Abstract
The chemical quality of soil carbon (C) inputs is a major factor controlling litter decomposition and soil C dynamics. Mycorrhizal fungi constitute one of the dominant pools of soil microbial C, while their litter quality (chemical proxies of litter decomposability) is understood poorly, leading to major uncertainties in estimating soil C dynamics. We examined litter decomposability of arbuscular mycorrhizal (AM) and ectomycorrhizal (EM) fungal species using samples obtained from in vitro cultivation. We showed that the chemical composition of AM and EM fungal mycelium differs significantly: EM fungi have higher concentrations of labile (water-soluble, ethanol-soluble) and recalcitrant (non-extractable) chemical components, while AM fungi have higher concentrations of acid-hydrolysable components. Our results imply that differences in decomposability traits among mycorrhizal fungal guilds represent a critically important driver of the soil C cycle, which could be as vital as is recognized for differences among aboveground plant litter.
Subject terms: Carbon cycle, Carbon cycle, Soil microbiology
Chemical profiles of arbuscular (AM) and ectomycorrhizal (EM) fungi reveal that differences in decomposability-relevant chemistry are larger between AM and EM fungi than across plant functional groups.
Introduction
The soil carbon (C) cycle is a critically important process for both ecosystem functioning and mitigation of climate change1,2. A major knowledge gap in this field is the lack of data on belowground influxes of C, and their fate in terms of contribution to stable C pools3,4. A particularly poorly understood aspect is the magnitude of C input into the soil pool of potentially decomposable C components as provided by belowground organisms, and the decomposability patterns of these organisms5. The chemical proxies of below-ground organisms’ litter decomposability (referred to as “decomposability” hereafter), for example, the amounts of components extractable with various extraction techniques, of belowground organisms are among the key factors that influence the soil C turnover process6–8. The decomposability of substrates could influence the growth efficiency of new microbial biomass9 and arguably mediate the ultimate fate of soil C, i.e., to be sequestered or respired10. However, due to the large uncertainty about the contribution of belowground organisms and their decomposability, until now, the largest known source of variability in the decomposability of C inputs into the soil has been associated with differences among plant species in terms of aboveground plant litter decomposability11–13. However, as soil organisms are excluded from these assessments we might be an underestimating of the true variability in the decomposability of C inputs.
Our knowledge about the factors that control the decomposability of C compounds entering into the soil pool through residues of microorganisms, especially so from widespread soil-borne fungi, is extremely limited. In soil ecosystems, mycorrhizal fungi living in symbiosis with plant roots are among the key soil microorganisms controlling the exchange of C and nutrients between soil and plants14,15. The living and dead biomass of these microorganisms constitute one of the most dominant pools of soil microbial C16,17. Depending on the soil ecosystem environment and mycorrhizal type, mycorrhizal hyphal biomass can constitute up to half of the standing mycelial biomass18 and one-third of total microbial biomass16. Mycorrhizal fungi are important C sinks of net primary production (NPP)19,20, and depending on the mycorrhizal guild, the annual mycelial accumulation can reach around 175–200 g C m−2, 21,22. This is particularly evident in some forest ecosystems, where the allocation of photosynthesized C into fungi can represent up to 30% of the NPP21,23. Yet, the magnitude of the potential contribution of mycorrhizal fungal pools to long-term soil C storage is unknown. Hence, a quantitative assessment of the chemical composition of microorganisms relevant for assessing the decomposability of microbial necromass is critically needed to narrow down the uncertainties in estimating belowground contributions to soil C pools24,25.
Among the four principal types of mycorrhiza, the two globally dominant ones are arbuscular mycorrhiza (AM) and ectomycorrhiza (EM)26. These soil fungi associate with the roots of most terrestrial plants26, and are predominant across the majority of the terrestrial vegetated areas27. Ecophysiological traits of these two main guilds of mycorrhizal fungi differ in many aspects (e.g., in the ability of enzymatic degradation of organic matter)28,29. Also, the microscopic structure of AM and EM fungal hyphae differs5. EM fungal hyphae have thicker walls, pigmentation, and septa between cells, and are generally believed to have a longer life span than AM fungal hyphae17,30. These differences in morphology could potentially determine the decomposability of EM and AM fungal litter, and have raised the hypothesis that dead EM hyphae are likely more recalcitrant to decomposition than AM hyphae5. However, our knowledge about the chemical differences among mycorrhizal fungal guilds, particularly on the chemical components that contribute differently to necromass decomposition, is remarkably limited.
Thus far, studies of the impacts of decomposition of fungal mycelium on soil organic matter (SOM) have focused primarily on EM and ericoid mycorrhizal fungi31, and have examined mostly the abundance of individual chemical components in the fungal mycelium, such as concentrations of nitrogen, chitin and melanin. The latter is known to be negatively correlated to necromass decomposition of mycorrhizal fungal biomass10,32. While the outcomes of these analyses shed new light on ecophysiological traits of mycorrhizal fungi at the individual level, they (1) do not provide a comprehensive assessment of the potential fate of fungal biomass in the process of organic matter decomposition, and (2) neglect the most ancient and widespread mycorrhizal fungal guild, currently associated with the largest part of Earth’s terrestrial vegetation – the AM fungi26,27.
The objective of this study was to fill a main knowledge gap in the soil C cycle by examining inherent differences between EM and AM fungi in terms of their ultimate decomposability potential. Similar to plant litter residues that have a variety of components that differ in recalcitrance33,34, variability of which among different types of plants, for instance among plant functional groups, have been recognized as the major factor controlling soil C dynamics for decades11–13, soil fungi also consist of components of distinct decomposability25. Most fungi contain relatively recalcitrant components, such as melanin, that require costly oxidative enzymes for further decomposition35, as well as relatively labile components (e.g., chitin) that are utilized as a source of C and N for the soil microbial community36. Upon release, these components enroll in principally different types of physical and chemical interactions with mineral surfaces and soil aggregates3,37. Yet the comprehensive understanding of principal differences among EM and AM fungi in terms of their decomposability is lacking.
