Abstract
Thermosensitive polymers are useful as intelligent materials. Dendrimers have well-defined structures, which can work as multifunctional polymers. In this study, we designed and synthesized various phenylalanine (Phe)-modified zwitterionic dendrimers as pH- and thermo-sensitive polymers. First, polyamidoamine (PAMAM) dendrimers were modified with Phe and succinic anhydride (Suc) to prepare carboxy-terminal Phe-modified dendrimers (PAMAM-Suc-Phe and PAMAM-Phe-Suc). Both these dendrimers showed upper critical solution temperature (UCST)-type thermosensitivity. Interestingly, PAMAM-Phe-Suc demonstrated lower critical solution temperature (LCST)-type thermosensitivity at lower pH, but PAMAM-Suc-Phe did not. This indicates that PAMAM-Phe-Suc can switch LCST/UCST-type thermosensitivity according to the solution's pH. PAMAM-Phe-SO3Na with sulfonic acid termini also demonstrated LCST/UCST-type thermosensitivity switched by pH, with a higher sensitivity than PAMAM-Phe-Suc. Coacervation occurred during the phase separation. The quaternized dendrimers (QPAMAM-Phe-Suc and QPAMAM-Phe-SO3Na) and dendrimers conjugating isoleucine or 4-(amino methyl)benzoic acid did not show the unique thermosensitive properties, indicating that the tertiary amines in the dendrimer core and the Phe residues at the termini are indispensable. PAMAM-Phe-SO3Na could separate a model compound (rose bengal) from an aqueous solution because of its encapsulation ability. This is the first report of pH-switchable LCST/UCST-type thermosensitive dendrimers.
We synthesized phenylalanine-modified zwitterionic dendrimers as pH- and thermo-responsive polymers. This is the first report of pH-switchable LCST/UCST-type thermosensitive dendrimers.
Introduction
Stimuli-responsive polymers have attracted significant attention from various fields, including nanotechnology, sensors, catalysts, and biomedical applications. There are many reports on smart polymers that respond to various stimuli – such as temperature, pH, light, ionic strength, redox systems, and host–guest interaction.1,2 Temperature is one of the most widely used stimuli, and many temperature-sensitive polymers that function in water have been studied, such as poly(N-isopropylacrylamide) (PNIPAM).3,4 There are two types of thermosensitive polymers: lower critical solution temperature (LCST)-type and upper critical solution temperature (UCST)-type. LCST-type thermo-responsive polymers are soluble at low temperatures but become insoluble above the LCST. In contrast, UCST-type thermo-responsive polymers are insoluble at low temperatures but become soluble above the UCST. It is known that UCST-type thermosensitive behaviors are based on intermolecular interactions such as hydrogen-bonding and electrostatic interactions. For example, poly(N-acryloyl glycinamide) with amide bonds and poly(sulfobetaine methacrylate) (SBMA) with zwitterionic groups show UCST-type thermosensitive behaviors through hydrogen-bonding and electrostatic interactions, respectively.5–7 Block copolymers bearing both LCST and UCST thermosensitive segments, such as PNIPAM-b-poly(3-[N-(3-methacrylamidopropyl)-N,N-dimethyl]ammoniopropane sulfonate [SPP]), were designed as unique polymers exhibiting both LCST- and UCST-type thermosensitive behaviors.8 These copolymers formed micelles whose structure depends on the solution temperature; the UCST segment assembled below the UCST, and the LCST segment assembled above the LCST. The thermosensitivity could be tuned by the monomer structure as well as the length of each segment.7 The addition of salt and the solvent change could induce the switching of the LCST/UCST behaviors of these LCST/UCST diblock copolymers.5,6,9 For example, the LCST/UCST behaviors of block copolymers composed of poly(SBMA) and poly(N-isopropyl methacrylamide) segments could be switched by adding salts.10 The LCST/UCST behaviors of poly(ethylene glycol diacrylate [OEGDA]-b-methacrylic acid [MAA]) were also switched by the content of alcohol in aqueous solution.11 Interestingly, the LCST-type thermosensitive behaviors of PNIPAM in water were changed into the UCST-type in a binary aqueous solution containing alcohol and DMSO.12 There are several reports on polymers with the LCST/UCST switching behaviors by changing solvents, like PNIPAM.13–15 Plamper et al. (2015) reported the LCST/UCST switching behaviors of poly(N,N-dimethylaminoethyl methacrylate) by changing pH in the presence of hexacyanocobaltate(iii).15 However, conditions to induce the switching of UCST/LCST behaviors are limited; therefore, they have little use in application.
Dendrimers are polymers with unique symmetrical branch structures. Dendrimers are synthesized by a stepwise reaction, and their molecular weight, shape, and size are highly controllable. In addition, dendrimers can load various types of molecules by attachment to their terminal groups or encapsulation into their interior.16 Thus, dendrimers are applicable as multifunctional nanocarriers as well as smart materials.17 Polyamidoamine (PAMAM) dendrimers are commercially available and have been well studied.16 LCST-type thermosensitive PAMAM dendrimers and dendritic polymers have been produced by modification with various compounds, oligo(ethylene glycol), N-isopropyl groups and elastin-like peptides, which are useful for drug delivery and substance separation.17–25 PAMAM dendrimers modified with phenylalanine (Phe) are also thermosensitive, but their thermosensitivity depends on their terminal groups. The amino-terminal Phe-modified PAMAM dendrimer (PAMAM-Phe) exhibited LCST-type thermosensitivity above pH 6.26 Conversely, the carboxy-terminal Phe-modified dendrimers showed UCST-type thermosensitivity at acidic pH.27 Carboxy-terminal Phe-modified PAMAM dendrimers are zwitterionic; they have anionic carboxy groups at the termini and tertiary amino groups in the dendrimer. It is most likely that the zwitterionic structure induces the UCST-type thermosensitive behaviors of the carboxy-terminal Phe-modified dendrimers.27
In this study, various zwitterionic Phe-modified dendrimers were designed and synthesized. First, two carboxy-terminal PAMAM dendrimers (PAMAM-Phe-Suc and PAMAM-Suc-Phe) were produced by changing the reaction order of acid anhydride and Phe. Interestingly, PAMAM-Phe-Suc exhibited both UCST-type thermosensitivity at pH 5.5 and LCST-type thermosensitivity at pH 4, indicating that the LCST/UCST thermosensitive behaviors could be switched the pH. To investigate the influence of the structures of anion and cation to the thermosensitivity of the dendrimer, a sulfonic acid-terminal dendrimer (PAMAM-Phe-SO3Na) and the quaternized dendrimers (QPAMAM-Phe-Suc and QPAMAM-Phe-SO3Na) were synthesized, and their temperature- and pH-sensitivities were characterized. Finally, the recovery of a model compound from an aqueous solution using the zwitterionic Phe-modified dendrimer was demonstrated.
