ABSTRACT
Background
Improvement of proteinuria as a marker for disease activity is associated with a better renal outcome in immunoglobulin A nephropathy (IgAN). Complement is an effector pathway in IgA-mediated kidney injury. Avacopan, a selective C5a receptor inhibitor, has previously shown efficacy in anti-neutrophil cytoplasmic antibody–associated vasculitis. The aim of this study was to evaluate the safety and efficacy of avacopan in patients with IgAN with persistent proteinuria despite a maximally tolerated dose of renin–angiotensin–aldosterone system blockade. The efficacy evaluation was based on the change in proteinuria.
Methods
This open-label pilot trial enrolled adult patients with biopsy-proven IgAN, urinary protein:creatinine ratio (UPCR) >1 g/g creatinine and an estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 or >45 mL/min/1.73 m2 if eGFR has not declined >10 mL/min/1.73 m2 over the previous 24 weeks. If the UPCR remained at >1 g/g creatinine after an 8-week run-in period, patients started avacopan 30 mg twice daily. The primary efficacy endpoint was the change in the slope of the UPCR from the 8-week run-in period to the slope in the 12-week avacopan dosing period.
Results
A total of 10 of 15 screened patients entered the run-in period. Seven patients with a UPCR >1 g/g creatinine received avacopan. Six of seven patients had numerical improvement in the UPCR during the avacopan treatment period, three of whom had a numerical improvement of ∼50% at week 12. At week 24, five of seven patients still showed numerical improvement in the UPCR compared with baseline. The urinary monocyte chemoattractant protein-1:creatinine ratio decreased numerically 30% by week 8, possibly reflecting the anti-inflammatory activity of avacopan. Avacopan was well tolerated. There was one serious adverse event of unstable angina, which was deemed to be unrelated to avacopan.
Conclusions
This short-term pilot study showed an improvement in the slope of the UPCR, with ∼50% improvement in three of seven patients with IgAN. Longer avacopan treatment duration may be indicated for maximal benefit.
Keywords: avacopan, C5a receptor, C5a receptor inhibitor, complement, IgA nephropathy, proteinuria
Graphical Abstract
Graphical Abstract.
BACKGROUND
Immunoglobulin A nephropathy (IgAN) was first described in 1968 by Berger. It is the most common glomerulonephritis worldwide and is associated with a wide spectrum of disease severity and rates of progression of renal failure [1–3].
The current understanding of the pathogenesis includes increased levels of circulating IgA1 with galactose deficiency and the synthesis of antibodies directed against galactose-deficient IgA. Immune complexes containing antibodies and IgA1 are formed, and these are subsequently accumulated in the mesangium of the glomeruli. This causes proliferation of mesangial cells and complement cascade activation, manifested by inflammation with urinary abnormalities such as haematuria and proteinuria leading to kidney injury [4].
In addition to kidney biopsy findings [mesangial (M) and endocapillary (E) hypercellularity, segmental sclerosis (S) and interstitial fibrosis/tubular atrophy (T); MEST score] and the risk of progression towards kidney failure in IgAN, the best-established risk factor for progressive IgAN with loss of renal function is the persistent presence of proteinuria >1 g/day, with some studies suggesting that the persistence of even lower levels of proteinuria (>500 mg/day) may be associated with worse prognosis [5–8].
Role of complement
Complement is an effector pathway in IgA-mediated kidney injury. The alternative and lectin pathways have been hypothesized to be of significance. Mesangial deposits of IgA often co-localize with C3 and other complement components. Increased renal expression of C3a, C5a and their receptors, implicating the alternative pathway, is correlated to the severity of IgAN [9]. Furthermore, lower expression of messenger RNA encoding for the complement inhibitory protein CD46 has been shown in patients with progressive IgAN, which may implicate a defective regulation of C3 convertase with uncontrolled complement activation [10]. Also, activation of the mannan-binding lectin complement pathway has been associated with increased proteinuria and worse renal outcome in IgAN [11, 12].
Despite the relative high prevalence of IgAN worldwide, the current evidence-based therapy available for the treatment of IgAN has been mainly empirical and thus insufficient. Current guidelines include initiating supportive care therapy in patients with hypertension and persistent proteinuria of at least 0.5 g/day with renin–angiotensin–aldosterone system (RAAS) blockade with either an angiotensin-conveerting enzyme inhibitor or an angiotensin receptor blocker to reach a level of proteinuria below that threshold. This recommendation is compatible with the kidney-protective effects of controlling blood pressure and reducing proteinuria for chronic kidney disease in general [13].
