Abstract
We investigated the pharmacokinetics and safety of an oral solution of itraconazole in two groups of neutropenic children stratified by age. Effective concentrations of itraconazole in plasma were reached quickly and maintained throughout treatment. The results indicate a trend toward higher concentrations of itraconazole in plasma in older children.
Invasive fungal infections are frequently observed in neutropenic patients (4). Several antifungal agents are available to treat and prevent invasive mycoses, but poor efficacy and a narrow spectrum of activity can limit their use (4, 17). Itraconazole is a broad-spectrum, orally active, triazole, which has favorable pharmacokinetics and is effective against many mycopathogens, including Candida and Aspergillus species (6, 15). An oral capsule formulation of itraconazole is as effective as intravenous amphotericin B (16), but it must be taken with food to be absorbed effectively. Additionally, children may experience difficulty in swallowing solid drug formulations, so itraconazole capsules are seldom used in children (2).
A 10-mg/ml oral solution of itraconazole in combination with hydroxypropyl-β-cyclodextrin has been developed, which is effective in adults receiving bone marrow autografts (11), adults in remission from acute myeloid leukemia (12), and HIV-positive adult patients with oral candidiasis (8, 10). Furthermore, the itraconazole oral solution prevents fungal infections in neutropenic adults (7, 9). Data regarding itraconazole oral solution in children are limited, although one study showed that it provided potentially therapeutic concentrations (3).
Children aged between 2 and 5 years (group 1) or between 6 and 12 years (group 2) were enrolled into this open-label, multicenter trial. All children were hospitalized for chemotherapy and were at risk of invasive fungal infection. Patients were excluded if they had received a bone marrow allograft or whole-body irradiation, had taken an inducer or inhibitor of hepatic metabolism within 2 weeks before the trial, had neutropenia that was expected to last for fewer than 14 days, or had been treated with astemizole, terfenadine, digoxin, or warfarin. The trial protocol was approved by the local ethics committee. Parental consent and, if possible, consent from the children were obtained. The trial was performed in accordance with the declaration of Helsinki and its amendments, and with French law.
All patients received itraconazole oral solution (2.5 mg/kg) twice daily. Treatment was started 7 to 10 days before expected onset of neutropenia and was discontinued 2 days after neutropenia had ended or if the investigator felt it clinically necessary to switch to amphotericin B. Itraconazole oral solution was given before breakfast and then 12 h later, before the evening meal. Predose blood samples (4 ml) were taken from an antecubital vein immediately before the morning administration of itraconazole (8 or 9 a.m.) on day 1, then every 2 days until the neutrophil count recovered, and then after a further 2 days. Additional blood samples were taken 4 h after administration on days 7 and 15, at the end of neutropenia, and 2 days after that. Plasma was separated from the cells and frozen at −20°C before analysis. Twenty-four-hour urine samples were collected on days 5 and 11; the whole samples were mixed thoroughly, and aliquots were frozen at −20°C before analysis.
Itraconazole and hydroxyitraconazole concentrations were determined using validated high-performance liquid chromatography with UV detection (18). The limits of quantitation were 50 ng/ml for itraconazole and hydroxyitraconazole in plasma and 25 ng/ml for both compounds in urine. Interassay accuracy and precision (coefficient of variation) were obtained from calibration curves ranging from 50 to 2,000 ng/ml for plasma and urine. The accuracy ranged from 99.9 to 101.1% for itraconazole and from 99.5 to 100.9% for hydroxyitraconazole. The precision ranged from 0.0 to 4.8% for itraconazole and from 0.1 to 8.1% for hydroxyitraconazole. Hydroxypropyl-β-cyclodextrin was measured by size-exclusion chromatography with postcolumn complexation (13). The limit of quantitation was 1 μg/ml; the accuracy ranged from 97.4 to 111.1%, and precision ranged from 1.9 to 7.4%, using calibration curves of 1 to 20 μg/ml for plasma and 2 to 50 μg/ml for urine.
