Abstract
The study evaluated the nutritional quality in muscle tissues of nine commercially important marine fish species. And the concentrations of trace metals (i.e. As, Hg, Cu, Pb, Cr, Cd and Zn) in the muscles (edible part) and tissues (gill and liver) of fishes caught from Dachen fishing ground, the coast of Zhejiang Province, East China Sea, were determined, and the values of target hazard quotient (THQ) and the carcinogenic risk (TR) were calculated for assessing human health risk. Significant differences(P < 0.05) were observed in the proximate chemical composition of fish muscles in these species. The muscle protein content of fish species ranged from 12.36 to 23.41%. The muscle lipid content of fishes ranged from 0.48 to 2.54%. The accumulation capacity of heavy metals (except Cr) in livers and gills was higher than that in muscles. In addition, the accumulation ability of most fishes is related to the water layer they live, the fishes living in the demersal layer showed more accumulation of heavy metals than the middle-upper layer(except Cu). Estimated daily intake (EDI), target hazard quotient (THQ), hazard index (HI) and the carcinogenic risk (TR) assessed for potential human health risk implications suggest that the values were within the acceptable threshold for human. However, the carcinogenic risk(TR) of As and Cr was close to the critical limit (10–4). Therefore, in order to ensure the health and safety of human consumption, the continuous monitoring of heavy metals in Dachen fishing ground area is suggested.
Subject terms: Ecology, Environmental sciences
Introduction
In recent decades, the consumption of fish worldwide has been growing rapidly due to their nutritional benefits and high quality proteins1. However, heavy metals contamination of fish has caused a great global concern2, which also poses a health threat to human health3,4. Fish can absorb heavy metals from surrounding water, sediment and their diet5, large or improper consumption is likely to cause adverse effects on human body, therefore, it is important and necessary to determine the accumulation of heavy metals contents in the widely consumed economical fish species.
Several methods have been proposed for estimation of the potential risks to human health of heavy metals in fish. Of course, we have never stopped studying this. Among these, carcinogenic and non-carcinogenic effects were extensively used to evaluate the impact on human health. The noncarcinogenic health risk is generally evaluated by estimating target hazard quotient (THQ). The carcinogenic effect is evaluated by providing cancer slope factor for As, Pb and Cd to determine the carcinogenic risk (TR) over a lifetime exposure to As, Pb and Cd. Some studies6–9 combined THQ and TR to assess the human health risks associated with consumption fish. These studies and experiments not only provide great help for the follow-up research, but also help to improve people's understanding of health and promote the improvement of human health.
Dachen Islands, known as the Pearl of the East China Sea, which is located in Taizhou Bay, the outer island off the coast of central and southern Zhejiang Province. Dachen Island, with a land area of 14.96 km2, is the midpoint of the western Pacific Ocean waterway, also one of the national first-class fishing ports and the second largest fishery in Zhejiang Province. It is an important industry on this island, which is also a tourism spot. Countless people come here and countless related researches are carried out here10. Due to unique natural resources and location advantages, there are many economical fish species such as Larimichthys crocea, Sebastiscus marmoratus, Lateolabrax maculatus, Muraenesox cinereus11,12. The concentrations of heavy metals in the East China Sea have been widely investigated and reported in previous literatures13–15, however, the heavy metal levels in economical fish from Dachen area have not yet been reported. The main purposes of this study are (1) to evaluate the nutritional value of nine commercial marine fish species collected from Dachen fishing ground by determining their proximate composition of protein and lipids; (2) to determine the concentrations of seven heavy metals (Cu, Zn, Pb, Cr, As, Hg, Cd) and compare variations of heavy metal contents and enrichment law in different tissues of fish species from Dachen fishing ground; (3) to conduct the non-carcinogenic and carcinogenic human health risk assessment of the consumption of heavy metals.
Material and methods
Study areas and sample collection
This study was carried out in the sea area of Dachen Islands (28° 28′ 12.00″ N–28° 22′ 12.00″ N, 121° 48′ 00.00″ E–121° 60′ 00.00″ E) (Fig. 1). The selection of fish species in this paper mainly considered economy and catch. And they represent different water layers. Samples of 9 different fish species were caught by bottom trawling in November, 2019(Fig. 2). Also they are carnivorous and have the potential to bioconcentrate contaminants, which would normally be present in the water or inside sediments. After the fishing ban, the biomass and biodiversity of the fishes are high in the sea area of Dachen Islands. For each species, six samples were collected for this study. The total length and weight for each individual was measured and recorded in Table 1. Sufficient amount of muscle, liver, gills from the fish of the same species were removed from each organism and dissected on-site by clean stainless steel knife. It should be mentioned here that only edible part (fish flesh and skin) were chosen as muscle tissue. Each collected tissue sample was preserved in clean plastic bags and frozen immediately until it was transported to the laboratory. The tissues samples of each individual fish were then air-dried to remove the extra water, and subsequently were dried at − 80 °C for 24 h using a vacuum freeze-drying instrument (Christ Alpha, Germany), rations of moisture were also accurately calculated by comparing the weight before and after drying. Then ground to powder, stored at – 20 °C for succeeding uses.
Figure 1.
Map of the study area.
Figure 2.
The experimental fish samples collected from Dachen fishing ground, East China Sea.
Table 1.
Characteristics of fish species from Dachen fishing ground.
| Scientific name | Number | Body weight (wet weight, g) | Body length (mm) | Habitat |
|---|---|---|---|---|
| Johnius belengeri (JB) | 6 | 3.75–14.72 | 70–120 | Demersal |
| Chrysochir aureus (CA) | 6 | 5.73–86.43 | 80–175 | Demersal |
| Collichthys lucidus (CL) | 6 | 7.06–28.50 | 100–153 | Demersal |
| Ctenotrypauchen chinensis (CC) | 6 | 8.19–16.49 | 90–160 | Demersal |
| Muraenesox cinereus (MC) | 6 | 11.92–550.50 | 246–692 | Demersal |
| Sebastiscus marmoratus (SM) | 6 | 23.91–52.59 | 111–147 | Demersal |
| Coilia macrognathos (CM) | 6 | 37.00–96.63 | 245–319 | Middle-upper |
| Larimichthys crocea (LC) | 6 | 52.50–84.20 | 180–204 | Demersal |
| Lateolabrax maculatus (LM) | 6 | 97.47–141.24 | 226–285 | Middle-upper |
ww wet weight.
Seawater samples for heavy metal determination were collected in acid washed polyethylene bottles. The bottles were rinsed thoroughly with deionised water after being washed in dilute nitric acid (HNO3). In the field the bottles were rinsed several times with the seawater and 1 L of water sample was then collected at about 50 cm below the water surface. And seawater samples were filtered through Whatman No. 45 filter paper. The seawater samples were acidified with concentrated nitric acid for preservation. The acid pretreatment ensured that heavy metals did not get absorbed to the surface of the container during transportation and storage.
A total of 10 surface sediment samples were collected at sampling sites distributed throughout the study area (Fig. 1). A stainless-steel grab sampler was used for collection, while a plastic shovel was used to excavate the sediments from the middle part of the sampler. Samples were kept in pre-cleaned polyethylene bags and frozen until lab analysis.