Results and discussion
Synthesis of two carboxy-terminal Phe-modified dendrimers
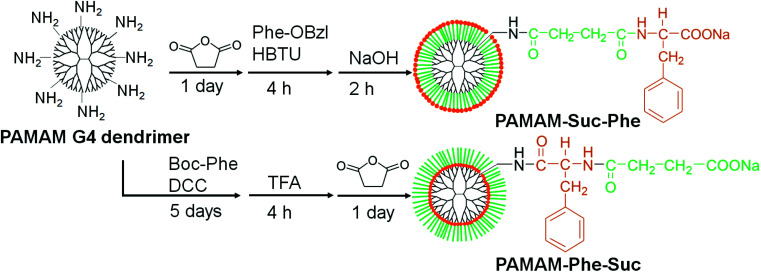
Two types of carboxy-terminal Phe-modified dendrimers were synthesized in accordance with Fig. 1. PAMAM-Suc-Phe was synthesized by reacting the amino-terminal PAMAM dendrimer of G4 with succinic anhydride and the subsequent phenylalanine benzyl ester (Phe-OBzl) prior to the deprotection of the OBzl group, in accordance with our previous report.27 In contrast, PAMAM-Phe-Suc was synthesized by reacting the amino-terminal PAMAM dendrimer with N-(tert-butoxycarbonyl)phenylalanine (Boc-Phe) and subsequent succinic anhydride after the deprotection of the Boc group. The bound numbers of succinic anhydride and Phe residues to the dendrimer were estimated from the proton nuclear magnetic resonance (1H NMR) spectra (Fig. S1†) and are listed in Table 1. The bound number of Phe residues to 64 amino termini was estimated as 56 and 49 from 1H NMR spectra of PAMAM-(Boc-Phe) and PAMAM-Phe-Suc, respectively. It is known that insoluble parts, such as hydrophobic units of micelles in D2O, were not detected in NMR spectra.28 It is possible that the low signal of the Phe residues in PAMAM-Phe-Suc came from the low solvation of the Phe residues in D2O. Because the digestion of Phe residues is negligible in the reaction with succinic anhydride, the bound number of Phe in PAMAM-Phe-Suc was estimated from the 1H NMR spectrum of PAMAM-(Boc-Phe).
Fig. 1. Synthetic scheme of PAMAM-Suc-Phe and PAMAM-Phe-Suc. HBTU, DCC, and TFA are 1-[bis(dimethylamino)methyliumyl]-1H-benzotriazole-3-oxide hexafluorophosphate, N,N′-dicyclohexylcarbodiimide, and trifluoroacetic acid, respectively.
List of dendrimers synthesized in the present study.
| Dendrimer | Bound number | |
|---|---|---|
| Phe/Ile/AMBA | Suc/SO3Na | |
| PAMAM-Suc-Phe | 57 | 64 |
| PAMAM-Phe-Suc | 56a | 57 |
| QPAMAM-Phe-Suc | 56a | 57 |
| PAMAM-Phe-SO3Na | 56a | 64 |
| QPAMAM-Phe-SO3Na | 56a | 64 |
| PAMAM-Ile-Suc | 63a | 59 |
| PAMAM-Suc-AMBA | 64b | 64b |
Estimated from the 1H NMR spectrum of PAMAM-(Boc-Phe) or PAMAM-(Boc-Ile).
Calculated.
pH- and thermo-sensitive behaviors of two carboxy-terminal Phe-modified dendrimers
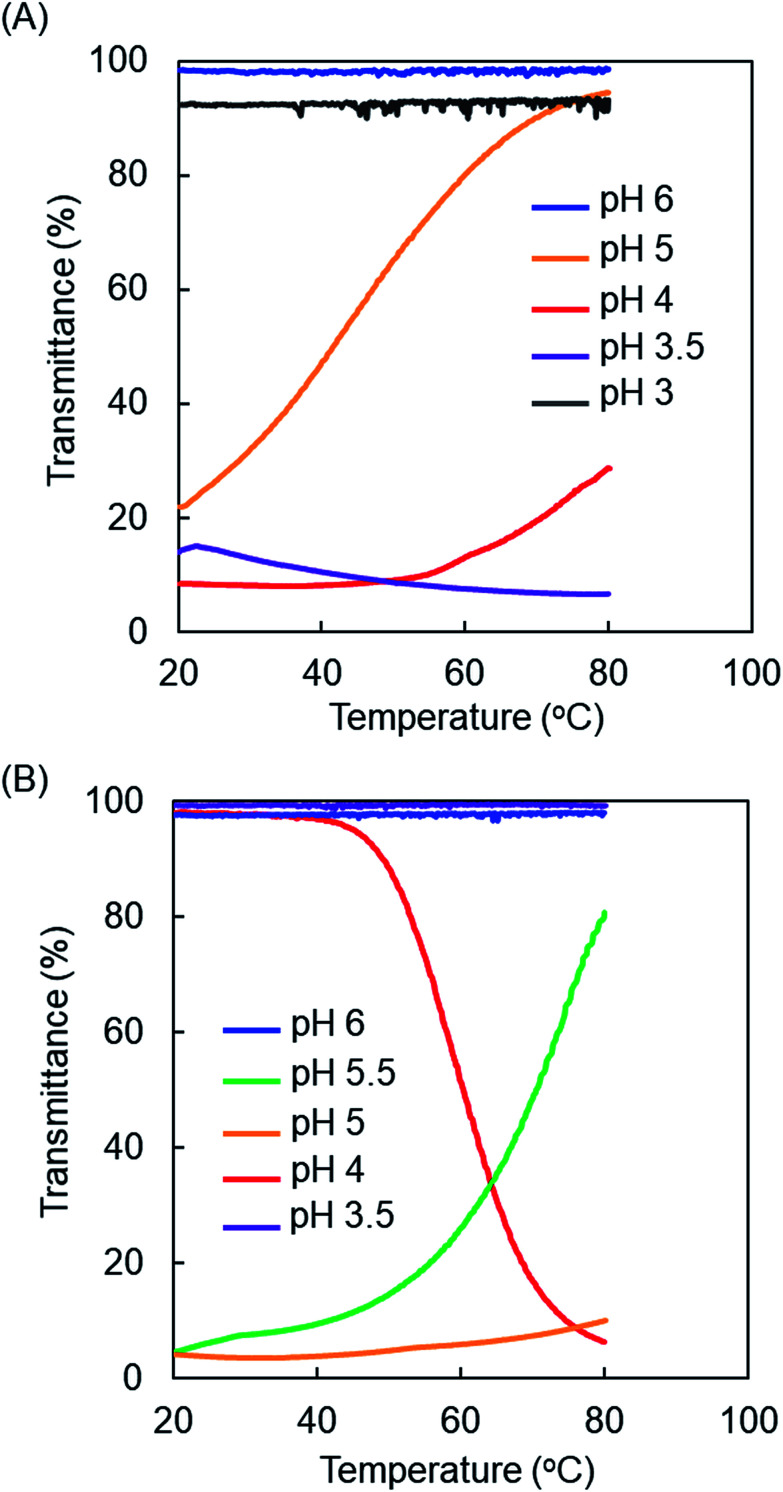
The temperature-dependent transmittances of PAMAM-Phe-Suc and PAMAM-Suc-Phe at various pHs were compared (Fig. 2). The aqueous solutions of both PAMAM-Suc-Phe and PAMAM-Phe-Suc were clear at pH 6. However, there were differences in the turbidity of these dendrimers at lower than pH 6. PAMAM-Suc-Phe and PAMAM-Phe-Suc showed UCST-type thermosensitivity at pH 5 and pH 5.5, respectively. The solution of PAMAM-Suc-Phe became clear at pH 5 by heating. The solution of PAMAM-Phe-Suc was turbid at pH 5 even when heated. Interestingly, LCST-type thermosensitivity was demonstrated in PAMAM-Phe-Suc at pH 4, but not in PAMAM-Suc-Phe. Additionally, PAMAM-Suc-Phe did not exhibit LCST-type thermosensitivity under any pH conditions. The thermosensitive behaviors and the phase transition temperature defined as the temperature at which the light transmittance was 50% were listed in Table 2. These results suggest that PAMAM-Phe-Suc and PAMAM-Suc-Phe demonstrate different pH- and thermo-sensitivities, although the chemical composition is almost the same. These results also reveal that PAMAM-Phe-Suc has both UCST-type and LCST-type thermosensitivity, which is switched by the solution's pH.
