Abstract
Intravenous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy usually starts with a 2.0 g/kg induction dose followed by 1.0 g/kg maintenance doses every 3 weeks. No dose-ranging studies with intravenous immunoglobulin maintenance therapy have been published.
The Progress in Chronic Inflammatory Demyelinating polyneuropathy (ProCID) study was a prospective, double-blind, randomized, parallel-group, multicentre, phase III study investigating the efficacy and safety of 10% liquid intravenous immunoglobulin (Panzyga®) in patients with active chronic inflammatory demyelinating polyneuropathy. Patients were randomized 1:2:1 to receive the standard intravenous immunoglobulin induction dose and then either 0.5, 1.0 or 2.0 g/kg maintenance doses every 3 weeks. The primary end point was the response rate in the 1.0 g/kg group, defined as an improvement ≥1 point in adjusted Inflammatory Neuropathy Cause and Treatment score at Week 6 versus baseline and maintained at Week 24. Secondary end points included dose response and safety. This trial was registered with EudraCT (Number 2015–005443-14) and clinicaltrials.gov (NCT02638207).
Between August 2017 and September 2019, the study enrolled 142 patients. All 142 were included in the safety analyses. As no post-infusion data were available for three patients, 139 were included in the efficacy analyses, of whom 121 were previously on corticosteroids. The response rate was 80% (55/69 patients) [95% confidence interval (CI): 69–88%] in the 1.0 g/kg group, 65% (22/34; CI: 48–79%) in the 0.5 g/kg group, and 92% (33/36; CI: 78–97%) in the 2.0 g/kg group. While the proportion of responders was higher with higher maintenance doses, logistic regression analysis showed that the effect on response rate was driven by a significant difference between the 0.5 and 2.0 g/kg groups, whereas the response rates in the 0.5 and 2.0 g/kg groups did not differ significantly from the 1.0 g/kg group. Fifty-six per cent of all patients had an adjusted Inflammatory Neuropathy Cause and Treatment score improvement 3 weeks after the induction dose alone. Treatment-related adverse events were reported in 16 (45.7%), 32 (46.4%) and 20 (52.6%) patients in the 0.5, 1.0 and 2.0 g/kg dose groups, respectively. The most common adverse reaction was headache. There were no treatment-related deaths.
Intravenous immunoglobulin (1.0 g/kg) was efficacious and well tolerated as maintenance treatment for patients with chronic inflammatory demyelinating polyneuropathy. Further studies of different maintenance doses of intravenous immunoglobulin in chronic inflammatory demyelinating polyneuropathy are warranted.
Keywords: chronic inflammatory demyelinating polyneuropathy, intravenous immunoglobulin, ProCID study, Panzyga®, randomized controlled trial
In a randomized, double-blind, phase III study, Cornblath et al. show that 1.0 g/kg intravenous immunoglobulin (panzyga®) is efficacious and well tolerated as maintenance treatment following a 2.0 g/kg induction dose in patients with CIDP, even in individuals previously treated successfully with corticosteroids.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare, auto-immune mediated polyneuropathy and can be diagnosed using guidelines from the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS).1,2 However, the diagnosis of CIDP is not straightforward,3 and a number of disorders can be mistaken for CIDP.4
Three treatments have been proven effective for CIDP: intravenous immunoglobulin (IVIg), corticosteroids, and plasma exchange.5 Where available, IVIg is the most frequently used first-line treatment due to its safety and efficacy. Based on several clinical studies, treatment with IVIg in CIDP usually starts with an induction dose of 2.0 g/kg over 2 to 5 days followed by maintenance dosing of 1.0 g/kg every 3 weeks.6-8
The optimal maintenance dose may vary between patients, and the EFNS/PNS guidelines recommend that the maintenance dose may need to be adjusted to suit an individual patient’s needs.2 Whilst tailored dosing has been long advocated and is supported by retrospective and real-life data,9–12 there have been no randomized, prospective trials evaluating different IVIg maintenance dose regimens in patients with CIDP. Only one small open, randomized study has evaluated different induction doses of IVIg for CIDP and multifocal motor neuropathy, and the results showed a potential dose-related effect 5 weeks after induction treatment.13 The PATH study evaluated two doses of subcutaneous immunoglobulin (SCIg), 0.2 and 0.4 g/kg every week, in patients with CIDP and showed no significant differences in outcomes between those two SCIg doses during a study period of 24 weeks.14
The Progress in Chronic Inflammatory Demyelinating polyneuropathy (ProCID) study investigated the efficacy and safety of IVIg after a 2.0 g/kg induction dose followed by a standard maintenance dose of 1.0 g/kg given every 3 weeks, and two other doses, 0.5 g/kg and 2.0 g/kg, on outcomes in patients with CIDP during a study period of 24 weeks.
Materials and methods
Study drug
IVIg (Panzyga®, Octapharma) is a glycine-stabilized 10% liquid human IVIg15,16 with proven efficacy and safety as replacement therapy in primary immunodeficiency17 and for immunomodulation in patients with immune thrombocytopenic purpura.18 This IVIg product is licensed for different indications (including CIDP) in the USA,19 Canada20 and the EU.21
Study design and participants
The ProCID study (EudraCT Number 2015–005443-14 and clinicaltrials.gov identifier NCT02638207) was designed in 2016/2017 and initiated in 2017.22 The trial protocol and statistical analysis plan were published previously.22
Patients were eligible if they were at least 18 years old and had been diagnosed with definite or probable CIDP according to published criteria1 and if they were dependent on treatment with immunoglobulins or corticosteroids. The main exclusion criteria included previous failure of immunoglobulin treatment, treatment with other immunomodulatory/suppressive agents in the prior 6 months, treatment with immuno-chemotherapeutic regimens, and clinical evidence of peripheral neuropathy from another cause. Full inclusion and exclusion criteria were published previously.22 All patients gave written informed consent prior to any study-related procedures. The ethics committees of all participating centres approved the study protocol.
