between the core target and the CX active compounds via applying the Swiss dock website (Figure 6). The previous literature proved that a binding affinity of < -4.25 kcal/mol indicated that the two molecules had a standard binding ability, < -5.0 kcal/mol meant good binding, while < -7.0 kcal/mol suggested strong binding activity (Saikia and Bordoloi, 2019). In our study, we docked two top targets in the MAPK pathway (MAPK1 and MAPK14) and TNF-α with seven active monomers of CX respectively. The results illustrated that most of results were < - 7 kcal/mol, and the binding energy between FA and MAPK1 as well as MAPK14 was < - 9 kcal/mol (Table 3). The docking results of FA and three targets were refined by exploring the specific binding sites and the spatial distance in UCFS chimera. The result showed that in MAPK1 (PDB ID:7AUV), FA had hydrogen bonds with amino acid residues of ASP-106 and MET-108, the distance was 1.803 Å and 2.145 Å, respectively. In MAPK14 (PDB ID:6SP9), FA and amino acid residues ALA-93 (2.458 Å) and ILE-346 (2.437 Å) had hydrogen bonds. In TNF-α (PDB ID:1A8M), FA had three hydrogen bonds with amino acid residues of GLU-127 (2.029 Å, 2.384 Å, and 2.109 Å).
FIGURE 6.
The molecular docking of FA and core targets. (A) FA docking with MAPK1. (B) FA docking with MAPK14. (C) FA docking with TNF-α.
TABLE 3.
The detailed molecular docking messages of seven CX compounds with MAPK1, MAPK14, and TNF-α.
| FA | Mandenol | Myricanone | Perlolyunone | Senkyunone | Sitosterol | Wallichilide | |
|---|---|---|---|---|---|---|---|
| MAPK1 | −9.24 | −7.96 | −6.98 | −7.39 | −7.82 | −7.97 | −7.93 |
| MAPK14 | −9.4 | −8.94 | −7.39 | −8.07 | −8.47 | −7.42 | −8.13 |
| TNF-a | −8.51 | −7.74 | −6.69 | −7.24 | −7.12 | −7.28 | −7.26 |
Discussion
Osteoarthritis is a common degenerative joint disease and the incidence rate of middle-aged and elderly people is increasing every year, which negatively impacts the health and quality of sufferers’ lives (Martel-Pelletier et al., 2016). Current treatment of osteoarthritis mainly focuses on relieving the pain and other discomfort symptoms of patients, which does little to stop or slow down the progression of the disease. The currently known functions of TCM are tonifying the liver and kidney, relaxing tendons and activating blood circulation, and dredging collateral and relieving pain (Xu et al., 2017). As an important blood-activating and detumescence drug, Ligusticum chuanxiong has a long history in the treatment of osteoarthritis, but the exploration of its specific molecular mechanism is far from sufficient (Luo Y. et al., 2020). There have been a few studies on osteoarthritis treatment by CX, most of them focused on the isolated compounds from CX and lacked systematic insights and depth (Zhou et al., 2019a). As we all know, many ingredients of Chinese herbal medicine have certain curative effects in experiments, but they have not been used in the clinic. The reason for the phenomenon is that those studies ignored the physicochemical property of CX as a drug (Anand et al., 2007). For example, many compounds have poor water solubility and oral availability, so it is difficult to effectively achieve the concentration required for treatment (Kunnumakkara et al., 2017).
With the emergence of modern bioinformatics, people are beginning to pay more attention to the combination of computer science and biology. Data mining can help us find valuable information from big data and guide us to carry out meaningful research (Gauthier et al., 2019). Based on a large number of experimental data and clinical trial results, network pharmacology could be used to study the potential mechanism of clinical treatment and prevention of diseases, and is especially suitable for the complex components and multi-target and multi mechanism features of TCM (Zhang et al., 2019). Therefore, this study was the first to systematically reveal the mechanism of CX anti-OA. In addition, the molecular docking model between the compound and the target also showed a good combination, which was further verified by the internal relationship between CX and OA.
