Abstract
Canine babesiosis is a life-threatening haemoparasitic disease in dogs that is prevalent worldwide. In this study, the prevalence of Babesia vogeli (B. vogeli) was investigated in dogs from Egypt by using Polymerase Chain Reaction (PCR) assay, and associated risk factors were evaluated. In addition, phylogenetic position of B. vogeli Egyptian isolate was determined by sequencing. A total of 275 blood samples were taken from dogs located in four governorates belonging to the north of Egypt. Samples were examined by PCR targeting the B. vogeli 18S rRNA gene and this species was also confirmed by sequencing. Overall, the prevalence of B. vogeli was 5.1% among the studied dogs and the highest prevalence rate was found in the Giza governorate. Univariate logistic regression was used to evaluate each variable individually. The results revealed a significant association between the prevalence of B. vogeli infection and whether or not dogs were infested with ticks and the type of floor used in dog shelters. Additionally, tick infestation (OR 6.1, 95% CI 1.2–31.4), and living in shelters with soil floors (OR 3.8, 95% CI 0.8–17.8) were identified as potential risk factors for B. vogeli infection. Phylogenetic analysis was performed using B. vogeli 18S rRNA partial sequences with the hypervariable V4 region from GenBank. The Egyptian isolate was assigned to second sub-cluster with B. vogeli isolates from Japan, Venezuela and Paraguay within the B. vogeli/B. canis cluster. The present data will be useful to improve the understanding of canine babesiosis epidemiology and ways to control the disease in companion dogs.
Subject terms: Molecular biology, Risk factors
Introduction
Babesiosis is a disease that affects dogs all over the world. It is caused by intracellular erythrocytic parasites of the genus Babesia1,2. The disease is spread by ixodid ticks that carry either small (1–2.5 µm) or large (4-5 µm) Babesia species. Large Babesia spp. were previously thought to be B. canis, but they are now classified into three independent species based on their genetic traits, the severity of the clinical signs that they cause, their tick vectors and their range geographical distribution3–5. These three species are Babesia canis, Babesia rossi and Babesia vogeli2,6,7.
The most common Babesia species in Europe is B. canis, which is transmitted by Dermacentor reticulatus. Haemaphysalis elliptica transmits B. rossi, which is the most common species in South Africa; B. vogeli is transmitted by Rhipicephalus sanguineus sensu lato, which is most commonly found in tropical and subtropical areas1,8–10.
Canine babesiosis has no specific symptoms; however, pyrexia, anorexia, epistaxis, petechiae and splenomegaly are common signs, along with hemoglobinuria, anemia and thrombocytopenia2. The disease is routinely diagnosed based on clinical signs, hematological findings and detection of intracellular parasites in blood smears11,12.
In order to confirm infection and start treatment, various laboratory procedures, such as serological tests and molecular methods, should be performed due to the lack of distinct clinical signs and the high frequency of false negatives that occur in analysis of blood smears, especially in cases of low parasitaemia13,14. Serological testing is a valuable diagnostic tool, although it has limits due to cross-reactivity between Babesia species and the inability to use this method to differentiate early from chronic infection15.
To directly detect hemoparasite DNA from clinical or environmental samples, a variety of molecular tools, such as loop-mediated isothermal amplification assay (LAMP), quantitative polymerase chain reaction (qPCR), and high resolution melting analysis (HRM), are used, providing relatively inexpensive, rapid molecular tests with high throughput. The PCR assay is more reliable than traditional methods for detection of piroplasms and has high sensitivity and specificity16–21.
Sparse research has been done on canine babesiosis or its associated risk factors in Egypt. A few studies have detected B. vogeli in dogs from Egypt based on microscopic smear and molecular techniques22,23. However, these researchers did not consider the risk factors associated with B. vogeli infection in dogs.
Therefore, the study described here aimed to determine the prevalence of canine babesiosis and its associated risk factors based on PCR assays and partial sequencing of the 18S rRNA gene.