Fig. 2. Temperature-dependent transmittance curves of PAMAM-Suc-Phe (A) and PAMAM-Phe-Suc (B) at various pHs. The results of PAMAM-Suc-Phe (except pH 3.5 and pH 3) were referred to in our previous report.27.
Thermosensitivity and pKa of two carboxy-terminal Phe-modified dendrimersa.
| Dendrimer | Clear | UCST | Turbid | LCST | 20 °C | 50 °C | ||
|---|---|---|---|---|---|---|---|---|
| pKa1 | pKa2 | pKa1 | pKa2 | |||||
| PAMAM-Suc-Phe | pH 6 | pH 5 (42 °C) | pH 3.5 | ND | 4.6 | 8.1 | 5.1 | 8.3 |
| pH 3 | pH 4 | |||||||
| PAMAM-Phe-Suc | pH 6 | pH 5.5 (71 °C) | pH 5 | pH 4 (60 °C) | 5.4 | 8.0 | 5.0 | 8.2 |
| pH 3.5 | ||||||||
ND: not detected.
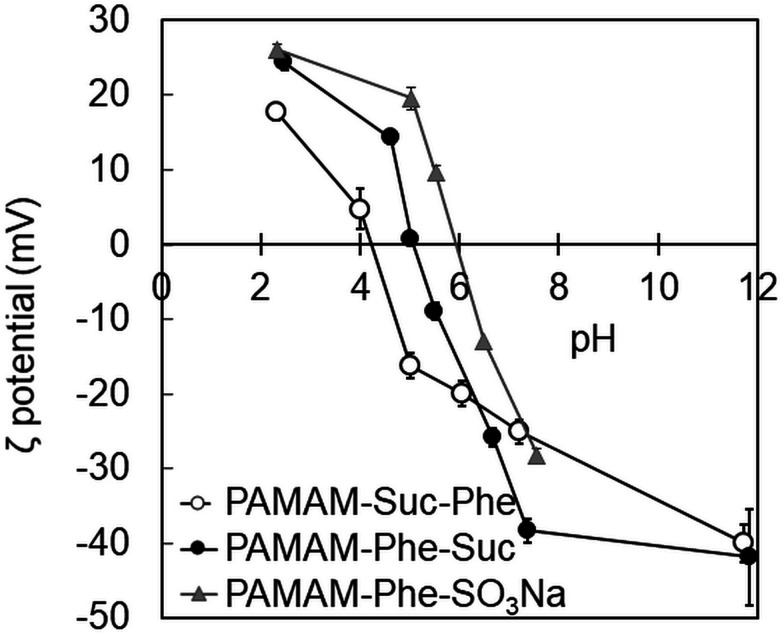
The ζ-potential of these dendrimers was measured at different pH (Fig. 3). The ζ-potential of PAMAM-Suc-Phe was −40 mV at pH 12, and it increased gradually with decreasing pH. It became neutral at pH 4.2 and then became positive at more acidic pH. These results suggest that terminal carboxy and tertiary amino groups of the dendrimer were deprotonated at high pH where the dendrimer was negatively charged. These dendrimer groups were protonated at low pH, by which the dendrimer was positively charged. When the surface charge was cancelled, the dendrimers easily aggregated each other. Thus, the solution of essentially non-charged PAMAM-Suc-Phe at pH 4 was turbid. The pKa values of PAMAM-Suc-Phe were estimated at 20 °C and 50 °C by titration (Fig. S2†),29 and the data are listed in Table 2. pKa of carboxylic acid (pKa1) and tertiary amine (pKa2) at 20 °C was evaluated as 4.6 and 8.1, respectively. The degree of protonation (α) is calculated from the following equation, whose relationship with pH is shown in Fig. S3.†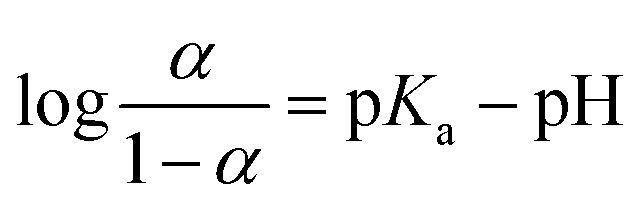
Fig. 3. ζ-potential of PAMAM-Suc-Phe (open circles), PAMAM-Phe-Suc (solid circles) and PAMAM-Phe-SO3Na (triangles) as a function of pH.
The numbers of the inner tertiary amine and terminal carboxylic acids in PAMAM-Suc-Phe are 62 and 64, respectively. Thus, the net charge of PAMAM-Suc-Phe became neutral at pH 7. However, these charges did not cancel, because the ζ-potential at pH 6 was still negative. The α value of terminal carboxylic acid was 0.7 at pH 4.2, at which the ζ-potential was neutral. These results suggest that PAMAM-Suc-Phe became hydrophobic when the terminal carboxylic acid was largely protonated. At pH 5, the ζ-potential was −16 mV and the α value of terminal carboxylic acid was 0.3. Because deprotonated terminal carboxylate anion and inner tertiary ammonium cation are zwitterionic at pH 5, PAMAM-Suc-Phe exhibited UCST-type thermosensitivity at pH 5.27 When almost all terminal carboxylic acid was protonated at pH 3, PAMAM-Suc-Phe became soluble at room temperature due to the positive surface charge. Although the protonated tertiary amines were located at the branched point of the dendrimer, the positive charge might have been exposed at the surface by the conformation change.