An important reason underpinning this knowledge gap is the need for samples of in vitro pure biomass of mycorrhizal fungi to examine their chemical composition. For the AM fungi, this constitutes a particular challenge due to their obligate symbiotic lifestyle, which requires a suitable host root established on a poor medium to avoid any contamination by unwanted microbes. Using unique methods of cultivation of mycorrhizal fungi38–40 established in the laboratory of mycology of the UCLouvain (Belgium), we cultivated multiple species of AM fungi under in vitro culture conditions and obtained amounts of fungal mycelia sufficient to examine their chemical compositions. To assess the differences in chemical traits between AM and EM fungal mycelium, we also cultivated EM fungi in vitro following standard laboratory techniques41. We subsequently assessed the chemical recalcitrance of AM and EM fungal mycelium. With this dataset, we tested two hypotheses crucial to understanding the contribution of major mycorrhizal fungal guilds to the soil C cycle:
AM and EM fungal guilds differ principally in their chemical composition traits relevant for decomposability.
Differences of decomposability between AM and EM fungal guilds are larger than the differences among litters from distinct plant functional types.
Results and discussion
Distinct chemical composition of AM and EM fungi
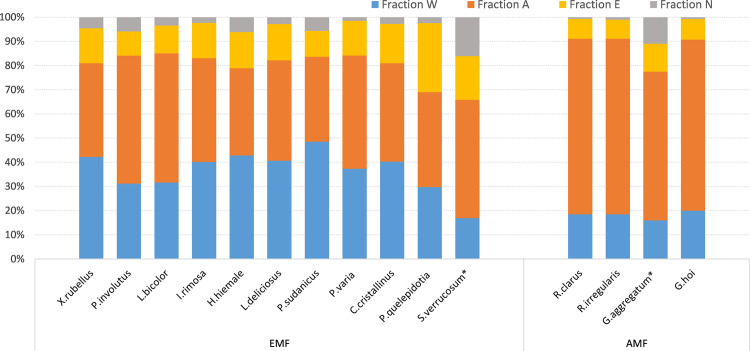
We used 11 species of EM fungi and 4 species of AM fungi from in vitro cultures (CBS/MUCL number see Supplementary Table S1): EM fungal species are Xerocomus rubellus, Paxillus involutus, Laccaria bicolor, Inocybe rimosa, Hebeloma hiemale, Lactarius deliciosus, Phaeogyroporus sudanicus, Peziza varia, Cortinarius cristallinus, Peziza quelepidotia, and Scleroderma verrucosum; AM fungal species are Rhizophagus clarus, Rhizophagus irregularis, Glomus aggregatum and Glomus hoi. Samples of dried fungal biomass were examined for water-soluble, acid-hydrolyzable, ethanol-soluble, and non-extractable components (hereafter W, A, E, N components, respectively), allowing direct comparison of AM and EM fungi for the entire suite of recalcitrance traits (Fig. 1).
Fig. 1. Chemical fractions of mycorrhizal biomass.
Relative abundance of water-soluble (W), acid-hydrolyzable (A), ethanol-soluble (E) and non-extractable (N) components in AM and EM fungi. Mycelia of S.verrucosum* and G.aggregatum* were assessed in a mixture with plant litter (details see Methods section).
We found that the biomass of AM fungi exhibits a distinct set of decomposability-related traits compared to that of EM fungi (outcomes of a perMANOVA test on the WAEN components: R2 = 0.617, p = 0.002, df = 1, for more details see Supplementary Table S3; dispersions of beta diversity, p = 0.294. In this analysis, data for individual fungal species were treated as replicates within AM (n = 4) and EM (n = 11) fungal guilds). The unambiguous difference between centroids of AM and EM fungi in the multidimensional space of WAEN components (Fig. 2) suggests that these two groups of fungi are likely to contribute to different pathways of soil C transformations as being direct sources of soil C compounds.
Fig. 2. Principal coordinate analysis of mycelium chemistry.
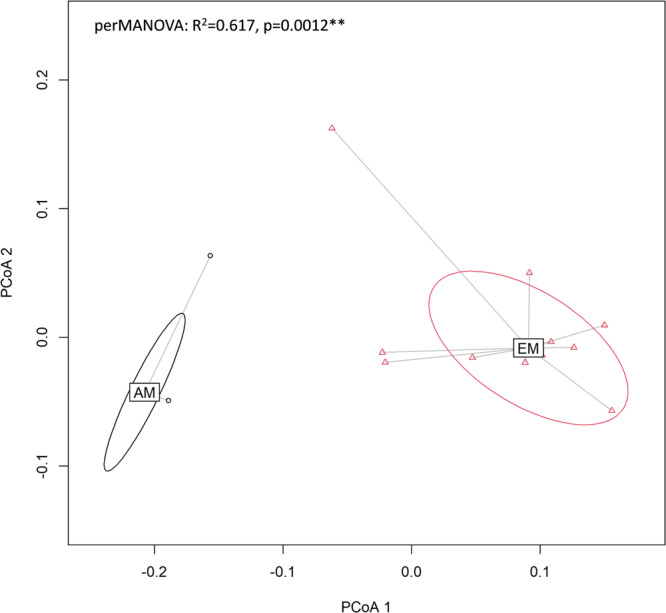
Clustering and centroids of AM and EM mycorrhizal fungal species in a multidimensional space of WAEN components.