Procedures
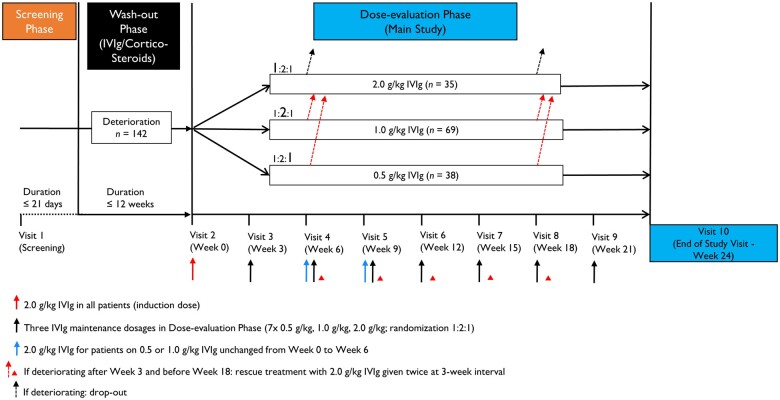
The study consisted of screening, wash-out, and dose-evaluation phases (Fig. 1). Patients were screened, and, if eligible, entered the wash-out phase, during which their current medication was reduced in a predefined standard manner, to determine whether they had active CIDP. For those previously on IVIg, the dose was reduced by 25% at each sequential infusion until deterioration. For those previously on corticosteroids, the dose was reduced at the discretion of the investigator at a rate to expect study entry within 6–12 weeks and to a dose of prednisolone ≤20 mg/day or equivalent when deterioration occurred. Low doses of corticosteroids at deterioration could be continued during the study. The wash-out phase had a maximum of 12 weeks, and only patients who deteriorated in this time frame were enrolled and randomized into the dose-evaluation phase. Patients who did not deteriorate in the wash-out phase were considered not to have active CIDP and were excluded from the study. Deterioration was determined by worsening of their overall status according to the Patients’ Global Impression of Change Scale and either an increase in adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) score by ≥1 point, or a decrease of ≥8 kPa on grip strength in one hand, or reached the Inflammatory Rasch-built Overall Disability Scale (I-RODS) minimum clinically important difference related to the varying standard errors cut-off of −1.96 or less.23
Figure 1.
Trial design.
All patients who deteriorated were randomly assigned 1:2:1 to maintenance doses of 0.5, 1.0 or 2.0 g/kg IVIg in the 24-week dose-evaluation phase. They then received an induction dose of IVIg 2.0 g/kg, followed by seven maintenance IVIg doses of 0.5, 1.0 or 2.0 g/kg every 3 weeks (± 4 days). The masked assignment of the correct dose and treatment for each study visit was managed using an interactive web response system. The treatment group assignment was only reported to the hospital pharmacist or designee by a dedicated email to which no other study personnel had access. The patient was masked to the medication by the use of an opaque (non-transparent) infusion line and overpouches. The evaluating investigator was not involved with administering medication to the patient. Dosing was based on actual body weight and administered over two consecutive days without pre-medication. Corticosteroids (prednisolone or equivalent) ≤20 mg/day as concomitant CIDP medication were permitted during the dose-evaluation phase for patients with prior corticosteroid therapy. For all patients in the 0.5 and 1.0 g/kg dose groups who were stable (unimproved) at Week 6 or deteriorated between Weeks 3 and 18, there was the option to administer rescue treatment with two consecutive infusions of IVIg 2.0 g/kg at 3-week intervals (± 4 days). Following the two IVIg rescue treatments, patients attended an end of study visit. Patients deteriorating after Week 18 or 21 dropped out and had their end of study visit at Week 21 or 24, respectively. Patients in the 2.0 g/kg arm were discontinued from the study if they were stable (unimproved) at Week 6 or deteriorated after Week 3 and before Week 21.
Outcomes
The primary outcome was the proportion of responders in the 1.0 g/kg arm at Week 24.8,24 A responder was defined as a subject who showed an improvement (decrease) ≥ 1 point in adjusted INCAT score at Week 6 compared with baseline (first visit of the dose-evaluation phase), completed the 24-week study, and maintained the response at Week 24. A non-responder was defined as a subject who did not achieve an improvement of ≥1 point in adjusted INCAT score by Week 6, or who deteriorated between Week 3 and Week 21, or who discontinued before Week 24, irrespective of adjusted INCAT score at termination.
The main secondary outcomes were the proportion of responders in the 0.5 g/kg and 2.0 g/kg arms at Week 24 relative to baseline compared with the 1.0 g/kg arm based on the adjusted INCAT score, grip strength, I-RODS score, and Medical Research Council (MRC) score, and time to first confirmed worsening on the adjusted INCAT score by ≥1 point from the value at baseline. Other secondary outcomes were the Pain Intensity Numeric Rating Scale and the sum of the distal evoked amplitude of eight motor nerves. To assess safety and tolerability, treatment-emergent adverse events (TEAEs) per infusion and the number of patients with TEAEs were recorded. An adverse event was defined as a TEAE if first onset or worsening occurred after the start of the first IVIg administration in the study. The full list of outcomes variables has previously been published.22
Statistical analysis
The sample size calculation was based on the expected proportion of responders based on the adjusted INCAT score in the 1.0 g/kg dose group. At the time this study was designed, experiences with this dosing regimen were available from the ICE6 and PRIMA7 studies. In the PRIMA study,7 the lower limit of the 95% Wilson-Score confidence interval (CI) for the proportion of responders (R) was 42%, and thus 42% was selected as the threshold for evaluation of the primary end point: null hypothesis (H0) R < 0.42; alternative hypothesis (H1) R ≥ 0.42. This was tested by comparing the lower limit of the 95% Wilson-Score CI for the observed proportion of responders with a pre-defined threshold of 0.42. We estimated the true percentage of responders in CIDP patients treated with IVIg as 60%. In comparison, 60.7% of patients were responders in the PRIMA study and 54.2% in the ICE study.6,7 We applied these parameters to a computer simulation using SAS® statistical analysis software and calculated that a minimum of 62 evaluable patients in the 1.0 g/kg dose group was needed to achieve a power of at least 80%. To account for possible dropouts, it was planned to enrol 70 patients into this group. To allow for the comparison between dose groups, it was planned to enrol half the number of evaluable patients into the 0.5 and 2.0 g/kg groups, resulting in a total of 124 evaluable patients and an enrolment target of 140 patients. The primary evaluation of efficacy end points was based on the full analysis set in an intention-to-treat analysis.