Steady-state plasma concentration (Cssmin) and time to reach Cssmin (TCss) for itraconazole and hydroxyitraconazole were estimated from the plasma concentration-time data for each patient. The plateau was estimated from visual examination of the predose through (Cmin 12h) plasma concentration-time profiles, and Cssmin was calculated as the average of the values during the plateau. To estimate the total exposure from each patient, the area under the concentration-time curve (AUCmin) was calculated using the trapezoidal rule from the Cmin 12h measured for the entire duration of itraconazole treatment. Because the treatment duration differed, AUCmin were reported as exposures per day, which was calculated by dividing AUCmin by the duration of treatment (AUCmin/d). The metabolic ratio was calculated from the ratio of AUCmin/d between hydroxyitraconazole and itraconazole. From the urine samples, 24-h urinary concentrations of itraconazole and hydroxyitraconazole were measured. Additionally, predose plasma concentrations and 24-h urinary concentrations of hydroxypropyl-β-cyclodextrin were measured. Adverse events were recorded throughout the trial. Blood chemistry abnormalities were recorded if a measure was normal before treatment and became abnormal during treatment.
A sample size of 20 was deemed sufficient to support conclusions about the pharmacokinetics of itraconazole in children. The Student t test was used to compare the pharmacokinetic data between the two groups, and the homogeneity of variances was tested with the Fisher exact test. The threshold for differences to be considered significant was set at the 5% level (i.e., P ≤ 0.05). The statistical package Pharm-Stat (Innaphase Corp., Philadelphia, Pa.) was used to perform data analysis.
A total of 17 patients were enrolled into the trial, with 10 in group 1 (aged 1.7 to 4.9 years; median, 1 year) and seven in group 2 (aged 6.2 to 14.3 years; median, 10 years). Overall, 59% of the patients were boys. A total of 12 patients (7 in group 1 and 5 in group 2) had hematologic malignancy (acute lymphocytic or myeloid leukemia or Burkitt's lymphoma) and 5 (3 in group 1 and 2 in group 2) had solid tumors (Ewing's sarcoma, germinal tumor, or neuroblastoma). Three patients stopped treatment prematurely, because of poor compliance (n = 1), coma (n = 1), and mucositis (n = 1). Data from one patient in group 1 were not used for pharmacokinetic analyses, because of extremely poor compliance. Three protocol deviations were recorded, including two patients who had liver enzyme concentrations above the present limits at inclusion and one patient who was 14 years old. One patient did not develop neutropenia. A total of 9 subjects in group 1 and 7 subjects in group 2 were evaluated for Cmin 12h; 5 patients in group 1 were excluded from the Cssmin calculation because of large intraindividual variability in their plasma concentrations (coefficient of variation of >30%).
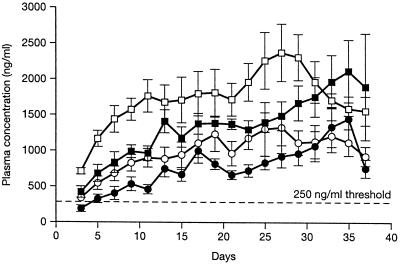
The pharmacokinetic parameters of itraconazole and its metabolite are shown in Table 1, and the Cmin 12h values for itraconazole and hydroxyitraconazole are shown in Fig. 1. Mean predose plasma concentrations of unchanged itraconazole were >250 ng/ml by day 3 in group 2 and day 5 in group 1 and >500 ng/ml by day 5 in group 2 and 15 in group 1. The concentration levels of 250 ng/ml (1) or 500 ng/ml (5) have been assumed to be as effective in clinical practice. These concentrations were sustained until the end of the trial. Concentrations of itraconazole in plasma were consistently higher in group 2 than in group 1. Although the Cssmin and TCss are not statistically different between groups 1 and 2, there are significant differences in Cmin 12h of itraconazole at days 3 and 21 and in peak plasma concentrations (Cmax 4h) of itraconazole at day 7. Furthermore, the Cmin 12h of hydroxyitraconazole at days 3, 5, 7, and 15, the Cmax 4h at days 7 and 15, and the AUCmin/d are significantly higher in group 2. The time to maximum concentration was shorter in group 2 for both compounds, but the difference was not statistically significant.
TABLE 1.