Analyses of individual chemical compositions
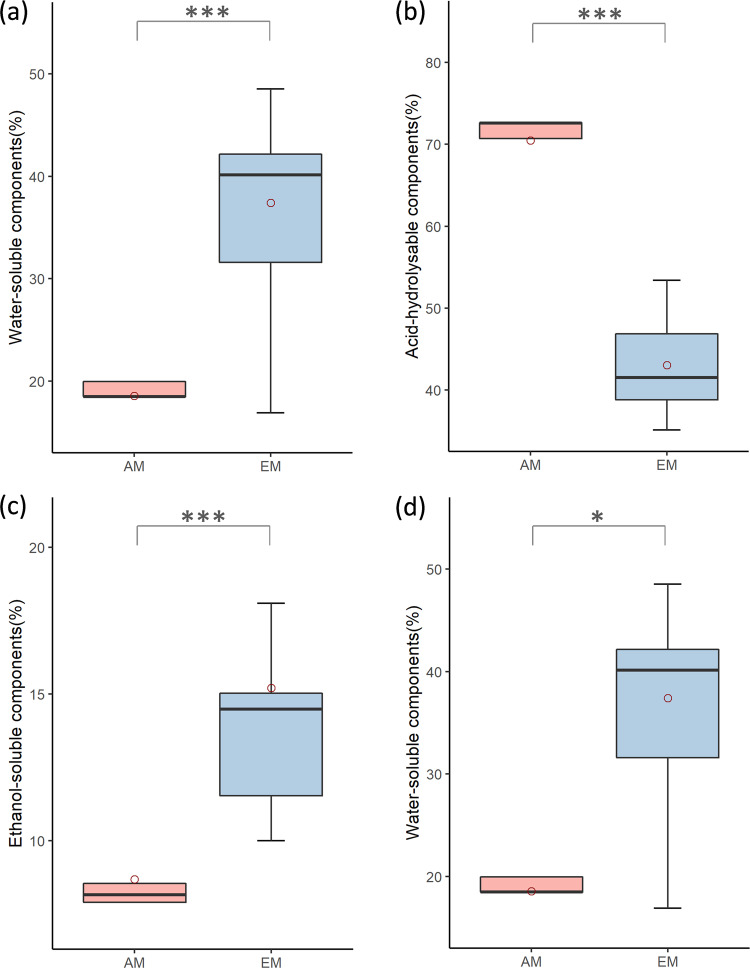
Subsequently, we examined which individual WAEN components differ among AM and EM fungi. We found that concentrations of the most easily decomposable component (W), the ethanol-soluble fraction (E) and the most recalcitrant component (N) were significantly higher in EM fungi (Mann–Whitney tests with data for individual fungi within a fungal guild treated as replicates; p < 0.001, p < 0.001, and p < 0.05, respectively; Fig. 3a,c, and d). In contrast, AM fungi had a significantly higher A fraction (Mann–Whitney test conducted in the same manner as for W, A, and N components: p < 0.001; Fig. 3b). While both A and E components are of intermediate recalcitrance, acid-hydrolysable components have been shown to have higher decomposability than ethanol-soluble components42. The higher relative amount of E and N components in EM fungi compared to that of AM fungi constitutes novel empirical evidence in support of previous suggestions24,43,44 that per fungal biomass units of EM fungi provide an important contribution to the soil pool of intact or partially oxidized mycorrhizal fungal biomass. At the same time, the high abundance of most easily decomposable W components supports empirical evidence of the high rate decomposability of EM fungal mycelium during the initial stages of decomposition45,46.
Fig. 3. Boxplots of WAEN components in AM and EM fungi.
a Water-soluble components, P < 0.001, b Acid- hydrolysable components, P < 0.001, c Ethanol-soluble components, P < 0.001, d Non-extractable components, P < 0.05. All P values refer to the Mann–Whitney test, AM (n = 4) and EM (n = 11). Upper and lower limits of the box- quartiles around the weighted-mean, horizontal lines within boxes- weighted-median values within each mycorrhiza group, and red circles - weighted-mean of each mycorrhiza group.
Hereto we compared four plant functional types: evergreen trees, deciduous trees, evergreen shrubs, herbaceous plants. The differences between AM and EM mycorrhizal fungal species in chemical recalcitrance of litter, measured as the relative abundance of WAEN components, was nearly twice as large as the differences in chemical recalcitrance of litter between plant species of distinct functional types. For the particular case of the water-soluble components, it was even three times higher (Table 1). The effect sizes (η2) of the difference between mycorrhizal fungal guilds for each individual WAEN component were higher. This was particularly evident for the major components of W and A (see Fig. 1) in the mycelium, which comprised the majority of the variation in chemical components. In contrast, a similar analysis conducted for plant species grouped into functional types (for details see Methods section) showed that the effect size of chemical differences of plant functional types was much smaller, and only acid-hydrolysable components contributed to the major variation in the group. Taken together, this suggests that the potential contribution to distinct pathways of C transformations differs markedly between mycorrhizal fungal guilds, and that differences in the decomposability pathways of mycorrhizal fungal material are even more striking than the differences observed in leaf litter among plant functional types, till now considered as one of the most important factors determining soil C circulation. Thus, chemical differences between mycorrhizal fungi types might be essential underestimated sources of (variation in) below-ground soil C dynamics.
Table 1.
Comparing effect sizes of WAEN conposition differences in plant litter and mycorrhizal litter.
| Water-soluble | Acid-extractable | Ethanol-soluble | Non-extractable | |
|---|---|---|---|---|
| Mycorrhizal fungal guilds | 0.68 | 0.76 | 0.46 | 0.26 |
| Plant functional types | 0.21 | 0.41 | 0.28 | 0.20 |
Effect sizes (η2, one-way ANOVA) of chemical composition differences in leaf litter within plant functional types and mycelial biomass within mycorrhizal fungal guilds.
Our test of chemical recalcitrance of mycorrhizal mycelium biomass of multiple EM and AM fungal species provides the first empirical evidence of the inherent difference between AM and EM fungi in terms of their chemical composition related to the decomposition pathway. Our study focussed on members of the globally most predominant family - Glomeraceae47. It remains to be determined whether less frequent AM fungal families with different life-history strategies potentially differ in their chemical composition.
Differences between AM and EM fungal guilds in decomposability support and mechanistically underpin previous speculations that EM fungi might contain a higher ratio of components recalcitrant to decomposition than AM fungi5. While microbiologists seek to specify the fungus-specific macromolecular compounds and basic chemical elements, these characters are difficult to link to soil C cycle mechanisms. Instead of analysing individual chemical components or complex chemical compounds of fungal biomass that are possibly a proxy for decomposability5,10,32, we opted to characterize fungal biomass through general traits of litter decomposability known to drive soil C cycling42,48,49. Recently, it has been suggested that labile and recalcitrant C compounds originating from decomposing organic matter might follow distinct pathways of stabilization depending on the abundance of soil saprotrophic organisms50,51. This suggests that C components originating from mycorrhizal fungi of distinct guilds are likely involved in distinct pathways of C transformations in soil. Moreover, through the differential release of labile and more recalcitrant C components, the temporal dynamics of contributions of different mycorrhizal fungal guilds to distinct soil C transformation pathways will also differ among EM and AM fungi.
