Abstract
Introduction
Resistant hypertension (RHT) is a phenotype of hypertension that is challenging to manage by medications alone. While high grade evidence supports physical activity (PA) and exercise to reduce blood pressure (BP) in hypertension, it is unclear whether these are also effective for RHT.
Aims
To determine the quality of evidence for the effectiveness of PA and exercise and the change of magnitude of 24-hour ambulatory BP (24hABP) in adults with RHT.
Methods
Scopus, MEDLINE, CINHAL, Web of Science, Embase and SPORTDiscus databases were searched. Cochrane risk of bias tools, Review Manager and Grading of the Recommendation Assessment, Development and Evaluation were used to assess the methodological quality, the clinical heterogeneity and quality of the evidence.
Results
Four studies comprising 178 individuals in total were included. A meta-analysis with random effects showed decreased 24hABP. The experimental group demonstrated grater mean differences for 24hABP following the PA and exercise programmes (systolic − 9.88 mmHg, 95% CI: − 17.62, − 2.14, I2 = 72%, p = 0.01; diastolic − 6.24 mmHg, 95% CI: − 12.65, 0.17, I2 = 93%,p = 0.06); and aerobic exercise (systolic − 12.06 mmHg, 95% CI: − 21.14, − 2.96, I2 = 77%, p = 0.009, diastolic − 8.19 mmHg, 95% CI: − 14.83, − 1.55, I2 = 92% ,p = 0.02). In the included studies, indirectness and publication bias were ‘moderate’ while inconsistency and imprecision were rated as ‘low’. Thus, the overall quality of the evidence was considered to be ‘low’.
Conclusions
Low certainty evidence suggests that PA and aerobic exercise added to usual care may be more effective in 24hABP reduction in RHT than usual care alone.
Registration
PROSPERO—2019 CRD42019147284 (21.11.2019).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40292-022-00517-6.
Keywords: Hypertension, Resistant hypertension, Physical activity, Exercise
Introduction
Controlling blood pressure (BP) in adults with hypertension using medication alone is becoming an increasing challenge. BP is adequately controlled by medications in only 25–62% of those with hypertension [1]. Resistant hypertension (RHT), a special phenotype of hypertension, first defined by the American Heart Association (AHA) in 2008 [2]. RHT is a multifactorial condition that may be related to non-compliance with medication, obstructive sleep apnoea, arterial stiffness, increased sympathetic activity, and sedentary life leading to physical inactivity [2, 3]. A combination of multiple medications in a single tablet has been considered as an approach to reduce BP, especially for poorly controlled hypertension [4], but is still at an experimental level [5, 6].
The concept of physical activity and exercise as a modality in the management of hypertension is well established [7]. Physical activity, exercise and dietary interventions are considered as adjunctive management strategies to pharmacological therapies [3, 8, 9]. Aerobic, dynamic resistance and isometric exercises, in combination or any two forms, has been shown to reduce BP at variable magnitude for people with hypertension [1, 10–14]. Aerobic exercise is the most highly recommended type of exercise to reduce BP [1, 14, 15]. A reduction in BP greater than 4.4 mmHg is considered to be a clinically significant change [16]. In randomised control trials that have explored the effects of exercise on BP in RHT, duration of exercise varied from a single bout (e.g. 45 min) [17, 18] to time periods of up to 6 months [13, 18–21]. Exercise interventions of more than 4 weeks duration have been shown to be more therapeutically effective than shorter term exercise [22].
Of the meta-analyses that have addressed the influence of physical activity or exercise in individuals with hypertension, only a few have considered 24 hour ambulatory blood pressure (24h ABP) as the key outcome measurement [10, 23, 24]. A recent network meta-analysis reported that the BP lowering effects in response to exercise were similar to the effects caused by the commonly used antihypertensive medications in individuals with hypertension [7]. For people with RHT the evidence regarding the effect of exercise or physical activity on change in magnitude of 24h ABP is unclear. Thus, the aim of this systematic review was to explore the effectiveness of physical activity and/or exercise on change in 24h ABP, by pooling the results of trials conducted in populations with RHT. The specific objectives were to: (1) compare the mean differences in 24h ABP in experimental and control groups in individuals with RHT; and (2) compare the change in magnitude of 24h ABP and/or office BP for individuals with RHT with different types of exercise (e.g. aerobic training, resistance training, isometric exercise or a combination) and/or physical activity.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) [25] was followed to develop the protocol and PRISMA guidelines were used to report this systematic review. The protocol [26] was prospectively registered with the PROSPERO registry (CRD42019147284).