Plasma concentration and pharmacokinetic parameters of itraconazole (ITR) and hydroxyitraconazole (OHI)a
| Parameter or concn | Group 1 (2–5 yr)
|
Group 2 (6–12 yr)
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | ITR | n | OHI | n | ITR | n | OHI | |
| Cmin 12h (ng/ml) | ||||||||
| Day 3 | 9 | 185 ± 129 | 9 | 465 ± 221 | 7 | 328 ± 98* | 7 | 700 ± 121* |
| Day 5 | 9 | 351 ± 172 | 9 | 732 ± 335 | 7 | 540 ± 241 | 7 | 1,164 ± 281* |
| Day 7 | 9 | 439 ± 255 | 9 | 915 ± 396 | 7 | 674 ± 285 | 6 | 1,427 ± 449* |
| Day 15 | 9 | 711 ± 251 | 9 | 1,275 ± 322 | 6 | 1,072 ± 408 | 6 | 1,964 ± 562** |
| Day 21 | 7 | 649 ± 145 | 7 | 1,351 ± 201 | 5 | 1,056 ± 380* | 5 | 1,799 ± 592 |
| Day 29 | 7 | 953 ± 462 | 7 | 1,659 ± 670 | 5 | 1,105 ± 385 | 5 | 2,306 ± 736 |
| Cmax 4h (ng/ml) | ||||||||
| Day 7 | 8 | 599 ± 231 | 7 | 1,008 ± 341 | 7 | 1,090 ± 383* | 7 | 1,658 ± 426** |
| Day 15 | 6 | 1,024 ± 351 | 6 | 1,358 ± 373 | 6 | 1,524 ± 770 | 6 | 2,180 ± 753* |
| Cssmin (ng/ml) | 5 | 877 ± 248 | 5 | 1,536 ± 334 | 7 | 1,085 ± 329 | 7 | 1,919 ± 535 |
| TCss (days) | 5 | 13 ± 4 | 5 | 14 ± 8 | 7 | 12 ± 6 | 7 | 11 ± 5 |
| AUCmin (ng · day/ml) | 9 | 23,888 ± 5,752 | 9 | 42,025 ± 9,615 | 7 | 27,938 ± 16,200 | 7 | 50,215 ± 25,822 |
| AUCmin/d (ng · day/ml) | 9 | 672 ± 130 | 9 | 1,187 ± 223 | 7 | 854 ± 302 | 7 | 15,35 ± 419* |
| Metabolic ratio | 9 | 1.85 ± 0.34 | 1.86 ± 0.26 | |||||
Cmin 12h, predose (trough) plasma concentrations; Cmax 4h, peak plasma concentration; Cssmin, average of plasma concentration at the plateau; TCss, time to reach Cssmin; AUCmin, area under the predose plasma concentration-time curve throughout the trial; AUCmin/d, AUCmin standardized to a day; metabolic ratio, ratio between AUCmin/d of hydroxyitraconazole and itraconazole. Data are indicated as the means ± the standard deviation where applicable. *, P < 0.05; **, P < 0.01 (versus group 1).
FIG. 1.
Linear mean (bars indicate the standard error of the mean) trough plasma concentration (Cmin 12h) over time for itraconazole and hydroxyitraconazole. Symbols: •, itraconazole group 1 (1.7 to 4.9 years, n = 10); ○, itraconazole group 2 (6.2 to 14.3 years, n = 7); ■, hydroxyitraconazole group 1; □, hydroxyitraconazole group 2.
Urinary concentrations of itraconazole measured on days 5 and 11 were below the limit of quantitation in all patients except for two patients in group 1 (309 and 348 ng/ml, both at day 11) and one patient in group 2 (73 ng/ml). For hydroxyitraconazole, the measurable concentrations were also low, ranging from 35 to 83 ng/ml and from 50 to 142 ng/ml in groups 1 and 2, respectively. The concentrations of hydroxypropyl-β-cyclodextrin in plasma on day 11 were below the lower limit of detection (<1.0 μg/ml) in both groups, with one exception (7.8 μg/ml). The mean concentrations of hydroxypropyl-β-cyclodextrin in urine on day 11 were 40 μg/ml in both groups.
Clinical adverse events and laboratory abnormalities were reported in all 17 participants and were mainly due to the underlying disease or concomitant treatments (including chemotherapy). One patient suffered coma, and another had coma and convulsions; in both cases, treatment was stopped, and the patients were withdrawn from the study. Abnormalities in the following hematologic parameters were reported: sodium (n = 14), aspartate transaminase (n = 11), total bilirubin (n = 11), total protein (n = 10), urea (n = 9), potassium (n = 9), chloride (n = 8), prothrombin time (n = 7), creatinine (n = 6), alanine transaminase (n = 5), calcium (n = 5), and conjugated bilirubin (n = 3).