For patients who fail to attain a reduction of proteinuria of <1 g/day, it has been suggested that patients should be treated with a 3- to 6-month course of glucocorticoids (GCs). Observational data from the European Validation Study of the Oxford Classofocation of IgA Nephropathy cohort were evaluated retrospectively to assess the effect of adding GCs to supportive care [14]. GCs reduced the risk of progression regardless of the initial estimated glomerular filtration rate (eGFR) when comparing 184 patients and matched controls, and the smaller randomized Pozzi trial showed protection against deterioration in renal function [15]. However, the Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN) trial, which randomized patients to either GC if the GFR was ≥60 mL/min/1.73 m2 or cyclophosphamide (CYC)/azathioprine/GC therapy if the GFR was <60 mL/min/1.73 m2, could not demonstrate a significant benefit on renal function, and adverse events in the intervention arm were more prevalent [16]. Recently, in a 10-year follow-up of the STOP-IgAN trial, GCs did not demonstrate differences in key clinical outcomes in IgAN patients between supportive care plus immunosuppression as compared with supportive care alone [17]. Although GCs were associated with a significant reduction in the rate of loss of eGFR, dialysis or death due to kidney failure in the randomized Therapeutic Evaluation of Steroids in IgA Nephropathy Global trial, adverse events led to early termination [18].
Thus there is an unmet need for therapies aimed at further reducing proteinuria and preserving renal function while limiting side effects in IgAN.
The interest in complement inhibition therapy has increased considerably, particularly in the field of anti-neutrophil cytoplasmic antibody–associated vasculitis since Xiao et al. [19] first demonstrated protection against myeloperoxidase-ANCA glomerulonephritis using C5a receptor (C5aR) knockout or inhibition with avacopan in an animal model.
The orally administered selective C5aR inhibitor avacopan, previously called CCX168, which selectively blocks the alternative pathway, given with low-dose or no prednisone, was compared with a standard-dose prednisone treatment in a Phase 2 study of ANCA-associated vasculitis. Patients also received either CYC or rituximab (RTX) for induction treatment. The results showed that avacopan significantly reduced disease activity and albuminuria as compared with standard prednisone [20]. A Phase 3 study confirmed the efficacy of avacopan in patients with ANCA-associated vasculitis [21].
Complement activation as a marker of disease progression has further augmented interest in prospective treatment studies in IgAN, and there are several trials ongoing or planned, including trials for narsoplimab (ClinicalTrials.gov NCT03608033) and iptacopan (ClinicalTrials.gov NCT04578834).
The aim of the current pilot study (ClinicalTrials.gov NCT02384317) was to evaluate the efficacy (based on proteinuria change) and safety of 30 mg avacopan twice daily over a 12-week treatment period in patients with IgAN and persistent proteinuria despite a maximally tolerated dose (MTD) of RAAS blockade.
MATERIALS AND METHODS
This open-label pilot Phase 2 trial enrolled adult patients in Sweden and the USA. Inclusion criteria were biopsy-proven IgAN performed for clinical purposes within 3 years prior to screening, urinary protein:creatinine ratio (UPCR) >1 g/g creatinine based on a first morning spot urine sample, eGFR >60 mL/min/1.73 m2 or >45 mL/min/1.73 m2 if eGFR has not declined >10 mL/min/1.73 m2 over the previous 24 weeks. Exclusion criteria were severe renal disease; pregnant or nursing; proteinuria >8 g/g creatinine or >8 g/day; systemic manifestations of Henoch–Schönlein purpura within 2 years prior to enrollment; patients with IgAN deemed secondary to another underlying disease; biopsy reported severe crescentic IgAN; history of treatment with GCs, CYC, azathioprine, mycophenolate mofetil or any biologic immunomodulatory agent with 24 weeks prior to enrollment; history of clinically significant cardiac conditions; history of cancer within 5 years prior to enrollment and any infection requiring antibiotic treatment that had not cleared prior to the study start.
All eligible patients participated in an 8-week run-in period during which a stable MTD of an RAAS blocker was either maintained or given to a target blood pressure <125/75 mmHg. If the UPCR was still >1 g/g creatinine at the end of the run-in period, patients started avacopan at 30 mg twice daily for 12 weeks, followed by a 12-week follow-up period without avacopan treatment (Figure 1).
