Abstract
A simple protocol for the clean preparation of heterocyclic compounds containing dibenzofuran's core via oxodefluorination of fluoroarenes on activated γ-Al2O3 is reported. Alumina can be considered as a reliable oxygen source enabling one-pot substitution of fluorine atoms and yielding benzoannulated furan derivatives. The corresponding C–F bond activation is selective towards less stable C–Br/C–I and occurs under metal- and solvent-free conditions.
A simple protocol for the clean preparation of heterocyclic compounds containing dibenzofuran's core via oxodefluorination of fluoroarenes on activated γ-Al2O3 is reported.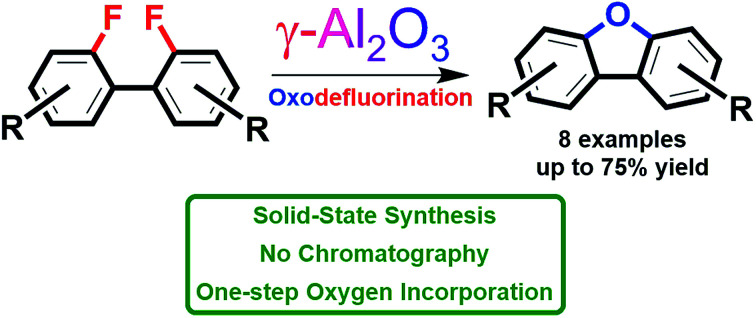
Dibenzo[b,d]furan is an important moiety as its derivatives constitute a long list of natural products.1,2 Moreover, multiple synthetic compounds bearing such a fragment also show biological activity3–5e.g. mimic fragments of complex biological structures.6,7 Due to its rigidity, dibenzofuran is oftentimes used as a bridge fixing fragment of the molecule at a desired distance giving rise to various chelating ligands,8–13 cryptands,14 and other supramolecular hosts.15–19 Furthermore, the stable dibenzofuran core enables different patterns of substitution. These features, in combination with appropriate electronic properties, make dibenzofuran and its derivatives potentially valuable components of phosphorescent OLEDs20–22 thermally activated delayed fluorescence emitters,23–25 perovskite solar cells26etc. Thus, functionalized dibenzofurans appear to be important building blocks.
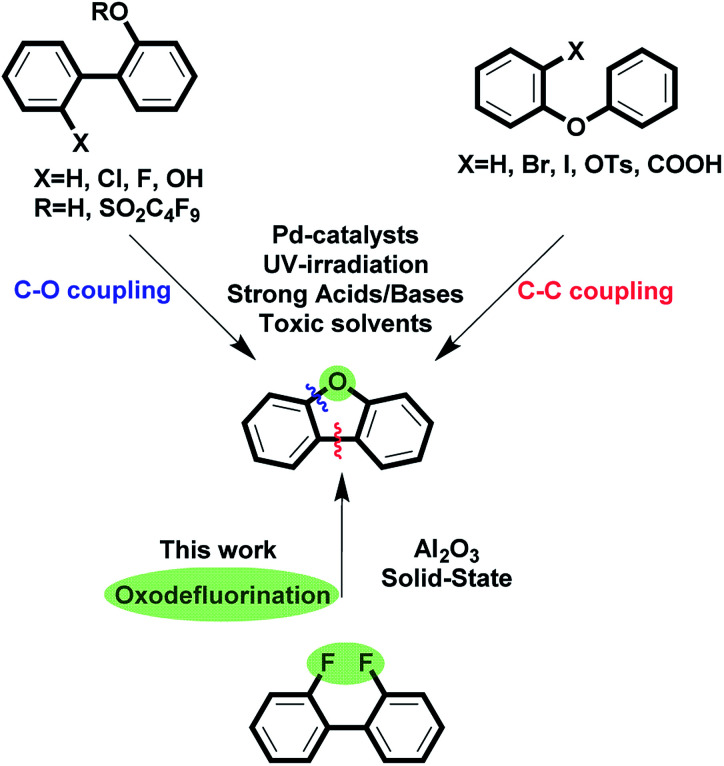
Despite numerous synthetic approaches to this class of compounds, the construction of functionalized dibenzofurans is far from being trivial since all existing methods are generally reduced to either C–O or C–C coupling (Fig. 1). This is mainly connected to the fact that oxygen has to be present in the precursor's structure because there is no reliable oxygen source that would enable double C–O bond incorporation. The majority of the available methods are based on Pd-catalysis,27–34 while others exploit strong bases/acids and toxic solvent.35–38 In addition, these methods rarely tolerate C–Br and C–I functionalities, which could have been used for further modifications.