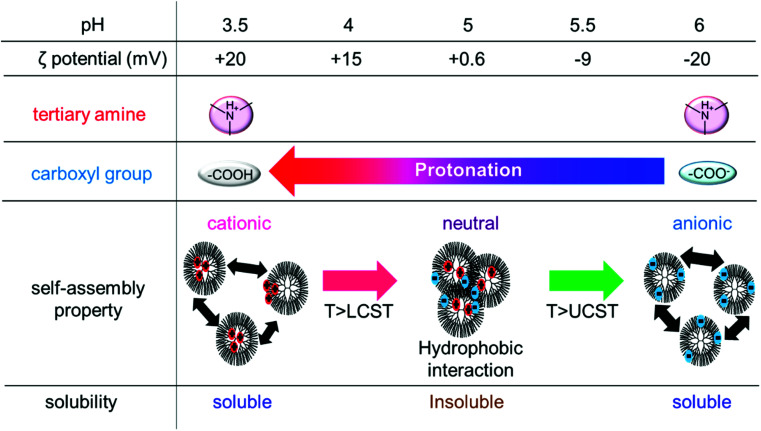
The same analyses were performed for PAMAM-Phe-Suc, and the ζ-potential, protonation state, solubility, and self-assemble property of PAMAM-Phe-Suc at different pH were summarized (Fig. 4). The pKa1 and pKa2 of PAMAM-Phe-Suc at 20 °C were evaluated as 5.4 and 8.0, respectively (Table 2 and Fig. S2†). Because the pKa1 of PAMAM-Phe-Suc was higher than that of PAMAM-Suc-Phe, PAMAM-Phe-Suc exhibited UCST-type thermosensitivity at a higher pH than PAMAM-Suc-Phe. The ζ-potential of PAMAM-Phe-Suc was −42 mV at pH 12 and was unchanged at pH 7 (Fig. 3), although the inner tertiary amines were protonated. This suggests that the terminal carboxy groups were not affected by the inner tertiary amino group in PAMAM-Phe-Suc. The α value of terminal carboxylic acid was 0.5 at pH 5.5 (Fig. S3†). The zwitterionic structure at pH 5.5 induced UCST-type thermosensitivity. When the solution of PAMAM-Phe-Suc was turbid at pH 5, the ζ-potential of PAMAM-Phe-Suc was neutral. When almost all terminal carboxylic acid was protonated at pH 4, PAMAM-Phe-Suc became soluble at room temperature due to the positive surface charge. Because the pKa1 at 50 °C was lower than that at 20 °C, fraction of deprotonated carboxylic acid was increased by heating at pH 4. The deprotonation led to a neutral surface charge, which became hydrophobic. Thus, PAMAM-Phe-Suc exhibited LCST behavior at pH 4. On the other hand, the pKa1 of PAMAM-Suc-Phe, which did not show LCST behavior, at 50 °C was higher than at 20 °C. Thus, the terminal carboxylic acid of PAMAM-Suc-Phe was not deprotonated by heating, unlike PAMAM-Phe-Suc. The temperature dependency of pKa1 might be a cause of their different thermosensitive behaviors of PAMAM-Suc-Phe and PAMAM-Phe-Suc.
Fig. 4. Relationship between the solubility and the protonation state of the ionic groups in PAMAM-Phe-Suc.
Effect of dendrimer structures in zwitterionic dendrimers to stimuli-sensitivity
Fig. 2 shows that these carboxy-terminal Phe-modified PAMAM dendrimers exhibit UCST-type thermosensitivity. The effects of Phe residues on thermosensitivity was investigated. Isoleucine (Ile) is a hydrophobic amino acid, and 4-(aminomethyl)benzoic acid (AMBA) is a phenyl compound. The carboxy-terminal Ile-modified and AMBA-modified PAMAM dendrimers (PAMAM-Ile-Suc and PAMAM-Suc-AMBA) were synthesized (Fig. 5, S1, S4† and Table 1). These dendrimers did not show any thermosensitive behaviors. PAMAM-Ile-Suc was soluble and PAMAM-Suc-AMBA was insoluble in water under any condition, respectively (Fig. S5†). Thus, the modification of Phe was important for these stimuli-responsive properties in the zwitterionic PAMAM dendrimers.
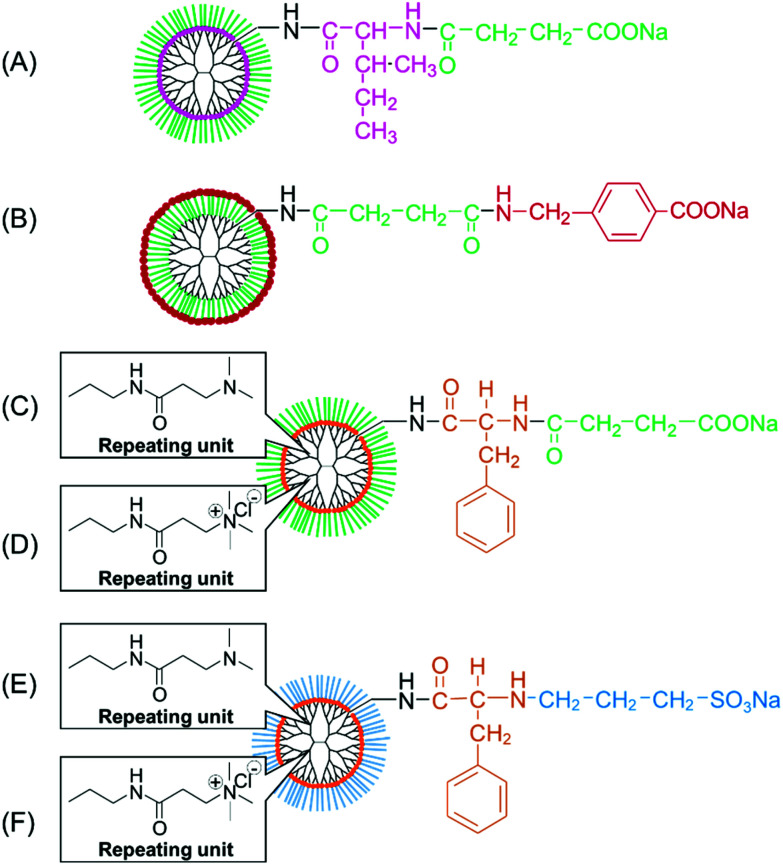
Fig. 5. Various dendrimers with different hydrophobic groups, cations, and anions. (A) PAMAM-Ile-Suc, (B) PAMAM-Suc-AMBA, (C) PAMAM-Phe-Suc, (D) QPAMAM-Phe-Suc, (E) PAMAM-Phe-SO3Na, and (F) QPAMAM-Phe-SO3Na.