FIGURE 1:
Study design.
Patients visited the study centres for one or more screening visits, then for the start of the titration period (4 weeks prior to the run-in period) and on days –63 (start of the run-in period), –35, –7 to –2 (two visits within this 5-day window), 1 (start of avacopan dosing), 8, 15, 29, 43, 57, 85 (end of the 12-week dosing period), 113, 141 and 169 (end of the follow-up period).
The primary efficacy endpoint was the change in slope of the UPCR from the 8-week run-in period through the 12-week avacopan dosing period. Secondary endpoints included the change from baseline in the urinary albumin:creatinine ratio (UACR), eGFR, urinary red blood cell count (URBC), urinary monocyte chemoattractant protein-1 (MCP-1):creatinine ratio (MCP-1 measured by enzyme immunoassay at Medpace Reference Laboratories), urinary epidermal growth factor (EGF):MCP-1 ratio (EGF measured by enzyme immunoassay at Medpace Reference Laboratories), and serum IgA:plasma C3 ratio (IgA and C3 measured by nephelometry at Medpace Reference Laboratories) with avacopan treatment.
The urinary MCP-1:creatinine ratio has been studied as an inflammatory marker in ANCA-associated vasculitis, where levels were higher in patients with active renal vasculitis compared with patients in remission and those with active extrarenal vasculitis and in primary glomerular diseases, including IgAN [22, 23].
The EGF:MCP-1 ratio has been shown to be independently associated with the severity of tubular atrophy and interstitial fibrosis, which is an important prognostic factor in primary glomerulonephritis [24].
Finally, the serum IgA:plasma C3 ratio has been associated with IgAN diagnosis, also in patients with proteinuria of ≤1 g/day, although neither severity nor outcome has been strongly linked to this relation yet [25].
The trial was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Ethics committees and institutional review boards approved the research protocol. All patients gave written informed consent before entry.
Statistical analysis
For the primary endpoint of change in the slope of UPCR, the slope of the linear regression line of the day –63, –35, –7 to –2 (two visits) and 1 (before the first dose of avacopan) time points were calculated for each patient for the period prior to avacopan dosing. The slope of the linear regression line of the day 1, 8, 15, 29, 57 and 85 time points was also calculated for each patient for the 12-week period of avacopan dosing. The change in the mean slope across all seven patients from the 8-week run-in period to the 12-week treatment period was calculated to evaluate the treatment effect of avacopan.
Summary statistics were calculated for the change from baseline in UPCR, UACR, eGFR, URBC count, urinary MCP-1:creatinine ratio, urinary EGF:MCP-1 ratio and serum IgA:plasma C3 ratio.
Baseline was defined generally as the last value prior to the start of dosing with avacopan (day 1 pre-dose value). However, for the UPCR change from baseline calculations, the baseline is the geometric mean of two UPCR measurements made in the day –7 to –2 window plus the day 1 (pre-dose) value. For the eGFR, UACR, MCP-1:creatinine ratio, EGF:MCP-1 ratio and URBC count, baseline will be the geometric mean of one measurement made in the day –7 to –2 window plus the day 1 (pre-dose) value.
RESULTS
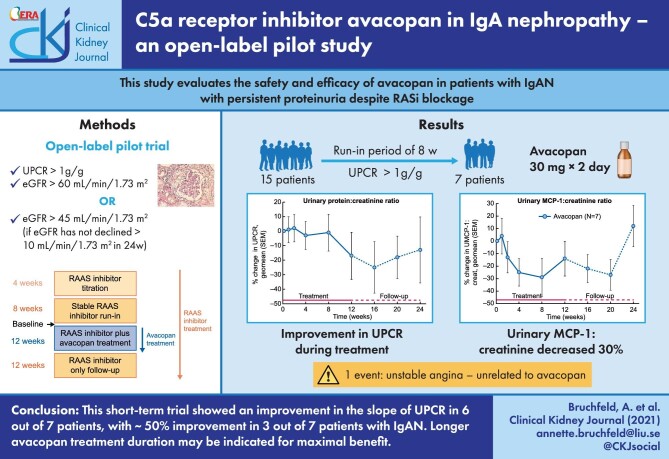
The patient’s disposition is shown in Figure 2. A total of 15 subjects underwent screening for the study. A total of 10 patients entered the run-in period. Seven patients were still eligible, with UPCR >1 g/g creatinine, at the end of the run-in period and were treated with open-label avacopan. All seven patients subsequently completed the study. The baseline characteristics are summarized in Table 1. The mean age was 44.1 years (range 25–59) with four females and three males.
