ABSTRACT
Despite the high prevalence of chronic kidney disease (CKD) and its high cardiovascular risk, patients with CKD, especially those with advanced CKD (stages 4–5 and patients on kidney replacement therapy), are excluded from most cardiovascular clinical trials. It is particularly relevant in patients with advanced CKD and heart failure (HF) who have been underrepresented in many pivotal randomized trials that have modified the management of HF. For this reason, there is little or no direct evidence for HF therapies in patients with advanced CKD and treatment is extrapolated from patients without CKD or patients with earlier CKD stages. The major consequence of the lack of direct evidence is the under-prescription of HF drugs to this patient population. As patients with advanced CKD and HF represent probably the highest cardiovascular risk population, the exclusion of these patients from HF trials is a serious deontological fault that must be solved. There is an urgent need to generate evidence on how to treat HF in patients with advanced CKD. This article briefly reviews the management challenges posed by HF in patients with CKD and proposes a road map to address them.
Keywords: advanced chronic kidney disease, heart failure, kidney failure, kidney replacement therapy
INTRODUCTION
Chronic kidney disease (CKD) is increasingly recognized as a global public health problem imposing substantial medical and financial burdens on societies and healthcare systems with an estimated global prevalence ranging from 9.1% [1] to 13.4% [2], depending on variations in methodological approach and data inclusion criteria, which corresponds to ∼850 million persons.
Patients with CKD exhibit a high to very high risk for cardiovascular disease (CVD). A meta-analysis of cohort studies involving >1.4 million individuals yielded an association of both low estimated glomerular filtration rate (eGFR) and higher urinary albumin:creatinine ratio (UACR) with CVD [3–5]. Half of all the patients with advanced CKD [including patients with severely decreased eGFR (<30 and ≥15 mL/min/1.73 m2 or stage 4 CKD), patients with kidney failure (eGFR <15 mL/min/1.73 m2 or stage 5 CKD) and patients undergoing kidney replacement therapy (KRT)] have CVD [6] and cardiovascular mortality accounts for ∼40–50% of all deaths in these patients compared with 26% in controls with normal kidney function [7]. According to the 2020 US Renal Data System (USRDS) Annual Report [8], heart failure (HF) is the most common cardiovascular manifestation in patients with CKD, especially in those with advanced CKD.
In the last 2 decades, most cardiovascular trials have excluded patients with CKD [9]. It is particularly evident in patients with advanced CKD and HF, as most of the randomized clinical trials have excluded these patients. Several reasons have been proposed to explain this issue [10]. Consequently, little evidence exists in support of treatment with HF pharmacological agents in patients with advanced CKD who are mostly undertreated.
In this article we briefly review some challenges posed by HF in advanced CKD that make it a true unresolved medical need and therefore deserve prompt and effective actions by the involved health professionals. In particular, we aim to create awareness among nephrologists and cardiologists that the treatment of HF in patients with advanced CKD is one of the biggest challenges they must face together right now.
CHALLENGES POSED BY ADVANCED CKD WITH HF
High incidence and prevalence
The incidence of de novo HF in persons with known CKD is in the range of 17–21% [11]. As demonstrated in the Atherosclerosis Risk in Communities (ARIC) study, a progressive decrease in eGFR and/or a progressive increase in the UACR are associated with a progressively increasing risk of incident HF after adjustment for multiple potential confounders [12]. Therefore patients with advanced CKD have the highest risk of incident HF among CKD patients.
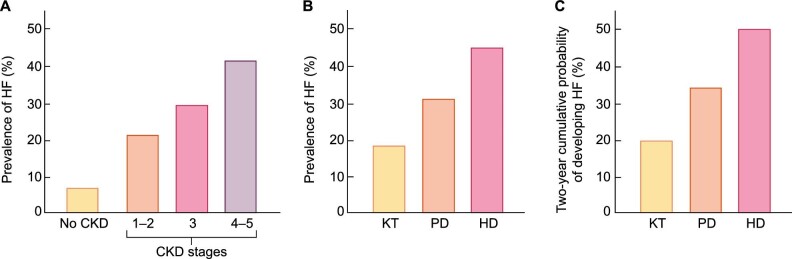
In the 2020 USRDS Annual Report, the prevalence of HF was 4-fold more common in patients with CKD (27.7%) than in patients without CKD (6.4%) [8]. Furthermore, HF was more common in patients with advanced CKD (41.3%) than in patients with CKD stage 3 (28.4%) and patients with CKD stages 1–2 (21.5%) [8] (Figure 1A). In patients with advanced CKD, HF with reduced ejection fraction (HFrEF) was slightly more common than HF with preserved EF (HFpEF) (18% versus 16%) [8].