Search Strategy
Search terms were piloted prior to the initial database search. Scopus, MEDLINE (Ovid), CINHAL, Web of Science, Embase, SPORTDiscus and Cochrane Central Register of Controlled Trials were used to search article abstracts and titles. For unpublished literature, Open Grey, Current Control Trials, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform were searched. The cut-off date for included literature was September 30, 2019. A subsequent update was undertaken on January 6, 2021. The steps in the search using keywords and combination of results is summarised below:
“Resistant hypertension”; “Refractory hypertension”; “Apparent resistant hypertension”; “Drug resistant hypertension”; “Uncontrolled hypertension”; “Uncontrolled blood pressure”, combining with Boolean logic ‘OR’
“Exercise”; “Physical activit*”; “Physical exercise”; “lifestyle modification*”; “Exercise training”; “Exercise protocol*”; “water based exercise”, combining with Boolean logic ‘OR’
The two search results were combined using Boolean logic ‘AND’
A manual search of the reference lists of included articles (n=4) was undertaken independently by each of the two reviewers (SD and EG), to search for additional studies not identified in the initial searches. A search in Google Scholar was undertaken for missing publications and compared with the first 100 records. No additional studies were found through screening of the reference lists. The initial search and screening were undertaken by the primary author (SD) and duplicates were removed (by SD). Two authors (SD & EG) then screened titles and abstracts for possible inclusion. Any disagreements were resolved by discussion between the reviewers and research team.
Inclusion and Exclusion Criteria
Inclusion criteria were met if studies included adult participants (age ≥18 years) with RHT, defined by the European Society of Cardiology and European Society of Hypertension (ESC/ESH) 2018 guideline [15], and without cardiovascular defects (such as congenital defects) or other diseases (such as kidney disease); compared exercise or physical activity to no exercise; used any type, duration and frequency of exercise, physical activity or lifestyle modification by means of physical activity and exercise; and used 24h ABP as the primary or secondary outcome. Studies were excluded if the period of intervention was less than 4 weeks and/or the studies that were considered included other non-pharmacological interventions (including acupuncture, diet, and surgery) without any exercise or physical activity intervention. Studies with comparisons of types of exercises were also excluded.
Data Extraction and Synthesis
Data were extracted by SD and checked by EG. The authors were contacted for missing or unpublished data. Extracted data included details of the interventions (type of exercise or physical activity, duration, frequency), populations (age, gender, ethnicity, and comorbidities), study methods and outcomes. The meta-analysis was conducted using Review Manager (RevManVersion 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014 [27]). Mean difference from baseline to follow up were calculated both to control and experimental groups [28]. Those mean differences were entered into RevMan. The statistical heterogeneity was assessed using I2 statistics (I2 ≥ 50% indicating significant heterogeneity) and the random effect model was used to determine the effect.
The RevMan Cochrane online resource was used for the sensitivity analysis. Forest plots were used to determine between-group mean differences and 95% confidence intervals for ambulatory SBP and DBP. The overall evidence quality was assessed with the Grading using the Recommendation Assessment, Development and Evaluation (GRADE) [29] tool. The methodological quality (risk of bias, consistency, directness, precision and publication bias) of the included full texts was independently assessed by the two reviewers (SD and EG) using the Cochrane risk of bias tools for randomised controlled trials (RoB -2, 2019 version) (Supplemental Digital Content 1.1) [30]. Any disagreements between the reviewers were resolved by consensus between the reviewers and the research team.