Materials and methods
Ethical statement
All procedures involving the handling and collection of blood samples were approved by the Benha University ethical committee for animal experiments. Informed consent and permissions were obtained from the dog's owners to collect samples. All procedures involving laboratory animals were performed in accordance with current standards and regulations, and approved by the ethics committee of the Faculty of Veterinary Medicine at Benha University. This study was carried out in compliance with the ARRIVE guidelines.
Study area
The study was conducted during 2019 in the four governorates of Giza, Kafr El Sheikh, Qalyubia and Gharbia located in the north of Egypt. According to the Köppen Geiger classification, the climate of the selected areas is that of a desert characterized by hot, dry summers and mild winters. The average annual temperature is 22 °C and the annual rainfall is 180 mm during the winter season.
Sampling
The number of samples to be taken in this study was determined based on an equation described by Thrusfield24 as follows:
where n is the sample size, pexp is the expected prevalence, which in this case was taken as 50%, and d2 is the precision, which was set at 5% in this study. To meet this requirement, a total of 275 blood samples were collected from saphenous and cephalic veins of dogs in sterile vacuum tubes and mixed with ethylenediamine tetraacetic acid (EDTA) buffer. All sampled dogs were owned by individuals. Some appeared to be healthy, while others showed signs of babesiosis.
To later take into account possible risk variables, information was collected regarding the locality in which each dog lived, its sex, breed, age, whether or not it was infested with ticks without determining the level of tick infestation, whether or not an acaricide had been applied and the type of floor it was sleeping on.
Molecular analysis
For molecular diagnosis, a commercial kit (Qiagen DNeasy-tissues-blood, Valencia, CA, USA) was used to extract DNA from whole blood samples according to the manufacturer's instructions.
DNA extraction controls were used to test newly extracted samples. The extracted DNA was stored at − 20 °C until the PCR assay could be performed. All samples were examined by using the conventional PCR assay that targeted the 18SrRNA gene and employing the BAB1 BAB4 primers, as previously described by Duarte et al.25. The sensitivity of this PCR assay was previously evaluated and can be able to detect one Babesia-infected blood cell per sample.
PCR amplification was carried out in a 25 µl volume including 12.5 µl of Dream Taq green PCR master mix (2×) (Thermo Scientific, Germany), 1 µl of each primer (20 pmol/µl), 5.5 μl of ddH2O and 5 μl (50–150 ng) of template DNA. In addition, distilled water and (DNA positive to B. vogeli) were used as negative and positive controls in order to confirm PCR results. The thermal conditions were as follows: 95 °C for 10 min, followed by 35 cycles at 95 °C for 15 s, 56 °C for 30 s and 72 °C for 1 min. The PCR products were electrophoresed in a 1.5% agarose gel with ethidium bromide staining.
PCR product based on the primers RIB-19 and RIB21 of Zahler et al.26 for one positive sample was purified by using the QIAquick PCR Purification Kit (QIAGEN, Valencia, USA), and sequenced with the ABI PRISM BigDye TM Terminators Kit (Applied Biosystems, USA) in accordance with the manufacturer's instructions.
The Bioedit program was used to trim and edit the obtained chromatogram, and sense and antisense sequences were used to create contigs and only overlapping sequences have been selected. The obtained sequence was deposited in GenBank with accession number LC651125.
The revealed sequence was compared and aligned with 18S rRNA partial sequences for B. vogeli and other Babesia species available in GenBank by using CLUSTAL W integrated in DNAMAN software (Version 5.2.2; Lynnon Biosoft, Que., Canada). The same software was used to construct a phylogenetic tree based on the Maximum-likelihood algorithm27 with bootstrap analysis of 1000 iterations28,29.