FIGURE 1:
Prevalence and incidence of HF in patients with CKD. (A) Prevalence of HF in patients without CKD and in patients with different stages of CKD. (B) Prevalence of HF in patients in the different modalities of KRT: HD, PD and KT. (C) Two-year cumulative incidence of HF in patients on the different modalities of KRT (adapted from USRDS [8] with permission).
In the same report, the prevalence of HF in patients with CKD stage 5 on KRT was 44.2% in haemodialysis (HD), 31.1% in peritoneal dialysis (PD) and 18.3% in kidney transplant (KT) recipients (Figure 1B) [8]. Beyond month 4 of KRT, the cumulative incidence of HF was higher in patients receiving HD than in patients receiving PD or patients with a KT [8]. The 2-year cumulative probability of developing HF was ∼50% for patients on HD, 34% for patients on PD and 20% for patients with a KT [8] (Figure 1C).
Complex pathophysiology
Due to the physiological connections between the heart and the kidney, the progressive loss of kidney function facilitates the impairment of cardiac function (Figure 2). In advanced CKD, haemodynamic risk factors for HF include excessive afterload due to long-standing hypertension and arterial stiffness and excessive preload due to salt and water retention [13–15]. In addition, advanced CKD is characterized by the concurrence of several non-haemodynamic factors such as neurohormonal activation, excess of reactive oxygen species, pro-inflammatory state, profibrotic factors, impaired iron utilization, anaemia, vitamin D deficiency, protein–energy wasting, retained uraemic toxins and decreased production of cardioprotective factors such as Klotho that may facilitate the microstructural and metabolic alterations of the myocardium [13–15]. Collectively, all these haemodynamic and non-haemodynamic factors contribute to adverse left ventricular (LV) remodelling and HF [13, 16].
FIGURE 2:
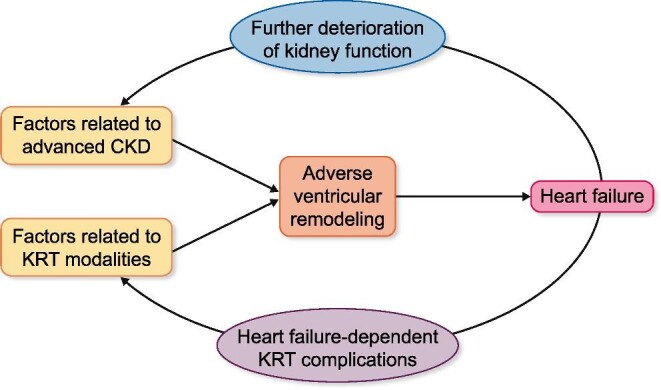
Simplified view of the pathophysiology of HF in patients with advanced CKD and impact of KRT. Note the bidirectionality of the detrimental impact of both CKD and KRT on the failing heart.
The mechanisms of organ injury and dysfunction in patients with advanced CKD that develop HF are bidirectional as the failing heart further deteriorates renal function (Figure 2). Cumulative evidence supports that when HF develops in the context of advanced CKD, both renal hypoperfusion due to low cardiac output and renal congestion due to elevated cardiac pressures and preload act as major haemodynamic determinants facilitating CKD progression to kidney failure [17, 18].
An additional aspect to consider in the complex pathophysiology of HF in patients with advanced CKD on KRT is the detrimental impact of replacement modalities on the heart, which would explain the high HF risk of these patients (Figure 2). Systemic circulatory stress or repeated hypotension episodes resulting from HD may amplify the previously mentioned mechanisms of HF operating in advanced CKD [19]. Additionally, arteriovenous fistulae or grafts may increase pulmonary pressures and facilitate adverse right ventricular remodelling and worsening HF in patients on HD [20]. Ultrafiltration failure occurs in about one-third of patients on PD and may easily lead to overhydration and hypertension, both factors contributing to HF development [21]. Additionally, some pharmacological agents used in KT patients (e.g. mammalian targets of rapamycin inhibitors, administered to offset the adverse effects of calcineurin inhibitors) may impair systolic cardiac function, thus being potential contributors to the long-term development of HFrEF [22].
On the other hand, it is also necessary to recognize the negative impact that HF can have on patient adaptation to KRT, especially to HD (Figure 2). Indeed, HF limits the ability of the left ventricle to increase cardiac output in response to hypotension, thus contributing to haemodynamic instability and organ ischaemia, including myocardial ischaemia during HD sessions [23]. Since cardiac function deteriorates even more [24] in HD patients who develop myocardial ischaemia during dialysis sessions, a vicious circle is established that aggravates the clinical situation of these patients.
Uncertain diagnosis and prevention
The 2021 European Society of Cardiology (ESC) HF Guidelines [10] define HF as a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles and peripheral oedema). In HFpEF patients, the definition also includes objective evidence of cardiac structural disease and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/elevated LV filling pressures, including elevated natriuretic peptides.