Sample analysis
The proximate composition of fish muscles (protein and lipids) was determined according to the standard methods of AOAC16. The metal extraction procedure is referred to a previous study17 and we have made corresponding improvements based on this research. Both fish and sediment samples were weighed in duplicate. For fish and sediment samples, about 0.5 g of each powdered sample was digested in microwave digester (MDS-6G, China) using the ratio of 2:1 (8 mL + 4 mL) of concentrated nitric acid (GR) and hydrogen peroxide (AR). After digestion process, one of them does not removed acid to determine arsenic and mercury. And the other transferred the sample to a 30 mL polytetrafluoroethylene beakers and heated it on hot plate at 170 °C until about 1 mL of solvent remains to determine other heavy metals. Then samples were diluted with deionized water in 25 mL polypropylene tubes. The concentration levels of Cu, Zn, Pb, Cd and Cr in the digested sample solutions were determined by using inductively coupled plasma optical emission spectrometer (ICP-OES, Optima8000, USA), and the Hg and As contents were determined by using atomic fluorescence spectrometry (AFS-3100, China). Certified Reference Material (Standard oyster Tissue 1566a) obtained from the U. S. Department of Commerce was also analyzed routinely every 10 samples and all runs were analyzed in triplicated as quality control. In addition, reagent blanks were analyzed to provide a baseline correction for the results of the samples. The mean recoveries of metals were between 91.8 and 105.2%, indicating a decent agreement between certified and measured values.
For seawater sample, detailed operating procedures are described in the Specifcation for Marine Monitoring of China (SMMC) (GB17378.4-2007, ICS 07.060 A 45)18.
Statistical analyses
Data were given as mean ± SD for each of the measured variables. All statistical analyzes were performed using SPSS version 21.0 version. The one-sample Kolmogorov–Smirnov (K–S) test was used to assess the data normality. All the concentration values for seven heavy metals in the tissues of fish species were normally distributed at the 95% confidence level. Therefore, a one-way ANOVA test was used to assess significant differences tissues. Turkey's Post hoc test was employed to test the significance of difference between single species in different organ.
Health risk assessment
Several methods have been proposed for estimating the potential risks to human health of heavy metals in fishes19–21. These methods were described in detail below. Mean concentrations of heavy metals for each species were used in all calculations.
Estimated daily intake (EDI)
Health risk was estimated considering the average concentrations of all fish muscles and daily heavy metal intake (EDI). The specific formula is as follows9:
| 1 |
where C is the concentration of heavy metals in the selected fish tissues (mg/kg, ww); FIR is the food ingestion rate, which is 31 g/person/day22; ED is the exposure duration 70 years23; EF is the frequency of exposure 365 days/year23; BW is the average body weight 55.9 kg24; AT is the average exposure time 25,500 days9.
Non-carcinogenic health risk
The target hazard quotient (THQ) and hazard index (HI) are a method proposed by the U. S. Environmental Protection Agency (USEPA) for assessing the risk of heavy metals caused by food intake by the human body23. The value of ratio < 1 implies a non-significant risk effects. The specific formula is as follows23:
| 2 |
| 3 |
RFDs of the different heavy metals for example As, Hg, Cd, Cu, Zn, Pb, and Cr are 0.0015, 0.00016, 0.001, 0.04, 0.3, 0.004 and 1.5, repectively23. Other factors have been mentioned as above.
The carcinogenic risk (TR)
As well as non-carcinogenic risks, there are also carcinogenic risks in human health risk assessment17. All trace metals do not have carcinogenic effects. However, As, Pb, Cd and Cr among the studied heavy metals are considered as carcinogens. For carcinogens, the individual risk assessment increases the probability of developing cancer due to exposure to potential carcinogens22,25. The acceptable risk levels of TR5,9 for carcinogens ranged from 10–6 to 10–4. The model formula is as follows26:
| 4 |
where the oral carcinogenic slope factor (CSFo) was obtained from the database of the U. S. Environmental Protection Agency26. Available CSFo values (mg/kg/day) are: As (1.5), Pb (0.0085), Cd (6.3) and Cr (0.5)26. Other factors have been mentioned as above. Assume that 10% of the total As can be assessed as inorganic state5,27,28 in this study.
Ethical approval
Fish used for this study were fresh but lifeless, however all procedures used conform to standard scientific research guidelines. All methods are reported in accordance with ARRIVE guidelines. All experimental protocols were approved by the Shanghai Ocean University. The study was approved by the ethic committee of the Shanghai Ocean University.
Results
Chemical composition of fish muscles
The chemical composition of fish muscles is shown in Table 2. The muscle protein content in Larimichthys crocea (23.41%) was significantly higher than that in the other species, followed by Muraenesox cinereus (21.28%) whereas the muscle protein content in Ctenotrypauchen chinensis (12.36%) was the lowest. The muscle lipid content of Sebastiscus marmoratus had significantly higher lipid content (2.54%), while Ctenotrypauchen chinensis had significantly lower lipid content (0.48%). And compared with other fishing grounds, the muscle protein content of fishes basically was the same (Table 2). The muscle lipid content of fishes ranged from 0.48 to 2.54% and lower than the reported levels in other fishing Ground (Table 2).
Table 2.
The chemical in the fish muscles of nine fish species collected from Dachen fishing ground.
| Scientific name | Site | Protein (%) | Lipid (%) |
|---|---|---|---|
| Chrysochir aureus (CA) | This study | 19.78 ± 0.42a | 0.54 ± 0.26a |
| Larimichthys crocea (LC) | This study | 23.41 ± 0.63c | 1.16 ± 0.34a |
| Zhoushan Fishing Ground29 | 21.46–23.80 | 4.34–9.76 | |
| Collichthys lucidus (CL) | This study | 14.42 ± 0.29b | 2.00 ± 0.71b |
| Zhoushan Fishing Ground30 | 14.47–15.33 | 3.45–3.95 | |
| Ctenotrypauchen chinensis (CC) | This study | 12.36 ± 0.31b | 0.48 ± 0.13a |
| Bohai Fishing Ground31 | 11.66–13.80 | 2.52–2.54 | |
| Coilia macrognathos (CM) | This study | 18.70 ± 0.48b | 1.45 ± 0.39c |
| Bohai Fishing Ground32 | 18.11–18.81 | 5.25–5.37 | |
| Muraenesox cinereus (MC) | This study | 21.28 ± 0.51c | 1.16 ± 0.29c |
| Southern Coastal Fishing Ground33 | 19.60 | 2.0 | |
| Johnius belengeri (JB) | This study | 15.38 ± 0.19a | 0.52 ± 0.12a |
| Southern Coastal Fishing Ground34 | 16.92 | 7.19 | |
| Sebastiscus marmoratus (SM) | This study | 19.05 ± 0.28c | 2.54 ± 0.77b |
| Zhoushan Fishing Ground30 | 17.90 ± 0.43 | 3.70 ± 0.25 | |
| Lateolabrax maculatus (LM) | This study | 15.87 ± 0.22a | 1.76 ± 0.67c |
| Bohai Fishing Ground35 | 16.51 ± 0.12 | 1.95 ± 0.08 |
Values in the same column having different superscript letters are significantly different(P < 0.05).