Statistical analysis
Univariable logistic regression was used for initial screening of investigated exposure factors associated with Babesia infection with P-value ≤ 0.20 were considered for multivariable logistic regression. Stepwise forward multivariable logistic regression was used to identify significant risk factor(s) associated with Babesia infection in dogs. Variable selection for stepwise forward multivariable logistic regression model was performed based on the lowest value for the Akaike information criterion (AIC). Confounding between risk factors retained in final models was examined by adding each of the variables to the model and assessing the changes in the POR (i.e., ≥ 20%) of the remaining variables in the model. Regression analysis was performed by using SAS 9.4 (SAS Inst. Inc., Cary, NC), and P < 0.05 was considered significant. The multicollinearity was assessed through correlation procedure, collinearity analysis (COLLIN) and Variance Inflation Factors (VIF). Application of acaricide variable was dropped to reduce collinearity with tick infestation.
Results
In the examined group of dogs, 5.1% of animals (n = 14/275) were found positive for B. vogeli by PCR targeting 18S rRNA. Of these animals, 6.7% lived in Giza, 6.3% in Qalyubia, 4.1% in Kafr El Sheikh and 3.2% in Gharbia (Table 1). Univariable logistic regression results showed that host-related variables such as sex and breed of each dog were non-significantly associated with the prevalence of B. vogeli infection (P > 0.05). Indeed, B. vogeli infection rate was higher in males (7.6%) and mongrel breeds (9.1%) compared to females (2.3%) and the German Shepherd and Rottweiler breeds (2.1%). Moreover, the dog age and the lack of acaricides application showed no statistically significant association with the prevalence of B. vogeli (P > 0.05) although the highest prevalence rate observed among dogs aged > 2 to ≤ 4 years (Table 1). According to the multivariable logistic regression analysis, the tick infestation (OR 6.1, 95% CI 0.8–17.8), and the presence of an earthen floor in the dog’s shelter (OR 3.8, 95% CI 1.2–31.4) were potential risk factors for occurrence of B. vogeli infection (Table 2).
Table 1.
Univariable logistic regression analysis for identification of risk factors associated with B. vogeli infection in 275 dogs in Egypt.
| Variable | Category | N | Positive | Prevalence (%) | POR (95% CI) | P-value | VIF |
|---|---|---|---|---|---|---|---|
| Geographic location | Gharbia | 62 | 2 | 3.2 | 1.0 (reference) | 0.299 | 1.2 |
| Giza | 75 | 5 | 6.7 | 2.1 (0.4–11.4) | |||
| Qalyubia | 64 | 4 | 6.3 | 2.0 (0.4–11.3) | |||
| Kafr ElSheikh | 74 | 3 | 4.1 | 1.3 (0.2–8.0) | |||
| Gender | Female | 130 | 3 | 2.3 | 1.0 (reference) | 0.06 | 1.0 |
| Male | 145 | 11 | 7.6 | 3.5 (0.9–12.7) | |||
| Breed | German Shepherd | 97 | 2 | 2.1 | 1.0 (reference) | 1.1 | |
| Rott weiler | 68 | 2 | 2.1 | 1.4 (0.2–10.5) | 0.719 | ||
| Mongrel | 110 | 10 | 9.1 | 4.8 (1.0–22.2) | 0.048 | ||
| Age | 6 to 12 months | 46 | 1 | 2.2 | 1.0 (reference) | 0.623 | 1.1 |
| > 1 to ≤ 2 years | 42 | 1 | 2.4 | 1.1 (0.1–18.1) | |||
| > 2 to ≤ 4 years | 109 | 10 | 9.2 | 4.5 (0.6–36.6) | |||
| > 4 years | 78 | 2 | 2.6 | 1.2 (0.1–13.4) | |||
| Tick infestation | No | 119 | 2 | 1.7 | 1.0 (reference) | 0.041 | 3.8 |
| Yes | 156 | 12 | 7.7 | 4.9 (1.1–22.2) | |||
| Application of Acaricide | Yes | 132 | 3 | 2.3 | 1.0 (reference) | 0.054 | 3.8 |
| No | 143 | 11 | 7.7 | 3.6 (1.0–13.1) | |||
| Shelter floor | Paved | 115 | 2 | 1.7 | 1.0 (reference) | 1.1 | |
| Soil + paved | 98 | 5 | 5.1 | 3.0 (0.6–16.0) | 0.190 | ||
| Soil | 52 | 7 | 11.3 | 7.2(1.4–35.8) | 0.016 |
Table 2.