Results
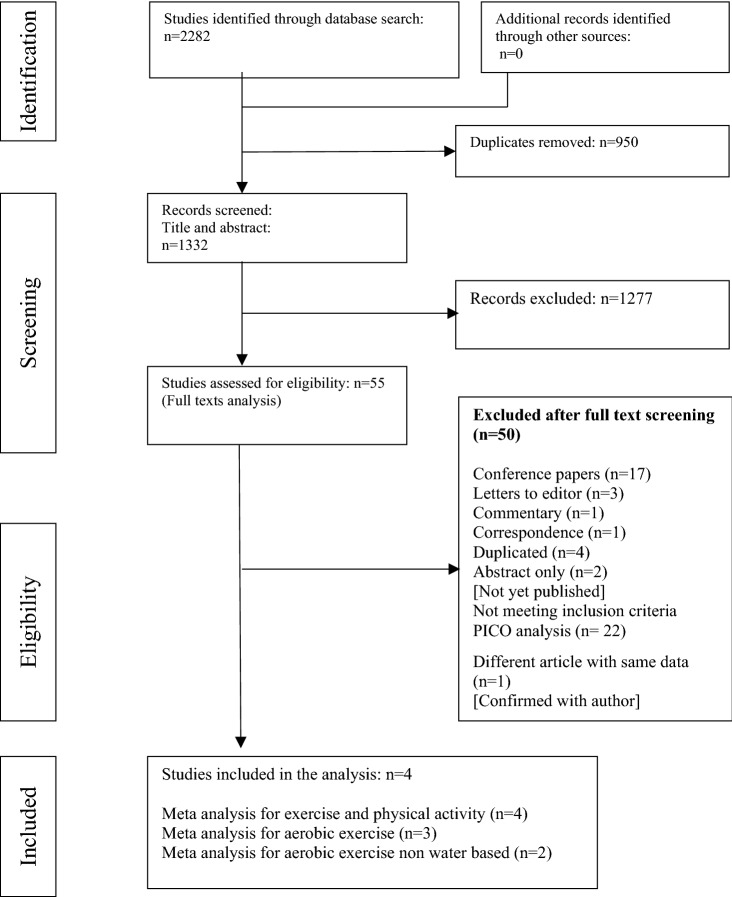
The initial search yielded 2282 studies (Fig. 1), 55 of which were included for the full text screening. Five articles were originally included in the study. The included studies were clinically homogenous, based on the inclusion criteria. Two of the five included articles [20, 31], appeared to have the same participant group, and one study [31] was removed following correspondence with the primary author. Thus, four articles [19, 20, 32, 33] were included in the review (178 participants) and the meta-analysis. Three studies [19, 20, 33] considered aerobic exercise and were included in the subgroup analysis I. One of the three studies [20], used heated water-based exercise. Thus, a separate analysis was conducted using the remaining two studies with land-based aerobic exercise.
Fig. 1.
Flow diagram for the study selection process
Overview of Studies
All trials with aerobic exercise were parallel group randomised control trials with land-based interval training [19] or endurance exercise [33], or heated water-based aerobic exercise [20]. In this review no studies were identified which investigated the effects of resistant or isometric exercise compared to a control group. Sample sizes ranged from 16 to 27 participants for the experimental groups. The period of intervention ranged from 8 to 16 weeks. Duration of the individual sessions was reported in only one study. The control groups received no exercise interventions, and were informed to continue with their usual medication and lifestyle, that is, followed usual care. The study characteristics and reported primary and secondary outcomes from the studies are summarised in Table 1.
Table 1.