The response rates in the alternative dose groups were compared descriptively and by an odds ratio analysis. All other end points were analysed by means of descriptive statistics. Safety was analysed in all patients who received any amount of IVIg in the study.
In addition, an exploratory logistic regression analysis was performed that included the pretreatment as well as the dose group as predictor variables for response based on adjusted INCAT score. The same model was applied to treatment responses based on grip strength, I-RODS scores and MRC sum score.
Data availability
Individual participant data collected during the trial will not be shared. The study protocol and main results are available at https://clinicaltrials.gov/ct2/show/study/NCT02638207 (Accessed 24 February 2022).
Results
Patient disposition
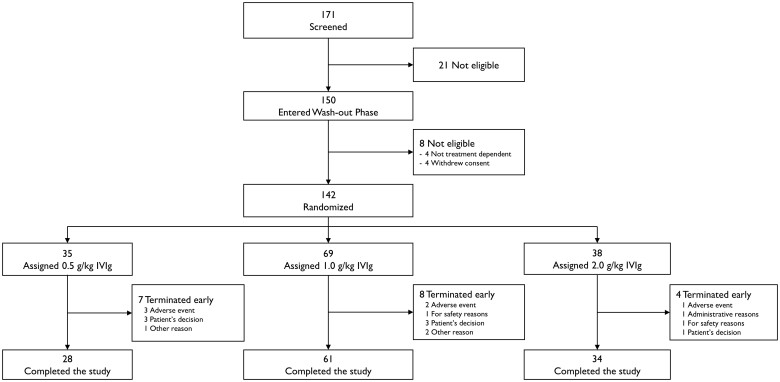
Patients were enrolled from 25 centres worldwide between 9 August 2017 and 5 September 2019. A total of 171 patients were screened, and 150 entered the wash-out phase. Eight patients were not eligible for inclusion into the dose-evaluation phase due to their disease not being treatment dependent (n = 4) or withdrawal of consent (n = 4). A total of 142 patients experienced deterioration during the wash-out phase and were randomized: 35 (24.6%) to IVIg 0.5 g/kg, 69 (48.6%) to IVIg 1.0 g/kg, and 38 (26.8%) to IVIg 2.0 g/kg. All 142 randomized patients received at least one infusion of IVIg and were included in the safety analysis set. The intention-to-treat population consisted of 139 patients because no post-infusion data were collected in three patients. The per-protocol set comprised 129 patients: five patients were excluded due to dosing errors, four patients withdrew from study, and one patient was lost to follow-up. In total, 123 patients (86.6%) completed the study (Fig. 2). Of the 19 patients (13.4%) who terminated early and were thus counted as non-responders, the most common reasons were patient’s decision (n = 7, 4.9%) and TEAEs (n = 6, 4.2%). The highest incidence of early terminations was seen in the 0.5 g/kg group (20.0%), compared with 11.6% in the 1.0 g/kg group and 10.5% in the 2.0 g/kg group.
Figure 2.
Trial profile.
Demographic and baseline characteristics
Demographic characteristics in the intention-to-treat population (Table 1) were similar across randomization strata and in the safety analysis set. In total, 91.4% of patients had a history of typical CIDP. Twelve (8.6%) patients had atypical CIDP: nine distal acquired demyelinating symmetric neuropathy and three multifocal acquired demyelinating sensory and motor neuropathy. One hundred and twenty-one patients (87.1%) had been treated previously with corticosteroids for their CIDP, and 18 patients (12.9%) with immunoglobulins. The percentage of patients previously treated for CIDP with immunoglobulins or with corticosteroids was distributed equally across the three dose groups. After deterioration and at the start of the dose-evaluation phase, 44/121 patients (36%) previously on corticosteroids entering this phase were still on corticosteroids, and this was balanced evenly across dose groups (Table 1). The corticosteroid dose was reduced to ≤20 mg/day in all except two of these 44 patients, one in the 0.5 g/kg group and one in the 1.0 g/kg group, who were on a dose of 25 mg/day prednisolone equivalent due to a miscalculation in the conversion of methylprednisolone to prednisolone. Neither of these patients were excluded from the analyses. Twelve of the 18 patients (67%) previously on IVIg were still on IVIg at the start of the dose-evaluation phase.
Table 1.
Baseline characteristics
| Characteristic | Treatment group (intention-to-treat) | ||
|---|---|---|---|
| 0.5 g/kg (n = 34) | 1.0 g/kg (n = 69) | 2.0 g/kg (n = 36) | |
| Female, n (%) | 13 (38) | 31 (45) | 13 (36) |
| Age, years | 57 (40–64) | 59 (51–67) | 61 (49–66) |
| Body weight, kg | 83 (72–97) | 80 (71–93) | 77 (66–89) |
| Body mass index, kg/m2 | 27 (25–31) | 27 (24–30) | 25 (23–29) |
| EFNS/PNS criteria, n (%) | |||
| Definite CIDP | 34 (100) | 68 (99) | 36 (100) |
| Probable CIDP | 0 | 1 (1) | 0 |
| Type of CIDP, n (%) | |||
| Typical | 33 (97) | 62 (90) | 32 (89) |
| Atypical | 1 (3) | 7 (10) | 4 (11) |
| Prior treatment, n (%) | |||
| Corticosteroids | 29 (85) | 60 (87) | 32 (89) |
| Immunoglobulins | 5 (15) | 9 (13) | 4 (11) |
| Patients still on corticosteroids at start of dose-evaluation phase, n (%) | 11 (38) | 20 (33) | 13 (41) |
| Prednisolone equivalent/day (mg) | |||
| Median (range) | 20 (2.5–25) | 18 (2.5–25) | 20 (2.5–20) |
| Mean ± SD | 14.8 ± 7.7 | 14.9 ± 7.1 | 14.8 ± 7.1 |
| Patients still on IVIg at start of dose-evaluation phase, n (%) | 2 (40) | 6 (67) | 4 (100) |
| IVIg (g/kg) | |||
| Median (range) | 0.9 (0.3–1.4) | 0.4 (0.3–0.8) | 0.4 (0.2–0.7) |
| Mean ± SD | 0.9 ± 0.8 | 0.5 ± 0.2 | 0.4 ± 0.2 |
| Efficacy scores at screening | |||
| Adjusted INCAT score (range 0–10) | 4 (4–5) | 4 (4–5) | 4 (4–5) |
| I-RODS (range 0–48) | 27 (20–32) | 25 (21–31) | 29 (21–32) |
| Maximum grip strength (kPa; range 0–160) | |||
| Dominant hand | 51 (32–68) | 53 (39–78) | 52 (41–64) |
| Non-dominant hand | 52 (28–70) | 54 (38–76) | 54 (38–67) |
| MRC sum score (total range 0–80) | 46 (42–50) | 46 (42–52) | 47 (43–53) |
All values are the median (interquartile range) unless otherwise stated (intention-to-treat).