Fig. 1. Synthesis of dibenzofuran. Existing approaches vs. oxodefluorination.
Herein, we report a transformation of fluorinated biphenyls into dibenzofuran's derivatives. The reaction occurs on the surface of activated γ-Al2O3 and does not require other reagents. The scope and limitations of the reactions are considered: the technique is compatible with C–Br and C–I functionalities, enables double oxodefluorination and incorporation of hexagons, thus yielding ladder-type π-conjugated heteroacenes and xanthene's derivatives, respectively.
As a part of our ongoing research on alumina mediated C–F activation,39,40 we have observed an interesting competition between C(aryl)–C(aryl) coupling and formation of oxygen-containing side products.41 In certain cases (i.e. when two fluorine atoms are dislocated in bay-region) the side products turned out to contain dibenzofuran's moiety.42 Some additional data on this competition are briefly discussed in ESI.†
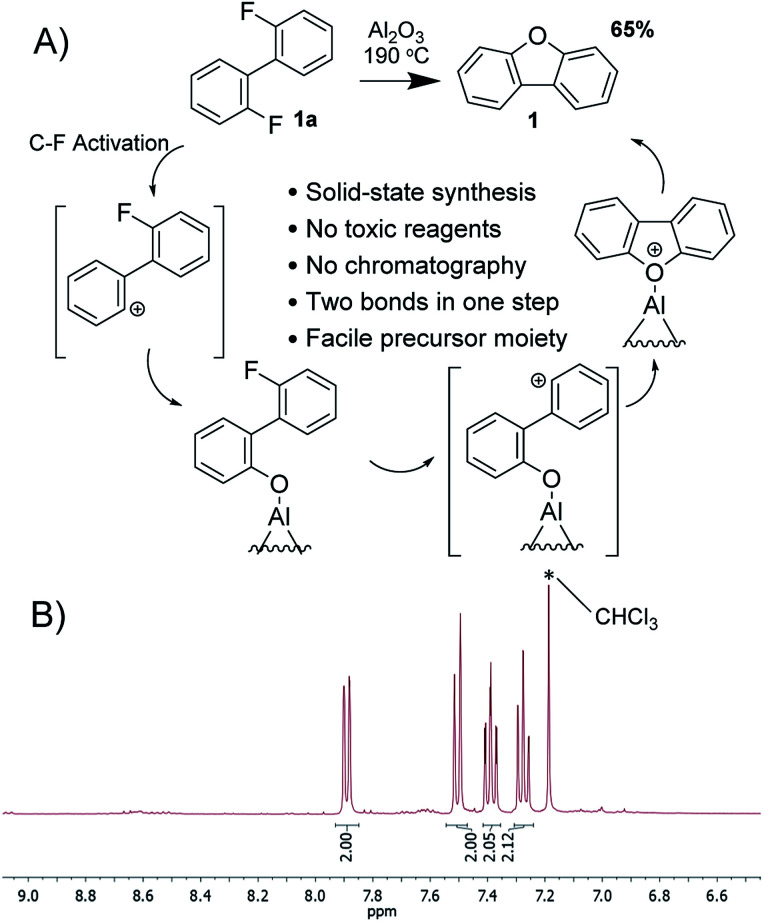
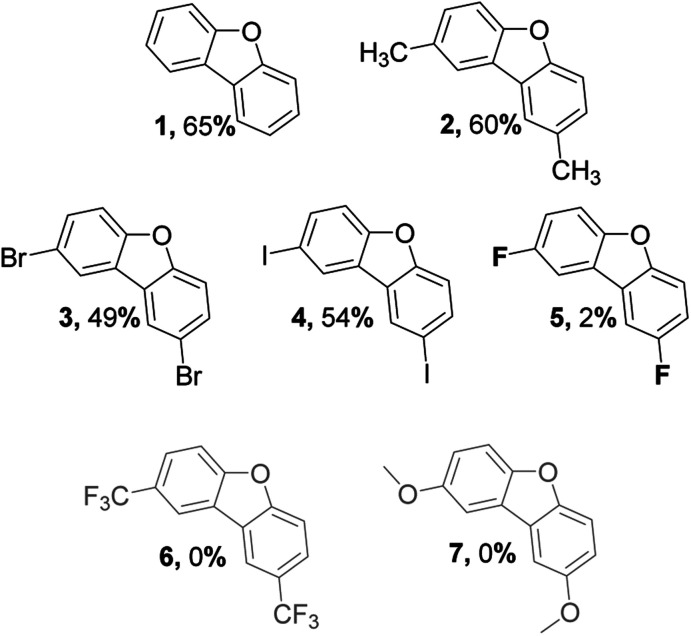
To investigate the formation of dibenzofuran's core in more details, we have excluded one of the competitors i.e. the possibility for intramolecular C(aryl)–C(aryl) coupling. The simplest model meeting the requirements is 2,2′-difluorobiphenyl (1a), which upon exposure to activated alumina transforms into dibenzofuran 1 in 65% yield. As a matter of fact, the desired 1 is the only product that can be extracted from the reactionary mixture with aprotic solvent such as toluene (Fig. 2). Thus, flash chromatography is not required, since reactionary alumina already plays the role of a plug enabling effective separation from intermediate and side products, which can be extracted with methanol afterwards (see ESI†). Notably, eliminated fluorine atoms are most likely to be bound to alumina in the form of extremely strong and naturally occurring Al–F bond yielding partially fluorinated alumina, which is of interest in multiple fields including catalysis.43,44
Fig. 2. (A) Synthesis of dibenzofuran and suggested mechanism of the reaction. (B) 1H NMR of the reactionary mixture as obtained after extraction with toluene.