FIGURE 2:
Patient disposition.
Table 1.
Demographics and baseline characteristics (avacopan, N = 7)
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD [range] | 44.1 ± 13.2 [25–59] |
| Sex (female/male), n/n | 4 / 3 |
| Race (White/Asian), n/n | 6 / 1 |
| Body mass index (kg/m2), mean ± SD | 30.1 ± 8.3 |
| Time since biopsy diagnosis (months), median (range) | 15.4 (1–42) |
| UPCR (mg/g), geometric mean (range) | 1801 (1181–3392) |
| UACR (mg/g), geometric mean (range) | 1425 (922–2898) |
| eGFR (mL/min/1.73 m2), mean ± SD | 65.9 ± 18.1 |
| Urinary MCP-1:creatinine ratio (pg/mg), geometric mean (range) | 514 (225–975) |
| Urinary RBC count (cells/high power field), geometric mean (range) | 6.21 (1.22–61.24) |
| Urinary EGF:MCP-1 ratio (pg/pg), geometric mean (range) | 15.14 (6.95–49.09) |
| Serum IgA:plasma C3 ratio (mg/mg), geometric mean (range) | 2.96 (2.21–4.81) |
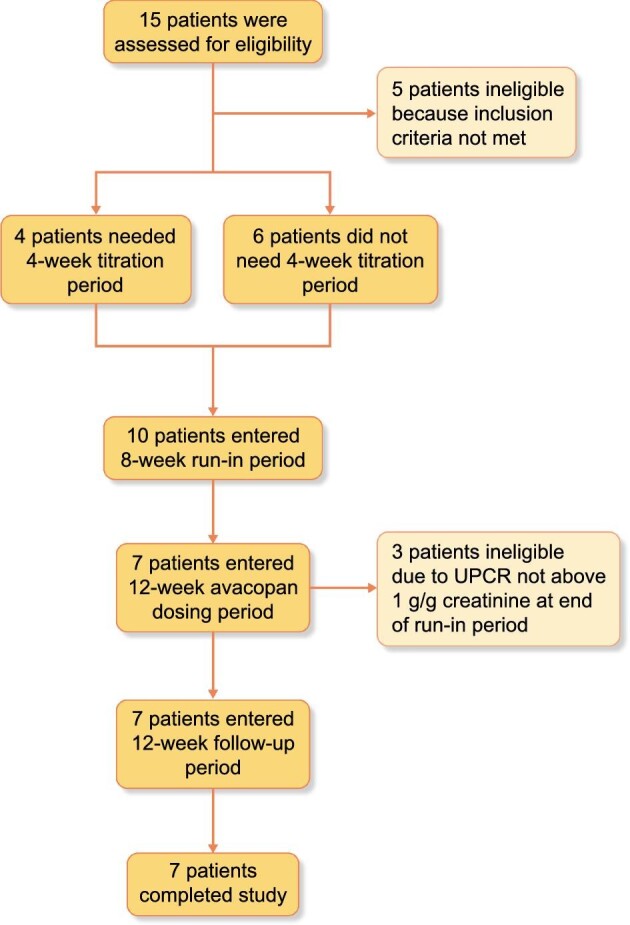
Regarding the primary endpoint, the median change in the UPCR slope from the 8-week run-in period to the 12-week treatment period was –79 g/g creatinine/week (range –105.4–280.5). Five of the seven subjects had a more negative slope during the 12-week avacopan treatment period compared with the 8-week run-in period (Table 2), indicating an improvement in proteinuria with avacopan treatment. Six of the seven subjects had a negative slope in the UPCR during the 12-week avacopan treatment period (Table 2). Three of the seven subjects had an improvement in the UPCR or UACR of ∼50% (Table 3) and three of the seven subjects had a UPCR ≤1 g/g creatinine at the end of the 12-week avacopan treatment period.
Table 2.