Through the D-C-T-P-D network, it was determined that FA, wallichilide, and senkyunone were the most important potential components of CX anti-OA. Previous studies have indicated that the folate receptor is overexpressed on activated macrophages, which are closely related to osteoarthritis (Tsuneyoshi et al., 2012). Some literature has also showed that folic acid deficiency (FD) can mediate synovial cell apoptosis by affecting the excessive production of reactive oxygen species (ROS) induced by mitochondrial complex II and NOX and the sharp release of intracellular calcium (Ca2 +) concentration (Hsu et al., 2016). Besides, Duan W et al. proved that a folate receptor-targeted nanocarrier system can effectively block the NF-κB signaling pathway and reduce the expression of proinflammatory cytokines, so as to significantly inhibit the progression of arthritis in a mouse model (Duan and Li, 2018). Gelmini F et al. conducted a small clinical trial and found that ointment containing sitosterol used to treat five patients with osteoarthropathy (three times a day for three consecutive weeks) reduced the inflammatory characteristics of hands and knees in all patients without obvious adverse reactions (Gelmini et al., 2016). Cheng BC et al. pointed out that sitosterol can target multiple different genes in the NF-κB signaling pathway, such as MMP and COX-2, to reduce inflammatory cytokines and chemokine TNF-α, IL-1 β, IL-6, and CCL5, thereby decreasing oxidative stress and inhibiting inflammation (Cheng et al., 2016). Besides, Shivnath N et al. found that sitosterol can bind to the chondrocyte membrane and inhibit the NF-κB pathway to downregulate the expression of MMP and inhibit the catabolism of the cartilage matrix (Shivnath et al., 2021). In general, the seven active components of CX have different degrees of therapeutic effects on OA, involving numerous inflammatory cells and signaling pathways.
Then we applied a PPI network. The results showed that there were abundant interactions among targets, which made it easier to produce cascade effects. The core targets were ALB, AKT1, IGF1, CASP3, MAPK1, ANXA5, MAPK14, CAT, and IGF1R. These targets corresponded to multiple components in CX; this point fully reflected the characteristics of multi-target and multi-component traditional Chinese medicine. Some studies have shown that the osteophyte formation of OA in AKT1-knockout mice was blocked in the animal model of osteoarthritis. Further studies have found that the mechanism was the inhibition of the expression of calcification inhibitor nucleotide pyrophosphatase/phosphodiesterase 1 (Fukai et al., 2010). And IGFs, including IGF1 and IGF1R, played an important role in chondrocyte growth and chondrogenesis (Claessen et al., 2012), while CASP3 was closely related to chondrocyte apoptosis (Wang et al., 2019). Deng Z et al. found that MAPK14 was highly expressed in patients with osteoarthritis as the death promoter of chondrocytes (Deng et al., 2021), while the overexpression of MAPK1 could reduce the inflammatory injury of chondrocytes, which was induced by IL-1 β (Hu et al., 2018). Annexin A5 (ANXA5) played an important role in matrix vesicle-mediated biomineralization during endochondral ossification and osteoarthritis (Grskovic et al., 2012). CAT (catalase) could clear ROS in chondrocytes and inhibit apoptosis induced by TNF-α, and then regulate the growth of chondrocytes by the death signaling pathway (van Gastel et al., 2020).
We discovered that the “PI3K-Akt signal pathway”, “MAPK signal pathway”, “osteological differentiation”, and “IL-17 signal pathway” were the potential key mechanisms of CX in the treatment of OA. In our study, MAPK1 and MAPK14 may be involved in the main mechanism of CX in the treatment of OA, as the most potential core molecules in the MAPK pathway. At the same time, the molecular docking results also proved that the active components of CX were well docked with MAPK1 and MAPK14. A variety of stimulating factors or toxic injury can activate the PI3K-Akt signaling pathway, and then regulate basic cell functions such as transcription, proliferation, and survival (Xue et al., 2017). ERK (MAPK1) and p38 (MAPK14) are the important ways for eukaryotes to regulate cell structure and function, and their activations were closely related to cartilage injury in OA. MMP, which is regulated by ERK and p38, is closely related to chondrocyte proliferation, apoptosis, and differentiation (Chen Y. et al., 2018). And the IL-17 signaling pathway played an important role in acute and chronic inflammatory and immune regulation (Liu et al., 2019). Osteoclasts are the only cells responsible for bone resorption in the body and play an important role in the process of OA. So, regulating osteoclast differentiation is a significant method to treat OA (Wang S. et al., 2020). Other pathways which we have been enriched, such as the TNF pathway and NF-κB pathway were also classic pathways highly related to OA.