As any other heterogeneous process, this reaction is quite likely to have a complicated manner. However, some pieces of evidence on C(aryl)–F polarization41,45,46 allow us to suggest the key steps of the transformation. Alumina induces a partial positive charge on carbon generating incipient phenyl cation species,47 which are to interact with proximal moieties. In the case of 2,2′-difluorobiphenyl, no aryl functionalities are available for intramolecular C(aryl)–C(aryl) coupling; therefore oxygen on alumina surface serves as an alternative nucleophile interacting with cation-like species. Such hydrolysis of C–F bond has already been observed as a side process.41 Unlike the reported transformation, the current precursor contains two fluorine atoms, whereas the polarization of the second C(aryl)–F bond induces the second C–O coupling, this time intramolecular, and subsequent covalent detachment of the product. Some other possible mechanisms occurring via aryne-particles or dehydration were excluded (see ESI†). Noteworthy, the outcome of the reaction resembles double SNAr, which can be generally achieved in activated arenes i.e. containing multiple electron-withdrawing groups. Such restriction does not apply to the alumina-promoted oxodefluorination due to its cationic nature.
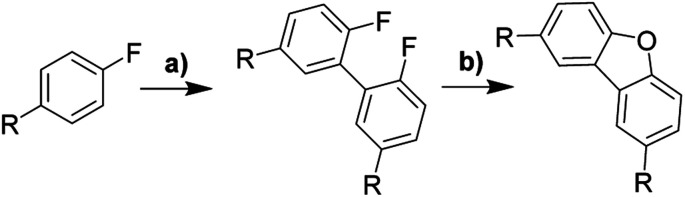
An interesting aspect of C(aryl)–F bond is that it can be used as both directing and functional groups, although it is seldom considered as the latter. In accordance with Table 1, para-substituted fluorobenzenes undergo directed ortho-metalation enabling simple one-pot synthesis of functionalized biphenyls bearing two fluorine atoms in 2,2′-positions. This gives a facile approach to several precursors, which were used to investigate the scope and limitation of the alumina-promoted oxodefluorination. After heating with activated alumina at 190 °C under vacuum, the reactionary mixture was extracted with toluene. The obtained extract containing the target molecule did not require flash chromatography unless stated otherwise.