Heavy metals in fish samples
The content of heavy metals in fish muscles
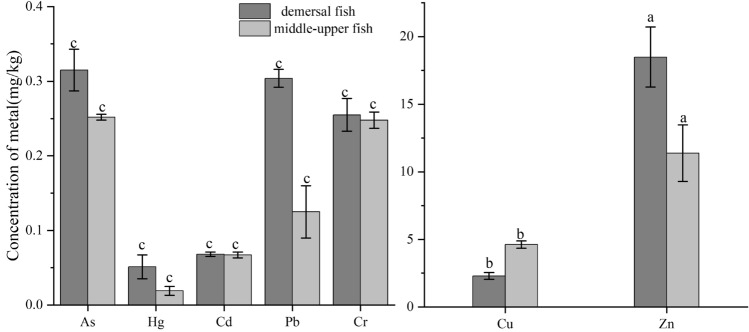
In the present study, the concentrations of seven heavy metals (As, Hg, Cd, Cu, Zn, Pb, and Cr) in the muscles of nine fish species were shown in Table 3. Metal concentrations were reported using wet-weight. The concentration of all the examine heavy metals in fish muscles remained well below the acceptable limits for human consumption established by FAO. The order of mean concentrations of heavy metals from high to low was shown as follows: Zn (16.910 mg/kg) > Cu (2.810 mg/kg) > As (0.301 mg/kg) > Pb (0.264 mg/kg) > Cr (0.074 mg/kg) > Cd (0.067 mg/kg) > Hg (0.044 mg/kg). It was found that the accumulating capacity of most fishes was linked to the water layer in which they lived (Fig. 3). The results obtained from this study were analyzed using the analysis of variance (ANOVA), and the differences between essential (Cu and Zn) and non essential metals (As, Hg, Cd, Pb and Cr) concentration levels were considered significant at 95% confidence interval (P < 0.05). The amount of heavy metals in the demersal fish was greater than the middle-upper fish (except Cu).
Table 3.
Concentration of heavy metals of fish and marine environment.
| Species | Tissues | Heavy metals (mg/kg, ww) | ||||||
|---|---|---|---|---|---|---|---|---|
| As | Hg | Cd | Cu | Zn | Pb | Cr | ||
| LC | Muscle | 0.140 ± 0.014 | 0.026 ± 0.005 | 0.067 ± 0.006 | 2.000 ± 0.028 | 12.550 ± 0.806 | 0.025 ± 0.003 | 0.036 ± 0.006 |
| Gill | 0.250 ± 0.085 | 0.014 ± 0.004 | 0.067 ± 0.001 | 3.300 ± 0.055 | 52.400 ± 3.408 | 0.350 ± 0.028 | 0.234 ± 0.015 | |
| Liver | 0.165 ± 0.010 | 0.023 ± 0.004 | 0.096 ± 0.007 | 15.900 ± 1.400 | 59.950 ± 1.838 | 0.100 ± 0.028 | 0.270 ± 0.014 | |
| MC | Muscle | 0.412 ± 0.014 | 0.066 ± 0.009 | 0.068 ± 0.007 | 2.300 ± 0.283 | 21.400 ± 1.853 | 0.100 ± 0.014 | 0.075 ± 0.008 |
| Gill | 0.220 ± 0.091 | 0.023 ± 0.006 | 0.069 ± 0.003 | 7.300 ± 0.360 | 47.800 ± 2.687 | 0.050 ± 0.001 | 0.253 ± 0.003 | |
| Liver | 0.802 ± 0.127 | 0.036 ± 0.003 | 0.096 ± 0.007 | 36.902 ± 4.667 | 77.410 ± 1.754 | 0.150 ± 0.016 | 0.260 ± 0.001 | |
| SM | Muscle | 0.245 ± 0.033 | 0.111 ± 0.018 | 0.067 ± 0.010 | 2.400 ± 0.141 | 27.150 ± 2.150 | 1.100 ± 0.042 | 0.092 ± 0.006 |
| Gill | 0.350 ± 0.085 | 0.009 ± 0.001 | 0.066 ± 0.011 | 1.700 ± 0.905 | 49.400 ± 1.131 | 0.050 ± 0.003 | 0.251 ± 0.004 | |
| Liver | 0.285 ± 0.016 | 0.037 ± 0.006 | 0.072 ± 0.006 | 11.950 ± 1.372 | 70.502 ± 2.192 | 0.051 ± 0.006 | 0.251 ± 0.003 | |
| JB | Muscle | 0.255 ± 0.013 | 0.037 ± 0.017 | 0.066 ± 0.004 | 1.300 ± 0.127 | 6.250 ± 0.311 | 0.650 ± 0.014 | 0.089 ± 0.011 |
| Gill | 0.271 ± 0.072 | 0.105 ± 0.008 | 0.089 ± 0.003 | 6.452 ± 0.170 | 34.900 ± 0.311 | 0.110 ± 0.014 | 0.207 ± 0.004 | |
| Liver | 0.255 ± 0.010 | 0.006 ± 0.002 | 0.067 ± 0.011 | 2.900 ± 0.156 | 74.804 ± 0.580 | 1.850 ± 0.014 | 0.198 ± 0.003 | |
| CA | Muscle | 0.430 ± 0.042 | 0.027 ± 0.011 | 0.067 ± 0.001 | 3.050 ± 0.099 | 17.300 ± 0.580 | 0.050 ± 0.003 | 0.104 ± 0.008 |
| Gill | 0.215 ± 0.083 | 0.021 ± 0.001 | 0.068 ± 0.004 | 3.550 ± 0.339 | 50.702 ± 2.418 | 0.100 ± 0.014 | 0.184 ± 0.003 | |
| Liver | 0.470 ± 0.071 | 0.038 ± 0.010 | 0.072 ± 0.004 | 9.352 ± 0.509 | 67.051 ± 1.470 | 0.102 ± 0.018 | 0.249 ± 0.003 | |
| CC | Muscle | 0.490 ± 0.099 | 0.082 ± 0.018 | 0.067 ± 0.003 | 2.200 ± 0.240 | 29.600 ± 3.309 | 0.100 ± 0.001 | 0.074 ± 0.001 |
| Gill | – | – | – | – | – | – | – | |
| Liver | – | – | – | – | – | – | – | |
| CL | Muscle | 0.235 ± 0.057 | 0.010 ± 0.004 | 0.067 ± 0.008 | 2.800 ± 0.283 | 15.150 ± 0.226 | 0.100 ± 0.016 | 0.065 ± 0.001 |
| Gill | 0.215 ± 0.071 | 0.009 ± 0.002 | 0.068 ± 0.003 | 3.752 ± 0.269 | 73.15 ± 2.546 | 0.150 ± 0.002 | 0.233 ± 0.004 | |
| Liver | 0.685 ± 0.033 | 0.018 ± 0.002 | 0.082 ± 0.011 | 9.306 ± 0.552 | 58.750 ± 1.428 | 0.053 ± 0.003 | 0.217 ± 0.004 | |
| CM | Muscle | 0.249 ± 0.018 | 0.023 ± 0.005 | 0.067 ± 0.007 | 4.160 ± 0.198 | 9.900 ± 0.283 | 0.150 ± 0.017 | 0.061 ± 0.006 |
| Gill | 0.350 ± 0.156 | 0.031 ± 0.001 | 0.069 ± 0.007 | 3.553 ± 0.297 | 13.600 ± 0.707 | 0.202 ± 0.014 | 0.267 ± 0.004 | |
| Liver | 0.455 ± 0.044 | 0.077 ± 0.002 | 0.092 ± 0.014 | 9.350 ± 0.156 | 20.902 ± 1.612 | 0.052 ± 0.004 | 0.249 ± 0.002 | |
| LM | Muscle | 0.255 ± 0.034 | 0.015 ± 0.005 | 0.067 ± 0.001 | 5.100 ± 0.707 | 12.85 ± 0.269 | 0.100 ± 0.003 | 0.076 ± 0.003 |
| Gill | 0.235 ± 0.048 | 0.048 ± 0.011 | 0.068 ± 0.007 | 2.850 ± 0.283 | 24.150 ± 1.216 | 0.352 ± 0.014 | 0.228 ± 0.003 | |
| Liver | 0.090 ± 0.012 | 0.006 ± 0.001 | 0.067 ± 0.001 | 2.602 ± 0.424 | 13.600 ± 1.414 | 0.360 ± 0.016 | 0.201 ± 0.001 | |
| Water(mg/l) × 10–3 | 1.28 ± 0.62 | 0.031 ± 0.007 | 0.051 ± 0.015 | 3.15 ± 1.50 | 15.67 ± 10.70 | 3.28 ± 1.89 | 0.84 ± 0.34 | |
| Sediment | 1.488 ± 0.980 | 0.029 ± 0.021 | 0.079 ± 0.015 | 28.27 ± 5.52 | 73.05 ± 7.65 | 19.95 ± 3.98 | 54.47 ± 15.21 | |
| FAO36 | 1.0 | 1.0 | 0.2 | 10 | 30 | 2.5 | 1.0 | |
The gills and liver of the CC were not sampled.