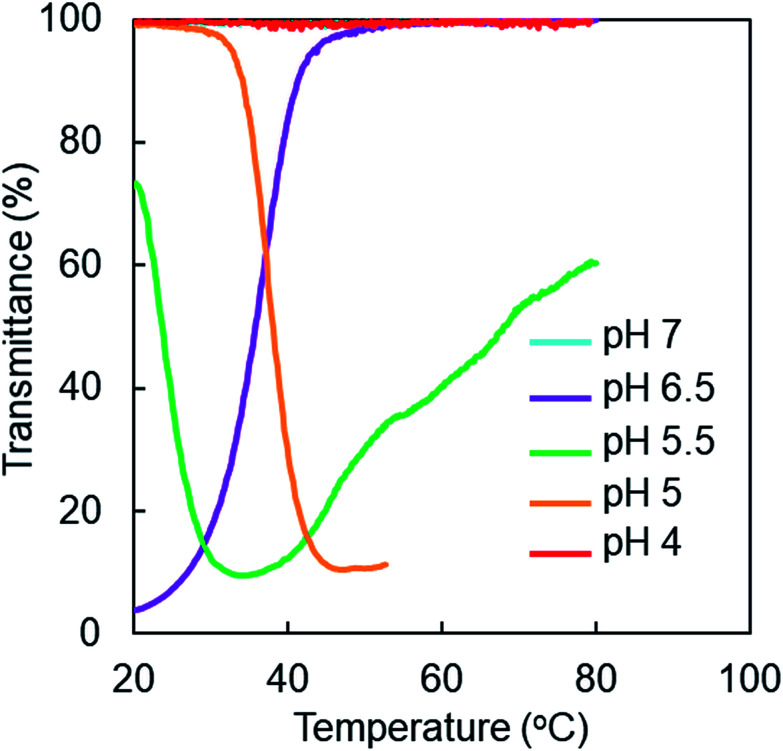
Fig. 2 shows that LCST/UCST thermosensitivity in PAMAM-Phe-Suc can be switched by pH. The pH-responsive internal amines and terminal carboxylic acids in the dendrimers were largely involved in their pH- and thermosensitivity. Therefore, we synthesized and compared various Phe-modified dendrimers with different anions and cations (Fig. 5). Sulfonic acid-terminal Phe-modified dendrimers (PAMAM-Phe-SO3Na) were synthesized by reacting PAMAM-Phe with 1.3-propanesultone,30 and quaternization of internal amines of PAMAM-Phe-Suc and PAMAM-Phe-SO3Na was conducted to produce QPAMAM-Phe-Suc and QPAMAM-Phe-SO3Na.31 These dendrimers were characterized by 1H NMR analyses (Fig. S1† and Table 1). Because PAMAM-Phe-SO3Na has secondary amines after the introduction of SO3Na, QPAMAM-Phe-SO3Na is more cationic than others. The temperature-dependent transmittance of these dendrimers at various pHs was examined (Fig. 6 and S6†). Fig. S6† shows the temperature-dependent transmittance of the quaternized dendrimers (QPAMAM-Phe-Suc and QPAMAM-Phe-SO3Na). The solutions of these dendrimers remained clear from pH 2 to pH 10 and did not exhibit any thermosensitive behaviors. Because internal quaternary amines are always cationic, these dendrimers are highly hydrophilic and do not associate with each other. This suggests that the protonation of the inner tertiary amino group is largely involved in pH- and temperature-responsive behaviors. Fig. 6 displays the temperature-dependent transmittance of PAMAM-Phe-SO3Na at various pHs. Table 3 summarizes the thermosensitivity of the zwitterionic Phe-modified PAMAM dendrimers. The aqueous solution of PAMAM-Phe-SO3Na remained clear at pH 7 and pH 4. UCST-type and LCST-type thermosensitivity was observed around 40 °C at pH 6.5 and pH 5, respectively. At pH 5.5, the dendrimer showed both LCST- and UCST-type phase transitions at 24 °C and 68 °C, respectively. Both PAMAM-Phe-Suc and PAMAM-Phe-SO3Na demonstrated pH-switchable UCST-type and LCST-type thermosensitivity. The sharper thermosensitivity was observed at PAMAM-Phe-SO3Na than PAMAM-Phe-Suc (Fig. 2B). The structure of PAMAM-Phe-SO3Na possibly induced the sharp phase transition, which is useful for their applications.
Fig. 6. Temperature-dependent transmittance curves of PAMAM-Phe-SO3Na at various pHs.
Thermo-responsive behaviors of various Phe-modified dendrimers with different ionic structures.
| Dendrimer | LCST | UCST | Responsiveness |
|---|---|---|---|
| PAMAM-Phe-Suc | pH 4 (60 °C) | pH 5.5 (71 °C) | Broad |
| QPAMAM-Phe-Suc | None | None | — |
| PAMAM-Phe-SO3Na | pH 5 (36 °C) | pH 6.5 (38 °C) | Sharp |
| QPAMAM-Phe-SO3Na | None | None | — |
Because it is thought that the electrostatic interaction is important in our system, the thermosensitivity of PAMAM-Phe-Suc and PAMAM-Phe-SO3Na was investigated in the presence of salt (Fig. S7†). Both LCST- and UCST-type thermosensitivity of these dendrimers was disappeared in the presence of NaCl. These dendrimers became soluble at low temperature in the presence of 150 mM NaCl under the condition to show the UCST-type thermosensitivity in the absence of NaCl. Because salts are known to shield the charge, the intra- and intermolecular electrostatic interactions between zwitterionic groups at the polymer chain were suppressed.5 In contrast, these dendrimers became insoluble at low temperature in the presence of 150 mM NaCl under the condition to show the LCST-type thermosensitivity in the absence of NaCl. Hydrophobic interaction is known to get strong in the presence of salt.32 This suggests that hydrophobic interactions play an important role to the LCST-type thermosensitivity of these dendrimers. We also investigated the effects of the dendrimer generation to the thermosensitivity. We additionally synthesized G3 and G5 of PAMAM-Phe-Suc and PAMAM-Phe-SO3Na (Table S1†). Their temperature-transmittance was measured at the same molar concentration. All synthesized dendrimers with carboxylates and sulfonates showed the pH-switchable LCST/UCST thermosensitivity (Fig. S8 and S9†). The LCST-type phase transition temperatures tended to decrease with increasing the dendrimer generation. However, the dependency of the dendrimer generation was different between PAMAM-Phe-Suc and PAMAM-Phe-SO3Na. The LCST-type phase transition temperature in PAMAM-Phe-Suc of G4 was similar to G3, but that in PAMAM-Phe-SO3Na of G4 was similar to G5. The dependency of the dendrimer generation in the UCST-type phase transition temperature was also different between PAMAM-Phe-Suc and PAMAM-Phe-SO3Na. The UCST-type phase transition temperatures in PAMAM-Phe-Suc with different generations were similar, but those in PAMAM-Phe-SO3Na of G4 showed the lowest the UCST-type phase transition temperature. The dependency of the generation possibly affected the clustering effects at the increased terminal groups in high generation and the molecular shape. It is known that PAMAM dendrimers of less than G3 are not spherical, but they became spherical at higher than G4.33 These suggest that the thermo-responsive mechanism was different between PAMAM-Phe-Suc and PAMAM-Phe-SO3Na.