Characteristics of the included studies
| Author/Year/Country | Study design (n) |
Type of exercise | Duration of exercise session | Frequency of and time period of intervention | Controls | Objective | Conclusions | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
Dimeo et al. [19] 2012 Germany |
Parallel group RCT (n=50) | Aerobic (Interval training) | Not Reported | 8–12 weeks (3 times per week) | Usual care without changing the medications or other treatment for hypertension | To investigate the hypothesis that an aerobic exercise programme is able to reduce blood pressure in RHT | Aerobic exercise on a regular basis is a helpful adjunct to control blood pressure and should be included in the therapeutic approach to RHT | 24h ABP* (Day time/Night time/ overall) |
| Office blood pressure | |||||||||
| Body weight | |||||||||
| BMI | |||||||||
| Maximal oxygen uptake | |||||||||
| Small and large artery compliance | |||||||||
| Cardiac index | |||||||||
| 2 |
Wang et al. [33] 2013 China |
Parallel group RCT (n=43) | Endurance exercise | Not reported | 16 weeks | Maintained their daily life style | To investigate the effects of endurance training on ABP and exercise capacity in refractory hypertension patients. | Sixteen week endurance training prescription could effectively lower blood pressure and enhance exercise capacity in refractory hypertension | ABP* |
| Body weight | |||||||||
| BMI | |||||||||
| artery compliance | |||||||||
| Maximal oxygen uptake | |||||||||
| Body fat% | |||||||||
| Resting heart rate | |||||||||
| 3 |
Guimaraes et al. [20] 2014 Brazil |
Parallel group RCT (n=32) | Heated water based exercise | 1 h | 8–12 weeks (3 times per week) | Non Hex and no change of usual antihypertensive treatment | To evaluate the effects of heated water based exercise training on BP in RHT | Heated water based exercise training tended to normalize the level of BP in patients with RHT | ABP* (Day/night/overall) |
| BMI | |||||||||
| heart rate | |||||||||
| peak oxygen uptake | |||||||||
| respiratory exchange rate | |||||||||
| BP (clinic) | |||||||||
| 4 |
Kruk et al. [32] 2018 Poland |
Control trial (n=53) | Physical activity | Not reported | 12 weeks (3 times a week: messaging) | Recommended physical activity and diet without advice and guidance by a physical therapist | To assess the effects of a programme of intensified physical activity introduced in primary health care combined with exercise training and short text message sent to the patient’s mobile phones or motivational telephone conversations on BP in patients with RHT | Individualized structured physical activity programme increases physical activity in the treatment of RHT in primary care but the effect on 24h ABP is transient. | Physical activity |
| Energy | |||||||||
| Number of steps | |||||||||
| MET average | |||||||||
| Energy expenditure | |||||||||
| Body composition | |||||||||
| Office BP | |||||||||
| Pulse pressure | |||||||||
| Total body water | |||||||||
| Lean body mass | |||||||||
| Fat mass | |||||||||
| Extracellular mass | |||||||||
| Body cell mass | |||||||||
| Basal metabolic rate | |||||||||
| Sleep time |
*Primary outcome: BP, blood pressure; ABP, ambulatory blood pressure; RHT, resistant hypertension; BMI, body mass index; HDL, high density lipids; LDL, low density lipids; TG, triglyceride; MET, metabolic equivalent; RCT, randomized control trial; Hex, heated water based exercise
The sample size and details of the participants for the included studies are summarised in Table 2. The mean ages of the participants in the studies ranged from 55 to 65 years. Three studies reported ethnicity: one study included Europeans [19], one included Brazilian participants [20], and one included Chinese participants [33]. The mean body mass indices (BMIs) were high (obese range) except in one study [33]. Most participants had a previous history of cardiovascular disease. Reported office BP values prior to the interventions were above 130/80 mmHg which came under ‘high normal’ (European guideline) [15] or Stage II hypertension (American guideline) [14] categories, despite all participants being on three or more medications.
Table 2.