The levels indicated by the FAO are for fish.
Figure 3.
Distribution of heavy metals in different habitats. Different letters are significantly different (P < 0.05).
Distribution of heavy metals in different tissues of fish
The concentrations of seven heavy metals in various tissues of nine marine fish species were shown in Fig. 4. In this study, the accumulation of heavy metals in most fish was higher in liver or gills than in muscle. The content of Zn in the liver of Muraenesox cinereus was the highest (77.410 mg/kg). And the content of Zn in muscle of Muraenesox cinereus was also relatively high. Moreover, the content of Hg in the liver of Johnius belengeri was the lowest (0.006 mg/kg). The accumulation ability of heavy metals varies among different tissues in the same fish. Our data indicated that the mean concentrations of heavy metals in muscle, liver and gills differed significantly (P < 0.05) in most fish species. The heavy metal Cu content in the liver and gill of Muraenesox cinereus exceeded that of the muscle. The accumulation ability of Cu in other fish was similar to Muraenesox cinereus. However, based on our results, it was found that the ability to accumulate Cr was different from that of Cu. Other heavy metals have the same accumulation capacity as Cu.
Figure 4.
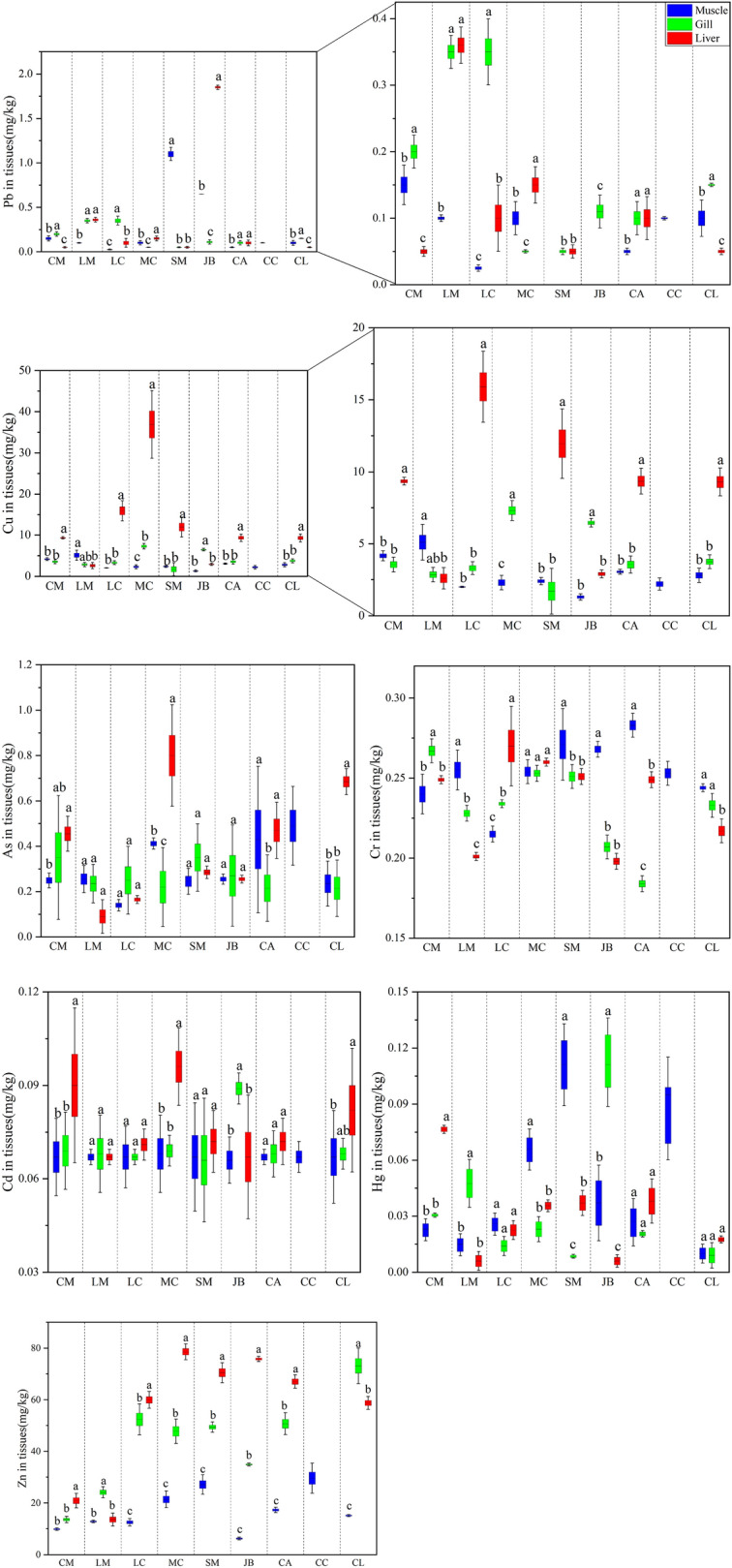
Concentrations of heavy metals (ww) in the muscles (blue), gills (green), and liver (red) of nine fish species collected from Dachen fishing ground, East China Sea. Different letters are significantly different within the same fish (P < 0.05).
Health risk assessment
Estimated daily intake (EDI)
The EDI values of seven heavy metals calculated for nine fish species were given in Table 4. The EDI value of Zn in all fish species were found higher, while those of Hg in most fish species were found lower. The EDI value of seven heavy metals calculated for each fish species was very lower than the tolerable daily intake (TDI) value, which suggested that the daily intake of seven heavy metals via the ingestion of nine fish species in this study would pose no detrimental health risk to humans.
Table 4.
Estimated daily intake (EDI) established for the consumption of fish from Dachen fishing ground, East China Sea (units ug/kg bw/day for EDI and TDI, ww).
| Species | EDI | ||||||
|---|---|---|---|---|---|---|---|
| As | Hg | Cd | Cu | Zn | Pb | Cr | |
| LC | 0.0233 | 0.0141 | 0.0372 | 1.1091 | 6.9597 | 0.0139 | 0.1192 |
| CM | 0.0415 | 0.0125 | 0.0372 | 2.3070 | 5.4902 | 0.0832 | 0.1331 |
| CL | 0.0391 | 0.0055 | 0.0372 | 1.5528 | 8.4016 | 0.0555 | 0.1353 |
| MC | 0.0686 | 0.0363 | 0.0377 | 1.2755 | 11.8676 | 0.0555 | 0.1409 |
| SM | 0.0408 | 0.0616 | 0.0372 | 1.3309 | 15.0564 | 0.6100 | 0.1503 |
| LM | 0.0425 | 0.0080 | 0.0372 | 2.8283 | 7.1261 | 0.0555 | 0.1414 |
| CA | 0.0716 | 0.0147 | 0.0372 | 1.6914 | 9.5939 | 0.0277 | 0.1569 |
| JB | 0.0425 | 0.0205 | 0.0366 | 0.7209 | 3.4660 | 0.3605 | 0.1486 |
| CC | 0.0816 | 0.0455 | 0.0372 | 1.2200 | 16.4150 | 0.0555 | 0.1403 |
| TDI | 2.1437 | 0.0838 | 0.839 | 50040 | 30040 | 1.541 | 30042 |
Non-carcinogenic health risk
The estimated target hazard quotient (THQ) for individual heavy metal from the consumption of various fish species was shown in Table 5. The THQ results for seven heavy metals in nine marine fishes revealed that THQ values for all metal in nine fishes were below one, and the THQ values of different heavy metal of the same species were quite different. And, hazard index (HI) values of combined heavy metals calculated for each fish species were below one. The THQ and HI results suggested that non-carcinogenic health risk from the intake of individual or combined heavy metals in the fish species were not expected for consumers.