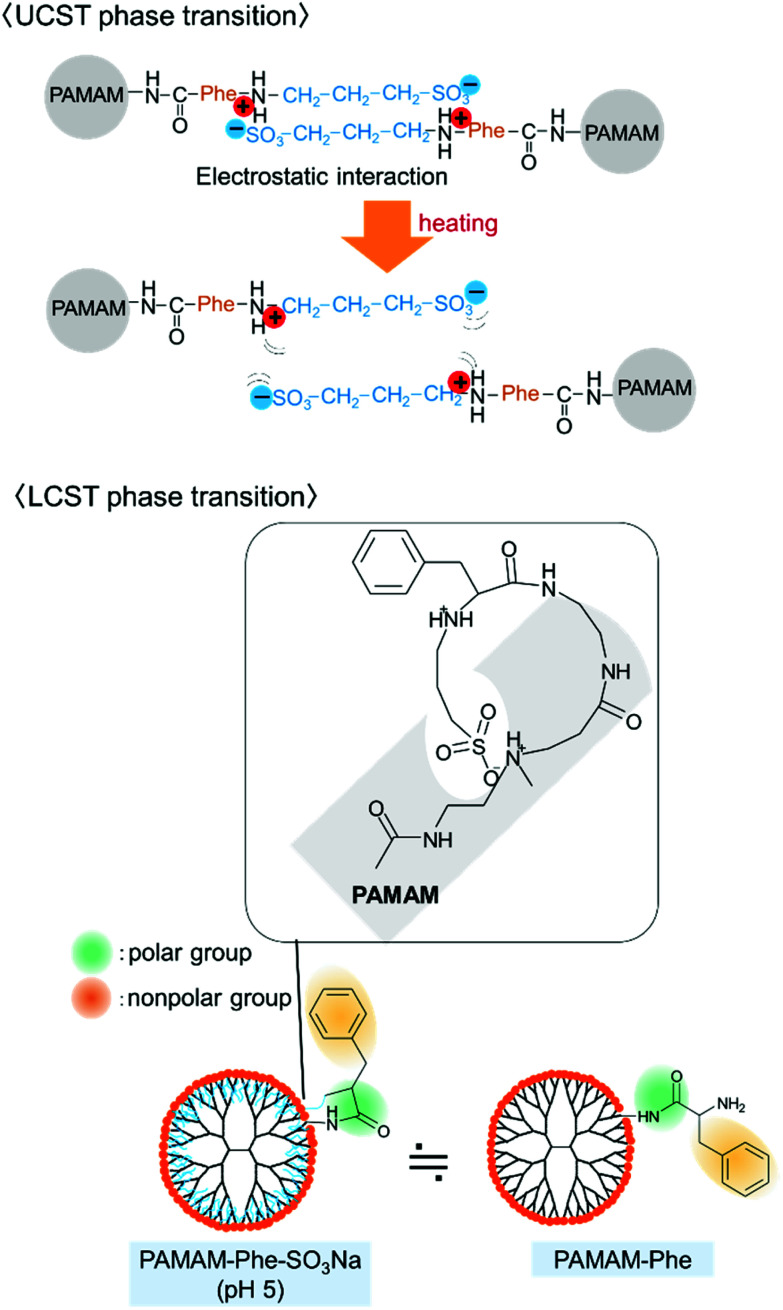
The pKa values of the tertiary and secondary amines (pKa1 and pKa2) in PAMAM-Phe-SO3Na were estimated at 20 °C and 50 °C by the titration (Fig. S2†).29 The pKa1 and pKa2 values of PAMAM-Phe-SO3Na were evaluated as 4.7, and 8.2 at 20 °C, and 5.0, and 8.4 at 50 °C, respectively. The protonation degree of the secondary amine in the terminal branch was 1 at pH 6.5 (Fig. S3†), at which PAMAM-Phe-SO3Na showed the UCST type thermosensitivity. The ζ-potential of PAMAM-Phe-SO3Na was also measured at different pH. The ζ potential was −28 mV at pH 8 and increased gradually with decreasing pH. It reached neutral around pH 6, which was higher than PAMAM-Suc-Phe and PAMAM-Phe-Suc. The ζ potential of PAMAM-Phe-SO3Na was changed into positive at more acidic pH (Fig. 3), although sulfonic acid is negatively charged under our experimental conditions. The number of protonated internal tertiary amines at pH 5 was calculated as 21 at 20 °C (Fig. S3†). These suggest that negatively charged sulfonic acid at the dendrimer termini was hindered by the positively charged internal tertiary amines. The zwitterionic structure was formed at each branch after the protonation of pKa2 in PAMAM-Phe-SO3Na at pH 6.2 or less, which could induce the sharp UCST-type thermosensitivity. Our results suggest that PAMAM G4 may have the optimal shape and surface density to interact each other. The LCST mechanism of PAMAM-Phe-SO3Na was different from that of PAMAM-Phe-Suc. It is because the pKa1 of PAMAM-Phe-Suc decreased at high temperature, but the pKa1 of PAMAM-Phe-SO3Na increased. The dehydration from the polymer is known as a main factor in LCST-type phase transitions. The LCST thermosensitivity in amino-terminal PAMAM-Phe is possibly based on the dehydration.26 At low pH, PAMAM-Phe-SO3Na became cationic although the terminal sulfate was stably negative. It is possible that the negatively sulfonate groups were got into the dendrimer inner space via electrostatic interaction. Consequently, the Phe residues may be exposed at the dendrimer surface, so that the surface structure of PAMAM-Phe-SO3Na may be similar to amino-terminal PAMAM-Phe with the LCST-type thermosensitivity (Fig. 7).26 The detailed molecular mechanisms involving the pH- and thermosensitive behaviors remain to be investigated.
Fig. 7. Schematic illustrations of thermosensitivity in PAMAM-Phe-SO3Na.