Synthesis of 2,8-substituted dibenzofurans. (a) n-BuLi, (1.5 eq.), tetramethylpiperidine (1.5 eq.), CuBr2 (1 eq.), PhNO2 (1 eq.), THF, −78 °C; (b) γ-Al2O3, 190 °C, 12 h.
|
|---|
|
While tolerance to methyl group comes as no surprise, selective C–F activation in presence of C–Br and more interestingly C–I is a quite notable feature since fluorine forms stronger bonds with carbon in comparison to other halogens. At the same time, the reaction has a rather poor selectivity to C(aryl)–F bonds since only traces of 5 were extracted, whereas the rest of the precursor has remained on the surface in forms of hydrolyzed side products. A similar, although anticipated, outcome was observed in the case of 6, where nothing could be extracted with toluene due to the rapid hydrolysis of CF3 groups.48 As of methoxy groups, the corresponding toluene extract revealed no desired product 7. Presumably, it is connected to the fact that OCH3 occupies reactive sites of alumina more rapidly than C–F polarization takes place.
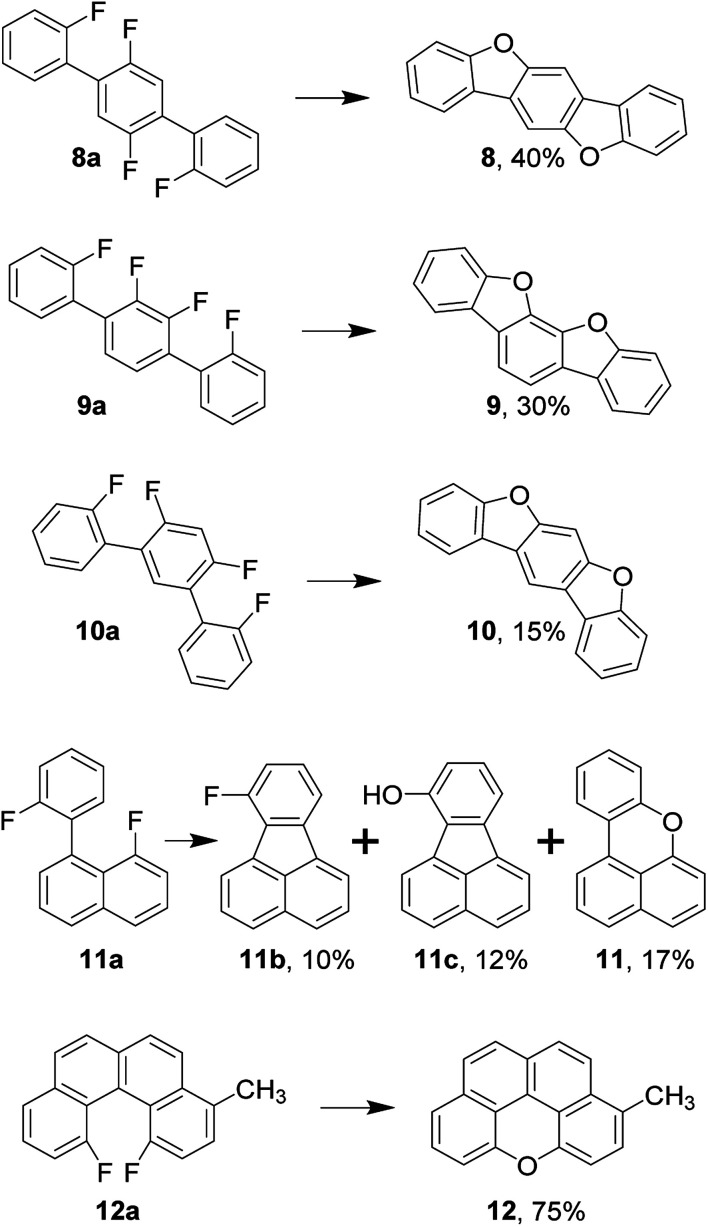
This observation is especially interesting in light of the following evidence. Oligophenylenes 8a, 9a, and 10a synthesized in one step via Suzuki coupling undergo double annulation yielding respective ladder-type π-conjugated compounds 8–10, which are promising candidates as organic semiconductors (Scheme 1).37 Here, the formation of the first dibenzofuran's moiety, apparently, does not prevent the second region from annulation.