Table 5.
The target quotients(THQ) and hazard index(HI) established for the consumption of fish from Dachen fishing ground, East China Sea(ww).
| Species | THQ | HI | ||||||
|---|---|---|---|---|---|---|---|---|
| As | Hg | Cd | Cu | Zn | Pb | Cr | ||
| LC | 0.0776 | 0.0884 | 0.0372 | 0.0277 | 0.0232 | 0.0035 | 8.93E−05 | 0.2576 |
| CM | 0.1381 | 0.0780 | 0.0372 | 0.0577 | 0.0183 | 0.0208 | 9.82E−05 | 0.3501 |
| CL | 0.1303 | 0.0347 | 0.0372 | 0.0388 | 0.0280 | 0.0139 | 1.01E−04 | 0.2829 |
| MC | 0.2285 | 0.2270 | 0.0377 | 0.0319 | 0.0396 | 0.0139 | 7.90E−05 | 0.5786 |
| SM | 0.1359 | 0.3847 | 0.0372 | 0.0333 | 0.0502 | 0.1525 | 1.02E−04 | 0.7938 |
| LM | 0.1414 | 0.0503 | 0.0372 | 0.0707 | 0.0238 | 0.0139 | 7.59E−05 | 0.3372 |
| CA | 0.2385 | 0.0918 | 0.0372 | 0.0423 | 0.0320 | 0.0069 | 1.19E−04 | 0.4488 |
| JB | 0.1414 | 0.1282 | 0.0366 | 0.0180 | 0.0116 | 0.0901 | 1.07E−04 | 0.4260 |
| CC | 0.2717 | 0.2842 | 0.0372 | 0.0305 | 0.0547 | 0.0139 | 8.24E−05 | 0.6922 |
The carcinogenic risk (TR)
Due to the high toxicity of As, Pb, Cd and Cr the target cancer risk (TR) for these four heavy metal elements was estimated and the results were shown in Table 6. The measured TR values of As, Pb and Cd were ranged from (2.33–8.15) × 10–5, (0.01–0.52) × 10–5, (4.68–4.75) × 10–5 and (5.96–7.85) × 10–5, respectively. The results showed calculated TR values were within the risk as acceptable range is 10–6 to 10–4. From the point of As, Pb, Cd and Cr TR values, the fish will not cause carcinogenic effects on humans through food consumption. Among them, two fishes (Sebastiscus marmoratus and Johnius belengeri) were higher TR values of Pb than other fishes, which should be given a great concern.
Table 6.
TR values of As, Pb, Cd and Cr for different species.
| Species | As (× 10–5) | Pb (× 10–5) | Cd (× 10–5) | Cr (× 10–5) |
|---|---|---|---|---|
| LC | 2.33 | 0.01 | 4.68 | 5.96 |
| CM | 5.41 | 0.07 | 4.68 | 6.66 |
| CL | 3.91 | 0.05 | 4.68 | 6.77 |
| MC | 6.92 | 0.05 | 4.75 | 7.04 |
| SM | 4.08 | 0.52 | 4.68 | 7.51 |
| LM | 4.24 | 0.05 | 4.68 | 7.07 |
| CA | 7.15 | 0.02 | 4.68 | 7.85 |
| JB | 4.24 | 0.31 | 4.61 | 7.43 |
| CC | 8.15 | 0.05 | 4.68 | 7.02 |
Discussion
In this study, the amount of muscle protein in fish species ranged from 12.36 to 23.41%. The study reported that the higher protein content observed in the fish was due to its feeding habits43. The protein contents of varies fish were found to be possibly due to their food availability44. And no correlation was observed between protein contents and heavy metals in fish muscle (using Pearson correlation test, P < 0.05). Studies45 also had shown that there was no correlation between heavy metals and protein content in muscle tissue. This is consistent with our study. And the muscle lipid content of fish ranged from 0.48 to 2.54%, where it varied significantly. Younis et al.46 stated lipid content is affected by feeding habits and the territorial food. Nath et al.47 reported that lipid content is influenced by the life cycle and environment. In the present study, no correlation was observed between lipid contents and heavy metals in fish muscle (P < 0.05). The differences in the chemical of marine fishes in this study may be due to difference in species, feeding habits, and this agrees with the study46,48.
This study found that the content of essential micronutrients (Zn, Cu) is higher than that of non-essential micronutrients (Pb, Cd, As, Hg, Cr). It might because the automatic adsorption of Zn and Cu is processed by organisms, which causes the content of Zn and Cu in fish muscles is greater than that of Pb, Cd, As, Hg and Cr. Zn is a component of a variety of enzymes in the body, and Cu is a component of various oxides in the body49.
The amount of heavy metals in the demersal fish was greater than the middle-upper fish (except Cu). This was consistent with the research by Sun et al.50. Demersal fish were easily exposed to heavy metals from sediments, which were seen as the main source of heavy metals in marine fish51,52. The content of Hg in fish muscle living in the demersal was significantly higher than that of the middle-upper layer. UNEP53 research showed that marine fish mercury is methyl mercury, mainly from the marine environment and the food chain transmission. Methyl mercury is primarily caused by the biological and abiotic methylation process of inorganic mercury54. Especially, the process of mercury bioremediation on the surface sediment might resulted in the bottom seawater with higher levels of methyl mercury. The corresponding mercury content of demersal fish was greater than that of middle-upper fish. The mercury content of heavy metals in water and sediment was simultaneously detected. The results suggested the mercury content in the sediment was higher than that in the seawater. However, Cu in demersal fish was lower than that in the middle-upper. The research by Yang et al.55 had also similar founding, the bioavailability of heavy metals in fish with varied habitats being different.
The accumulation ability of heavy metals in different tissues of the same fish was different. Our data indicated that the mean concentrations of heavy metals in muscle, liver and gills differed significantly (P < 0.05) in most fish species. Different fish have different ability to accumulate heavy metals. This may be linked to the lifespan of fish in the water body and the difference in physiological metabolism56. This was in line with the research by Monroy et al.57 on the concentration and distribution of heavy metals in fish. It indicated that the liver and gills were the main organs for heavy metal accumulation in fish. The unique structure of the gills is conducive to the penetration of ions in the water, making the gills the central part of fish to directly absorb heavy metals from the water environment58,59. The enrichment of heavy metals in the liver was mainly associated with the induction and bonding of metallothionein. The liver is a tissue that continually accumulates, biotransforms, and detoxifies. Therefore, measuring the content of heavy metals in the liver was beneficial to assess the level of heavy metal pollutants in the environment60,61. This demonstrated that the primary means of heavy metals entering fish include food intake, branchial membrane adsorption, liver digestion and absorption, and accumulation. However, based on our results, it was found that the ability to accumulate Cr was different from that of Cu. Generally, the content of Cr in muscle was higher than that of the gills and the liver. Since the low absorption rate and a relatively high excretion rate of Cr, the retention rate of Cr in fish is low62, resulting the reduction of Cr to some extent in the liver and gills.