This definition of HF by the ESC has limitations when applied to patients with advanced CKD, in particular those with HFpEF. On the one hand, almost all patients with advanced CKD who do not receive KRT develop signs and symptoms consistent with HF and the severity of dyspnoea in patients on intermittent HD changes with volume removal. On the other hand, structural heart disease is highly prevalent in patients with CKD. For instance, the prevalence of LV hypertrophy increases progressively with the loss of renal function and is present in 75–90% of patients with advanced CKD [25]. Finally, advanced CKD is characterized by elevated natriuretic peptide levels and this may weaken their diagnostic utility in HFpEF [26]. Therefore, there is a need to explore a more specific definition of HF in patients with advanced CKD, especially in those on HD [27].
A reverse epidemiology of classic cardiovascular risk factors has been described in patients with advanced CKD, especially in patients with kidney failure and those on HD [28], raising substantial concern about extrapolation of evidence-based HF prevention and management strategies in patients without CKD or with earlier stages of CKD to patients with advanced CKD. For example, in contrast to the general population, a higher body-mass index is associated with better survival in patients with kidney failure [29]; similar findings have been reported for high cholesterol and high BP in patients on HD [30].
Poor outcomes and high costs
Reduced eGFR is associated with increased risks of all-cause mortality, cardiovascular mortality and hospitalization for HF (HFH) in patients with HF [31–33]. Additionally, a graded relationship exists between CKD stage and the risk of death in patients with HF. In fact, the 2-year adjusted survival probability following an HF diagnosis in patients without CKD was comparable to the 19-month survival probability in patients with stage 3 CKD and the 11-month survival probability in patients with advanced CKD [4].
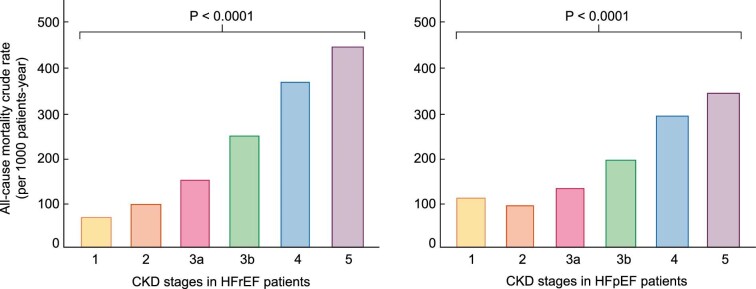
A large meta-analysis of almost 21 000 HF patients (77% with HFrEF and 23% with HFpEF) found that both groups had a stepwise increase in all-cause mortality rate with the stage of CKD that was independent of several confounding factors including age, sex, ischaemic aetiology, anaemia, hypertension, diabetes and atrial fibrillation [34]. In patients with advanced CKD, the increase in the all-cause mortality rate was higher in patients with HFrEF than in patients with HFpEF [34] (Figure 3).
FIGURE 3:
All-cause mortality crude rates in patients with different stages of CKD and HFrEF and in patients with CKD and HFpEF. Patients in CKD stage 5 (eGFR <15 mL/min/1.73 m2) were not on dialysis (adapted from McAlister et al. [34] with permission).
Within two large US CKD populations, higher rates of HFH were observed across categories of lower eGFR [35, 36] and higher UACR [36]. The rates of HFH were higher in patients with advanced CKD than in patients with earlier stages of CKD even after adjusting by many potential confounding factors, including EF [35] (Figure 4). Conversely, HFH was associated with greater risks of CKD progression and death [36].
FIGURE 4:
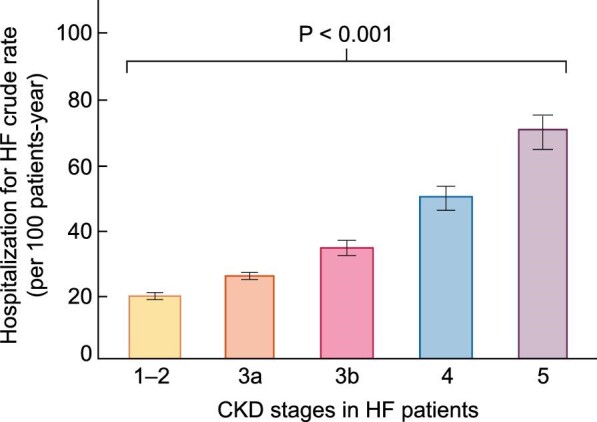
Hospitalization for HF crude rates in patients with different stages of CKD and HF. Patients in CKD stage 5 (eGFR <15 mL/min/1.73 m2) were not on dialysis. Error bars represent 95% confidence limits (adapted from Go et al. [35] with permission).