Scheme 1. Synthesis of ladder-type π-conjugated compounds, benzo[kl]xanthene and incorporation of oxygen into cove region.
To investigate whether the approach is applicable to the synthesis of xanthene's derivatives such as benzo[kl]xanthene (11), we have designed the corresponding precursor 11a having a choice between C–F and C–C couplings. Due to this competition, several products were extracted with toluene in this particular case. Therefore, toluene and methanol extracts were combined and separated by means of flash chromatography to reveal three major products 11b, 11c, and 11. To preclude the variability, we have synthesized rigid precursor 12a with two fluorine atoms in cove region. Thus, 12a transforms into 12 in considerable 75% yield enabling the synthesis of elusive naphtho[2,1,8,7-klmn]xanthene's core.49
Conclusions
In summary, we have occasionally come across an attractive method enabling the incorporation of oxygen in place of two C(aryl)–F bonds, where alumina serves as a reliable oxygen source. The simplicity of the required fluorinated moiety makes precursors, in general, readily available via facile one-step synthesis. The method allows the synthesis of dibenzofuran's, xanthene's, and ladder-type π-conjugated derivatives under metal- and solvent-free conditions. In principle, alumina-promoted oxodefluorination tolerates such useful functionalities as C–Br and C–I. The synthetic procedure is straightforward and requires activated γ-Al2O3 as a reagent and simple extraction to yield high purity target products without flash chromatography and respective solvent and silica wastes.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
Funded by the Deutsche Forschungsgemeinschaft (DFG) – Projektnummer 182849149 – SFB 953, AM407.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01369b
References
- Lin C.-H. Chang H.-S. Liao C.-H. Ou T.-H. Chen I.-S. Tsai I.-L. J. Nat. Prod. 2010;73:1628–1631. doi: 10.1021/np100200s. [DOI] [PubMed] [Google Scholar]
- Millot M. Dieu A. Tomasi S. Nat. Prod. Rep. 2016;33:801–811. doi: 10.1039/C5NP00134J. [DOI] [PubMed] [Google Scholar]
- Yurttaş L. Abu Mohsen U. Ozkan Y. Cobanoglu S. Levent S. Kaplancikli Z. A. J. Enzyme Inhib. Med. Chem. 2016;31:1177–1183. doi: 10.3109/14756366.2015.1108971. [DOI] [PubMed] [Google Scholar]
- Ma Y. Wei H.-Y. Zhang Y.-Z. Jin W.-Y. Li H.-L. Zhou H. Cheng X.-C. Wang R.-L. Oncotarget. 2017;8(24):38466–38481. doi: 10.18632/oncotarget.16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal N. Donahue J. P. Do C. Perry T. Bongay-Williams K. Foroozesh M. IUCrData. 2018;3:x181306. doi: 10.1107/S2414314618013068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean F. Buisine E. Melnyk O. Drobecq H. Odaert B. Hugues M. Lippens G. Tartar A. J. Am. Chem. Soc. 1998;120:6076–6083. doi: 10.1021/ja972346o. [DOI] [Google Scholar]
- Petrassi H. M. Johnson S. M. Purkey H. E. Chiang K. P. Walkup T. Jiang X. Powers E. T. Kelly J. W. J. Am. Chem. Soc. 2005;127:6662–6671. doi: 10.1021/ja044351f. [DOI] [PubMed] [Google Scholar]