Because the content of heavy metal As in marine organisms is generally relatively high, in marine organisms, arsenic occurs mainly in the fasts of organic compounds, as arsenobetaine27. Its toxicity is expected to be very low, and it can be quickly eliminated from the body after swallowing. The toxic inorganic arsenic generally only accounts for 0.1–10% of the total arsenic content27. Assume that the maximum rate of 10% was used to estimate the level of inorganic arsenic content in fish in the Dachen Sea. In this case, the As content of all the samples in this study was comparatively low. The source of arsenic pollution may be domestic sewage and the use of arsenic-containing pesticides63. However, mean arsenic content (0.301 mg/kg) of this study differs from that of Peng et al.14 (1.600 μg/g, total arsenic). Future studies should address the As forms accumulated in the edible muscle.
Moreover, Pb is toxic to organisms, can disrupt normal metabolic activities, transmitted through the food chain, enriched and accumulated, and can be transformed into more toxic organic compounds under certain conditions64. Lee et al.65 had found that long-term exposure to high levels of Pb can damage the brain, liver and kidneys and even reduce the function of the nervous system, eventually leading to death. This study found that the enrichment trend of Pb was similar to that of Cu, and its content in the gills and liver was higher than that muscle. The average content of Pb (0.264 mg/kg) in this study was consistent with that of Li66 (0.128 mg/kg) but different from that of Peng et al.14 (5.700 mg/kg). It may be caused by the development of coastal industries in Zhejiang province over the past few years. The concentration of Pb in the aquatic environment is higher, which exceeds the fish body’s discharge capacity, resulting in a parallel accumulation of Pb in the fish body (gills, liver, muscle). Studies63,67 have reported that the industrial wastewater and domestic sewage generated by the pharmaceutical, chemical and electronic electroplating industries in the Jiaojiang Estuary water were directly discharged into the water, resulting in high lead content. Dachen Island is located in the southeast of Jiaojiang River estuaries and is highly susceptible to lead pollution from the Jiaojiang River estuaries. Besides, due to the development and utilization of fishing and ship transportation in the Dachen sea area in recent years, the wastewater produced by the local maritime transport has also caused lead pollution to a certain extent.
The heavy metal pollution is one of important environmental factors that can considerably affect human health. The muscles of fish may enter into human metabolism through food consumption, leading to serious health risks68. Therefore, this study assessed the impact on human health by studying heavy metal pollution in fish in the Dachen Island region. Moreover, it is also conducive to the construction and management of marine ranches10.
The EDI reflects the daily exposure to the heavy metal, and is executed to avoid any harmful effect on human health28. The EDI values lower than TDI guidelines suggested a lower possible health risk of the heavy metals to the consumers. However, it would not be wise to take it as a permanent measurement to reach a final conclusion28,69.
The THQ and HI values below one. There was no potential non-carcinogenic effect for the consumers due to intake of the fish species. Studies carried out by several authors in similar conditions were in line with our results7,9,28. However, due to multiple simultaneous pollutants, human could dramatically suffer in the long run70. The THQ values of different heavy metal of the same species were quite different. It was found that the risk of Cr was relatively low. Many kinds of literature71,72 have suggested that the potential risk of Cr was low, which was also proved by this study, and the potential health risk of Cr was the lowest, which may be ascribed to its higher RFD value.
The results showed calculated TR values were within the risk as acceptable range is 10–6 to 10–4, and consumers were less prone to carcinogenic. In fact, 90% of the carcinogenic risk is observed in the As contaminated acquatic food items28. The inorganic form of As is more lethal than organic one27,73,74, and only 10% of the total As can be assessed as inorganic state5,28. In this study, although the TR value of As was within an acceptable range, regular monitoring of fish in this fishery was still essential.
In this study, we assumed that the intake of heavy metals was equivalent to the absorption of heavy metals, without considering the time and residual content of heavy metals in the human body. This hypothesis improves the calculation of carcinogen risk. Some heavy metals may be excreted. The heavy metals levels in blood, and urine are suggested to be measured in the future.
Conclusion
In this study, the results showed that all nine fish species were a good source of protein and lipids. The concentrations of seven heavy metals in various tissues of nine marine fish species were compared and found to be varied considerably among tissues and species. Maximum and minimum of heavy metals concentrations in the fish respectively were determined as Zn and Hg. The accumulation capacity of heavy metal (except Cr) in the liver and gills was higher than that of muscle. The cumulative capacity of heavy metals in fish muscle was linked to the water layer they live, that was, the demersal fish contained more heavy metal than the middle-upper ones (except Cu). From the perspective of human health, the EDI of each element was lower than the respective recommended tolerable daily intake. The THQ and HI values of fish indicated that fish in the Dachen fishing ground were safe to eat. From the point of As, Pb, Cd and Cr TR values, the fish may not cause carcinogenic effects on humans through food consumption. However, the carcinogenic risk (TR) of As and Cr was close to the critical limit (10–4). Therefore, regular and long-term monitoring of the heavy metal content of fish in this fishery is recommended.
Acknowledgements
The authors thank all the participants.
Author contributions
H.H.: supervision, conceptualization, methodology, writing-review and editing, resources and funding acquisition. Y.L.: investigation, data curation, original draft. X.Z.: investigation, data curation. Z.W.: investigation, data curation. Z.W.: investigation, writing-review and editing. X.C.: investigation.
Funding
This work was funded by National key R&D Program of China (Grants 2019YFD0901302, 2018YFD0900704).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO Food and Agriculture Organization) Fishery Information Data and Statistics Unit. FISHSTAT + Databases and Statistics. Food and Agriculture Organization of the United Nation; 2016. [Google Scholar]