Efficacy
For the primary end point, the response rate in the 1.0 g/kg group was 80% (55/69 patients; 95% CI: 69–88%) (Table 2). The lower CI limit of 69% exceeded the predefined threshold of 42%, thus the primary end point was met.
Table 2.
Proportion of responders at the end of study
| Parameter | Treatment group (intention-to-treat) | Overall P-value | ||
|---|---|---|---|---|
| 0.5 g/kg (n = 34) | 1.0 g/kg (n = 69) | 2.0 g/kg (n = 36) | ||
| Adjusted INCAT score | 65 (48–79) | 80 (69–88) | 92 (78–97) | 0.040 |
| Grip strength | 56 (40–71) | 65 (53–75) | 83 (68–92) | 0.047 |
| I-RODS | 38 (24–55) | 55 (43–66) | 72 (56–84) | 0.038 |
| MRC sum score | 59 (42–74) | 72 (61–82) | 86 (71–94) | 0.066 |
All values are % of patients (95% Cl). The primary end point (adjusted INCAT score response in patients treated with 1.0 g/kg IVIg) is indicated in italics. The overall P-value is calculated using a type 3 logistic regression analysis of effects modelling response from treatment, randomization stratum, CIDP variant, and baseline score without interactions.
The adjusted INCAT score response rates in the 0.5 and 2.0 g/kg maintenance dose groups were 65% (22/34 patients; 95% CI: 48–79%) and 92% (33/36 patients; 95% CI: 78–97%) at Week 24, respectively. In patients previously on corticosteroids, the response rate across all three dose groups was 82% (99/121 patients; 95% CI: 74–88%) and 66%, 83% and 94% in the 0.5, 1.0 and 2.0 g/kg groups, respectively. In patients previously on immunoglobulin therapy, the response rate across all three dose groups was 61% (11/18 patients; 95% CI: 39–80%) and 60%, 56% and 75% in the 0.5, 1.0 and 2.0 g/kg groups, respectively.
Responder rates in the 1.0 g/kg dose group for grip strength, I-RODS and MRC sum score were 65%, 55% and 72%, respectively (Table 2). Response rates were 56%, 38% and 59% for the 0.5 g/kg cohort and 83%, 72% and 86% for the 2.0 g/kg cohort, respectively (Table 2).
Using logistic regression analysis, the dose group had an effect on the treatment outcomes of adjusted INCAT score (P = 0.040), grip strength (P = 0.047), and I-RODS (P = 0.038), but not MRC sum score (P = 0.066). Odds ratio analyses comparing the 0.5 and 2.0 g/kg groups to the 1.0 g/kg group showed overlapping CIs (Table 3). Further analyses showed that the statistical differences were not driven in a stepwise fashion by dose, but due to the differences between the 0.5 g/kg and 2.0 g/kg dose groups (Table 3), with an odds ratio of 5.8 (95% CI: 1.4–23.6) for adjusted INCAT score.
Table 3.
Odds ratio analysis of the effect of treatment group on response
| Parameter | Odds ratio (95% CI) (intention-to-treat) | ||
|---|---|---|---|
| 0.5 g/kg versus 1.0 g/kg | 2.0 g/kg versus 0.5 g/kg | 2.0 g/kg versus 1.0 g/kg | |
| Adjusted INCAT score | 0.5 (0.2–1.2) | 5.8 (1.4–23.6) | 2.7 (0.7–10.2) |
| Grip strength | 0.6 (0.3–1.4) | 4.2 (1.4–13.3) | 2.5 (0.9–7.0) |
| I-RODS | 0.5 (0.2–1.3) | 3.9 (1.4–10.8) | 2.1 (0.8–5.0) |
| MRC sum score | 0.6 (0.2–1.4) | 4.1 (1.2–13.2) | 2.3 (0.8–6.7) |
An improvement in least square means change from baseline to end of study was achieved in all dose groups for adjusted INCAT score, grip strength, I-RODS, and MRC sum score (data not shown).
Of the 29 non-responders across the dose groups, 22 were on corticosteroids prior to study enrolment and 11 were still on corticosteroids during the study. Of the 29 non-responders, 13 received rescue treatment; six in the 0.5 g/kg group and seven in the 1.0 g/kg group (Supplementary Table 1). The three remaining non-responders were in the 2.0 g/kg group and therefore not eligible for rescue treatment. Three out of six (50%) patients in the 0.5 g/kg group and 4 of 7 (57%) patients in the 1.0 g/kg group had an improved adjusted INCAT score following rescue treatment (Supplementary Table 1). In the 0.5 g/kg group, one of these patients received rescue treatment despite the adjusted INCAT score not returning to baseline. In the 1.0 g/kg group, another patient received rescue therapy in error. The other 16 non-responders dropped out of the study for various reasons and were considered study non-responders, irrespective of whether their adjusted INCAT score had improved at the time they dropped out: six in the 0.5 g/kg group, seven in the 1.0 g/kg group, and three in the 2.0 g/kg group. At the time of early termination, an improvement of ≥1 point in adjusted INCAT score had been achieved by all six patients in the 0.5 g/kg group, five of the seven patients in the 1.0 g/kg group, and none of the three patients in the 2.0 g/kg group.