Individual subject UPCR slopes and change in slope (mg/g/week)
| Patient | Slope, RAAS inhibitor 8-week run-in perioda | Slope, avacopan + RAAS inhibitor 12-week treatment periodb | Change in slope from run-in period to avacopan treatment period |
|---|---|---|---|
| 1 | 73 | –6 | –79 |
| 2 | –91 | 189 | 280 |
| 3 | 66 | –23 | –89 |
| 4 | 66 | –39 | –105 |
| 5 | –3 | –4 | –1 |
| 6 | 53 | –50 | –103 |
| 7 | –104 | –25 | 79 |
The slope of the run-in period was based on the linear regression of the data from the day –63, –35, –7 to –2 (two visits) and day 1 (before the first dose of avacopan) time points.
The slope of the treatment period was based on the linear regression of the data from the day 1, 8, 15, 29, 57 and 85 time points.
Table 3.
Percent change from baselinea to week 12 in UPCR and UACR
| Patient | UPCR change at week 12 (%) | UACR change at week 12 (%) |
|---|---|---|
| 1 | 17 | 51 |
| 2 | 59 | 38 |
| 3 | –15 | –3 |
| 4 | –47 | –52 |
| 5 | 26 | 32 |
| 6 | –49 | –51 |
| 7 | –48 | –49 |
Baseline was calculated as the geometric mean of three UPCR and UACR measurements, the two measurements made within the day –7 to –2 time frame and the day 1 (pre-dosing) measurement.
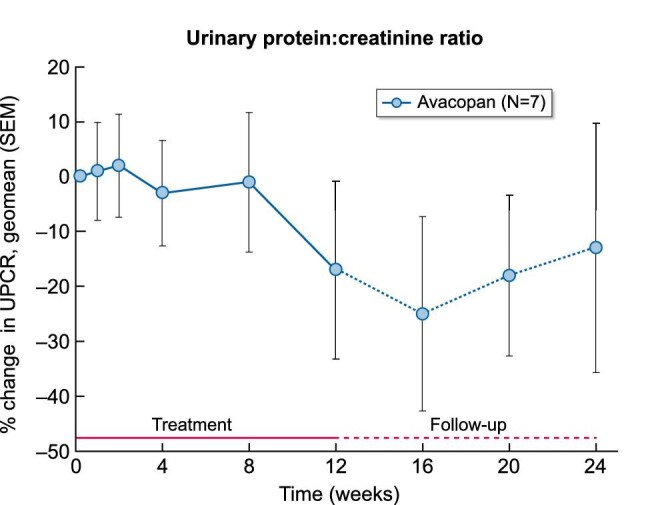
The percent change from baseline in the UPCR over the course of the 12-week treatment period and the 12-week follow-up period is shown in Figure 3. The mean eGFR did not change meaningfully from baseline {mean 65.9 [standard deviation (SD) 18.1]} to week 12 [mean 62.1 (SD 12.8)]. The URBC count decreased from a geometric mean of 6.2 cells per high power field (range 1–61) at baseline to 2.6 (range 0–16) at week 12. The change from baseline in the urinary MCP-1:creatinine ratio is shown in Figure 4. The urinary MCP-1:creatinine ratio decreased 24% numerically by week 4, 29% by week 8 and 14% by week 12, possibly reflecting the anti-inflammatory activity of avacopan. The IgA:C3 ratio remained unchanged [geometric mean 2.96 (range 2.21–4.81) at baseline and 3.06 (range 2.56–5.10) at week 12]. The eGFR:MCP-1 ratio did not change meaningfully [geometric mean 15.14 (range 6.95–49.09) at baseline and 16.90 (range 4.10–43.40) at week 12].
FIGURE 3:
The mean percent change from baseline in UPCR during the 12-week avacopan treatment period and 12-week follow-up period.
FIGURE 4:
The mean percent change from baseline in the urinary MCP-1:creatinine ratio during the 12-week avacopan treatment period and 12-week follow-up period.
By-patient listings for all efficacy measurements and all RAAS inhibitors are provided in the Supplementary data. None of the patients received any systemic immunosuppressive medications or GCs during the study.
Blood pressure remained relatively stable throughout the 12-week dosing period: mean systolic blood pressure was 128.9 mmHg (SD 13.15) on day 1 (prior to the start of avacopan dosing) and 127.4 mmHg (SD 12.01) at week 12 and mean diastolic blood pressure was 80.1 mmHg (SD 12.98) on day 1 and 82.3 mmHg (SD 9.67) at week 12.