- 2.Ke P, Wang WX. Trace metal contamination in estuarine and coastal environments in China. Sci. Total Environ. 2012;421–422(Apr.1):3–16. doi: 10.1016/j.scitotenv.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Jarup L. Hazards of heavy metal contamination. Brit. Med. Bull. 2003;68(1):167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 4.Golden C, Allison EH, Cheung W, Dey MM, Halpern BS, Mccauley DJ, et al. Nutrition: Fall in fish catch threatens human health. Nature. 2016;534(7607):317–320. doi: 10.1038/534317a. [DOI] [PubMed] [Google Scholar]
- 5.Baki AM, Khan FM, Akter J, et al. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018;159:153–163. doi: 10.1016/j.ecoenv.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Saha N, Mollah M, Alam MF, Rahman MS. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70:110–118. doi: 10.1016/j.foodcont.2016.05.040. [DOI] [Google Scholar]
- 7.Gu YG, Lin Q, Huang HH, Wang LG, Ning JJ, Du FY. Heavy metals in fish tissues/stomach contents in four marine wild commercially valuable fish species from the western continental shelf of south china sea. Mar. Pollut. Bull. 2017;114(2):1125–1129. doi: 10.1016/j.marpolbul.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Korkmaz C, Özcan A, Ersoysal Y, Köroğlu MA, Erdem C. Heavy metal levels in muscle tissues of some fish species caught from north-east mediterranean: Evaluation of their effects on human health. J. Food Compos. Anal. 2019;81:1–9. doi: 10.1016/j.jfca.2019.04.005. [DOI] [Google Scholar]
- 9.Rahman MS, Hossain MS, Ahmed MK, Akther S, Jolly YN, Akhter S, et al. Assessment of heavy metals contamination in selected tropical marine fish species in Bangladesh and their impact on human health. Environ. Nanotechnol. Monit. Manage. 2019;11:25. [Google Scholar]
- 10.Zhou XJ, Zhao X, Zhang SY, Lin J. Marine ranching construction and management in East China Sea: Programs for sustainable fishery and aquaculture. Water. 2019;6:25. [Google Scholar]
- 11.Lu C. Thoughts on promoting the construction of Dachen Ecological Island. Decis. Mak. Consult. 2017;03:80–83. doi: 10.1002/bdm.1922. [DOI] [Google Scholar]
- 12.Liu YY, Ren M, Gu Y. Study on the planning and construction of Taizhou Dachen Marine ecological special reserve. Mar. Dev. Manage. 2012;29(05):113–115. [Google Scholar]
- 13.Wang JY, Wang YC, Lou JH. Analysis on heavy metal pollution in major seafoods from Zhoushan Fishery, China. Chin. J. Epidemiol. 2012;33(10):1001–1004. [PubMed] [Google Scholar]
- 14.Peng F, Yin J, Wang Q, Ni TH, Lin J, Li JY. Occurrence and risk assessment of heavy metals and polycyclic aromatic hydrocarbons in marine organisms from Yuwai Fishing Ground. Asian J. Ecotoxicol. 2019;14(01):168–179. [Google Scholar]
- 15.Liu Q, Liao Y, Xu X, Shi X, Shou L. Heavy metal concentrations in tissues of marine fish and crab collected from the middle coast of Zhejiang Province, China. Environ. Monit. Assess. 2020;192:5. doi: 10.1007/s10661-019-7972-4. [DOI] [PubMed] [Google Scholar]
- 16.AOAC . Association of Official Analytical Chemists. Official Methods of Analysis. 16. Arlington; 2016. [Google Scholar]
- 17.Varol M, Kaya GK, Sünbül MR. Evaluation of health risks from exposure to arsenic and heavy metals through consumption of ten fish species. Environ. Sci. Pollut. Res. 2019;26(32):33311–33320. doi: 10.1007/s11356-019-06450-x. [DOI] [PubMed] [Google Scholar]
- 18.SOA . GB 17378–2007. Standardization Administration of the People’s Republic of China (SAC); 2007. [Google Scholar]
- 19.Yi Y, Yang Z, Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River Basin. Environ. Pollut. 2011;159(10):2575–2585. doi: 10.1016/j.envpol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Lie A, Poa A, Aaec D, It A, Eob D. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin river, Nigeria. Toxicol. Rep. 2019;6:1–9. doi: 10.1016/j.toxrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Lin J, Pan Q, Ma Y, Zhang S, Li Y. Review on the evaluation methods of food safety of edible fish in Meijiang River. Guangdong Chem. Ind. 2019;46(11):122–123. [Google Scholar]
- 22.Yue DD, Wang LM, Fang H, Wang Q, Ji WW, Ruan W, Xiao L, Zheng L. Relationship between aquatic product consumption and income gap between Chinese urban and rural residents. Fish. Inf. Strat. 2018;33(337):4–11. [Google Scholar]
- 23.USEPA (U.S. Environmental Protection Agency) Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Volume II. Risk Assessment and Fish Consumption Limits. (EPA 823-B-00-008) United States Environmental Protection Agency; 2000. [Google Scholar]
- 24.Wang L, Chen F, Ma Q, Yao L, Xu Z, Zhao X, Liang R. Heavy metal pollution and health risk assessment of fish in the Huizhou section of the Dongjiang River. J. Ecol. Rural Environ. 2017;33(01):70–76. [Google Scholar]
- 25.Wang X, Sato T, Xing B, Tao S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350(1/3):28–37. doi: 10.1016/j.scitotenv.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 26.USEPA . Risk-Based Concentration Table. United States Environmental Protection Agency; 2009. [Google Scholar]
- 27.Shang D, Zhao Y, Guo Y, Zhai Y, Ning J, Sheng X, Zhang M. Safety evaluation of arsenic and arsenic compounds in food. Chin. Fish. Qual. Std. 2012;04:21–32. [Google Scholar]
- 28.Ahmed ASS, Sultana S, Habib A, Ullah H, Sarker MSI. Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS One. 2019;14(10):e0219336. doi: 10.1371/journal.pone.0219336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quanyou G, et al. Quality differences of large yellow croaker (Pseudosciaena crocea) cultured in deep-water sea cages of two China Regions. Spine. 2018;9(9):1–8. [Google Scholar]
- 30.Zhu AY, Xie JY, Jiang LH, Lou B. The nutritional composition and evaluation in muscle of S. marmoratus. Acta Nutr. Sin. 2011;33(06):621–623. [Google Scholar]
- 31.Xu XH, Liu X, Yan BL, Xu JT, Xu GC, Shao YZ, Shen C. Analysis and quality evaluation of the muscle nutrients of Wild Pirate Goby in Lianyungang Sea. Jiangsu Agric. Sci. 2012;1:261–265. [Google Scholar]
- 32.Jiang XH, Yang PM. Nutritional composition analysis in muscle of Tapertail Anchovy Coilia nasus from Dayyang River before and after reproduction. Fish. Sci. 2021;40(06):835–842. [Google Scholar]
- 33.Zeng SK, Zhang CY, Jiang ZH. Study on the comparison of the food nutrient contents between the muscle and head of Muraenesox cinereus. Mar. Sci. 2002;05:13–15. [Google Scholar]
- 34.Guo H, Xu M, Shen YC, Ye N, Cao YT. Analysis and evaluation of nutritional composition in the muscle of Johnius belangerii. Feed Ind. 2016;37(18):24–26. [Google Scholar]
- 35.Wang YH, Lv ZH, Gao TX, Zheng GX. Research on nutritional components of Lateolabrax sp and L. japonicus. Progress Fish. Sci. 2003;02:35–39. [Google Scholar]
- 36.Nauen, C. E. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular (FAO) no 764. (1983).