One responder in each dose group did not receive rescue medication (0.5 g/kg and 1.0 g/kg group) or was not discontinued (2.0 g/kg group) according to protocol despite being stable at Week 6 and subsequently had a response at Week 9 (0.5 and 1.0 g/kg patients) or Week 12 (2.0 g/kg patient) that was maintained at Week 24. In the per protocol set, which excluded 10 intention-to-treat patients including those three responders, the response rate was 72% (21/29 patients; 95% CI: 54–85%) in the 0.5 g/kg group, 83% (54/65 patients; 95% CI: 72–90%) in 1.0 g/kg group, and 91% (32/35 patients; 95% CI: 78–97%) in the 2.0 g/kg group (Supplementary Table 2).
More than half of all patients (n = 78; 56.1%) showed an improvement of ≥1 adjusted INCAT score point after the induction dose of 2.0 g/kg: 23 of 34 (67.6%) in the 0.5 g/kg group, 35 of 69 (50.7%) in the 1.0 g/kg group and 20 of 36 (55.6%) in the 2.0 g/kg group. By Week 6, 121 of 139 (87.0%) patients had an improvement of ≥1 adjusted INCAT score point: 30 of 34 (88.2%) in the 0.5 g/kg group, 59 of 69 (85.5%) in the 1.0 g/kg group and 32 of 36 (88.9%) in the 2.0 g/kg group.
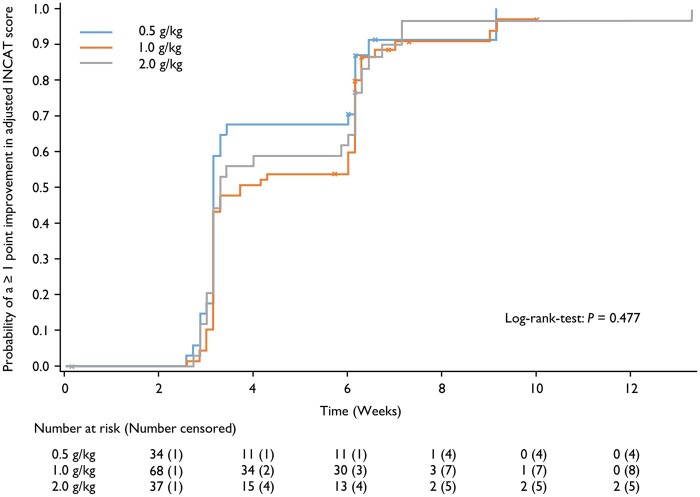
Median time to an improvement of ≥1 adjusted INCAT score point from baseline was 22, 26 and 23 days in the 0.5, 1.0 and 2.0 g/kg groups, respectively (Fig. 3). Overall, 91.2% of patients in the 0.5 g/kg group, 88.4% of patients in the 1.0 g/kg group and 91.7% of patients in the 2.0 g/kg group had an improvement of ≥1 adjusted INCAT score point at some point in the study. These values are higher than in the primary analysis because patients could show an improvement in the score during the study, but subsequently declined or dropped out and were then considered non-responders. Median time to response in I-RODS was 63, 64, and 43.5 days in the 0.5, 1.0, and 2.0 g/kg groups, respectively.
Figure 3.
Time to improvement of ≥1 point in adjusted INCAT score. Data by maintenance dose group in patients who achieved such an improvement at any time during the study (safety analysis set).
The time to first confirmed worsening in adjusted INCAT score by at least 1 point from the value at baseline (Week 0) revealed that only a single patient in the 1.0 g/kg dose group met this criterion, and therefore this analysis was not possible. This was also the case for the time to first confirmed worsening in I-RODS scores as this occurred in only three patients: one in the 0.5 g/kg group and two in the 1.0 g/kg group.
The proportion of patients deteriorating during the dose-evaluation phase of the study was low, one each in the 0.5 g/kg and 1.0 g/kg groups, as was the number of patients who were stable at Week 6: 13 (9.4%) patients in total, with five in the 0.5 g/kg group, six in the 1.0 g/kg group, and two in the 2.0 g/kg group (Supplementary Table 1).
Mean pain intensity according to Pain Intensity Numeric Rating Scale score increased during the wash-out phase, then tended to decrease through Week 24. Mean ± standard deviation (SD) changes from baseline were −2.3 ± 3.0, −2.2 ± 2.9 and −2.2 ± 3.3 in the 0.5, 1.0 and 2.0 g/kg groups, respectively.
There were no clear trends over time in the nerve conduction studies.
Safety
Median doses of IVIg administered per patient per infusion, including the induction dose, were 70 g (0.7 g/kg) in the 0.5 g/kg group, 101 g (1.1 g/kg) in the 1.0 g/kg group, and 155 g (2.0 g/kg) in the 2.0 g/kg group. Dosing was based on actual body weight, and there was no maximum daily dose.
In the 0.5 g/kg group, 16 (45.7%) patients experienced 37 related TEAEs over 230 infusions (Table 4). In the 1.0 g/kg group, 32 (46.4%) patients experienced 80 related TEAEs over 482 infusions, and in the 2.0 g/kg group 20 (52.6%) patients experienced 56 related TEAEs over 270 infusions. Overall, the most commonly reported related TEAEs were headache in 20 patients (14.1%), allergic dermatitis in 13 patients (9.2%), pyrexia in 11 patients (7.7%), increased blood pressure in seven patients (4.9%), and increased body temperature in seven patients (4.9%). Headache was the most prevalent related TEAE in the 1.0 and 2.0 g/kg groups (14.5% and 23.7%, respectively), while the most common related TEAE in the 0.5 g/kg group was allergic dermatitis (11.4% of patients). Generally, the incidence of related TEAEs was similar across the treatment groups. The only TEAE where a dose effect was apparent was headache, with an incidence of 2.9% in the 0.5 g/kg group, 14.5% in the 1.0 g/kg group, and 23.7% in the 2.0 g/kg group.
Table 4.