As reported in the 2020 USRDS Annual Report, expenditures for patients with advanced CKD, even excluding those on KRT, are higher than for patients with earlier stages and costs also increased more over the last decade for patients with advanced CKD [8]. The same report shows that spending for CKD patients with HF is higher than for CKD patients without HF and it increased gradually with the stage of CKD, thus being highest in patients with advanced CKD [8]. Excluding patients on KRT, mean annual expenditures for patients with advanced CKD and HF were 88% higher than in patients with advanced CKD without HF [8].
Lack of evidence for HF therapy
Patients with advanced CKD are generally excluded from cardiovascular clinical trials conducted in the general population or in populations at risk [9]. Many reasons (e.g. potential for diminished treatment effects, high risk of clinical events unmodifiable by the intervention, complex pathophysiology with many potential mechanisms contributing to HF, poor understanding of albuminuria, incomplete information on optimal dosing schedules, safety concerns, difficult recruitment and retention in trials) contribute to the exclusion of patients with advanced CKD from HF trials [37, 38]. In addition, there are no universally agreed upon designs and outcomes for HF trials conducted in patients on KRT and, specifically, in patients on HD [39].
It should be noted that the percentage of HF trials that exclude patients with CKD ranges from 52% to 78% [10] (Figure 5). Consequently, the pharmacological and device-based treatment of HF in patients with CKD is not based on evidence, but rather it is empirical, with a lack of consensus on optimal management that collectively gives rise to under treatment [13]. This is particularly relevant for the use of HF-modifying therapies in patients with advanced CKD and concomitant HF. For instance, it has been reported that angiotensin-converting enzyme inhibitors and beta-blockers are associated with similar reductions in mortality in HFrEF with and without advanced CKD but are less often prescribed in patients with advanced CKD [40]. Later, these findings were expanded to HF patients across the spectrum of EF values [41].
FIGURE 5:
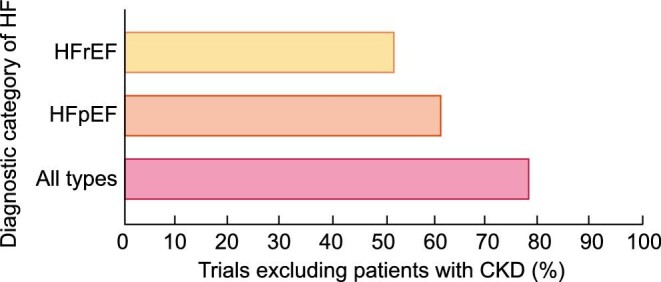
Percentage of trials on all types of HF, HFrEF and HFpEF that excluded patients with any stage of CKD (adapted from Konstantinidis et al. [10] with permission).
However, it must be recognized that the criteria for the inclusion or exclusion of patients with advanced CKD in randomized clinical trials of HF are changing. In fact, eGFR cut-off values for inclusion were lower in recent trials in HFrEF patients: 25 mL/min/1.73 m2 in the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease trial [42], 20 mL/min/1.73 m2 in the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and Reduced Ejection Fraction [43] and the Global Approach to Lowering Adverse Cardiac outocmes Through Improving Contractility in Heart Failure trial with omecamtiv mecarbil [44] and 15 mL/min/1.73 m2 in the Vericiguat in Participants with Heart Failure with Reduced Ejection Fraction trial [45]. Although differences were found in baseline characteristics between patients with severely impaired renal function and other patients, no interactions between drug effects across renal function status were found in the subgroup analyses of these trials [42–45].
Worrisome epidemiological projections
The worldwide increase in the prevalence of CKD is accompanied by the increasing incidence and prevalence of advanced CKD [1]. The increased incidence of CKD stages 4–5 is due to, among other reasons, the increased ageing of populations, increasing prevalence of type 2 diabetes and hypertension [46] and a low detection rate and therapeutic inertia in the early stages of CKD [47–49]. The prevalence of KRT has also increased worldwide, likely due to improving kidney failure survival, population demographic shifts, a higher prevalence of kidney failure risk factors and increasing KRT access in countries with growing economies [50].
On the other hand, HF is a growing public health problem worldwide. Although the incidence of HF is stable, the prevalence is constantly increasing due to the ageing of the population and better survival rates in treated patients with HFrEF [51].
Since Spain is the country with a longer projected life expectancy this century [52], it is worth exploring the national projections for CKD and HF. At the current rate of population ageing, and assuming constant incidence rates, it is estimated that in 2040 the prevalence of CKD and HF will be ∼18% and 4.2%, respectively [51, 53–55]. These figures represent an increase in the prevalence of CKD (Figure 6A) and HF (Figure 6B) of 100% and 90%, respectively, with respect to the prevalence of each of the two diseases in the first decade of the century [51, 53–55]. Therefore it is reasonable to anticipate that the prevalence of patients with advanced CKD complicated by HF will also increase in Spain throughout the next decades.