In conclusion, our study used network pharmacology to elaborate the potential mechanism of CX in the treatment of osteoarthritis, and intuitively verified its effectiveness through molecular docking. Our study showed that CX can act on chondrocytes, synovial cells, and other cells closely related to the occurrence and development of OA. Meanwhile, CX was effective in the treatment of osteoarthritis by multi-target and multi-channel properties. However, there are still some deficiencies in this study, and the reliability of these conclusions needs further basic research to verify. Our research provided a new direction for the further research of CX in the treatment of osteoarthritis.
Conclusion
Our research, based on bioinformatics, found that Ligusticum chuanxiong was effective in the treatment of osteoarthritis with the characteristics of multi-component, multi-target, and multi-pathway properties. Our results also guide directions for subsequent basic experiments.
Acknowledgments
We sincerely appreciate the databases of TCMSP, STRING, Metascape, bioinformatics, and Swiss dock and Cytoscape, Openbabel, and UCSF chimera software for providing the data and making the charts.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
ZC and YL designed the study, CX wrote the manuscript, and ZZ derived the datasets. YX and PW performed statistical analyses, TX revised the manuscript, and BX and AL generated all figures and prepared the supplementary information.
Funding
This article was funded by the National Nature Science Foundation of China (grant number 82072977), Research Project of Human Health Commission (grant number 202204073071), the Nature Science Foundation of Hunan (grant number 2019JJ50861) and the Fundamental Research Funds for the Central Universities of Central South University (grant numbers 2021zzts0380, 2021zzts0371, 2021zzts0368).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.854215/full#supplementary-material
References
- Abramoff B., Caldera F. E. (2020). Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. North. Am. 104 (2), 293–311. 10.1016/j.mcna.2019.10.007 [DOI] [PubMed] [Google Scholar]
- Ai Z., Zhou S., Li W., Wang M., Wang L., Hu G., et al. (2020). "Fei Yan No. 1" as a Combined Treatment for COVID-19: An Efficacy and Potential Mechanistic Study. Front. Pharmacol. 11, 581277. 10.3389/fphar.2020.581277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. (2007). Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 4 (6), 807–818. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- Carugo O. (2021). Random Sampling of the Protein Data Bank: RaSPDB. Sci. Rep. 11 (1), 24178. 10.1038/s41598-021-03615-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shou K., Gong C., Yang H., Yang Y., Bao T. (2018). Anti-Inflammatory Effect of Geniposide on Osteoarthritis by Suppressing the Activation of P38 MAPK Signaling Pathway. Biomed. Res. Int. 2018, 8384576. 10.1155/2018/8384576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang C., Gao F., Fu Q., Fu C., He Y., et al. (2018). A Systematic Review on the Rhizome of Ligusticum Chuanxiong Hort. (Chuanxiong). Food Chem. Toxicol. 119, 309–325. 10.1016/j.fct.2018.02.050 [DOI] [PubMed] [Google Scholar]
- Cheng B. C., Fu X. Q., Guo H., Li T., Wu Z. Z., Chan K., et al. (2016). The Genus Rosa and Arthritis: Overview on Pharmacological Perspectives. Pharmacol. Res. 114, 219–234. 10.1016/j.phrs.2016.10.029 [DOI] [PubMed] [Google Scholar]