For decades, foliage litter and its variability among species or plant functional types has been considered as one of the main factors controlling soil C cycle process11. Our analysis shows that the magnitude of differences in decomposability traits between fungi of distinct mycorrhizal guilds is much higher than that of the leaf litter of plant species belonging to distinct functional groups. This suggests that the decomposability of mycorrhizal fungal biomass is a critically important factor for pathways of soil C transformation processes. Such pathways have been previously hypothesized5,25 but in practice neglected or underestimated due to the high uncertainty associated with this phenomenon. This comparison of decomposability between mycorrhizal fungi and plant foliage litter is the first attempt at examining such characters of different substrates of decomposition litter. Future research with more fungal species may reveal information beyond the limited data used in this study. Given that plants allocate a significant part (up to 30%) of NPP to mycorrhizal fungal biomass21,23, an amount comparable to the allocation into plant leaves in some ecosystems52,53, the differential contributions of mycorrhizal fungal guilds to the processes of soil C turnover should be considered as a critical SOM formation factor. As mycorrhizal fungal necromass is among the most important sources of below ground soil C input, our results provide decomposability information of soil C inputs which is essential in narrowing down major uncertainties in estimating soil C fluxes dynamics.
Methods
Cultivation of AM and EM fungi
We selected available AM fungal species strains from the Glomeraceae family, as this family is globally the most dominant family of AM fungi47, while they can be grown in vitro producing reasonably large amounts of fungal biomass. We selected EM fungal species to cover relatively abundant strains of various families. In addition, we opted to use a higher number of EM fungi species compared to AM fungi, because (1) EM fungi consist of ca. 20,000–25,000 species54,55 which entail high diversity of chemical traits, while AM fungi have been known to exhibit lower diversity with ca. 300 species identified within this fungal phylum56–58, (2) mass-production of AM fungi to reach the amounts of biomass necessary for the recalcitrance assessments is complicated, necessitating hundreds of Petri plates. We have opted to use laboratory cultivation protocols adapted to each fungal species to assure that each species develops in a most healthy way during mass cultivation. This ensures that the chemical composition of fungal material examined is representative for each mycorhizal type in general, while it may differ from specific local soil conditions. Through cultivation, all manipulations were conducted under sterile conditions to prevent contamination of fungal material, by using a laminar flow hood, and with sterile or sterilized laboratory material.
EM fungi cultivation and sample preparation
Original cultures of EM fungal species were obtained from Westerdijk Fungal Biodiversity Institute (the Netherlands), which also provided standard laboratory instructions for EM fungi cultivation (except strain of Scleroderma verrucosum, which was obtained from the collection of GINCO). We assumed that the chemistry of fungi cultivated following standard cultivation protocols is only affected by the species morphology. Each species was inoculated in 30–80 Petri plates (90 mm, diameter), containing species-specific medium (Supplementary Table S1) solidified with bacteriological agar, then sealed with film and incubated in climate rooms (temperature 21–27 °C according to the preference of each strain, in the dark) for 4–5 weeks (Supplementary Fig. S1). Harvested fresh mycelium of EM fungi was washed with distilled water for 10 s, collected by filtration, and stored at −20 °C. The frozen fungi biomass samples were dried using a freeze dryer or oven under 55 °C for at least 12 h (weighed after another 4 h until the weight is stable, drying methods see Supplementary Table S1), then stored at −20 °C before chemical recalcitrance assessments.
AM fungi cultivation and sample preparation
All AM fungal strains were obtained from the Glomeromycota in vitro Collection (GINCO, Belgium). The cultivation protocol of AM fungi followed the methods well-established in the laboratory of mycology of UCLouvain (Belgium). As AM fungi are relatively slow-growing, and there was no prior knowledge on biomass output among in vitro cultivation approaches. We recruited a combination of different cultivation characteristics to maximize biomass productivity, and each strain was cultivated using four different systems (Fig. 4): autotrophic whole plants system either with a Petri (S1) or a mesh (S2) root compartment (RC), transformed root organ culture (ROC) system in bi- (S3) or mono-compartmented (S4) Petri plates. In the end, we established over 600 AM systems, all biomass produced by each strain in the four different in vitro systems described below was needed to fulfill the standard amount required for the chemical analysis.
Fig. 4. In vitro cultivation of arbuscular mycorrhizal fungi.
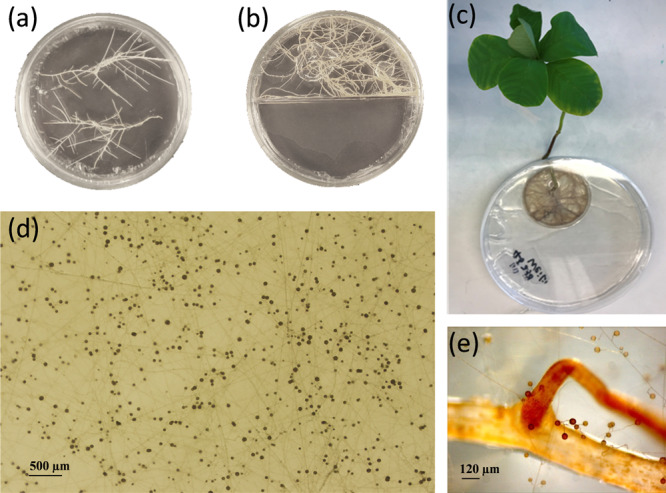
Cultivation system in (a) mono-compartmented or (b) bi-compartmented Petri dish on excised Ri T-DNA transformed root organs of carrot. c Cultivation with the whole plant of Crotalaria Spectabilis in a bi-compartment Petri dish system; d Mycelium and spore production of the AM fungus (Rhizophagus irregularis, MUCL 41833) grown in a bi-compartmented Petri dish and (e) a mono-compartment Petri dish.