FIGURE 6:
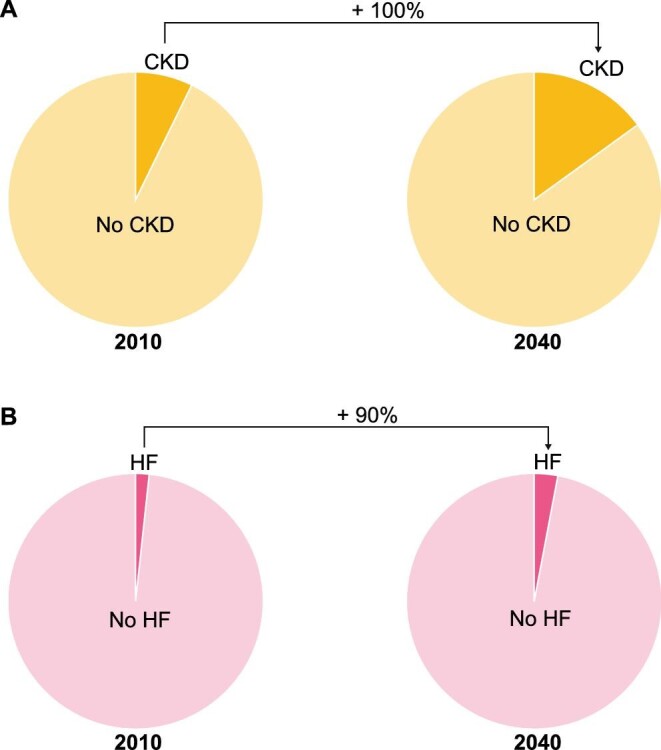
(A) Prevalence of CKD in the adult population of Spain as estimated in 2010 and as projected for 2040. (B) Prevalence of HF in the adult population of Spain as estimated in 2010 and as projected for 2040 (adapted from Savarese and Lund [51], Otero et al. [53], Ortiz [54] and Gomez-Soto et al. [55]).
ROAD MAP FOR FACING THE CHALLENGES OF MANAGING PATIENTS WITH ADVANCED CKD AND HF
Although patients with CKD and HF represent probably the highest cardiovascular risk population, the ESC guidelines for the treatment of HF recently recognized that there is little direct evidence to support any recommendation for the treatment of these patients [56]. Therefore there is an urgent need to generate evidence on managing this population with life-improving and life-saving therapies.
To this aim, several issues must be addressed [57] (Table 1). High-quality data are lacking on all aspects of HF (epidemiology, pathophysiology, diagnosis, prevention and treatment) specific to the population of patients with advanced CKD, and particularly those on KRT. Testing of prevention and treatment strategies will require the design and conduct of adequately powered clinical trials with careful adverse event monitoring and follow-up. These trials must cover the complex set of clinical conditions that HF in CKD appears to be. One key issue is how to define HF in patients with advanced CKD and whether the available standard definitions are well suited for this population. In particular, what criteria should be used to reliably distinguish a real HFH from the unfortunate but common occurrence of transient fluid overload related to dry weight overestimation or non-adherence to diet in dialysis patients? Methods to identify patients with advanced CKD who are more likely to have adverse cardiovascular outcomes rather than other common outcomes in this population (e.g. cancer or sepsis-related deaths) should be optimized, as current ones are inadequate. Enrolling patients at higher risk for HF-related events in HF outcome clinical trials (prognostic enrichment) improves the ability to detect a treatment effect. Identification of high-risk patients and of patients that may benefit from specific forms of therapy requires the validation of traditional and potentially non-traditional cardiac biomarkers in the population of patients with advanced CKD. The trials should include patient-oriented outcomes when evaluating therapeutic strategies, particularly in patients undergoing HD in which the cyclic nature of volume overload and correction and the predominant modes of cardiovascular death might differ from those observed in other patients. Finally, beyond face-to-face care, virtual remote healthcare services may improve the quality of life of patients with CKD and influence their attitudes and behaviours [58], which can be of special relevance in patients with advanced CKD participating in HF clinical trials [59].
Table 1.