Participant characteristics and baseline blood pressure measurements
| Author/ Year | N | Mean Age (years) |
Ethnicity | Anti HT Med | Concomitant diseases | Past History of CVD |
Body Weight (kg) |
Body Mass Index (kg/m2) |
Office/ Clinic SBP (mmHg) |
Office/ Clinic DBP (mmHg) |
24h ASBP (mmHg) |
24h ADBP (mmHg) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | CVD | CHD | HyLp | |||||||||||||
| 1 |
Dimeo et al. 2012[19] |
Total:50 | Exp: 62.8 ± 8 |
100% European |
4 | Exp: 4 | Exp: 0 | NR | Exp: 15 | Exp: 14 | Exp: 85.7 ± 17.1 | Exp: 28.9 ± 4.4 | Exp: 141.8 ± 16.3 | Exp: 78.1 ± 9.1 | Exp: 135.3 ± 15.2 | Exp: 75.4 ± 9.5 |
|
(Exp: 29) (Ctrl: 21) |
Ctrl: 67.9 ± 6.2 | Ctrl:6 | Ctrl: 3 | Ctrl: 18 | Ctrl: 14 | Ctrl: 84.0 ± 14.1 | Ctrl: 29.9 ± 4.7 | Ctrl: 140.2 ± 19.5 | Ctrl: 74.6 ± 10.7 | Ctrl: 128.7 ± 12.2 | Ctrl: 70.2 ± 9.1 | |||||
| 2 |
Wang et al. 2013[33] |
Total: 43 | Exp: 65.4 ± 5.4 |
100% Chinese |
NR | NR | NR | NR | NR | NR | Exp: 72.4 ± 7.8 | Exp: 25.6 ± 2.8 | Exp: 142 ± 21 | Exp: 79 ± 12 | Exp: 135 ± 9 | Exp: 76 ± 5 |
|
(Exp: 23) (Ctrl: 20) |
Ctrl: 62.8 ± 4.9 | Ctrl: 69.8 ± 6.6 | Ctrl: 24.3 ± 3.2 | Ctrl: 140 ± 25 |
Ctrl: 75 ± 14 |
Ctrl 132 ± 7 |
Ctrl: 74 ± 4 | |||||||||
| 3 | Guimaraes et al. 2014[20] | Total: 32 | Exp: 55.0 ± 5.9 |
69% Black 31% White Brazilian |
4 | NR | NR | NR | NR | NR | NR | Exp: 29.2 ± 4.9 | Exp: 160.2 ± 26.5 | Exp: 82.8 ± 15.4 | Exp: 139.4 ± 22.7 | Exp: 82.6 ± 13 |
|
(Exp: 16) (Ctrl: 16) |
Ctrl: 52.4 ± 5.9 | Ctrl: 30.1 ± 4.5 | Ctrl: 157.6 ± 18.1 | Ctrl: 86.3 ± 10.5 |
Ctrl: 140.8 ± 22.7 |
Ctrl: 81.1 ± 10.1 | ||||||||||
| 4 | Kruk et al. 2018[32] | Total: 53 |
Exp: 55.5 ± 9 Ctrl: 54.8 ± 9 |
NR | NR | Exp: 11 | NR | Exp: 3 | NR | Exp: 25 | Exp: 89.4 ± 13.6 | Exp: 32.5 ± 5.1 | Exp: 150 ± 24 | Exp: 90 ± 14 | Exp: 127 ± 17 | Exp: 75.4 ± 11 |
|
(Exp: 27) (Ctrl: 26) |
Ctrl: 8 | Ctrl: 0 | Ctrl: 22 | Ctrl: 76.3 ± 11.6 | Ctrl: 28.2 ± 4.3 | Ctrl: 132 ± 85 | Ctrl: 80.5 ± 7.5 | Ctrl: 119 ± 12 | Ctrl: 72.6 ± 7.1 | |||||||
Ant HT Meds: Anti-hypertensive medications, ABP : Ambulatory blood pressure, CVD: Cardio vascular disease, DM: Diabetes Mellitus, CHD: Chronic heart disease, HyLp: Hyper-lipidemia , Exp: Experimental group, Ctrl: Control group, AT: Aerobic training group, RT: Resistant training group, NR : Not reported or no data available, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, ASBP: Ambulatory systolic blood pressure, ADBP: Ambulatory diastolic blood pressure
Risk of Bias and Quality Assessment
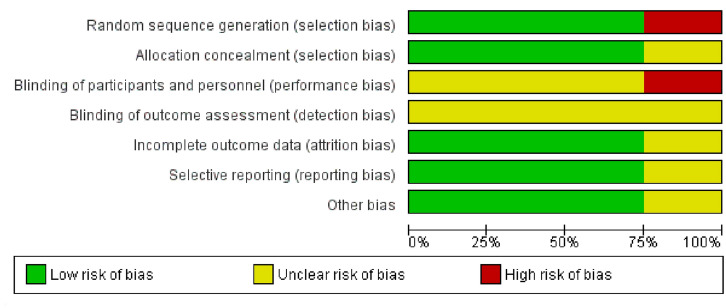
Two studies had low [19, 20] and one study had moderate [33] risk of bias while one study [32] had a high risk of bias (Supplemental Digital Content 1.1). One study (high risk of bias) [32] used physical activity while the other three studies used exercise [19, 20, 33]. The categorisation of moderate or high risk of bias was based on the domain ‘blinding’ of both the participants and assessor (Fig. 2). Kappa correlation coefficient for the reviewers’ agreement of risk of bias assessment was 0.69 which implied a ‘moderate’ interrater agreement [34]. Publication bias was not assessed as the number of studies was less than 10 [26]. Following the GRADE assessment criteria indirectness and publication bias were marked as ‘moderate’. Risk of bias, inconsistency and imprecision were rated as ‘low’. Thus, the overall quality of the evidence was considered to be ‘low’ (Supplemental Digital Content 1.2).