- Caradoc-Davies P. L. Hanton L. R. Dalton Trans. 2003:1754–1758. doi: 10.1039/B300761H. [DOI] [Google Scholar]
- Hlavinka M. L. Hagadorn J. R. Chem. Commun. 2003:2686. doi: 10.1039/B309732C. [DOI] [PubMed] [Google Scholar]
- Deschamps E. Deschamps B. Laure Dormieux J. Ricard L. Mézailles N. Le Floch P. Dalton Trans. 2006:594–602. doi: 10.1039/B508678G. [DOI] [PubMed] [Google Scholar]
- He S. Wang F. Tong W.-L. Yiu S.-M. Chan M. C. W. Chem. Commun. 2016;52:1017–1020. doi: 10.1039/C5CC08794E. [DOI] [PubMed] [Google Scholar]
- Li S. T. Braun-Cula B. Hoof S. Limberg C. Dalton Trans. 2018;47:544–560. doi: 10.1039/C7DT03752J. [DOI] [PubMed] [Google Scholar]
- Itoh K. Sibi M. P. Org. Biomol. Chem. 2018;16:5551–5565. doi: 10.1039/C8OB01010B. [DOI] [PubMed] [Google Scholar]
- Bazzicalupi C. Bencini A. Ciattini S. Denat F. Désogère P. Goze C. Matera I. Valtancoli B. Dalton Trans. 2010;39:11643. doi: 10.1039/C0DT00948B. [DOI] [PubMed] [Google Scholar]
- Asakawa M. Ashton P. R. Brown C. L. Fyfe M. C. T. Menzer S. Pasini D. Scheuer C. Spencer N. Stoddart J. F. White A. J. P. Williams D. J. Chem.–Eur. J. 1997;3:1136–1150. doi: 10.1002/chem.19970030720. [DOI] [Google Scholar]
- Pistorio B. J. Chang C. J. Nocera D. G. J. Am. Chem. Soc. 2002;124:7884–7885. doi: 10.1021/ja026017u. [DOI] [PubMed] [Google Scholar]
- Kadish K. M. Frémond L. Ou Z. Shao J. Shi C. Anson F. C. Burdet F. Gros C. P. Barbe J.-M. Guilard R. J. Am. Chem. Soc. 2005;127:5625–5631. doi: 10.1021/ja0501060. [DOI] [PubMed] [Google Scholar]
- Garner L. E. Zhu H. Hlavinka M. L. Hagadorn J. R. Chen E. Y.-X. J. Am. Chem. Soc. 2006;128:14822–14823. doi: 10.1021/ja066401h. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. Luckett T. D. Hodgkiss J. M. Nocera D. G. J. Am. Chem. Soc. 2006;128:6546–6547. doi: 10.1021/ja058731s. [DOI] [PubMed] [Google Scholar]
- May F. Al-Helwi M. Baumeier B. Kowalsky W. Fuchs E. Lennartz C. Andrienko D. J. Am. Chem. Soc. 2012;134:13818–13822. doi: 10.1021/ja305310r. [DOI] [PubMed] [Google Scholar]
- Yun J. H. Kang Y. J. Han S. H. Lee J. Y. J. Mater. Chem. C. 2018;6:320–325. doi: 10.1039/C7TC03336B. [DOI] [Google Scholar]
- Yun J. H. Kang Y. J. Han S. H. Lee J. Y. Dyes Pigm. 2019;162:1–7. doi: 10.1016/j.dyepig.2018.10.003. [DOI] [Google Scholar]
- Yu J. G. Han S. H. Lee H. L. Hong W. P. Lee J. Y. J. Mater. Chem. C. 2019;7:2919–2926. doi: 10.1039/C9TC00214F. [DOI] [Google Scholar]
- Jung M. Lee K. H. Hong W. P. Lee J. Y. J. Mater. Chem. C. 2019;7:7760–7767. doi: 10.1039/C9TC01577A. [DOI] [Google Scholar]
- Tao W.-W. Wang K. Chen J.-X. Shi Y.-Z. Liu W. Zheng C.-J. Li Y.-Q. Yu J. Ou X.-M. Zhang X.-H. J. Mater. Chem. C. 2019;7:4475–4483. doi: 10.1039/C9TC00419J. [DOI] [Google Scholar]
- Shi Y. Hou K. Wang Y. Wang K. Ren H. Pang M. Chen F. Zhang S. J. Mater. Chem. A. 2016;4:5415–5422. doi: 10.1039/C6TA00976J. [DOI] [Google Scholar]
- Parisien M. Valette D. Fagnou K. J. Org. Chem. 2005;70:7578–7584. doi: 10.1021/jo051039v. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K. Nakano K. Nozaki K. J. Org. Chem. 2007;72:5119–5128. doi: 10.1021/jo070427p. [DOI] [PubMed] [Google Scholar]