Multiple stepwise logistic regression analysis of potential risk factors associated with B. vogeli infection in dogs from Egypt.
| Variable | Categories | Estimate | SE | P-value | PORadj | 95% CIOR |
|---|---|---|---|---|---|---|
| Intercept | − 6.6 | 1.3 | < 0.001 | – | – | |
| Shelter floor | Paved | Reference | ||||
| Soil + paved | 0.9 | 0.87 | 0.284 | 2.5 | 0.5–13.8 | |
| Soil | 1.8 | 0.84 | 0.031 | 6.1 | 1.2–31.4 | |
| Tick infestation | No | Reference | ||||
| Yes | 1.3 | 0.79 | 0.041 | 3.8 | 0.8–17.8 |
SE Standard error, POR Odds ratio, CI confidence interval.
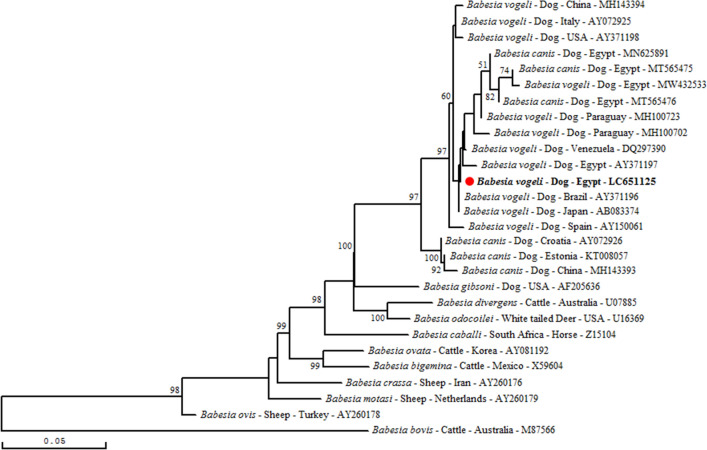
Babesia vogeli infection was confirmed by sequencing of the portion of 18SrRNA gene for one randomly selected positive dog sample. When compared our revealed sequence to those published in GenBank, the identity rates were ranged from 98 to 100%. Phylogenetic analysis was performed using Babesia vogeli 18S rRNA partial sequences with the hypervariable V4 region from GenBank. The Egyptian isolate was assigned to second sub-cluster with B. vogeli isolates from Japan, Venezuela and Paraguay within the B. vogeli/B. canis cluster closely related to that of Babesia gibsoni (Fig. 1).
Figure 1.
Maximum-likelihood tree based on the alignment of partial 18S rRNA sequences (531 bp) of B. vogeli and other sequences of Babesia spp. Multiple sequence alignments were generated with DNAMAN program (Version 5.2.2; Lynnon Biosoft, Que., Canada). Numbers associated with nodes represent the percentage of 1000 bootstrap iterations supporting the nodes (only percentages greater than 50% were represented). The novel sequence of B. vogeli obtained in the present study is represented in bold and by a circle colored in red. One Babesia bovis 18S rRNA partial sequence was added as out-group. The host, the country of origin and the GenBank accession number are indicated.
Discussion
Babesia vogeli is one of the most important pathogens among Babesia species found in dogs. However, few epidemiological studies have been performed on canine babesiosis in Egypt, particularly on B. vogeli. Many epidemiological research studies have revealed that molecular and serological approaches produce different outcomes and PCR data have been shown to be more accurate14,30.
The identification of DNA in many infections in vectors and hosts, particularly by haemoparasites, requires the use of molecular biology tools. The use of the PCR technique increases the ability to diagnose canine babesiosis and to provide taxonomic classification of Babesia species.