Fig. 2.
Risk of bias items across the included studies
Meta-Analysis
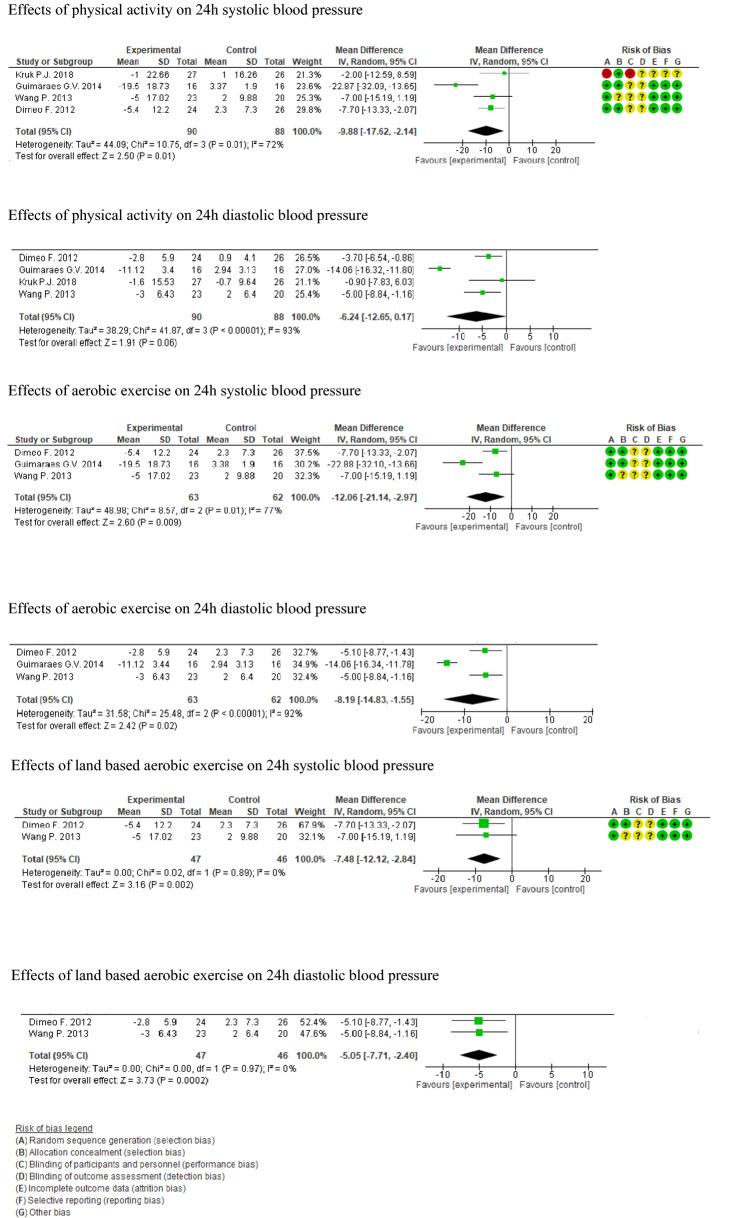
The meta-analysis for effectiveness of physical activity and exercise compared to usual care on 24h ABP included 178 participants (90 experimental and 88 control). Day and night measurements were not used in the meta-analysis as those has been undertaken at different time intervals and durations across the studies. Thus, the clinical heterogeneity of the studies could have been high.