A three-step road map for improving the outcomes of patients with advanced CKD and HF
| 1. Improve our understanding of the epidemiology, pathophysiology, diagnosis and risk stratification of HF in patients with advanced CKD and particularly in those on different modalities of KRT |
| a. Adapt the definitions of HF and HFH to patients with advanced CKD |
| b. Validate traditional and potentially non-traditional cardiac biomarkers in patients with advanced CKD |
| c. Validate and/or develop methods for risk stratification that allow the enrichment of clinical trials with patients with advanced CKD at higher risk for HF-related events |
| 2. Design and conduct adequately powered clinical trials to address questions related to the optimization of prevention and treatment strategies specific to patients with advanced CKD |
| a. Use adapted definition of HF and HFH |
| b. Use validated methods and risk stratification methods to enrich the high HF-risk patient and adapt the trial population to the mechanism of action of the intervention: aim at precision therapy |
| c. Careful adverse event monitoring |
| d. Include patient-oriented outcomes and adapt outcomes to the advanced CKD reality |
| e. Implement virtual remote healthcare services that facilitate compliance and patient retention |
| 3. Extend the nephrology–cardiology collaboration into the development of consensus documents and clinical guidelines that facilitate the rapid uptake and implementation of therapeutic advances. |
CONCLUSION
It is time for a multidisciplinary approach to overcome the challenges posed by HF in advanced CKD. Nephrologists and cardiologists must share both knowledge and skills [60] in cardiorenal clinical programmes [61] to develop the details and implement the road map delineated above to improve the outcomes of HF in patients with advanced CKD. The major goal of this collaboration must be to design and perform randomized clinical trials aimed at providing evidence-based therapy to treat HF in patients with advanced CKD. The collaboration should then extend to the development of consensus documents and clinical guidelines that facilitate the rapid uptake and implementation of therapeutic advances.
Contributor Information
Alberto Ortiz, Division of Nephrology IIS-Fundacion Jimenez Diaz, Department of Medicine, Universidad Autónoma de Madrid, Madrid, Spain; RICORS2040, Carlos III Institute of Health, Madrid, Spain.
Juan F Navarro-González, RICORS2040, Carlos III Institute of Health, Madrid, Spain; Division of Nephrology and Research Unit, University Hospital Nuestra Señora de Candelaria, and Universitary Institute of Biomedical Technologies, University of La Laguna, Santa Cruz de Tenerife, Spain.
Julio Núñez, Division of Cardiology, University Hospital, INCLIVA, Universitat de Valencia, Valencia, Spain; Department of Medicine, University of Valencia, Valencia, Spain; Centro de Investigación Biomédica en Red de las Enfermedades Cardiovasculares, Carlos III Institute of Health, Madrid, Spain.
Rafael de la Espriella, Division of Cardiology, University Hospital, INCLIVA, Universitat de Valencia, Valencia, Spain.
Marta Cobo, Centro de Investigación Biomédica en Red de las Enfermedades Cardiovasculares, Carlos III Institute of Health, Madrid, Spain; Division of Cardiology, University Hospital Puerta de Hierro, University Autónoma of Madrid, Madrid, Spain.
Rafael Santamaría, RICORS2040, Carlos III Institute of Health, Madrid, Spain; Division of Nephrology, University Hospital Reina Sofia, Cordoba, Spain; Maimonides Biomedical Research Institute of Cordoba, Cordoba, Spain.
Patricia de Sequera, Department of Nephrology, University Hospital Infanta Leonor, University Complutense of Madrid, Madrid, Spain.
Javier Díez, Centro de Investigación Biomédica en Red de las Enfermedades Cardiovasculares, Carlos III Institute of Health, Madrid, Spain; Departments of Nephrology and Cardiology, University of Navarra Clinic, Pamplona, Spain; Program of Cardiovascular Diseases, Center of Applied Medical Research, University of Navarra, Pamplona, Spain.
AUTHORS’ CONTRIBUTIONS
A.O., J.F.N.-G., R.S., P.S. and J.D. developed the concept and design of the manuscript and J.D. drafted and wrote it. J.N., R.E. and M.C. revised and edited the manuscript. All authors approved the final version.
CONFLICT OF INTEREST STATEMENT
A.O. has received consultancy or speaker fees or travel support from AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma–UAM of diabetic kidney disease and the Catedra AstraZeneca–UAM of chronic kidney disease and electrolytes. A.O. is the Editor-in-Chief of CKJ. J.F.N.-G. has served as a consultant and has received speaker fees or travel support from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Eli Lilly, MSD, Mundipharma, Novartis, NovoNordisk, Sanofi-Genzyme, Servier, Shire and Vifor Fresenius Medical Care Renal Pharma. J.N. has received consultancy or speaker fees or travel support from AstraZeneca, Vifor Pharma, Novartis, Rovi, Pfizer, NovoNordisk, Bayer and Boehringer Ingelheim. R.d.l.E. has received consultancy or speaker fees or travel support from AstraZeneca, Vifor Pharma, Novartis, Rovi, Pfizer, Novo Nordisk, Bayer, Daiichi Sankyo and Boehringer Ingelheim. M.C. has received consultancy or speaker fees or travel support from AstraZeneca, Vifor Pharma, Novartis, Rovi, Pfizer, Bayer, Eli Lilly and Boehringer Ingelheim. R.S. has received consultancy or speaker fees or travel support from AstraZeneca, Vifor Fresenius Medical Care Renal Pharma and Boehringer Ingelheim. P.S. has received consultancy or speaker fees or travel support from Vifor Pharma, Amgen, Fresenius, AstraZeneca, Nipro, Alexion, Astellas, Braun and Baxter. J.D. has received consultancy or speaker fees or travel support from AstraZeneca, Bayer and Vifor Pharma. This article has not been published previously in whole or part.