Kidney biopsies
Patients who were included in the study were required to have a kidney biopsy consistent with IgAN during the last 3 years or be eligible after a new biopsy. The protocol also included an optional post-treatment kidney biopsy, which was carried out in two patients after the 3-month follow-up period. One patient, who had a 49% reduction in proteinuria, showed some improvement with regard to endocapillary proliferation and the presence of crescents (Oxford classification M1 E0 S1 T0) as compared with a kidney biopsy performed ∼4 years prior (Oxford classification M0 E1 S1 T1 with 10–15% crescents). The other patient did not show significant changes in histology.
Side effects
Avacopan was well tolerated. There were no early withdrawals during the 12-week avacopan treatment period. None of the observed adverse events were severe. One serious adverse event of unstable angina in a patient with a medical history of coronary artery disease and angina was considered unrelated to avacopan treatment by the investigator. Headache, nasopharyngitis and peripheral swelling were the most commonly reported adverse events (each reported in three subjects). All of these events were mild and were considered not related to avacopan by the investigators, except for one event of headache.
DISCUSSION
An interest in new therapies for IgAN has increased lately based on the current understanding of the complement system in the pathogenesis and the unmet need for improved care with more effective therapy and fewer adverse events. Although widely used in patients with significant proteinuria despite best supportive care, the benefit of GCs varies widely and short- and long-term toxicity is a concern.
Results from this pilot study in patients with IgAN on stable RAAS treatment showed that proteinuria improved in the majority of patients and that 43% of patients had an improvement in proteinuria of ∼50% to a level <1 g/g creatinine. The urinary MCP-1:creatinine ratio, a marker of renal inflammation, decreased with avacopan treatment, which is consistent with results from the recent Phase 3 avacopan randomized study in patients with ANCA-associated vasculitis [25]. Interestingly, the decrease in MCP-1 preceded the decrease in UPCR, which is biologically in line with a reduction in renal inflammation as a prerequisite for a reduction of proteinuria. As anticipated with such a short-term study, eGFR did not change over the course of the study and neither did the eGFR:MCP-1 ratio nor the IgA:C3 ratio.
The main finding of this short-term study was that proteinuria improved in most patients and substantially in several of them. Sustained improvement of proteinuria as a surrogate marker for disease activity has been shown to be associated with better renal outcomes [26, 27].
The current Kidney Disease: Improving Global Outcomes standard for IgAN studies is stable maximal supportive care, including RAAS blockade for a minimum of 3 months before assessing proteinuria and adding additional therapy [13]. In this open-label pilot study, the run-in was 8 weeks, in addition to the 4-week titration period preceding it, which at the time was considered reasonable and practical. The proteinuria slope during the run-in period was subsequently compared with the slope during the treatment period, which was pre-specified in the protocol.
Of the 15 patients who were screened, 10 were entered into the titration phase and 7 were eventually eligible for treatment. In most cases, the proteinuria did not decrease further during the 8-week run-in and remained stable during the first 4–8 weeks of treatment, as compared with the fairly early decrease in the urinary MCP-1:creatinine ratio.
The main limitations of this study were the small number of patients and the short treatment duration. However, as the subjects were their own controls, additional information was accessible during the run-in and follow-up. Avacopan appeared to be safe and well tolerated in this group of patients and there seemed to be no safety issues that would prevent further study of avacopan in IgAN. Longer avacopan treatment duration may be indicated for maximal benefit.
CONCLUSIONS
Avacopan treatment showed an improvement in the slope of UPCR, with clinically meaningful improvement in three of seven patients with IgAN. Avacopan appeared to be safe and well tolerated. A randomized controlled study to assess the long-term effect of avacopan, avoiding or reducing exposure to GCs, in IgAN should be explored.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
Contributor Information
Annette Bruchfeld, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden; Department of Renal Medicine, Karolinska University Hospital and CLINTEC Karolinska Institutet, Stockholm, Sweden.
Hasan Magin, Department of Renal Medicine, Karolinska University Hospital and CLINTEC Karolinska Institutet, Stockholm, Sweden.
Patrick Nachman, Division of Renal Diseases and Hypertension, Minneapolis, University of Minnesota, Minneapolis, MN, USA.