- Claessen K. M., Ramautar S. R., Pereira A. M., Smit J. W., Biermasz N. R., Kloppenburg M. (2012). Relationship between Insulin-like Growth Factor-1 and Radiographic Disease in Patients with Primary Osteoarthritis: a Systematic Review. Osteoarthritis Cartilage 20 (2), 79–86. 10.1016/j.joca.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Deng Z., Hu X., Alahdal M., Liu J., Zhao Z., Chen X., et al. (2021). High Expression of MAPK-14 Promoting the Death of Chondrocytes Is an Important Signal of Osteoarthritis Process. PeerJ 9, e10656. 10.7717/peerj.10656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragos D., Gilca M., Gaman L., Vlad A., Iosif L., Stoian I., et al. (2017). Phytomedicine in Joint Disorders. Nutrients 9 (1). 10.3390/nu9010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Li H. (2018). Combination of NF-kB Targeted siRNA and Methotrexate in a Hybrid Nanocarrier towards the Effective Treatment in Rheumatoid Arthritis. J. Nanobiotechnology 16 (1), 58. 10.1186/s12951-018-0382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H., Gu Z. Y., Li Q., Liu Q. H., Yang X. Y., Zhang J. J. (2019). Identification of Significant Genes with Poor Prognosis in Ovarian Cancer via Bioinformatical Analysis. J. Ovarian Res. 12 (1), 35. 10.1186/s13048-019-0508-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai A., Kawamura N., Saito T., Oshima Y., Ikeda T., Kugimiya F., et al. (2010). Akt1 in Murine Chondrocytes Controls Cartilage Calcification during Endochondral Ossification under Physiologic and Pathologic Conditions. Arthritis Rheum. 62 (3), 826–836. 10.1002/art.27296 [DOI] [PubMed] [Google Scholar]
- Gauthier J., Vincent A. T., Charette S. J., Derome N. (2019). A Brief History of Bioinformatics. Brief Bioinform 20 (6), 1981–1996. 10.1093/bib/bby063 [DOI] [PubMed] [Google Scholar]
- Gelmini F., Ruscica M., Macchi C., Bianchi V., Maffei Facino R., Beretta G., et al. (2016). Unsaponifiable Fraction of Unripe Fruits of Olea Europaea: An Interesting Source of Anti-inflammatory Constituents. Planta Med. 82 (3), 273–278. 10.1055/s-0035-1558155 [DOI] [PubMed] [Google Scholar]
- Grosdidier A., Zoete V., Michielin O. (2011). SwissDock, a Protein-Small Molecule Docking Web Service Based on EADock DSS. Nucleic Acids Res. 39 (Web Server issue), W270–W277. 10.1093/nar/gkr366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grskovic I., Kutsch A., Frie C., Groma G., Stermann J., Schlötzer-Schrehardt U., et al. (2012). Depletion of Annexin A5, Annexin A6, and Collagen X Causes No Gross Changes in Matrix Vesicle-Mediated Mineralization, but Lack of Collagen X Affects Hematopoiesis and the Th1/Th2 Response. J. Bone Miner Res. 27 (11), 2399–2412. 10.1002/jbmr.1682 [DOI] [PubMed] [Google Scholar]
- Hopkins A. L. (2008). Network Pharmacology: the Next Paradigm in Drug Discovery. Nat. Chem. Biol. 4 (11), 682–690. 10.1038/nchembio.118 [DOI] [PubMed] [Google Scholar]
- Hsu H. C., Chang W. M., Wu J. Y., Huang C. C., Lu F. J., Chuang Y. W., et al. (2016). Folate Deficiency Triggered Apoptosis of Synoviocytes: Role of Overproduction of Reactive Oxygen Species Generated via NADPH Oxidase/Mitochondrial Complex II and Calcium Perturbation. PLoS One 11 (1), e0146440. 10.1371/journal.pone.0146440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Ji X., Yang M., Fan S., Wang J., Lu M., et al. (2018). Cdc42 Is Essential for Both Articular Cartilage Degeneration and Subchondral Bone Deterioration in Experimental Osteoarthritis. J. Bone Miner Res. 33 (5), 945–958. 10.1002/jbmr.3380 [DOI] [PubMed] [Google Scholar]
- Ju X. D., Deng M., Ao Y. F., Yu C. L., Wang J. Q., Yu J. K., et al. (2010). The Protective Effect of Tetramethylpyrazine on Cartilage Explants and Chondrocytes. J. Ethnopharmacol 132 (2), 414–420. 10.1016/j.jep.2010.08.020 [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y. (2020). KEGG Mapper for Inferring Cellular Functions from Protein Sequences. Protein Sci. 29 (1), 28–35. 10.1002/pro.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. N., Arant K. R., Loeser R. F. (2021). Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. Jama 325 (6), 568–578. 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B., Bordoloi D., Padmavathi G., Monisha J., Roy N. K., Prasad S., et al. (2017). Curcumin, the golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 174 (11), 1325–1348. 10.1111/bph.