The experimental group had a greater mean difference in 24h ABP than the control group, namely − 9.88 mmHg (95% CI: − 17.62, − 2.14) for SBP and − 6.24 mmHg (95% CI: − 12.65, 0.17) for DBP at follow up. The effect of physical activity or exercise on reducing ambulatory SBP was statistically significant (z = 2.50; p < 0.01) while DBP did not show a statistically significant difference (z = 1.91; p < 0.06). The heterogeneity was reported as ‘high’ in the analysis for both systolic I2 = 72% (chi2 = 10.75, df = 3; p = 0.01) and diastolic I2 = 93% (chi2 = 41.87, df = 3; p = 0.00001) 24h ABP (Fig. 3).
Fig. 3.
Forest plots—effectiveness of physical activity and aerobic exercise on 24h ambulatory blood pressure
The sub-group analysis-I of three studies determined the effect of aerobic exercise (land-based or water-based) on the 24h ABP (n = 125; experimental 63, control 62) [19, 20, 33]. The intervention groups had greater reductions in 24h ABP than the controls: a between-group difference of − 12.06 mmHg in SBP and − 8.19 mmHg in DPB following exercise. The effects were statistically significant with respective effect sizes being z = 2.60 (p < 0.009), z = 2.42 and p values < 0.02. The heterogeneity of the subgroup formed was high with I2 = 77% (chi2 = 8.57, df = 2; p = 0.01) and I2 = 92% (chi2 = 25.48, df = 2; p = 0.00001) (Fig. 3).
After excluding the study on heated water-based exercise by Guimarease et al. [20], sub-group analysis-II (experimental group 47 and control group 46) was carried out using two studies [19, 33] that considered land-based exercise only. The analysis showed statistical homogeneity, I2 = 0% (chi2 = 0.02, df = 1; p = 0.89) for SBP and I2 = 0% (chi2 < 0.001, df = 1; p = 0.97) greater mean difference for both SBP (− 7.48 mmHg, z = 3.16; p = 0.002) and DBP (− 5.05 mmHg, z = 3.73; p = 0.0002) in experimental groups and control groups at follow up (Fig. 3).
Discussion
The meta-analysis showed that aerobic exercise and/or physical activity programmes led to greater decreases for 24h ABP in experimental groups than in control groups. When comparing baseline to follow-up changes within a group, differences were considered clinically meaningful if the difference SBP > 4.5–7.5 and DBP > 5 mmHg and in previous studies [11, 16, 35–37], considering the mean difference from baseline to follow up for the physical activity or exercise groups in this meta-analysis, the forest plots indicated that most of them had a reduction of around 5 mmHg or more for SBP. Those differences compare well with the previous meta-analysis for people with hypertension [24]. The findings of the present systematic review provided evidence that an 8–12 week exercise programme when added to usual care is more effective in reducing 24h ABP than usual care alone for individuals with RHT [19, 20, 38]. However, the quality of evidence in the review was rated as low.
Specific Effects of Heated Water-Based Exercise on Blood Pressure
A large reduction in 24h ABP was reported in the heated water based exercise study [20]. A recent systematic review [39] reported that heated water based exercise reduced BP in hypertensive groups, supporting the hypothesis that there is a potential to induce desirable haemodynamic responses to heated water immersion (passive heat) [40, 41]. The haemodynamic responses related to decreased vascular tone (arterial stiffness) and pulse wave velocity may be explained as the leading mechanism for BP reduction in heated water immersion [20, 41]. Heated water immersion or heating the tissues passively induces positive haemodynamic effects in both central and peripheral arterial systems, especially through vasodilatation which in turn reduces the peripheral resistance [21, 40, 41].
Arterial stiffness is described as both structural (determined by the anatomical structures such as elastin, collagen) and functional (caused by sympathetic activity, vasoconstrictive substances). Resulting stiffness will reduce the cross-sectional area of the arteries and increase the peripheral resistance, leading to increased load on the myocardium and, thereby, BP [21, 40, 41]. In particular the passive heat deals mainly with functional arterial stiffness [21, 40, 41]. Passive heat will help endogenous production of vasodilators such as nitric oxide and reduce the vascular tone which reduces the resistance to blood flow through the arterial system [21, 40]. The decrease in vascular tone will increase the antegrade shear rate which will in turn improve the blood flow through the arteries leading to reduction in peripheral resistance [21, 40, 41].