In dogs from four governorates in northern Egypt, the overall prevalence of B. vogeli infection was 5.1%. The prevalence rate varied non-significantly among localities however it was higher in Giza than other governorates. This lack of difference might be due to similarities in geo-climatic circumstances. Comparing the results with those of previous research, we found that the reported prevalence rate of B. vogeli was consistent with those reported in Iraq (5.1%)31 and in Recife, Brazil (4.8%)32. In contrast, the prevalence rate found in this study was lower than that estimated by Paulino et al.33, Khanmohammadi et al.34, Ćoralić et al.1 and Obeta et al.35, who reported B. vogeli infection rates of 15.6, 9.3, 85 and 10.8% in Brazil, Iran, Bosnia and Herzegovina, and Nigeria, respectively. Disparities between these prevalence rates could be caused by differences in experimental design, geographical or environmental factors, study duration, season of the year in which the studies were performed, sanitary measures that were applied and differences between used diagnostic tests6,34,36–38.
Regarding dog breeds, a higher prevalence rate of B. vogeli infection was recorded in mongrels (9.1%) compared to the purebreed of German Shepherd (2.1%) and Rottweiler (2.1%). This finding was in accordance with previous findings reported by Obeta et al.35. Interestingly, mongrels are more susceptible to tick infestation and other potential risk factors. In contrast, other research has reported that babesiosis is more common and severe in imported dogs than in native breeds. However, according to Mellanby et al.39, not all dog breeds are equally susceptible to babesiosis; they reported that Toy types were at lower risk than other breeds.
Nonetheless, we believe that mongrel breeds are inexpensive to purchase and their owners often ignore them and allow them to stray and scavenge, exposing them to ticks. The present findings confirm that males are more likely to be infected with B. vogeli than females. Similar results were found by Daniel et al.40, who reported that males were more susceptible to Babesia infection than females. The higher susceptibility of male dogs to canine babesiosis may be attributed to differences in environmental exposure to tick infestation, such as that caused by a strong tendency to roam, or sex-related genetic or hormonal effects on disease. Females were also considered to be better managed by their owners in order to get more money from their puppies39,40. However, other studies have shown babesiosis to be more prevalent in females than males, and researchers have attributed this to increased sitting behavior in female dogs, especially while nursing their puppies, which makes them more susceptible to tick vector infestations41,42. Additionally, unusual reproductive behaviors of females may cause stress, leading to reduced their immunity and increased tolerance of tick-borne diseases.
The current findings show that the prevalence rate of B. vogeli was highest in dogs aged from > 2 to ≤ 4 years. This result was consistent with previous results from Obeta et al.35,43. This is most likely due to decreased maternal immunity and resistance in dogs of this age, as well as repeated tick infestations35,44. We believe that dogs in this category are active and, if given the opportunity, like to roam randomly, which predisposes them to tick infestations.
The prevalence of B. vogeli infection was significantly associated with the presence of ticks. This result is consistent with findings of previous research33, which reported that B. vogeli was transmitted with R. sanguineus s. l. ticks. Therefore, the distribution of ticks among dogs in both urban and rural areas of Egypt would lead to increased susceptibility of dogs to B. vogeli infection45.
Moreover, the most detectable tick species was observed among examined dogs was R. sanguineus s.l. which come in agreement with previous report by Hassanen23. As expected, dogs that did not receive adequate care or regular application of suitable acaricides and repellents to reduce the number of ticks they carried were generally associated with a higher prevalence of vector-borne diseases such as babesiosis. These findings are directly in line with those of Araujo et al.46, who found a strong correlation between levels of B. vogeli infection and lack of veterinary care. The material from which the shelter floor was made was also found to be a significant risk factor in this investigation. Compared with dogs housed in shelters with paved floors, the risk of B. vogeli infection was considerably higher in dogs raised in earthen-floor shelters. Paved floors are easier to clean than earth floors and provide less favorable conditions for vector larvae to spread47.
Understanding the evolutionary relationships between B. vogeli isolates is essential to conduct an in-depth intra-specific diversity analysis that will help to improve prevention and management of the spread of this bacterium. Sequence analysis of partial 18S rRNA gene from an Egyptian isolate obtained in this study showed a high degree of similarity with 18S rRNA partial sequences isolated from different B. vogeli isolates infecting dogs from various countries. Indeed, a phylogenetic tree was generated and the position of our Egyptian isolate confirmed that the positive dogs were infected with B. vogeli, as previously reported by Hassanen23 in the same country.