REFERENCES
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill NR, Fatoba ST, Oke JLet al. . Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, van der Velde M, Astor BCet al. . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Velde M, Matsushita K, Coresh Jet al. . Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79: 1341–1352 [DOI] [PubMed] [Google Scholar]
- 5. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. . Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 6. Stevens PE, O'Donoghue DJ, de Lusignan Set al. . Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007; 72: 92–99 [DOI] [PubMed] [Google Scholar]
- 7. Thompson S, James M, Wiebe Net al. . Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015; 26: 2504–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 9. Maini R, Wong DB, Addison Det al. . Persistent underrepresentation of kidney disease in randomized, controlled trials of cardiovascular disease in the contemporary era. J Am Soc Nephrol 2018; 29: 2782–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konstantinidis I, Nadkarni GN, Yacoub Ret al. . Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med 2016; 176: 121–124 [DOI] [PubMed] [Google Scholar]
- 11. Kottgen A, Russell SD, Loehr LRet al. . Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 2007; 18: 1307–1315 [DOI] [PubMed] [Google Scholar]
- 12. Waheed S, Matsushita K, Sang Yet al. . Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2012; 60: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. House AA, Wanner C, Sarnak MJet al. . Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1304–1317 [DOI] [PubMed] [Google Scholar]
- 14. Rangaswami J, McCullough PA. Heart failure in end-stage kidney disease: pathophysiology, diagnosis, and therapeutic strategies. Semin Nephrol 2018; 38: 600–617 [DOI] [PubMed] [Google Scholar]
- 15. Rangaswami J, Bhalla V, Blair JEAet al. . Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: e840–e878 [DOI] [PubMed] [Google Scholar]
- 16. Kaesler N, Babler A, Floege Jet al. . Cardiac remodeling in chronic kidney disease. Toxins (Basel) 2020; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verbrugge FH, Guazzi M, Testani JMet al. . Altered hemodynamics and end-organ damage in heart failure: impact on the lung and the kidney. Circulation 2020; 142: 998–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Husain-Syed F, Gröne H-J, Assmus Bet al. . Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail 2021; 8: 183–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canaud B, Kooman JP, Selby NMet al. . Dialysis-induced cardiovascular and multiorgan morbidity. Kidney Int Rep 2020; 5: 1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reddy YNV, Obokata M, Dean PGet al. . Long-term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur Heart J 2017; 38: 1913–1923 [DOI] [PubMed] [Google Scholar]
- 21. Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 2010; 6: 451–460 [DOI] [PubMed] [Google Scholar]
- 22. Tsujimura K, Ota M, Chinen Ket al. . Supplementary administration of everolimus reduces cardiac systolic function in kidney transplant recipients. Ann Transplant 2017; 22: 315–322 [DOI] [PubMed] [Google Scholar]
- 23. Douvris A, Zeid K, Hiremath Set al. . Mechanisms for hemodynamic instability related to renal replacement therapy: a narrative review. Intensive Care Med 2019; 45: 1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burton JO, Jefferies HJ, Selby NMet al. . Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 2009; 4: 1925–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Lullo L, Gorini A, Russo Det al. . Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med 2015; 5: 254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maisel A, Mueller C, Adams K Jret al. . State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008; 10: 824–839 [DOI] [PubMed] [Google Scholar]
- 27. Chawla LS, Herzog CA, Costanzo MRet al. . Proposal for a functional classification system of heart failure in patients with end-stage renal disease: proceedings of the Acute Dialysis Quality Initiative (ADQI) XI workgroup. J Am Coll Cardiol 2014; 63: 1246–1252 [DOI] [PubMed] [Google Scholar]
- 28. Ortiz A, Covic A, Fliser Det al. . Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014; 383: 1831–1843 [DOI] [PubMed] [Google Scholar]
- 29. Park J, Ahmadi SF, Streja E. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 2014; 56: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossignol P, Cridlig J, Lehert Pet al. . Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension 2012; 60: 339–346 [DOI] [PubMed] [Google Scholar]
- 31. Hillege HL, Nitsch D, Pfeffer MAet al. . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678 [DOI] [PubMed] [Google Scholar]