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwic A., Edwards M. H., Dennison E. M., Cooper C. (2013). Epidemiology and burden of Osteoarthritis. Br. Med. Bull. 105, 185–199. 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ouyang S., Yu B., Liu Y., Huang K., Gong J., et al. (2010). PharmMapper Server: a Web Server for Potential Drug Target Identification Using Pharmacophore Mapping Approach. Nucleic Acids Res. 38 (Web Server issue), W609–W614. 10.1093/nar/gkq300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Qu Y., Liu L., Zhao H., Ma H., Si M., et al. (2019). PPAR-γ Agonist Pioglitazone Protects against IL-17 Induced Intervertebral Disc Inflammation and Degeneration via Suppression of NF-Κb Signaling Pathway. Int. Immunopharmacol 72, 138–147. 10.1016/j.intimp.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Luo E., Zhang D., Luo H., Liu B., Zhao K., Zhao Y., et al. (2020). Treatment Efficacy Analysis of Traditional Chinese Medicine for Novel Coronavirus Pneumonia (COVID-19): an Empirical Study from Wuhan, Hubei Province, China. Chin. Med. 15, 34. 10.1186/s13020-020-00317-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Wang C. Z., Sawadogo R., Tan T., Yuan C. S. (2020). Effects of Herbal Medicines on Pain Management. Am. J. Chin. Med. 48 (1), 1–16. 10.1142/S0192415X20500019 [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Barr A. J., Cicuttini F. M., Conaghan P. G., Cooper C., Goldring M. B., et al. (2016). Osteoarthritis. Nat. Rev. Dis. Primers 2, 16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- O'Boyle N. M., Banck M., James C. A., Morley C., Vandermeersch T., Hutchison G. R., et al. (2011). Open Babel: An Open Chemical Toolbox. J. Cheminform 3, 33. 10.1186/1758-2946-3-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., et al. (2004). UCSF Chimera-Aa Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 25 (13), 1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Piñero J., Saüch J., Sanz F., Furlong L. I. (2021). The DisGeNET Cytoscape App: Exploring and Visualizing Disease Genomics Data. Comput. Struct. Biotechnol. J. 19, 2960–2967. 10.1016/j.csbj.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran X., Ma L., Peng C., Zhang H., Qin L. P. (2011). Ligusticum Chuanxiong Hort: a Review of Chemistry and Pharmacology. Pharm. Biol. 49 (11), 1180–1189. 10.3109/13880209.2011.576346 [DOI] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., et al. (2014). TCMSP: a Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform 6, 13. 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia S., Bordoloi M. (2019). Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 20 (5), 501–521. 10.2174/1389450119666181022153016 [DOI] [PubMed] [Google Scholar]
- Sharma L. (2021). Osteoarthritis of the Knee. N. Engl. J. Med. 384 (1), 51–59. 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- Shivnath N., Siddiqui S., Rawat V., Khan M. S., Arshad M. (2021). Solanum Xanthocarpum Fruit Extract Promotes Chondrocyte Proliferation In Vitro and Protects Cartilage Damage in Collagenase Induced Osteoarthritic Rats (Article Reference Number: JEP 114028). J. Ethnopharmacol 274, 114028. 10.1016/j.jep.2021.114028 [DOI] [PubMed] [Google Scholar]
- Sue Y. M., Cheng C. F., Chang C. C., Chou Y., Chen C. H., Juan S. H. (2009). Antioxidation and Anti-inflammation by Haem Oxygenase-1 Contribute to protection by Tetramethylpyrazine against Gentamicin-Induced Apoptosis in Murine Renal Tubular Cells. Nephrol. Dial. Transpl. 24 (3), 769–777. 10.1093/ndt/gfn545 [DOI] [PubMed] [Google Scholar]
- Tao W., Xu X., Wang X., Li B., Wang Y., Li Y., et al. (2013). Network Pharmacology-Based Prediction of the Active Ingredients and Potential Targets of Chinese Herbal Radix Curcumae Formula for Application to Cardiovascular Disease. J. Ethnopharmacol 145 (1), 1–10. 10.1016/j.jep.2012.09.051 [DOI] [PubMed] [Google Scholar]