Conclusion
Overall, epidemiological analysis performed in this study by multivariable logistic regression showed a strong association between the prevalence of B. vogeli in dogs and whether or not they were infested with ticks and the type of floor used for their shelters. Phylogenetic analysis confirmed that the partial 18S rRNA sequence identified herein indicated that the revealed isolate of B. vogeli were related to those previously infecting Egyptian dogs and other B. vogeli worldwide isolates.
Supplementary Information
Acknowledgements
The author would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Author contributions
Conceptualization, A.S. A.M., A.A. M.BS. and M.S.A.; methodology, A.S.; formal analysis, A.S.; investigation, A.S.; resources, A.M., A.A., M.BS.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.S., A.M., A.A. M.BS. and M.S.A.; project ad-ministration, A.M., A.A., M.S.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data that were generated or analysed during this study are included in this published article and its additional files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelfattah Selim, Email: Abdelfattah.selim@fvtm.bu.edu.eg.
Abdullah D. Alanazi, Email: aalanazi@su.edu.sa
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11079-x.
References
- 1.Ćoralić A, et al. First molecular detection of Babesia canis in dogs from Bosnia and Herzegovina. Ticks Tick-Borne Dis. 2018;9:363–368. doi: 10.1016/j.ttbdis.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: The European perspective. Parasit. Vectors. 2016;9:1–18. doi: 10.1186/s13071-015-1291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selim, A., Abdelhady, A. & Alahadeb, J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak. Vet. J.41, 117–121 (2020).
- 4.Selim A, Alanazi AD, Sazmand A, Otranto D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasit. Vectors. 2021;14:1–11. doi: 10.1186/s13071-020-04505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selim A, Almohammed H, Abdelhady A, Alouffi A, Alshammari FA. Molecular detection and risk factors for Anaplasma platys infection in dogs from Egypt. Parasit. Vectors. 2021;14:1–6. doi: 10.1186/s13071-020-04505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panti-May JA, Rodríguez-Vivas RI. Canine babesiosis: A literature review of prevalence, distribution, and diagnosis in Latin America and the Caribbean. Vet. Parasitol. Reg. Stud. Rep. 2020;21:100417. doi: 10.1016/j.vprsr.2020.100417. [DOI] [PubMed] [Google Scholar]
- 7.Vishwakarma, P. & Nandini, M. Veterinary Medicine and Pharmaceuticals 1–17 (IntechOpen, 2019).
- 8.Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet. Parasitol. 2006;138:103–111. doi: 10.1016/j.vetpar.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Goo Y-K, Xuan X. New molecules in Babesia gibsoni and their application for diagnosis, vaccine development, and drug discovery. Korean J. Parasitol. 2014;52:345. doi: 10.3347/kjp.2014.52.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razi, J. M. et al. Babesia infection in urban and rural dogs in Ahvaz district, Southwest of Iran. Arch. Razi. Inst. 68, 37–42 (2013). [DOI] [PubMed]
- 11.Mittal M, et al. Canine babesiosis among working dogs of organised kennels in India: A comprehensive haematological, biochemical, clinicopathological and molecular epidemiological multiregional study. Prev. Vet. Med. 2019;169:104696. doi: 10.1016/j.prevetmed.2019.104696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dos Santos FB, et al. Microscopic detection, hematological evaluation and molecular characterization of piroplasms from naturally infected dogs in Rio de Janeiro, Brazil. Acta Parasitol. 2021;66:1–13. doi: 10.1007/s11686-020-00254-7. [DOI] [PubMed] [Google Scholar]