Samir Parikh, Department of Nephrology, Ohio State University Wexner Medical Center, Columbus, OH, USA.
Richard Lafayette, Department of Nephrology, Stanford University, Palo Alto, CA, USA.
Antonia Potarca, ChemoCentryx, San Carlos, CA, USA.
Shichang Miao, ChemoCentryx, San Carlos, CA, USA.
Pirow Bekker, ChemoCentryx, San Carlos, CA, USA.
REFERENCES
- 1. Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968; 74: 694–695 [PubMed] [Google Scholar]
- 2. Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int 2004; 66: 920–923 [DOI] [PubMed] [Google Scholar]
- 3. Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414 [DOI] [PubMed] [Google Scholar]
- 4. Hitoshi S, Krzysztof K, Jan Net al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 2011; 22: 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbour SJ, Espino-Hernandez G, Reich HNet al. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 6. Coppo R, D'Arrigo G, Tripepi Get al. Is there long-term value of pathology scoring in immunoglobulin A nephropathy? A validation study of the Oxford classification for IgA nephropathy (VALIGA) update. Nephrol Dial Transplant 2020; 35: 1002–1009 [DOI] [PubMed] [Google Scholar]
- 7. Moriyama T, Tanaka K, Iwasaki Cet al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 2014; 9: e91756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Radford MG Jr, Donadio JV Jr, Bergstralh EJ. et al. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 1997; 8: 199–207 [DOI] [PubMed] [Google Scholar]
- 9. Liu L, Zhang Y, Duan Xet al. C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy. J Clin Immunol 2014; 34: 224–232 [DOI] [PubMed] [Google Scholar]
- 10. Coppo R, Peruzzi L, Loiacono Eet al. Defective gene expression of the membrane complement inhibitor CD46 in patients with progressive immunoglobulin A nephropathy. Nephrol Dial Transplant 2019; 34: 587–596 [DOI] [PubMed] [Google Scholar]
- 11. Roos A, Rastaldi MP, Calvaresi Net al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 2006; 17: 1724–1734 [DOI] [PubMed] [Google Scholar]
- 12. Segarra A, Romero K, Agraz Iet al. Mesangial C4d deposits in early IgA nephropathy. Clin J Am Soc Nephrol 2018; 13: 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021; 100 (4 Suppl): S1–S276 [DOI] [PubMed] [Google Scholar]
- 14. Tesar V, Troyanov S, Bellur Set al. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol 2015; 26: 2248–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pozzi C, Bolasco PG, Fogazzi GBet al. Corticosteroids in IgA nephropathy: a randomized controlled trial. Lancet 1999; 353: 883–887 [DOI] [PubMed] [Google Scholar]
- 16. Rauen T, Eitner F, Fitzner Cet al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 17. Rauen T, Wied S, Fitzner Cet al. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int 2020; 98: 1044–1052 [DOI] [PubMed] [Google Scholar]
- 18. Lv J, Zhang H, Wong MGet al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 2017; 318: 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao H, Dairaghi DJ, Powers JPet al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 2014; 25: 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayne DR, Bruchfeld AN, Harper Let al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017; 28: 2756–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayne DRW, Merkel PA, Schall TJet al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 2021; 384: 599–609 [DOI] [PubMed] [Google Scholar]
- 22. Moran SM, Monach PA, Zgaga L. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2020; 35: 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myriam K, Arnaud B, Dominique SGet al. Clinical use of complement, inflammation, and fibrosis biomarkers in autoimmune glomerulonephritis. Kidney Int Rep 2020; 5: 1690–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Worawichawong S, Worawichawong S, Radinahamed Pet al. Urine epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as biomarkers for interstitial fibrosis and tubular atrophy in primary clomerulonephritis. Kidney Blood Press Res 2016; 41: 997–1007 [DOI] [PubMed] [Google Scholar]
- 25. Gong WY, Liu M, Luo Det al. High serum IgA/C3 ratio better predicts a diagnosis of IgA nephropathy among primary glomerular nephropathy patients with proteinuria ≤ 1 g/d: an observational cross-sectional study. BMC Nephrol 2019; 20: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson A, Carroll K, Inker LAet al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 2019; 14: 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canney M, Barbour SJ, Zheng Yet al. Quantifying duration of proteinuria remission and association with clinical outcome in IgA nephropathy. J Am Soc Nephrol 2021; 32: 436–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.