System S1 consisted of a lid of a small 50-mm-diameter Petri dish placed inside a large 145-mm-diameter Petri dish, to create an RC inside a mycelial compartment (MC) (Fig. 4c). System S2 was similar to S1 with the difference that a 55-mm-diameter cap made with 40 µm nylon mesh and filled with cotton was used as RC instead (Supplementary Fig. S2). The RC contained roots of mycorrhizal plants to sustain fungal growth into the MC. Both compartments were filled with modified Strullu–Romand (MSR59) medium without sucrose and vitamins. The large plates were covered with black plastic foil to minimize light exposure. In each large plate, the plant shoot grew outside through a 2-mm-diameter lateral opening sealed with sterile silicon grease as described60. The systems were kept in a growth chamber with a 16 h photoperiod, 130 μmol m−2 s−1 light intensity, 27 °C temperature, and 80% relative humidity. Each RC was refilled with medium every 2–3 weeks.
System S3 (Fig. 4b) consisted of 94-mm-diameter bi-compartmented Petri plates with RC and MC. The RC contained mycorrhizal Ri T-DNA transformed roots clone DC2 of Daucus carota growing in MSR medium to sustain the fungal growth into the MC. The MC was filled with MSR medium without sucrose and vitamins. These bi-compartmented plates were incubated inverted in the dark at 27 °C for 6 months; The system S4 (Fig. 4a) consisted of a 145-mm-diameter mono-compartmented Petri plate with mycorrhizal Ri T-DNA transformed roots of D. carota clone DC2 growing in MSR medium59. The plates were incubated inverted in the dark at 27 °C for 4–5 months.
For systems S1, S2, and S3, roots were trimmed before invading the MC to keep the MC root-free. Once the MC was full with mycelium, the medium was harvested to extract the mycelium as described below, and the MC was re-filled with medium to allow fungal re-growth. The harvesting procedure was repeated for each plate every 4–6 weeks until another 6–10 months according to the productivity of each plate; For system S4, only the sections of the medium without any roots were harvested once to exclude roots and root exudates after incubation.
The absence of roots in the harvested medium was carefully evaluated and confirmed using a stereomicroscope. For all different systems and strains, the harvested medium which only contained mycelium was immediately liquefied inside a beaker in a water bath at 70 °C for 2 h—this procedure also killed the mycelium. The mycelium was then collected using a 38-µm filter, washed with demineralized water for 10 s to remove any remnants of medium and root exudates (only possibly exist in the harvested medium from S4), and stored at −20 °C until further use. Prior to chemical analyses, all mycelia were dried using the same procedure as for the EM fungi described above (Supplementary Table S1).
Decomposability analysis
Chemical composition of mycelia was examined in the laboratory of Natural Resources Institute (Finland). We examined the fungal samples for different extractable components based on their solubility in distinct solvents which has been widely used in investigating compositions of plant litter and soil organic matter61: The W-fraction is largely composed of carbohydrates and nitrogen-containing compounds, and is frequently used as a measure for potentially bioavailable SOM and thus for the readily decomposable C pool62; The A-fraction: acid hydrolysis can extract carbohydrate and protein materials by disruption of hydrolytic bonding, leaving the more biologically recalcitrant alkyl and aryl materials largely intact63,64; The E-fraction: dichloromethane was used for extracting nonpolar extractives65 (e.g., fatty acids, long-chain alcohols, wax esters, oils, resins, etc.); The N-fraction is the residue remaining after hydrolysis in sulfuric acid (also known as Klason lignin). The amounts of extractable substances were determined gravimetrically by incubating samples with a solvent and weighing the samples after filtration and drying. Mass loss during each extraction was considered to be equal to the amount of a compound being extracted. For details of the protocols, see Ryan et al. (1990)66 and Wieder and Starr (1998)67.
The WAEN components extracted through different chemical methods represent the suite of decomposability traits61 related to the extraction capacity of different types of enzymes potentially excreted by saprotrophic organisms68. These components with different decomposability are key to determining the dynamics of litter decomposition and soil C cycling in soil C modeling42,48,69. The decomposability order W-A-E-N was determined based on the Yasso soil C model42,48, which is coherent with the findings of real plant litter decomposition experiments (see Supplementary Fig. S3).
The raw measurement results corresponding to Fig. 1 are provided in Supplementary Table S2. Samples that did not reach 0.5 g were measured with a mixture of plant litter (with a known content of WAEN components) to reach the necessary quantity for analysis, and the fungal chemical composition (Gi) was calculated based on the proportion of fungal biomass in the samples according to the following equations:
| 1 |
| 2 |
where WT is the total weight of the mixture of dried plant litter and fungi; Wp is the weight of dried plant litter; Wf is the weight of dried fungal biomass; is the measured value of each chemical composition (WAEN) in the mixture.
We calculated the WAEN fractions for fungi and estimated the corresponding accuracy with different portions of fungi and standard litter mixture (Table 2). Based on the relatively high proportion of N-fraction in standard plant litter, which increases the uncertainty in N-fraction estimates in mixtures, data for fungal species assessed in a mixture with plant material was assigned a lower weight (0.5 instead of 1 as default weight value for other data) in the data analysis (next section).
Table 2.
Accuracy assessment for experiments measured with mixture.
| Mycelium proportion in the mixture with leaf litter | Mixture AWEN value from experiment | Mycorrhizal AWEN fraction from the calculation | ||||||
|---|---|---|---|---|---|---|---|---|
| W’ (mix) | A’ (mix) | E’ (mix) | N’ (mix) | W | A | E | N | |
| (0 g fungal mycelium + 0.5 g leaf) 0% | 9.29 | 10.60 | 45.81 | 34.30 | \ | \ | \ | \ |
| (0.10 g fungal mycelium + 0.40 g leaf) 20% | 15.32 | 11.76 | 42.99 | 29.92 | 39.46 | 16.40 | 31.73 | 12.42 |
| (0.20 g fungal mycelium + 0.30 g leaf) 40% | 23.65 | 13.38 | 38.41 | 24.56 | 45.18 | 17.55 | 27.31 | 9.96 |
| (0.30 g fungal mycelium + 0.20 g leaf) 60% | 33.25 | 14.90 | 33.51 | 18.34 | 49.23 | 17.77 | 25.31 | 7.69 |
| (0.40 g fungal mycelium + 0.10 g leaf) 80% | 42.70 | 16.18 | 29.66 | 11.45 | 51.06 | 17.58 | 25.62 | 5.74 |
| (0.50 g fungal mycelium + 0 g leaf)100% | 51.86 | 17.95 | 26.05 | 4.14 | 51.86 | 17.95 | 26.05 | 4.14 |
Accuracy assessment of the fungal chemical composition calculated from WAEN test results of a mixture with plant litter. EM Fungi of Hebeloma hiemale biomass and a typical northern conifer tree branch were used for this estimation.