Coacervation
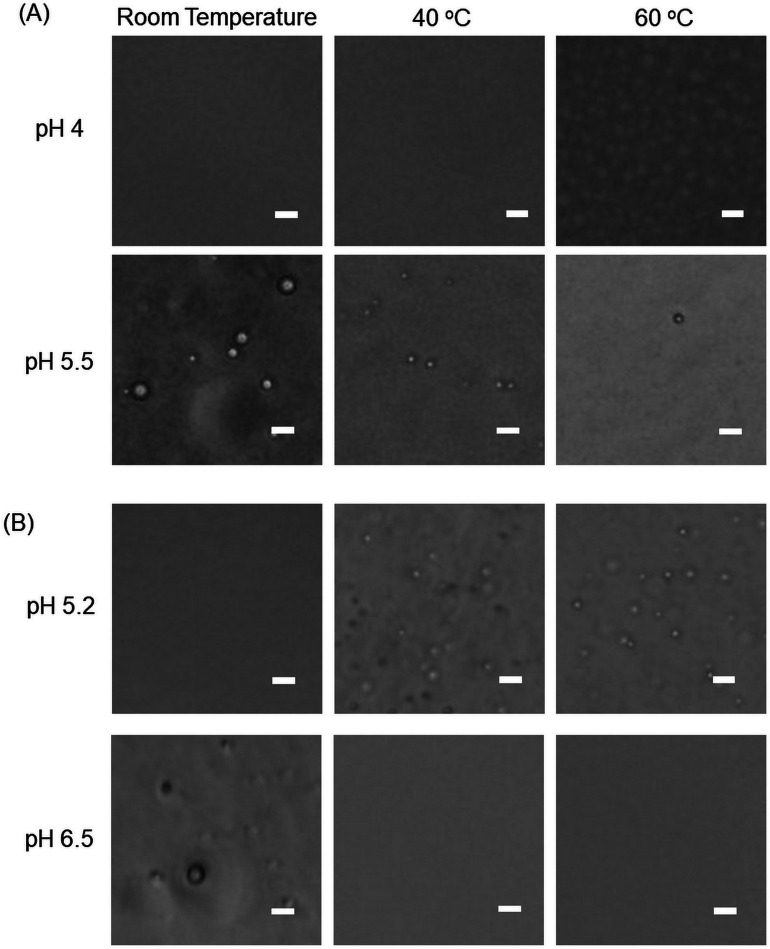
We then examined the phase transition phenomena using an optical microscope. Fig. 8 shows microscopic images of PAMAM-Phe-Suc and PAMAM-Phe-SO3Na during LCST- and UCST-type phase transitions. When the solutions were turbid, spherical droplets were observed in both solutions containing PAMAM-Phe-Suc and PAMAM-Phe-SO3Na. This suggests that coacervation (liquid–liquid phase separation) occurred during the phase transition, similar to UCST-type phase transition of PAMAM-Suc-Phe.27
Fig. 8. Optical microscopic images of the solutions containing (A) PAMAM-Phe-Suc and (B) PAMAM-Phe-SO3Na under different conditions. Bar 10 μm.
Separation of RB from aqueous solutions
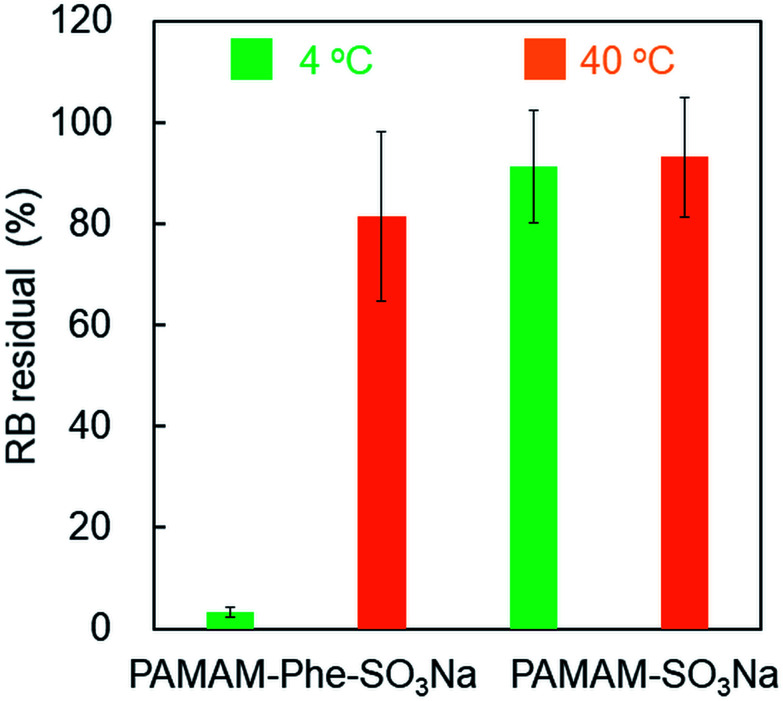
Finally, we demonstrated the material separation from the aqueous solutions using a zwitterionic thermosensitive dendrimer. Various materials including biomolecules could be separated using UCST-type thermosensitive polymers.34,35 It has been reported that PAMAM dendrimers can encapsulate anionic compounds, such as rose bengal (RB).27 Thus, we used RB as a model compound for separation. PAMAM-Phe-SO3Na showed thermosensitivity even in the presence of RB, but the phase transition temperature shifted to higher due to the enhanced hydrophobic interaction of the dendrimer/RB complex (Fig. S10†). RB was mixed with PAMAM-Phe-SO3Na at pH 6.5 and incubated at 4 °C and 60 °C for 30 min. Before and after centrifugation at 4 °C and 40 °C, the absorbance of the dendrimer-RB solutions was measured. The residual RB (%) was 3% and 82% at 4 °C and 40 °C, respectively, in the presence of PAMAM-Phe-SO3Na (Fig. 9). Because PAMAM-Phe-SO3Na was insoluble at 4 °C, the RB molecules in the solution were encapsulated and condensed into the dendrimer droplets. However, the RB encapsulated in PAMAM-Phe-SO3Na was not condensed effectively at 40 °C because PAMAM-Phe-SO3Na was mostly dissolved. Non-thermosensitive PAMAM-SO3Na was also used instead of thermosensitive PAMAM-Phe-SO3Na. The residual RB in the presence of PAMAM-SO3Na was 91% and 93% at 4 °C and 40 °C, respectively. Since PAMAM-SO3Na remains soluble even in the presence of RB (Fig. S10†), the RB molecules could not be separated. Therefore, our results suggest that some compounds can be separated from aqueous solutions by changing the temperature in the thermosensitive Phe-modified zwitterionic dendrimer.
Fig. 9. Separation of RB in the presence of PAMAM-Phe-SO3Na and PAMAM-SO3Na from aqueous solutions at 4 °C (green) and 40 °C (orange) (n = 3).
Conclusions
We synthesized various Phe-modified zwitterionic dendrimers as pH- and thermo-responsive polymers. Interestingly, PAMAM-Phe-Suc and PAMAM-Phe-SO3Na showed both LCST- and UCST-type thermosensitive behaviors that could be switched by pH. PAMAM-Phe-SO3Na showed a sharper temperature response than PAMAM-Phe-Suc owing to the zwitterionic branches with stable negative charge. These pH- and temperature-sensitive properties disappeared by quaternizing the PAMAM dendrimer and by changing Phe into Ile or AMBA. Thus, the inner tertiary amine and the Phe residue in the dendrimer are indispensable for their stimuli-sensitive behaviors. These dendrimers induced the coacervation during temperature changes. PAMAM-Phe-SO3Na was able to separate RB from aqueous solutions by using its thermosensitive properties. This paper is a first report of pH-switchable LCST/UCST thermosensitive dendrimers. These kinds of materials are useful in the design of smart materials.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We would like to thank Enago (www.enago.jp) for the English language review. This manuscript was supported by JSPS KAKENHI Grant Number JP17K06004.