- Tsuneyoshi Y., Tanaka M., Nagai T., Sunahara N., Matsuda T., Sonoda T., et al. (2012). Functional Folate Receptor Beta-Expressing Macrophages in Osteoarthritis Synovium and Their M1/M2 Expression Profiles. Scand. J. Rheumatol. 41 (2), 132–140. 10.3109/03009742.2011.605391 [DOI] [PubMed] [Google Scholar]
- Uszkoreit J., Winkelhardt D., Barkovits K., Wulf M., Roocke S., Marcus K., et al. (2021). MaCPepDB: A Database to Quickly Access All Tryptic Peptides of the UniProtKB. J. Proteome Res. 20 (4), 2145–2150. 10.1021/acs.jproteome.0c00967 [DOI] [PubMed] [Google Scholar]
- van Gastel N., Stegen S., Eelen G., Schoors S., Carlier A., Daniëls V. W., et al. (2020). Lipid Availability Determines Fate of Skeletal Progenitor Cells via SOX9. Nature 579 (7797), 111–117. 10.1038/s41586-020-2050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. W., Jiang Y., Yao Z. L., Chen P. S., Yu B., Wang S. N. (2019). Aucubin Protects Chondrocytes against IL-1β-Induced Apoptosis In Vitro and Inhibits Osteoarthritis in Mice Model. Drug Des. Devel Ther. 13, 3529–3538. 10.2147/DDDT.S210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Gao Y., Zhang Z., Chi Q., Liu Y., Yang L., et al. (2020). Safflower Yellow Alleviates Osteoarthritis and Prevents Inflammation by Inhibiting PGE2 Release and Regulating NF-Κb/sirt1/AMPK Signaling Pathways. Phytomedicine 78, 153305. 10.1016/j.phymed.2020.153305 [DOI] [PubMed] [Google Scholar]
- Wang M., Yao M., Liu J., Takagi N., Yang B., Zhang M., et al. (2020). Ligusticum Chuanxiong Exerts Neuroprotection by Promoting Adult Neurogenesis and Inhibiting Inflammation in the hippocampus of ME Cerebral Ischemia Rats. J. Ethnopharmacol 249, 112385. 10.1016/j.jep.2019.112385 [DOI] [PubMed] [Google Scholar]
- Wang S., Deng Z., Ma Y., Jin J., Qi F., Li S., et al. (2020). The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int. J. Biol. Sci. 16 (14), 2675–2691. 10.7150/ijbs.46627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Chen B., Wang Y., Wang X., Han D., Ding D., et al. (2017). The Effectiveness of Manual Therapy for Relieving Pain, Stiffness, and Dysfunction in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Physician 20 (4), 229–243. [PubMed] [Google Scholar]
- Xue J. F., Shi Z. M., Zou J., Li X. L. (2017). Inhibition of PI3K/AKT/mTOR Signaling Pathway Promotes Autophagy of Articular Chondrocytes and Attenuates Inflammatory Response in Rats with Osteoarthritis. Biomed. Pharmacother. 89, 1252–1261. 10.1016/j.biopha.2017.01.130 [DOI] [PubMed] [Google Scholar]
- Ye H. Z., Zheng C. S., Xu X. J., Wu M. X., Liu X. X. (2011). Potential Synergistic and Multitarget Effect of Herbal Pair Chuanxiong Rhizome-Paeonia Albifora Pall on Osteoarthritis Disease: a Computational Pharmacology Approach. Chin. J. Integr. Med. 17 (9), 698–703. 10.1007/s11655-011-0853-5 [DOI] [PubMed] [Google Scholar]
- Yuan Q. L., Wang P., Liu L., Sun F., Cai Y. S., Wu W. T., et al. (2016). Acupuncture for Musculoskeletal Pain: A Meta-Analysis and Meta-Regression of Sham-Controlled Randomized Clinical Trials. Sci. Rep. 6, 30675. 10.1038/srep30675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhu X., Bai H., Ning K. (2019). Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 10, 123. 10.3389/fphar.2019.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chen Y., Jiang H., Yang J., Wang Q., Du Y., et al. (2020). Integrated Strategy for Accurately Screening Biomarkers Based on Metabolomics Coupled with Network Pharmacology. Talanta 211, 120710. 10.1016/j.talanta.2020.120710 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Ming J., Li Y., Deng M., Chen Q., Ma Y., et al. (2019a). Ligustilide Attenuates Nitric Oxide-Induced Apoptosis in Rat Chondrocytes and Cartilage Degradation via Inhibiting JNK and P38 MAPK Pathways. J. Cel Mol Med 23 (5), 3357–3368. 10.1111/jcmm.14226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A. H., Tanaseichuk O., et al. (2019b). Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 10 (1), 1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.