- 13.Vismaya K, et al. Clinico-haematological evaluation and molecular identification of Babesia gibsoni, Babesia canis vogeli, Ehrlichia canis and Trypanosoma evansi in dogs. J. Vet. Parasitol. 2020;34:17–26. doi: 10.5958/0974-0813.2020.00004.2. [DOI] [Google Scholar]
- 14.Kubelová M, Sedlák K, Panev A, Široký P. Conflicting results of serological, PCR and microscopic methods clarify the various risk levels of canine babesiosis in Slovakia: A complex approach to Babesia canis diagnostics. Vet. Parasitol. 2013;191:353–357. doi: 10.1016/j.vetpar.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso L, et al. Babesia canis canis and Babesia canis vogeli infections in dogs from northern Portugal. Vet. Parasitol. 2008;156:199–204. doi: 10.1016/j.vetpar.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Kivrane A, et al. Development of a real-time PCR method for rapid diagnosis of canine babesiosis and anaplasmosis. Parasit. Vectors. 2021;14:1–12. doi: 10.1186/s13071-021-04756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyamada M, et al. Detection of Babesia canis rossi, B. canis vogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies. Clin. Vaccine Immunol. 2005;12:1343–1346. doi: 10.1128/CDLI.12.11.1343-1346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargas-Hernández G, et al. Molecular and serological detection of Ehrlichia canis and Babesia vogeli in dogs in Colombia. Vet. Parasitol. 2012;186:254–260. doi: 10.1016/j.vetpar.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Selim A, Ali A-F, Moustafa SM, Ramadan E. Molecular and serological data supporting the role of Q fever in abortions of sheep and goats in northern Egypt. Microb. Pathog. 2018;125:272–275. doi: 10.1016/j.micpath.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Selim A, Ali A-F, Ramadan E. Prevalence and molecular epidemiology of Johne’s disease in Egyptian cattle. Acta Trop. 2019;195:1–5. doi: 10.1016/j.actatropica.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Selim A, Attia K, Ramadan E, Hafez YM, Salman A. Seroprevalence and molecular characterization of Brucella species in naturally infected cattle and sheep. Prev. Vet. Med. 2019;171:104756. doi: 10.1016/j.prevetmed.2019.104756. [DOI] [PubMed] [Google Scholar]
- 22.Salem N, Farag H. Clinical, hematologic, and molecular findings in naturally occurring Babesia canis vogeli in Egyptian dogs. Vet. Med. Int. 2014;2014:1–6. doi: 10.1155/2014/270345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassanen EA. Clinical, morphological and molecular characterization of canine babesiosis and its compatible tick vector in naturally infected dogs, in Egypt. Zagazig Vet. J. 2020;48:242–253. doi: 10.21608/zvjz.2020.24982.1103. [DOI] [Google Scholar]
- 24.Thrusfield, M. Veterinary Epidemiology. (Wiley, 2018).
- 25.Duarte SC, Linhares GFC, Romanowsky TN, da Silveira Neto OJ, Borges LMF. Assessment of primers designed for the subspecies-specific discrimination among Babesia canis canis, Babesia canis vogeli and Babesia canis rossi by PCR assay. Vet. Parasitol. 2008;152:16–20. doi: 10.1016/j.vetpar.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Zahler M, et al. ‘Babesia gibsoni’ of dogs from North America and Asia belong to different species. Parasitology. 2000;120:365–369. doi: 10.1017/S0031182099005557. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 28.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrljak V, et al. Prevalence and geographic distribution of vector-borne pathogens in apparently healthy dogs in Croatia. Vector Borne Zoonotic Dis. 2017;17:398–408. doi: 10.1089/vbz.2016.1990. [DOI] [PubMed] [Google Scholar]
- 31.Badawi NM, Yousif AA. Babesia canis spp. in dogs in Baghdad Province, Iraq: First molecular identification and clinical and epidemiological study. Vet. World. 2020;13:579. doi: 10.14202/vetworld.2020.579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva VCL, et al. Parasitological and molecular detection of Babesia canis vogeli in dogs of Recife, Pernambuco and evaluation of risk factors associated. Semina Ciências Agrárias. 2016;37:163–171. doi: 10.5433/1679-0359.2016v37n1p163. [DOI] [Google Scholar]