Summary of related TEAEs observed in ≥5% of patients
| TEAE | Treatment group (safety analysis set) | |||||
|---|---|---|---|---|---|---|
| 0.5 g/kg (n = 35) | 1.0 g/kg (n = 69) | 2.0 g/kg (n = 38) | ||||
| Patients, (% of patients) | Events, (% of infusions) | Patients, (% of patients) | Events, (% of infusions) | Patients, (% of patients) | Events, (% of infusions) | |
| Any related TEAE | 16 (45.7) | 37 (16.1) | 32 (46.4) | 80 (16.6) | 20 (52.6) | 56 (20.8) |
| Headache | 1 (2.9) | 1 (0.4) | 10 (14.5) | 12 (2.5) | 9 (23.7) | 14 (5.2) |
| Somnolence | 2 (5.7) | 3 (1.3) | 0 | 0 | 1 (2.6) | 1 (0.4) |
| Allergic dermatitis | 4 (11.4) | 4 (1.7) | 5 (7.2) | 10 (2.1) | 4 (10.5) | 5 (1.9) |
| Urticaria | 3 (8.6) | 4 (1.7) | 0 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 0 | 0 | 2 (5.3) | 3 (1.1) |
| Pyrexia | 3 (8.6) | 4 (1.7) | 6 (8.7) | 6 (1.2) | 2 (5.3) | 3 (1.1) |
| Chills | 3 (8.6) | 4 (1.7) | 2 (2.9) | 2 (0.4) | 1 (2.6) | 1 (0.4) |
| Blood pressure increased | 2 (5.7) | 3 (1.3) | 5 (7.2) | 6 (1.2) | 0 | 0 |
| Body temperature increased | 2 (5.7) | 2 (0.9) | 2 (2.9) | 5 (1.0) | 3 (7.9) | 3 (1.1) |
| Blood lactate dehydrogenase increased | 0 | 0 | 5 (7.2) | 5 (1.0) | 0 | 0 |
| Nausea | 1 (2.9) | 1 (0.4) | 1 (1.4) | 2 (0.4) | 3 (7.9) | 5 (1.9) |
Five patients (3.5%) experienced TEAEs that led to discontinuation of the study drug, one of which, allergic dermatitis in a patient receiving 1.0 g/kg IVIg, was considered related.
Eleven serious TEAEs were reported in six patients (4.2%). Two of these (headache and vomiting) were reported in one patient in the 1.0 g/kg group and considered related to IVIg treatment, but did not lead to study discontinuation.
Two patients (1.4%) had TEAEs leading to death, but neither event was considered related to the study drug. One patient aspirated leading to respiratory arrest, and one developed meningoencephalitis.
During the study, no haemolysis or thromboembolic events were reported. Laboratory analyses of haematology, clinical chemistry, urinalysis and viral markers did not indicate any safety concerns. There were no findings of note on physical examination or in the vital signs data.
Discussion
The ProCID study is the largest randomized study to show that IVIg is effective as maintenance therapy in patients with CIDP and the first to examine systematically a lower and higher IVIg maintenance dose. The primary objective was to assess the efficacy of IVIg administered as a 2.0 g/kg induction dose followed by seven maintenance doses of 1.0 g/kg every 3 weeks. The response rate on this regimen was 80%, and the lower 95% CI limit of 69% exceeded the predefined threshold of 42%, indicating that the primary end point had been met. This threshold was also exceeded in the 0.5 and 2.0 g/kg maintenance groups. Similar responses were seen in other outcome measures.
Published guidelines recommend that the maintenance dose of IVIg may need to be adjusted individually.2 The ProCID study was the first to examine systematically a lower (0.5 g/kg) and a higher (2.0 g/kg) maintenance dose of IVIg in CIDP and compare efficacy with standard dosing of 1.0 g/kg. The adjusted INCAT score response rates were 65, 80 and 92% in the 0.5, 1.0 and 2.0 g/kg maintenance dose groups, respectively. Similar incremental improvements in response rates were observed for secondary efficacy variables. Despite the study not being powered to show a statistically significant dose response, a statistically significant effect of dose on response was observed across all dose groups using logistic regression analysis. However, statistically significant differences between any two of the individual dose groups were only seen between the 0.5 and 2.0 g/kg maintenance groups. Our data therefore suggest that a lower maintenance dose of 0.5 g/kg is sufficient to achieve and maintain a response in 65% of patients but that a higher dose may be beneficial to improve those not responding to low or standard dosing.
The PATH study examined two different doses of SCIg maintenance therapy, 0.2 and 0.4 g/kg every week, the equivalent of 0.6 and 1.2 g/kg IVIg every 3 weeks, in patients with CIDP.14 The study evaluated a 2-fold dose difference and found no differences in either safety or efficacy between these two doses with a study period of 24 weeks.14 In the PATH extension study, the overall relapse rates were 10% in the 0.4 g/kg/week and 48% in the 0.2 g/kg/week group.24 After dose reduction from 0.4 to 0.2 g/kg/week, 51% (27/53) of patients relapsed, of whom 92% (24 of 26) improved after re-initiation of the 0.4 g/kg/week dose.24 In our study, a 2-fold dose difference, low versus standard, or standard versus high dosing, also did not result in a significant difference in response rate. Only the 4-fold dose difference between low (0.5 g/kg) and high (2.0 g/kg) maintenance dosing did, with an odds ratio of 5.8 (95% CI: 1.4–23.6) for adjusted INCAT score. Thus, we postulate that a 2-fold dose difference might be too small to detect significant differences in response rate. Therefore, a larger study, statistically powered to compare different dose ratios, e.g. 2-fold versus 3-fold versus 4-fold, would be needed to confirm a clear dose relationship and to identify potential patient characteristics that might help to predict the required maintenance dose for an individual patient.