The data generated by this trial are consistent with the good bioavailability of itraconazole oral solution in children. This is supported by the plasma concentration data, which show a steady increase in predose and peak concentrations of itraconazole and hydroxyitraconazole in all patients to reach steady state at about 2 weeks. Decreasing concentrations of itraconazole or metabolite observed after day 30 are due to the small number of remaining patients. The threshold of 250 ng/ml of itraconazole was reached after 3 to 5 days of treatment in younger children and at day 3 in older children. The elevated concentrations of itraconazole in plasma in older children could be due to a higher clearance in younger children. Data from several studies of lipophilic drugs metabolized by hepatic enzymes suggest higher clearance in infants than in older children (14). Similar trends were observed by De Repentigny et al. in a pediatric study: children aged 6 months to 2 years had lower maximum concentrations, reduced AUC levels, and a tendency toward lower minimum concentrations of itraconazole and hydroxyitraconazole than older children (aged 2 to 5 years or 5 to 12 years) (3).
Although the time to reach Cssmin is similar (∼12 days), our results differ from those of De Repentigny et al. (3) despite equivalent daily dosages and similar underlying disorders. The average Cmin 12h values of itraconazole and hydroxyitraconazole on day 15 in our study are much greater than those reported on day 14 by De Repentigny et al. (3). The different dosage schemes (2.5 mg/kg twice daily versus 5 mg/kg once daily) and sample times (12 h versus 24 h) cannot totally explain the differences observed. The lower concentrations previously reported in children (3) could be related to the dose-dependent bioavailability of itraconazole, as previously demonstrated following oral administration to healthy volunteers (11).
A previous clinical trial in adult bone marrow autograft recipients receiving a daily 5-mg/kg dose of itraconazole oral solution reported plasma concentrations consistently lower than those of group 2 in our study (12). The significance of the higher concentration in children is not fully understood but could be related to reduced bioavailability in the population of the Prentice et al. trial. The low concentration of hydroxypropyl-β-cyclodextrin in the plasma samples and the higher concentration in urine is consistent with the negligible oral bioavailability of the agent and the rapid renal elimination of the unchanged agent.
Safety data indicate that all patients experienced some adverse events, and blood chemistry abnormalities were equally frequent across the two groups. Any conclusion about the cause of these adverse events is speculative, given the serious nature of the underlying diseases and the amount of concomitant medication.
In conclusion, our data have shown that itraconazole oral solution is well tolerated in neutropenic children, and effective plasma concentrations of the parent drug and its active metabolite are reached within 3 to 5 days of the start of treatment and are maintained for the duration of treatment, without undue accumulation.
Acknowledgments
This study was supported by a grant from Janssen Research Foundation, Beerse, Belgium.