Electronic supplementary information (ESI) available: Experimental section; summary of synthesized dendrimers with different generations; NMR and FT-IR spectra of synthesized dendrimers; the pH titration profiles; and temperature-dependent transmittance curves of obtained dendrimers under various conditions. See DOI: 10.1039/d0ra00499e
References
- Wei M. Gao Y. Li X. Serpe M. J. Polym. Chem. 2017;8:127–143. doi: 10.1039/C6PY01585A. [DOI] [Google Scholar]
- Zhao J. Lee V. E. Liu R. Priestley D. R. Annu. Rev. Chem. Biomol. Eng. 2019;10:361–382. doi: 10.1146/annurev-chembioeng-060718-030155. [DOI] [PubMed] [Google Scholar]
- Kim Y.-J. Matsunaga Y. T. J. Mater. Chem. B. 2017;5:4307–4321. doi: 10.1039/C7TB00157F. [DOI] [PubMed] [Google Scholar]
- Sponchioni M. Capasso Palmiero U. Moscatelli D. Mater. Sci. Eng., C. 2019;102:589–605. doi: 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- Niskanen J. Tenhu H. Polym. Chem. 2017;8:220–232. doi: 10.1039/C6PY01612J. [DOI] [Google Scholar]
- Seuring J. Agarwal S. Macromol. Rapid Commun. 2012;33:1898–1920. doi: 10.1002/marc.201200433. [DOI] [PubMed] [Google Scholar]
- Kotsuchibashi Y. Ebara M. Aoyagi T. Narain R. Polymers. 2016;8:380. doi: 10.3390/polym8110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arotçaréna M. Heise B. Ishaya S. Laschewsky A. J. Am. Chem. Soc. 2002;124:3787–3793. doi: 10.1021/ja012167d. [DOI] [PubMed] [Google Scholar]
- Papadakis C. M. Müller-Buschbaum P. Laschewsky A. Langmuir. 2019;35:9660–9676. doi: 10.1021/acs.langmuir.9b01444. [DOI] [PubMed] [Google Scholar]
- Vishnevetskaya N. S. Hildebrand V. Nizardo N. M. Ko C.-H. Di Z. Radulescu A. Barnsley L. C. Müller-Buschbaum P. Laschewsky A. Papadakis C. M. Langmuir. 2019;35:6441–6452. doi: 10.1021/acs.langmuir.9b00241. [DOI] [PubMed] [Google Scholar]
- Cao H. Guo F. Chen Z. Kong X. Z. Nanoscale Res. Lett. 2018;13:209. doi: 10.1186/s11671-018-2610-6. doi: 10.1186/s11671-018-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. O. R. Freitas R. F. S. Polymer. 2002;43:5879–5885. doi: 10.1016/S0032-3861(02)00507-4. [DOI] [Google Scholar]
- Zhang Q. Hoogenboom R. Prog. Polym. Sci. 2015;48:122–142. doi: 10.1016/j.progpolymsci.2015.02.003. [DOI] [Google Scholar]
- Amemori S. Kokado K. Sada K. J. Am. Chem. Soc. 2012;134:8344–8347. doi: 10.1021/ja301688u. [DOI] [PubMed] [Google Scholar]
- Plamper F. A. Ballauff M. Müller A. H. E. J. Am. Chem. Soc. 2007;129(47):14538–14539. doi: 10.1021/ja074720i. [DOI] [PubMed] [Google Scholar]
- Tomalia D. A. Naylor A. M. Goddard W. A. Angew. Chem., Int. Ed. 1990;29:138–175. doi: 10.1002/anie.199001381. [DOI] [Google Scholar]
- Kojima C. Expert Opin. Drug Delivery. 2010;7:307–319. doi: 10.1517/17425240903530651. [DOI] [PubMed] [Google Scholar]
- Haba Y. Kojima C. Harada A. Kono K. Angew. Chem., Int. Ed. 2007;46:234–237. doi: 10.1002/anie.200603346. [DOI] [PubMed] [Google Scholar]
- Haba Y. Kojima C. Harada A. Kono K. Macromolecules. 2006;39:7451–7453. doi: 10.1021/ma061019a. [DOI] [Google Scholar]
- Haba Y. Harada A. Takagishi T. Kono K. J. Am. Chem. Soc. 2004;126:12760–12761. doi: 10.1021/ja047755g. [DOI] [PubMed] [Google Scholar]
- Li X. Haba Y. Ochi K. Yuba E. Harada A. Kono K. Bioconjugate Chem. 2013;24:282–290. doi: 10.1021/bc300190v. [DOI] [PubMed] [Google Scholar]
- Liu H. Chen Y. Shen Z. J. Polym. Sci., Part A: Polym. Chem. 2007;45:1177–1184. doi: 10.1002/pola.21878. [DOI] [Google Scholar]
- Liu Y. Li W. Hou L. Wu P. RSC Adv. 2014;4:24263–24271. doi: 10.1039/C4RA02242D. [DOI] [Google Scholar]
- Luzon M. Boyer C. Peinado C. Corrales T. Whittaker M. Tao L. Davis T. P. J. Polym. Sci., Part A: Polym. Chem. 2010;48:2783–2792. doi: 10.1002/pola.24027. [DOI] [Google Scholar]
- Zheng K. Ren J. He J. Macromolecules. 2019;52:6780–6791. doi: 10.1021/acs.macromol.9b00920. [DOI] [Google Scholar]
- Tono Y. Kojima C. Haba Y. Takahashi T. Harada A. Yagi S. Kono K. Langmuir. 2006;22:4920–4922. doi: 10.1021/la060066t. [DOI] [PubMed] [Google Scholar]
- Tamaki M. Fukushima D. Kojima C. RSC Adv. 2018;8:28147–28151. doi: 10.1039/C8RA05381B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa S. Fukuda K. Yamamoto T. Ishihara K. Morishima Y. Biomacromolecules. 2005;6:663–670. doi: 10.1021/bm0495553. [DOI] [PubMed] [Google Scholar]
- Niu Y. Sun L. Crooks R. M. Macromolecules. 2003;36:5725–5731. doi: 10.1021/ma034276d. [DOI] [Google Scholar]
- Chen H.-T. Neerman M. F. Parrish A. R. Simanek E. E. J. Am. Chem. Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- Lee J. H. Lim Y. B. Choi J. S. Lee Y. Kim T. I. Kim H. J. Yoon J. K. Kim K. Park J. S. Bioconjugate Chem. 2003;14:1214–1221. doi: 10.1021/bc034095g. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Furyk S. Bergbreiter D. E. Cremer P. S. J. Am. Chem. Soc. 2005;127:14505–14510. doi: 10.1021/ja0546424. [DOI] [PubMed] [Google Scholar]
- Maiti P. K. Çaǧın T. Wang G. Goddard W. A. Macromolecules. 2004;37:6236–6254. doi: 10.1021/ma035629b. [DOI] [Google Scholar]
- Ohnishi N. Furukawa H. Hideyuki H. Wang J.-M. An C.-I. Fukusaki E. Kataoka K. Ueno K. Kondo A. NanoBiotechnology. 2006;2:43–49. doi: 10.1007/s12030-006-0006-7. [DOI] [Google Scholar]
- Shimada N. Nakayama M. Kano A. Maruyama A. Biomacromolecules. 2013;14:1452–1457. doi: 10.1021/bm400120y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.