The adjusted INCAT score overall response rate of 80% with a 1.0 g/kg maintenance dose in our study was higher than reported in other IVIg studies: ICE 54%, PRIMA 61% and PRISM 76%.6–8 It is of interest to compare groups of patients in the different studies that have been treated previously with corticosteroids or IVIg. Selection of patients who had previously responded to IVIg may impact the IVIg response rate in a trial.14 Our study is the first IVIg study to include a high percentage of patients who were previously treated with corticosteroids and not with IVIg. The higher proportion of patients having been treated with corticosteroids might be due to lower availability of IVIg in some of the countries. The PATH study preselected patients based on IVIg response.14 Corticosteroids are a first-line treatment option for CIDP.2 Corticosteroids are widely available and inexpensive, but long-term use has potentially serious side effects.5,25,26 Unlike the ProCID study, patients previously on corticosteroids (>10 mg/day prednisolone or equivalent) were excluded from the ICE study,6 patients previously on ‘high-dose’ corticosteroids were excluded from the PRISM study8 and, although corticosteroid use was not specifically excluded, no information on prior corticosteroid use was provided in the PRIMA study.7 Furthermore, the percentage of patients classified as IVIg-naïve, but potentially on prior corticosteroids, was 23%,6 54%7 and 55%8 in the ICE, PRIMA and PRISM studies, respectively, compared with 87% who were IVIg-naïve and on prior corticosteroids in our study. These differences in prior treatment, along with other differences in patient and study characteristics, might have influenced response rates across studies and make comparisons between the studies difficult. Nevertheless, since the ProCID study clearly differentiated between patients previously successfully treated with IVIg and those previously treated with corticosteroids, with most subjects being in the latter stratum, the study provided a unique opportunity to assess the effects of switching from corticosteroids to IVIg. The high response rates in the corticosteroids stratum, 66%, 83% and 94% in the 0.5 g/kg, 1.0 g/kg and 2.0 g/kg arms, respectively, clearly show that subjects previously on corticosteroids can successfully and safely be transitioned to IVIg.
The incidence of treatment-related TEAEs, except for headache, was similar across the dose groups. Treatment was well tolerated even in IgG-naïve patients and in those regularly treated with high dose IVIg. These data show that patients can switch from corticosteroids to IVIg with confidence to potentially avoid the side effects associated with corticosteroid use.
In this study, 56% of all patients and 62% of responders showed an improvement of ≥1 adjusted INCAT score point after the induction dose alone, i.e. by Week 3. In comparison, the percentage of responders who had an improved adjusted INCAT score after the induction dose was 44% in the ICE study,27 50% in the PRIMA study7 and 22% in the PRISM study.8 This has important implications for clinical practice. Our study and the ICE trial27 show that nearly all patients who will respond to IVIg do so within 6–8 weeks, that is, a single induction dose and two maintenance doses. Given the issues above with actual patient inclusion, the translation of this to the treatment-naïve patient is less clear. When to decide that a patient is not responding to IVIg remains a clinical decision, but these data suggest that treating longer than 3 months while waiting for a significant response is not useful.2
A limitation of the study as detailed above, in common with other studies evaluating IVIg in patients with CIDP, is that it evaluates rescue treatment mainly of previously successfully treated CIDP patients whose condition has been allowed to deteriorate prior to treatment, and not newly diagnosed and untreated CIDP patients. Given that the study period was 24 weeks, the longer-term efficacy, safety and relapse rate with the different maintenance doses were not assessed in this study. However, the study demonstrated a low rate of deterioration (1.4%) after Week 6 across all dose groups. Other limitations of the study were that it was not placebo controlled and was not statistically powered to detect a dose response between the three dosing regimens.
In summary, the ProCID study demonstrated that 1.0 g/kg IVIg is efficacious and well tolerated as maintenance treatment following a 2.0 g/kg induction dose in patients with active CIDP, even in those patients having been successfully treated with corticosteroids previously. A lower or higher maintenance dose may be beneficial in some patients, although further studies are needed to confirm this finding.
Supplementary Material
Acknowledgements
This study was sponsored by Octapharma Pharmazeutika Produktionsges.m.b.H. (Vienna, Austria) who thank investigators, trial personnel and patients for their participation. Medical writing assistance was provided by nspm ltd, Meggen, Switzerland, and funded by Octapharma.
Funding
This study was funded by Octapharma Pharmazeutika Produktionsges.m.b.H. (Vienna, Austria).
Competing interests
D.R.C. reports consulting for Amgen, Annexon Biosciences, argenx SE, Biotest Pharmaceuticals, Cigna Health Management, CSL Behring, Grifols, Johnson & Johnson, Momenta Pharma, New Enterprise Associates, Octapharma, Pfizer, Pharnext, Polyneuron Pharmaceuticals, Seattle Genetics, and UCB. D.R.C. is on the Data Safety Monitoring Board for the following: Alnylam Pharmaceuticals, Anavex, PledPharma, Hansa Medical, and Mitsubishi Tanabe Pharma Corporation. D.R.C. was involved with technology licensing for AstraZeneca Pharmaceuticals, LP Pharmaceuticals (Xiamen), Genentech, Levicept, Seattle Genetics, Merrimack Pharmaceuticals, and Disarm Therapeutics, outside the submitted work. P.A.v.D. reports grants from Sanquin Blood Supply and Prinses Beatrix Spierfonds, during the conduct of the study; and grants from Grifols, Takeda, Annexion, Argenx, Commonwealth Serum Laboratories, Octapharma, and Hansa, outside the submitted work. H.P.H. reports consulting CSL Behring, Sanofi Genzyme, and UCB. H.P.H. received payments or honoraria from CSL Behring and Octapharma. H.D.K. is on Steering Committees for Octapharma and Sanofi Genzyme. I.S.J.M. reports grants from Talecris Talents program, GBS/CIDP Foundation International and FP7 EU program, outside the submitted work; Furthermore, a research foundation at the University of Maastricht received honoraria on behalf of him for participation in steering committees of the Talecris Immune Globulin Intravenous For Chronic Inflammatory Demyelinating Polyneuropathy Study, Commonwealth Serum Laboratories, Behring, Octapharma, LFB, Novartis, Union Chimique Belge, outside the submitted work. H.D.K. reports travel support and consulting fees from Octapharma in relation to a study design advisory board. H.D.K. reports consulting for UCB, Terumo, Akcea, Alnylam, and CSL Behring. H.D.K. is on Data Safety Monitoring Boards or Advisory Boards for UCB and Octapharma. H.D.K. received a grant from Takeda for investigator-initiated research. D.H. and E.C. are employees of Octapharma PPG, Vienna, Austria.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- CIDP
chronic inflammatory demyelinating polyneuropathy
- I-RODS
Inflammatory Rasch-built Overall Disability Scale
- INCAT
Inflammatory Neuropathy Cause and Treatment
- IVIg
intravenous immunoglobulin
- MRC
Medical Research Council
- ProCID
progress in chronic inflammatory demyelinating polyneuropathy; TEAE = treatment-emergent adverse events
Appendix I
ProCID study investigators
Full details are provided in the Supplementary material.