- 37.JECFA (Joint FAO/WHO Expert Committee on Food Additives) Evaluation of Certain Food Additives and Contaminants. Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, No 776. World Health Organization; 1989. [PubMed] [Google Scholar]
- 38.FAO . The State of the World Fisheries and Aquaculture. FAO Fisheries and Aquaculture Dept; 2014. [Google Scholar]
- 39.JECFA (Joint FAO/WHO Expert Committee on Food Additives) Evaluation of Certain Food Additives and Contaminants. Seventythird Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, No 960. World Health Organization; 2011. [Google Scholar]
- 40.JECFA (Joint FAO/WHO Expert Committee on Food Additives) Evaluation of Certain Food Additives and Contaminants. Twenty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No 683. World Health Organization; 1982. [Google Scholar]
- 41.EFSA (European Food Safety Authority) Scientific opinion on lead in food. EFSA J. 2010;8(4):1570. [Google Scholar]
- 42.EFSA (European Food Safety Authority) Scientific opinion on dietary reference values for chromium. EFSA J. 2014;12(10):3845. doi: 10.2903/j.efsa.2014.3845. [DOI] [Google Scholar]
- 43.Younis EM, Abdel-Warithl-Shayia AAAS. Chemical composition and mineral contents of six commercial fish species from the Arabian Gulf coast of Saudi Arabia. J. Anim. Vet. Adv. 2011;10(23):3063–3069. [Google Scholar]
- 44.Jakhar K, Jakhar JK, Pal AK, Reddy AD, Vardia HK. Fatty acids composition of some selected Indian fishes. Afr. J. Basic Appl. Sci. 2012;4(5):155–160. [Google Scholar]
- 45.Patrizia C, Francesca T, Rosaria S. Heavy metal bioaccumulation and metallothionein content in tissues of the sea bream Sparus aurata from three different fish farming systems. Environ. Monit. Assess. 2010;20:1–4. doi: 10.1007/s10661-009-0948-z. [DOI] [PubMed] [Google Scholar]
- 46.Younis EM, Abdel-Warith A, Al-Asgah NA, Elthebite SA, Rahman MM. Nutritional value and bioaccumulation of heavy metals in muscle tissues of five commercially important marine fish species from the red sea. Saudi J. Biol. Sci. 2020;20:20. doi: 10.1016/j.sjbs.2020.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nath AK, Patra A, Sen B, Dey D, Das I, Mukherjee I, Paul S. Fatty acid compositions of four edible fishes of Hooghly Estuary, West Bengal, India. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:208–218. [Google Scholar]
- 48.Saeed S. Impact of environmental parameters on fish condition and quality in Lake Edku, Egypt. Egypt. J. Aquat. Biol. Fish. 2013;17(1):101–112. [Google Scholar]
- 49.Xiao MS, Wang S, Bao FY, Feng C. Enrichment of heavy metals in economic aquatic animals in huaihe river segment of Bengbu sampling points. Res. Environ. Sci. 2011;24(8):942–948. [Google Scholar]
- 50.Sun WP, Liu XY, Pan JM, Weng HX. Levels of heavy metals in commercial fish species from the near-shore of Zhejiang Province. J. Zhejiang Univ. (Sci. Ed.) 2012;26:1–21. [Google Scholar]
- 51.Djikanovic V, Skoric S, Spasic S, Naunovic Z, Lenhardt M. Ecological risk assessment for different macrophytes and fish species in reservoirs using biota-sediment accumulation factors as a useful tool. Environ. Pollut. 2018;241(4):1167. doi: 10.1016/j.envpol.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 52.Wuana R, Ogbodo C, Itodo UAPD, Eneji I. Ecological and human health risk assessment of toxic metals in water, sediment and fish from Lower Usuma Dam Abuja, Nigeria. J. Geosci. Environm. Protect. 2020;08:82–106. doi: 10.4236/gep.2020.85006. [DOI] [Google Scholar]
- 53.UNEP Chemicals . Inter-Organization Programm for the sound Management of Chemicals, Global Mercury Assessment. UNEP Chemicals; 2002. [Google Scholar]
- 54.Hammerschmidt CR, Fitzgerald W. Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ. Sci. Technol. 2004;38(5):1487–1495. doi: 10.1021/es034528q. [DOI] [PubMed] [Google Scholar]
- 55.Yang YF, Liu JR, Lin CR, Liang HL, Xie WQ, Feng JX. Heavy metal characterization of fish species in a typical sea area of Guangdong-Hong Kong-Macau Greater Bay Area. Trans. Oceanol. Limnol. 2021;43(03):107–116. [Google Scholar]
- 56.Wang H, Fang F, Xie H. Research situation and outlook on heavy metal pollution in water environment of China. Guangdong Trace Elem. Sci. 2010;17:14–18. [Google Scholar]
- 57.Monroy M, Maceda-Veiga A, Sostoa AD. Metal concentration in water, sediment and four fish species from lake Titicaca reveals a large-scale environmental concern. Sci. Total Environ. 2014;487(15):JUL.15–244. doi: 10.1016/j.scitotenv.2014.03.134. [DOI] [PubMed] [Google Scholar]
- 58.Canli M, Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six mediterranean fish species. Environ. Pollut. 2003;121(1):129–136. doi: 10.1016/S0269-7491(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 59.Makedonski L, Peycheva K, Stancheva M. Determination of heavy metals in selected black sea fish species. Food Control. 2017;72:313–318. doi: 10.1016/j.foodcont.2015.08.024. [DOI] [Google Scholar]
- 60.Shinn C, Dauba F, Grenouillet G, Guenard G, Lek S. Temporal variation of heavy metal contamination in fish of the river lot in Southern France. Ecotoxicol. Environ. Saf. 2009;72(7):1957–1965. doi: 10.1016/j.ecoenv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Nabavi SF, Nabavi SM, Latifi AM, Eslami S, Ebrahimzadeh MA. Determination of trace elements level of pikeperch collected from the Caspian sea. Bull. Environ. Contam. Toxicol. 2012;88(3):401–405. doi: 10.1007/s00128-011-0513-7. [DOI] [PubMed] [Google Scholar]
- 62.Chen HX, Xing WU, Ran BI, Li-Xia LI, Gao M, Dan LI, et al. Mechanisms of cr(VI) toxicity to fish in aquatic environment: A review. Chin. J. Appl. Ecol. 2015;10:3226–3234. [PubMed] [Google Scholar]
- 63.Ding X, Si YE, Jing L. The heavy metals distribution pattern and geochemical provinces of the surficial sediments offshore Zhejiang. Mar. Geol. Front. 2010;26(12):1–8. [Google Scholar]
- 64.Dai W. Research progress on the toxicity of lead in aquatic animals. J. Anhui Agric. Sci. 2010;38(011):5819–5820. [Google Scholar]
- 65.Lee KG, Kweon HY, Yeo JH, Woo SO, Han SM, Kim JH. Characterization of tyrosine-rich antheraea pernyi silk fibroin hydrolysate. Int. J. Biol. Macromol. 2011;48(1):223–226. doi: 10.1016/j.ijbiomac.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 66.Li TF. Heavy metal content and food risk of aquatic organisms in Jiaojiang district of Taizhou city. J. Food Saf. Qual. 2019;10(16):5561–5567. [Google Scholar]
- 67.Wu ZY, Yang SY, Su N, Guo YL, Bi L. Distribution and pollution assessment of heavy metals in the sediments of Jiaojiang River. Mar. Geol. Q. Geol. 2018;38(01):96–107. [Google Scholar]
- 68.Bravo AG, Loizeau JL, Bouchet S, Richard A, Rubin JF, Ungureanu VG, et al. Mercury human exposure through fish consumption in a reservoir contaminated by a chlor-alkali plant: Babeni reservoir (Romania) Environ. Sci. Pollut. R. 2010;17(8):1422–1432. doi: 10.1007/s11356-010-0328-9. [DOI] [PubMed] [Google Scholar]
- 69.Vu CT, Lin C, Yeh G, Villanueva MC. Bioaccumulation and potential sources of heavy metal contamination in fish species in Taiwan: Assessment and possible human health implications. Environ. Sci. Pollut. Res. 2017;24:19422–19434. doi: 10.1007/s11356-017-9590-4. [DOI] [PubMed] [Google Scholar]
- 70.Li PH, Kong SF, Geng CM, Han B, Lu B, Sun RF, et al. Assessing the hazardous risks of vehicle inspection workers’ exposure to particulate heavy metals in their work places. Aerosol. Air Qual. Res. 2013;13:255–265. doi: 10.4209/aaqr.2012.04.0087. [DOI] [Google Scholar]
- 71.Saha N, Zaman MR. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013;185(5):3867–3878. doi: 10.1007/s10661-012-2835-2. [DOI] [PubMed] [Google Scholar]
- 72.Miri M, Akbari E, Amrane A, Jafari SJ, Taghavi M. Health risk assessment of heavy metal intake due to fish consumption in the Sistan Region, Iran. Environ. Monit. Assess. 2017;189(11):583. doi: 10.1007/s10661-017-6286-7. [DOI] [PubMed] [Google Scholar]
- 73.Kalantzi I, Pergantis SA, Black KD, Shimmield TM, Papageorgiou N, Tsapakis M, et al. Metals in tissues of seabass and seabream reared in sites with oxic and anoxic substrata and risk assessment for consumers. Food Chem. 2016;194(1):659–670. doi: 10.1016/j.foodchem.2015.08.072. [DOI] [PubMed] [Google Scholar]
- 74.Zhong W, Zhang Y, Wu Z, Yang R, Chen X, Yang J, et al. Health risk assessment of heavy metals in freshwater fish in the central and Eastern North China. Ecotoxicol. Environ. Saf. 2018;157(AUG):343–349. doi: 10.1016/j.ecoenv.2018.03.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.