Statistics and reproducibility
We assessed the significance of the overall differences in recalcitrance between AM and EM fungi, with the permutational analysis of variance – perMANOVA70, performed with 999 permutations in the Vegan package (Bray–Curtis function) in R. Data distribution and homogeneity of variance of original WAEN values are provided in Supplementary Figs. S4, S5 (include the information about log-transformed values). Dispersions of beta diversity (the distance from an individual measure to the group’s centroid) were calculated for each beta diversity metric within AM and EM fungal groups for estimating within-group variation across individuals. Significant differences in beta diversity variation71 were tested using permutational statistical tests for the homogeneity of group dispersions with 999 permutations in Vegan. We used Principal Coordinates Analysis (PCoA) for visualization of the data present in the beta diversity distance matrix (Fig. 2).
Subsequently, we tested the hypothesis that AM fungi exhibit higher amounts of easily soluble, and acid-hydrolysable compounds, while EM fungi have higher amounts of compounds that are neither soluble nor hydrolysable, by a non-parametric Mann–Whitney U test (with package sjstats in R) to determine if there were statistically significant differences in each chemical component between the two mycorrhizal groups. To account for the fact that WAEN of two fungal strains were assessed in a mixture with plant litter, all statistical analyses of fungal WAEN were conducted as weighted analyses according to the accuracy assessment for the results of sample from a mixture.
We examined the magnitude of the difference between the recalcitrance of mycorrhizal fungal types vs. the recalcitrance of plant material (Hypothesis 2) comparing the effect size- Eta square (η2)72 of ANOVAs on WAEN values of AM vs EM fungi to the effect sizes of ANOVAs on WAEN of plant functional types (other effect size indices, dispersions, and variation across groups of plant litter are provided in Supplementary Note S1, Table S5, Fig. S6). To meet the normality assumptions, WAEN values were log-transformed.
The data on plant functional types used for this analysis were obtained as follows: We gathered plant leaf WAEN chemical composition data for 57 species from CIDET33 and LIDET34 datasets (details see Supplementary Note S1, Supplementary Table S4). Those data were grouped into the evergreen tree, deciduous tree, evergreen shrub, and herb, based on plant growth form information from the TRY database73. Species with multiple form definitions were defined according to the highest occurrence frequency74.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research was supported by the Vidi grant 016.161.318 (issued to N.A.S. by The Netherlands Organization for Scientific research) and China Scholarship Council (CSC, grant No. 201706040071 issued to W.H.). M.C. was supported by the European Commission’s grant H2020-MSCA-IF-2018 ‘SYMBIO-INC’ (GA 838525). T.V., J.L., and J.H. were supported by the Strategic Research Council at the Academy of Finland (decision 327214, 327342) and the Nessling foundation TWINWIN project. We appreciate the Natural Resources Institute Finland and Prof. Hannu Fritze for supporting chemical analysis. We would like to thank colleagues of the Soil-process group and Chen Li (CML, Leiden University) for discussions. We also thank the anonymous reviewers for their constructive comments and suggestions.
Author contributions
W.H., P.M.B., and N.A.S. conceived the original idea and planned the project. S.D. and J.L. were involved in planning the project. W.H. carried out the cultivation with assistance from S.D. and M.C. on AM fungi. J.H. provided the sample measurements and processed the experimental data. W.H. performed the numerical calculations and analysed the data. P.M.B, N.A.S, J.H., and T.V. aided in interpreting the results. W.H. and N.A.S. wrote the manuscript in consultation with P.V.B, S.D., M.C., J.H., T.V., and J.L. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Communications Biology thanks Adriana Romero-Olivares and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Meritxell Riquelme and Caitlin Karniski. Peer reviewer reports are available.
Data availability
All data generated or analyzed during this study are included in this article (and its supplementary information file).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03341-9.
References
- 1.Melillo JM, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- 2.Stockmann U, et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013;164:80–99. doi: 10.1016/j.agee.2012.10.001. [DOI] [Google Scholar]
- 3.Sokol NW, Sanderman J, Bradford MA. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019;25:12–24. doi: 10.1111/gcb.14482. [DOI] [PubMed] [Google Scholar]
- 4.Krull ES, Baldock JA, Skjemstad JO. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. 2003;30:207–222. doi: 10.1071/FP02085. [DOI] [PubMed] [Google Scholar]
- 5.Langley JA, Hungate BA. Mycorrhizal controls on belowground litter quality. Ecology. 2003;84:2302–2312. doi: 10.1890/02-0282. [DOI] [Google Scholar]
- 6.Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009;23:627–636. doi: 10.1111/j.1365-2435.2008.01515.x. [DOI] [Google Scholar]
- 7.Cou ^teaux MM, Bottner P, Berg B. Litter decomposition, climate and litter quality. Trends Ecol. Evol. 1995;10:63–66. doi: 10.1016/S0169-5347(00)88978-8. [DOI] [PubMed] [Google Scholar]
- 8.Prescott CE. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry. 2010;101:133–149. doi: 10.1007/s10533-010-9439-0. [DOI] [Google Scholar]
- 9.Frey SD, Lee J, Melillo JM, Six J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Chang. 2013;3:395–398. doi: 10.1038/nclimate1796. [DOI] [Google Scholar]