- 32. Smith DH, Thorp ML, Gurwitz JHet al. . Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes 2013; 6: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lofman I, Szummer K, Dahlstrom Uet al. . Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail 2017; 19: 1606–1614 [DOI] [PubMed] [Google Scholar]
- 34. McAlister FA, Ezekowitz J, Tarantini Let al. . Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease–Epidemiology Collaboration Group formula. Circ Heart Fail 2012; 5: 309–314 [DOI] [PubMed] [Google Scholar]
- 35. Go AS, Yang J, Ackerson LMet al. . Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006; 113: 2713–2723 [DOI] [PubMed] [Google Scholar]
- 36. Bansal N, Zelnick L, Baht Zet al. . Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol 2019; 73: 2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation 2017; 135: 1769–1771 [DOI] [PubMed] [Google Scholar]
- 38. Hein AM, Scialla JJ, Edmonston Det al. . Medical management of heart failure with reduced ejection fraction in patients with advanced renal disease. JACC Heart Fail 2019; 7: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Lone E, Viecelli AK, Craig JCet al. . Cardiovascular outcomes reported in hemodialysis patients. J Am Coll Cardiol 2018; 71: 2802–2810 [DOI] [PubMed] [Google Scholar]
- 40. Shlipak MG, Browner WS, Noguchi Het al. . Comparison of the effects of angiotensin converting-enzyme inhibitors and beta-blockers on survival in elderly patients with reduced left ventricular function after myocardial infarction. Am J Med 2001; 110: 425–433 [DOI] [PubMed] [Google Scholar]
- 41. McAlister FA, Ezekowitz J, Tonelli Met al. . Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009 [DOI] [PubMed] [Google Scholar]
- 42. McMurray JJV, Solomon SD, Inzucchi SEet al. . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008 [DOI] [PubMed] [Google Scholar]
- 43. Packer M, Anker SD, Butler Jet al. . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424 [DOI] [PubMed] [Google Scholar]
- 44. Teerlink JR, Diaz R, Felker GMet al. . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021; 384: 105–116 [DOI] [PubMed] [Google Scholar]
- 45. Armstrong PW, Pieske B, Anstrom KJet al. . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883–1893 [DOI] [PubMed] [Google Scholar]
- 46. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 2016; 12: 73–81 [DOI] [PubMed] [Google Scholar]
- 47. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–S266 [PubMed] [Google Scholar]
- 48. Ma I, Guo M, Muruve Det al. . Sociodemographic associations with abnormal estimated glomerular filtration rate (eGFR) in a large Canadian city: a cross-sectional observation study. BMC Nephrol 2018; 19: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nissenson AR, Collins AJ, Hurley Jet al. . Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol 2001; 12: 1713–1720 [DOI] [PubMed] [Google Scholar]
- 50. Thurlow J, Joshi M, Yan Get al. . Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol 2021; 52: 98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Foreman KJ, Marquez N, Dolgert Aet al. . Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Otero A, de Francisco A, Gayoso Pet al. . Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrología 2010; 30: 78–86 [DOI] [PubMed] [Google Scholar]
- 54. Ortiz A. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2022; 10.1093/ckj/sfab170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomez-Soto FM, Andrey JL, Garcia-Egido AAet al. . Incidence and mortality of heart failure: a community-based study. Int J Cardiol 2011; 151: 40–45 [DOI] [PubMed] [Google Scholar]
- 56. McDonagh TA, Metra M, Adamo Met al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726 [DOI] [PubMed] [Google Scholar]
- 57. Rossignol P, Pitt B, Thompson Aet al. . Roadmap for cardiovascular prevention trials in chronic kidney disease. Lancet 2016; 388: 1964–1966 [DOI] [PubMed] [Google Scholar]
- 58. Bodington R, Kassianides X, Bhandari S. Point-of-care testing technologies for the home in chronic kidney disease: a narrative review. Clin Kidney J 2021; 14: 2316–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bekfani T, Fudim M, Cleland JGFet al. . A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur J Heart Fail 2021; 23: 175–185 [DOI] [PubMed] [Google Scholar]
- 60. Díez J, Navarro-González JF, Ortiz Aet al. . Developing the subspecialty of cardio-nephrology: the time has come. A position paper from the coordinating committee from the Working Group for Cardiorenal Medicine of the Spanish Society of Nephrology. Nefrología 2021; 41: 391–402 [DOI] [PubMed] [Google Scholar]
- 61. de la Espriella R, González M, Górriz JLet al. . Setting up a cardiorenal clinic. Consensus document of the cardiorenal working groups of the Spanish Society of Cardiology and the Spanish Society of Nephrology. CardioClinics 2021; 56: 284–295 [Google Scholar]