- Liégault B. Lee D. Huestis M. P. Stuart D. R. Fagnou K. J. Org. Chem. 2008;73:5022–5028. doi: 10.1021/jo800596m. [DOI] [PubMed] [Google Scholar]
- Wang C. Piel I. Glorius F. J. Am. Chem. Soc. 2009;131:4194–4195. doi: 10.1021/ja8100598. [DOI] [PubMed] [Google Scholar]
- Xiao B. Gong T.-J. Liu Z.-J. Liu J.-H. Luo D.-F. Xu J. Liu L. J. Am. Chem. Soc. 2011;133:9250–9253. doi: 10.1021/ja203335u. [DOI] [PubMed] [Google Scholar]
- Nervig C. S. Waller P. J. Kalyani D. Org. Lett. 2012;14:4838–4841. doi: 10.1021/ol302166n. [DOI] [PubMed] [Google Scholar]
- Okazaki T. Nakagawa M. Futemma T. Kitagawa T. J. Phys. Org. Chem. 2016;29:107–111. doi: 10.1002/poc.3505. [DOI] [Google Scholar]
- Kaida H. Satoh T. Nishii Y. Hirano K. Miura M. Chem. Lett. 2016;45:1069–1071. doi: 10.1246/cl.160496. [DOI] [Google Scholar]
- Yamato T. Hideshima C. Prakash G. K. S. Olah G. A. J. Org. Chem. 1991;56:3192–3194. doi: 10.1021/jo00009a051. [DOI] [Google Scholar]
- Nakanishi K. Fukatsu D. Takaishi K. Tsuji T. Uenaka K. Kuramochi K. Kawabata T. Tsubaki K. J. Am. Chem. Soc. 2014;136:7101–7109. doi: 10.1021/ja502209w. [DOI] [PubMed] [Google Scholar]
- Truong M. A. Nakano K. Beilstein J. Org. Chem. 2016;12:805–812. doi: 10.3762/bjoc.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo Solórzano P. Brigante F. Pierini A. B. Jimenez L. B. J. Org. Chem. 2018;83:7867–7877. doi: 10.1021/acs.joc.8b00742. [DOI] [PubMed] [Google Scholar]
- Papaianina O. Akhmetov V. A. Goryunkov A. A. Hampel F. Heinemann F. W. Amsharov K. Y. Angew. Chem., Int. Ed. 2017;56:4834–4838. doi: 10.1002/anie.201700814. [DOI] [PubMed] [Google Scholar]
- Akhmetov V. Amsharov K. Phys. Status Solidi. 2019;256:1900254. doi: 10.1002/pssb.201900254. [DOI] [Google Scholar]
- Akhmetov V. Feofanov M. Papaianina O. Troyanov S. Amsharov K. Chem.–Eur. J. 2019;25:11609–11613. doi: 10.1002/chem.201902586. [DOI] [PubMed] [Google Scholar]
- Akhmetov V. Feofanov M. Troyanov S. Amsharov K. Chem.–Eur. J. 2019;25:7607–7612. doi: 10.1002/chem.201901450. [DOI] [PubMed] [Google Scholar]
- Skapin T. J. Mater. Chem. 1995;5:1215–1222. doi: 10.1039/JM9950501215. [DOI] [Google Scholar]
- Padhye R. Aquino A. J. A. Tunega D. Pantoya M. L. ACS Appl. Mater. Interfaces. 2017;9:24290–24297. doi: 10.1021/acsami.7b05372. [DOI] [PubMed] [Google Scholar]
- Allemann O. Baldridge K. K. Siegel J. S. Org. Chem. Front. 2015;2:1018–1021. doi: 10.1039/C5QO00170F. [DOI] [Google Scholar]
- Lu M. Allemann O. Xu J. Linden A. Baldridge K. K. Siegel J. S. Org. Chem. Front. 2019;6:2640–2646. doi: 10.1039/C9QO00633H. [DOI] [Google Scholar]
- Sharapa D. Steiner A.-K. Amsharov K. Phys. Status Solidi. 2018;1800189:1800189. doi: 10.1002/pssb.201800189. [DOI] [Google Scholar]
- Papaianina O. Amsharov K. Y. Chem. Commun. 2016;52:1505–1508. doi: 10.1039/C5CC08747C. [DOI] [PubMed] [Google Scholar]
- Donovan P. M. Scott L. T. J. Am. Chem. Soc. 2004;126:3108–3112. doi: 10.1021/ja038254i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.