- 10.Fernandez CW, Heckman K, Kolka R, Kennedy PG. Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming. Ecol. Lett. 2019;22:498–505. doi: 10.1111/ele.13209. [DOI] [PubMed] [Google Scholar]
- 11.Brovkin V, et al. Plant-driven variation in decomposition rates improves projections of global litter stock distribution. Biogeosciences. 2012;9:565–576. doi: 10.5194/bg-9-565-2012. [DOI] [Google Scholar]
- 12.Aponte C, García LV, Marañón T. Tree species effect on litter decomposition and nutrient release in mediterranean oak forests changes over time. Ecosystems. 2012;15:1204–1218. doi: 10.1007/s10021-012-9577-4. [DOI] [Google Scholar]
- 13.Hättenschwiler S, Jørgensen HB. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010;98:754–763. doi: 10.1111/j.1365-2745.2010.01671.x. [DOI] [Google Scholar]
- 14.van der Heijden MG, Martin FM, Selosse M-A, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. N. Phytol. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- 15.Lin G, McCormack ML, Ma C, Guo D. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. N. Phytol. 2017;213:1440–1451. doi: 10.1111/nph.14206. [DOI] [PubMed] [Google Scholar]
- 16.Högberg MN, Högberg P. Extramatrical ectomycorrhizal mycelium contributes one‐third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. N. Phytol. 2002;154:791–795. doi: 10.1046/j.1469-8137.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 17.Leake J, et al. Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004;82:1016–1045. doi: 10.1139/b04-060. [DOI] [Google Scholar]
- 18.Bååth E, Nilsson LO, Göransson H, Wallander H. Can the extent of degradation of soil fungal mycelium during soil incubation be used to estimate ectomycorrhizal biomass in soil? Soil Biol. Biochem. 2004;36:2105–2109. doi: 10.1016/j.soilbio.2004.06.004. [DOI] [Google Scholar]
- 19.Kaiser C, et al. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. N. Phytol. 2015;205:1537–1551. doi: 10.1111/nph.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konvalinková T, Püschel D, Řezáčová V, Gryndlerová H, Jansa J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil. 2017;419:319–333. doi: 10.1007/s11104-017-3350-6. [DOI] [Google Scholar]
- 21.Ouimette AP, et al. Accounting for carbon flux to mycorrhizal fungi may resolve discrepancies in forest carbon budgets. Ecosystems. 2019;23:715–729. doi: 10.1007/s10021-019-00440-3. [DOI] [Google Scholar]
- 22.Wallander H, Nilsson LO, Hagerberg D, Bååth E. Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. N. Phytol. 2001;151:753–760. doi: 10.1046/j.0028-646x.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen MF, Kitajima K. Net primary production of ectomycorrhizas in a California forest. Fungal Ecol. 2014;10:81–90. doi: 10.1016/j.funeco.2014.01.007. [DOI] [Google Scholar]
- 24.Godbold DL, et al. Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil. 2006;281:15–24. doi: 10.1007/s11104-005-3701-6. [DOI] [Google Scholar]
- 25.Frey SD. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019;50:237–259. doi: 10.1146/annurev-ecolsys-110617-062331. [DOI] [Google Scholar]
- 26.Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. N. Phytol. 2018;220:1108–1115. doi: 10.1111/nph.14976. [DOI] [PubMed] [Google Scholar]
- 27.Soudzilovskaia NA, et al. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019;10:5077. doi: 10.1038/s41467-019-13019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips RP, Brzostek E, Midgley MG. The mycorrhizal‐associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. N. Phytol. 2013;199:41–51. doi: 10.1111/nph.12221. [DOI] [PubMed] [Google Scholar]
- 29.Miyauchi S, et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020;11:5125. doi: 10.1038/s41467-020-18795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harley JL. Fungi in ecosystems. J. Ecol. 1971;59:653. doi: 10.2307/2258131. [DOI] [Google Scholar]
- 31.Fernandez CW, Langley JA, Chapman S, McCormack ML, Koide RT. The decomposition of ectomycorrhizal fungal necromass. Soil Biol. Biochem. 2016;93:38–49. doi: 10.1016/j.soilbio.2015.10.017. [DOI] [Google Scholar]
- 32.Fernandez CW, Koide RT. Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol. Biochem. 2014;77:150–157. doi: 10.1016/j.soilbio.2014.06.026. [DOI] [Google Scholar]
- 33.Trofymow, J. A. The Canadian Institute Decomposition Experiment (CIDET): project and site establishment report / J.A. Trofymow and the CIDET Working Group. (1998).
- 34.Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000;6:751–765. doi: 10.1046/j.1365-2486.2000.00349.x. [DOI] [Google Scholar]
- 35.Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002;34:139–162. doi: 10.1016/S0038-0717(01)00158-4. [DOI] [Google Scholar]
- 36.Zeglin LH, Myrold DD. Fate of decomposed fungal cell wall material in organic horizons of old-growth douglas-fir forest soils. Soil Sci. Soc. Am. J. 2013;77:489–500. doi: 10.2136/sssaj2012.0204. [DOI] [Google Scholar]
- 37.Kleber M, et al. Mineral-organic associations: formation, properties, and relevance in soil environments. in. Adv. Agron. 2015;130:1–140. doi: 10.1016/bs.agron.2014.10.005. [DOI] [Google Scholar]
- 38.Fortin JA, et al. Arbuscular mycorrhiza on root-organ cultures. Can. J. Bot. 2002;80:1–20. doi: 10.1139/b01-139. [DOI] [Google Scholar]
- 39.Declerck, S., Séguin, S. & Dalpé, Y. The monoxenic culture of Arbuscular Mycorrhizal fungi as a tool for germplasm collections. in In Vitro Culture of Mycorrhizas 17–30 (Springer-Verlag, 2005).
- 40.Lalaymia, I. & Declerck, S. The Mycorrhizal Donor Plant (MDP) in vitro culture system for the efficient colonization of whole plants. 2146, (Springer US, 2020). [DOI] [PubMed]
- 41.Crous, P. W., Verkley, G. J. M., Groenewald, J. Z. & Houbraken, J. Westerdijk Laboratory Manual Series 1: Fungal Biodiversity. (2019).