S. Kastrev, V. Rizova, R. Massie, R. Talab, M. Bednar, P. Ridzon, J. Schmidt, J. Zschüntzsch, C. Rózsa, L. Vécsei, K. Rejdak, M. Koszewicz, S. Budrewicz, A. Dulamea, M. Marian, A. Kadar, L. Zecheru-Lapusneanu, V. Mikhailov, D. Zakharov, N. Suponeva, M. Piradov, N. Smolko, D Smolko.
Contributor Information
the ProCID Investigators:
S Kastrev, V Rizova, R Massie, R Talab, M Bednar, P Ridzon, J Schmidt, J Zschüntzsch, C Rózsa, L Vécsei, K Rejdak, M Koszewicz, S Budrewicz, A Dulamea, M Marian, A Kadar, L Zecheru-Lapusneanu, V Mikhailov, D Zakharov, N Suponeva, M Piradov, N Smolko, and D Smolko
References
- 1. van den Bergh PYK, Hadden RDM, Bouche P, et al. ; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010;17(3):356–363. [DOI] [PubMed] [Google Scholar]
- 2. van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force - second revision. J Peripher Nerv Syst. 2021;26(3):242–268. [DOI] [PubMed] [Google Scholar]
- 3. Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498–504. [DOI] [PubMed] [Google Scholar]
- 4. Neligan A, Reilly MM, Lunn MP. CIDP: Mimics and chameleons. Pract Neurol. 2014;14(6):399–408. [DOI] [PubMed] [Google Scholar]
- 5. Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): An overview of systematic reviews. Cochrane Database Syst Rev. 2017;1:CD010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes RAC, Donofrio P, Bril V, et al. ; ICE Study Group. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144. [DOI] [PubMed] [Google Scholar]
- 7. Léger J-M, Bleecker JL, de Sommer C, et al. ; PRIMA study investigators. Efficacy and safety of Privigen® in patients with chronic inflammatory demyelinating polyneuropathy: Results of a prospective, single-arm, open-label Phase III study (the PRIMA study). J Peripher Nerv Syst. 2013;18(2):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nobile‐Orazio E, Pujol S, Kasiborski F, et al. An international multicenter efficacy and safety study of IqYmune in initial and maintenance treatment of patients with chronic inflammatory demyelinating polyradiculoneuropathy: PRISM study. J Peripher Nerv Syst. 2020;25(4):356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajabally YA, Seow H, Wilson P. Dose of intravenous immunoglobulins in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst. 2006;11(4):325–329. [DOI] [PubMed] [Google Scholar]
- 10. Rajabally YA, Afzal S. Clinical and economic comparison of an individualised immunoglobulin protocol vs. standard dosing for chronic inflammatory demyelinating polyneuropathy. J Neurol. 2019;266(2):461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuitwaard K, Fokkink W-JR, Brusse E, et al. Maintenance IV immunoglobulin treatment in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2017;22(4):425–432. [DOI] [PubMed] [Google Scholar]
- 12. Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM. A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripher Nerv Syst. 2016;21(1):33–37. [DOI] [PubMed] [Google Scholar]
- 13. Kubori T, Mezaki T, Kaji R, et al. The clinical usefulness of high-dose intravenous immunoglobulin therapy for chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy. No to Shinkei. 1999;51(2):127–135. [PubMed] [Google Scholar]
- 14. van Schaik IN, Bril V, van Geloven N, et al. ; PATH study group. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35–46. [DOI] [PubMed] [Google Scholar]
- 15. Mersich C, Ahrer K, Buchacher A, et al. Biochemical characterization and stability of immune globulin intravenous 10% liquid (Panzyga®). Biologicals. 2017;45:33–38. [DOI] [PubMed] [Google Scholar]
- 16. Radomski KU, Lattner G, Schmidt T, Römisch J. Pathogen safety of a new intravenous immune globulin 10% liquid. BioDrugs. 2017;31(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borte M, Melamed IR, Pulka G, et al. Efficacy and safety of human intravenous immunoglobulin 10% (Panzyga®) in patients with primary immunodeficiency diseases: A two-stage, multicenter, prospective, open-label study. J Clin Immunol. 2017;37(6):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arbach O, Taumberger AB, Wietek S, Cervinek L, Salama A. Efficacy and safety of a new intravenous immunoglobulin (Panzyga®) in chronic immune thrombocytopenia. Transfus Med. 2019;29(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.panzyga® USPI. 2021. https://www.fda.gov/media/115397/download. Accessed 16 July 2021.
- 20.panzyga® Canadian Product Monograph. 2017. https://pdf.hres.ca/dpd_pm/00041520.PDF. Accessed 16 July 2021.
- 21.panzyga® SmPC. 2019. https://www.medicines.org.uk/emc/product/7317/smpc#gref. Accessed 16 July 2021.
- 22. Cornblath DR, Hartung H-P, Katzberg HD, Merkies ISJ, van Doorn PA. A randomised, multi-centre phase III study of 3 different doses of intravenous immunoglobulin 10% in patients with chronic inflammatory demyelinating polyradiculoneuropathy (ProCID trial): Study design and protocol. J Peripher Nerv Syst. 2018;23(2):108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Draak THP, Vanhoutte EK, van Nes SI, et al. ; PeriNomS Study Group. Changing outcome in inflammatory neuropathies: Rasch-comparative responsiveness. Neurology. 2014;83(23):2124–2132. [DOI] [PubMed] [Google Scholar]
- 24. van Schaik IN, Mielke O, Bril V, et al. ; PATH study group. Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bunschoten C, Jacobs BC, van den Bergh PYK, Cornblath DR, van Doorn PA. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019;18(8):784–794. [DOI] [PubMed] [Google Scholar]
- 26. Hughes RA, Mehndiratta MM, Rajabally YA. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2017;11:CD002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latov N, Deng C, Dalakas MC, et al. ; IGIV-C CIDP Efficacy (ICE) Study Group. Timing and course of clinical response to intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol. 2010;67(7):802–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data collected during the trial will not be shared. The study protocol and main results are available at https://clinicaltrials.gov/ct2/show/study/NCT02638207 (Accessed 24 February 2022).