REFERENCES
- 1.Boogaert M A, Verhoef G E, Zachee P, Demuynck H, Verbist L, De Beule K. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses. 1989;32(Suppl. 1):103–108. doi: 10.1111/j.1439-0507.1989.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 2.Castagnola E, Bucci B, Montinaro E, Viscoli C. Fungal infections in patients undergoing bone marrow transplantation: an approach to a rational management protocol. Bone Marrow Transplant. 1996;18(Suppl. 2):97–106. [PubMed] [Google Scholar]
- 3.De Repentigny L, Ratelle J, Leclerc J-M, Cornu G, Sokal E, Jacqmin P, De Beule K. Repeated-dose pharmacokinetics of an oral solution of itraconazole in infants and children. Antimicrob Agents Chemother. 1998;42:404–408. doi: 10.1128/aac.42.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning D W. Evolving etiology of fungal infection in the 1990s. In: Rinaldi M G, Dixon D M, editors. The evolving etiologies of invasive mycoses. Infect. Dis. Clin. Pract. 3(Suppl. 2):S50–S55. 1994. [Google Scholar]
- 5.Glasmacher A, Hahn C, Molitor E, Marklein G, Sauerbrucht T, Schmidt-Wolf I G H. Itraconazole through concentrations in antifungal prophylaxis with six different dosing regimens using hydroxypropyl-β-cyclodextrin oral solution or coated-pellet capsules. Mycoses. 1999;42:591–600. doi: 10.1046/j.1439-0507.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Heykants J, Michiels M, Meuldermans W, Monbaliu J, Lavrijsen K, van Peer A, Levron J C, Woestenborghs R, Cauwenbergh G. The pharmacokinetics of itraconazole in animals and man: an overview. In: Fromtling R A, editor. Recent trends in the discovery, development and evaluation of antifungal agents. Barcelona, Spain: JR Prous Science Publishers; 1987. pp. 223–249. [Google Scholar]
- 7.Kageyama S, Masuya M, Tanaka I, Oka K, Merita K. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J Infect Chemother. 1999;5:213–216. doi: 10.1007/s101560050038. [DOI] [PubMed] [Google Scholar]
- 8.Levron J C, Reynes J, Bazin C, Ajana F, Datry A, Le Moing J P, Chwetzoff E, De Beule K. Bioavailability of itraconazole oral solution (IOS) during treatment of oropharyngeal candidosis in HIV+ patients. Montreal, Quebec, Canada: 19th International Congress of Chemotherapy; 1995. [Google Scholar]
- 9.Morgenstern G R, Prentice A G, Prentice H G, Ropner J E, Schey S A, Warnock D W. A randomized controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies. Br J Haematol. 1999;105:901–911. doi: 10.1046/j.1365-2141.1999.01465.x. [DOI] [PubMed] [Google Scholar]
- 10.Murray P A, Koletar S L, Mallegol I, Wu J, Moskovitz B L. Itraconazole oral solution versus clotrimazole troches for the treatment of oropharyngeal candidiasis in immunocompromised patients. Clin Ther. 1997;19:471–480. doi: 10.1016/s0149-2918(97)80131-2. [DOI] [PubMed] [Google Scholar]
- 11.Prentice A G, Warnock D W, Johnson S A, Phillips M J, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J Antimicrob Chemother. 1994;34:247–252. doi: 10.1093/jac/34.2.247. [DOI] [PubMed] [Google Scholar]
- 12.Prentice A G, Warnock D W, Johnson S A, Taylor P C, Oliver D A. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J Antimicrob Chemother. 1995;36:657–663. doi: 10.1093/jac/36.4.657. [DOI] [PubMed] [Google Scholar]
- 13.Szathmary S C. Determination of hydroxypropyl-beta-cyclodextrin in plasma and urine by size-exclusion chromatography with post-column complexation. J Chromatogr. 1989;487:99–105. doi: 10.1016/s0378-4347(00)83011-x. [DOI] [PubMed] [Google Scholar]
- 14.Tréluyer J M, Rey E, Pons G. Pharmacokinetics principles in paediatric pharmacology: proof of differences beyond the neonatal period. Baillieres Clin Paediatr. 1998;6:399–418. [Google Scholar]
- 15.Van Cutsem J, Van Gerven F, Janssen P A J. The in vitro and in vivo antifungal activity of itraconazole. In: Fromtling R A, editor. Recent trends in the discovery, development and evaluation of antifungal agents. Barcelona, Spain: JR Prous Science Publishers; 1987. pp. 177–192. [Google Scholar]
- 16.van't Wout J W, Novakova I, Verhagen C A, Fibbe W E, de Pauw B E, van der Meer J W. The efficacy of itraconazole against systemic fungal infections in neutropenic patients: a randomised comparative study with amphotericin B. J Infect. 1991;22:45–52. doi: 10.1016/0163-4453(91)90954-q. [DOI] [PubMed] [Google Scholar]
- 17.Walsh T J, Gonzalez C, Lyman C A, Chanock S J, Pizzo P A. Invasive fungal infections in children: recent advances in diagnosis and treatment. Adv Pediatr Infect Dis. 1996;11:187–290. [PubMed] [Google Scholar]
- 18.Woestenborghs R, Lorreyne W, Heykants J. Determination of itraconazole in plasma and animal tissues by high-performance liquid chromatography. J Chromatogr. 1987;413:332–337. doi: 10.1016/0378-4347(87)80249-9. [DOI] [PubMed] [Google Scholar]