- 42.Tuomi M, et al. Leaf litter decomposition-Estimates of global variability based on Yasso07 model. Ecol. Modell. 2009;220:3362–3371. doi: 10.1016/j.ecolmodel.2009.05.016. [DOI] [Google Scholar]
- 43.Clemmensen KE, et al. Carbon sequestration is related to mycorrhizal fungal community shifts during long‐term succession in boreal forests. N. Phytol. 2015;205:1525–1536. doi: 10.1111/nph.13208. [DOI] [PubMed] [Google Scholar]
- 44.Averill C, Turner BL, Finzi AC. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature. 2014;505:543–545. doi: 10.1038/nature12901. [DOI] [PubMed] [Google Scholar]
- 45.Staddon PL, Ramsey CB, Ostle N, Ineson P, Fitter AH. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science. 2003;300:1138–1140. doi: 10.1126/science.1084269. [DOI] [PubMed] [Google Scholar]
- 46.Adamczyk B, Sietiö O, Biasi C, Heinonsalo J. Interaction between tannins and fungal necromass stabilizes fungal residues in boreal forest soils. N. Phytol. 2019;223:16–21. doi: 10.1111/nph.15729. [DOI] [PubMed] [Google Scholar]
- 47.Davison J, et al. Plant functional groups associate with distinct arbuscular mycorrhizal fungal communities. N. Phytol. 2020;226:1117–1128. doi: 10.1111/nph.16423. [DOI] [PubMed] [Google Scholar]
- 48.Liski J, Palosuo T, Peltoniemi M, Sievänen R. Carbon and decomposition model Yasso for forest soils. Ecol. Modell. 2005;189:168–182. doi: 10.1016/j.ecolmodel.2005.03.005. [DOI] [Google Scholar]
- 49.Guendehou GHS, et al. Decomposition and changes in chemical composition of leaf litter of five dominant tree species in a West African tropical forest. Trop. Ecol. 2014;55:207–220. [Google Scholar]
- 50.Paterson E, et al. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008;40:1103–1113. doi: 10.1016/j.soilbio.2007.12.003. [DOI] [Google Scholar]
- 51.Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013;19:988–995. doi: 10.1111/gcb.12113. [DOI] [PubMed] [Google Scholar]
- 52.Xia J, et al. Global patterns in Net Primary Production allocation regulated by environmental conditions and forest stand age: a model‐data comparison. J. Geophys. Res. Biogeosciences. 2019;124:2039–2059. doi: 10.1029/2018JG004777. [DOI] [Google Scholar]
- 53.Malhi Y, Doughty C, Galbraith D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. B Biol. Sci. 2011;366:3225–3245. doi: 10.1098/rstb.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tedersoo L, May TW, Smith ME. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza. 2010;20:217–263. doi: 10.1007/s00572-009-0274-x. [DOI] [PubMed] [Google Scholar]
- 55.Rinaldi AC, Comandini O, Kuyper TW. Ectomycorrhizal fungal diversity: separating the wheat from the chaff. Fungal Divers. 2008;33:1–45. [Google Scholar]
- 56.Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. N. Phytol. 2012;193:970–984. doi: 10.1111/j.1469-8137.2011.03962.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee E-H, Eo J-K, Ka K-H, Eom A-H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology. 2013;41:121–125. doi: 10.5941/MYCO.2013.41.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schüβler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 2001;105:1413–1421. doi: 10.1017/S0953756201005196. [DOI] [Google Scholar]
- 59.Declerck S, Strullu DG, Plenchette C. Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia. 1998;90:579. doi: 10.2307/3761216. [DOI] [Google Scholar]
- 60.Voets L, et al. Extraradical mycelium network of arbuscular mycorrhizal fungi allows fast colonization of seedlings under in vitro conditions. Mycorrhiza. 2009;19:347–356. doi: 10.1007/s00572-009-0233-6. [DOI] [PubMed] [Google Scholar]
- 61.von Lützow M, et al. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007;39:2183–2207. doi: 10.1016/j.soilbio.2007.03.007. [DOI] [Google Scholar]
- 62.Davidson EA, Galloway LF, Strand MK. Assessing available carbon: Comparison of techniques across selected forest soils. Commun. Soil Sci. Plant Anal. 1987;18:45–64. doi: 10.1080/00103628709367802. [DOI] [Google Scholar]
- 63.Trumbore SE, Vogel JS, Southon JR. AMS 14C measurements of fractionated soil organic matter: an approach to deciphering the soil carbon cycle. Radiocarbon. 1989;31:644–654. doi: 10.1017/S0033822200012248. [DOI] [Google Scholar]
- 64.Henriksen T, Breland T. Evaluation of criteria for describing crop residue degradability in a model of carbon and nitrogen turnover in soil. Soil Biol. Biochem. 1999;31:1135–1149. doi: 10.1016/S0038-0717(99)00031-0. [DOI] [Google Scholar]
- 65.Schnitzer M, Schuppli P. Method for the sequential extraction of organic matter from soils and soil fractions. Soil Sci. Soc. Am. J. 1989;53:1418–1424. doi: 10.2136/sssaj1989.03615995005300050019x. [DOI] [Google Scholar]
- 66.Ryan MG, Melillo JM, Ricca A. A comparison of methods for determining proximate carbon fractions of forest litter. Can. J . Res. 1990;20:166–171. doi: 10.1139/x90-023. [DOI] [Google Scholar]
- 67.Wieder RK, Starr ST. Quantitative determination of organic fractions in highly organic, Sphagnum peat soils. Commun. Soil Sci. Plant Anal. 1998;29:847–857. doi: 10.1080/00103629809369990. [DOI] [Google Scholar]
- 68.Xu G, et al. Differential responses of soil hydrolytic and oxidative enzyme activities to the natural forest conversion. Sci. Total Environ. 2020;716:136414. doi: 10.1016/j.scitotenv.2019.136414. [DOI] [PubMed] [Google Scholar]
- 69.Viskari T, et al. Improving Yasso15 soil carbon model estimates with ensemble adjustment Kalman filter state data assimilation. Geosci. Model Dev. 2020;13:5959–5971. doi: 10.5194/gmd-13-5959-2020. [DOI] [Google Scholar]
- 70.Anderson, M. J. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online, 10.1002/9781118445112.stat07841 (2014).
- 71.Anderson MJ, Ellingsen KE, McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006;9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 72.Tomczak M, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014;1:19–25. [Google Scholar]
- 73.Kattge J, et al. TRY - a global database of plant traits. Glob. Chang. Biol. 2011;17:2905–2935. doi: 10.1111/j.1365-2486.2011.02451.x. [DOI] [Google Scholar]
- 74.Engemann K, et al. A plant growth form dataset for the New World. Ecology. 2016;97:3243. doi: 10.1002/ecy.1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its supplementary information file).