- 33.Paulino PG, et al. Molecular epidemiology of Babesia vogeli in dogs from the southeastern region of Rio de Janeiro, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2018;13:160–165. doi: 10.1016/j.vprsr.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Khanmohammadi M, Zolfaghari-Emameh R, Arshadi M, Razmjou E, Karimi P. Molecular Identification and Genotyping of Babesia canis in Dogs from Meshkin Shahr County, Northwestern Iran. J. Arthropod Borne Dis. 2021;15:97–107. doi: 10.18502/jad.v15i1.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obeta SS, et al. Prevalence of canine babesiosis and their risk factors among asymptomatic dogs in the federal capital territory, Abuja, Nigeria. Parasite Epidemiol. Control. 2020;11:e00186. doi: 10.1016/j.parepi.2020.e00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selim A, Marawan MA, Ali A-F, Manaa E, AbouelGhaut HA. Seroprevalence of bovine leukemia virus in cattle, buffalo, and camel in Egypt. Trop. Anim. Health Prod. 2020;52:1207–1210. doi: 10.1007/s11250-019-02105-8. [DOI] [PubMed] [Google Scholar]
- 37.Selim, A., Radwan, A., Arnaout, F. & Khater, H. The recent update of the situation of West Nile Fever among Equids in Egypt after three decades of missing information. Pak. Vet. J.40, 390–393 (2020).
- 38.Selim A, et al. Seroprevalence and risk factors associated with canine leishmaniasis in Egypt. Vet. Sci. 2021;8:236. doi: 10.3390/vetsci8100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellanby R, et al. Breed and sex risk factors for canine babesiosis in South Africa. J. Vet. Intern. Med. 2011;25:1186–1189. doi: 10.1111/j.1939-1676.2011.00779.x. [DOI] [PubMed] [Google Scholar]
- 40.Daniel, L., Kujul, N., Kemza, S. & Ibukunoluwa, M. Retrospective study of the risk factors associated with canine babesiosis diagnosed at Veterinary Clinic Federal College of Animal Health and Production Technology, Vom, North-central Nigeria, 1999–2006. Int. J. Sci. Appl. Res. (ISSN: 2504–9070)1 (2016).
- 41.Okubanjo O, Adeshina O, Jatau I, Natala A. Prevalence of Babesia canis and Hepatozoon canis in Zaria, Nigeria. Sokoto J. Vet. Sci. 2013;11:15–20. [Google Scholar]
- 42.Jegede O, Obeta S, Faisal B. Infection of dogs with Babesia canis in Gwagwalada metropolis of Federal Capital Territory, Abuja, Nigeria. Sokoto J. Vet. Sci. 2014;12:37–41. doi: 10.4314/sokjvs.v12i3.7. [DOI] [Google Scholar]
- 43.Hornok S, Edelhofer R, Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasitol. Res. 2006;99:638–642. doi: 10.1007/s00436-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 44.Egege S, Okolocha E, Nwanta J, Mosimabable F. Prevalence and seasonality of babesiosis in dogs treated at a Univerisity Veterinary Clinic in Kaduna, Nigeria from 1990–1999. Niger. Vet. J. 2008;29:21–26. [Google Scholar]
- 45.Nasr A, El Hariri M, Ghafar MW. Detection of Anaplasma platys and Ehrlichia canis in Rhipicephalus sanguineus ticks attached to dogs from Egypt; a public health concern. Vet. Med. J. Giza. 2020;66:1–9. [Google Scholar]
- 46.Araujo AC, et al. Babesia canis vogeli infection in dogs and ticks in the semiarid region of Pernambuco, Brazil. Pesquisa Veterinária Brasileira. 2015;35:456–461. doi: 10.1590/S0100-736X2015000500012. [DOI] [Google Scholar]
- 47.de Almeida Leal GG, et al. Risk profile for Leishmania infection in dogs coming from an area of visceral leishmaniasis reemergence. Prev. Vet. Med. 2018;150:1–7. doi: 10.1016/j.prevetmed.2017.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that were generated or analysed during this study are included in this published article and its additional files.