Thus, immersion of the lower body in heated water improves the conductance of vascular beds in the skin and muscles of the lower limbs inducing vasodilatation in both muscles and the skin. This reduces the resistance to the blood flow, increasing the pressure gradient by which the heart needs less force to pump blood in to peripheries [21, 40, 41]. Vasodilatation in peripheries, reduces the central blood volume and thereby the ventricular filling pressure, causing reduced myocardial contractility (ionotropic effect) required to maintain cardiac output and thus a smaller load on the myocardium [40]. Further, the pressure waves generated with left ventricular systole (pulse wave velocity) are also reduced by passive heat generated in heated water immersion [21, 40, 41]. Decrease pulse wave velocity leads to reduced myocardial work and increased perfusion in the coronary arteries. This helps to maintain the cardiac output with optimal contractions, whilst preventing BP elevations [21, 40, 41]. In addition to the physiological effects mentioned, the up thrust of the water will be helpful to overcome the axial loading of the joint and overcome the barriers such as excess weight, involved in exercise [39].
Thus, the reported large reduction in BP could be due to the reduction of peripheral resistance induced by the vasodilator effect of heated water [20, 21, 39]. The hydrostatic pressure may also improve the venous return which would lead to a reflexive decrease of heart rate thereby reducing BP [39, 42].
Additional Benefits of Exercise
A slight reduction in body weight and subsequently in the BMI as a result of exercise or physical activity was reported in two included studies in the present review [33, 43]. Exercise can influence reduction of weight and excess body fat, leading to reduced abdominal and visceral adiposity [44, 45]. Strengthening exercise in particular, increases the lean body mass and muscular and general endurance [46]. Exercise improves the blood circulation to the key organs, cardio-respiratory fitness, insulin sensitivity, lipid profile and sleep quality, thereby reducing comorbidities associated with those parameters [44, 47, 48].
The findings of the present study suggest that exercise and physical activity can be used as a modality to reduce BP in RHT to clinically meaningful levels. The magnitude of reduction in BP identified in this review may reduce cardio-vascular disease risk by 6-14%, all-cause mortality by 13%, and cardiovascular events' (viz. stroke and ischaemic heart diseases) risk by around 20% [22, 49, 50].
Strengths, Limitations and Recommendations
To the best of our knowledge, this was the first systematic review to determine the effectiveness of exercise/physical activity in blood pressure reduction in adults with RHT. The review included a meta-analysis with sub-group analyses. Several databases were used to identify the published studies and no language restrictions were applied at the stage of inclusion of the studies. Thus, there was a low risk of relevant publications not being included.
A major limitation of this review was that the number of studies (n = 4) and the combined number of participants (n = 178) were small compared to other systematic reviews for hypertension and exercise/physical activity.[10, 23, 24] As the heterogeneity of the selected studies was high we cannot yet have full confidence in the findings. Application of the results may therefore be limited. Thus, further investigations of the effect of exercise/physical activity on BP in people with RHT involving larger numbers are needed.
In the systematic review the ‘blinding of participants’ domain contributed to a higher risk of bias. However, as the intervention modality was ‘exercise’, it would be difficult to make a study single or double blinded. Methodologically reducing the potentially high risk of bias is a challenge to be consider in any similar intervention. Though there is evidence that resistance exercise and isometric exercise have positive effects on hypertension [1, 10–12], no study has been conducted to determine the effects of various types of exercise in cohorts with RHT. Only one study, by de Carvalho [18] has compared the effects of aerobic and resistance exercise. Although the study had a small sample size (n = 11), its results suggest that aerobic and strengthening exercise may reduce BP in people with RHT. Thus, it is recommended that future studies be developed to determine the effects of such exercise types on individuals with RHT and a control group.
Conclusions
This systematic review showed that an 8-12 week physical activity or aerobic exercise programme may lead to greater reductions in 24h ABP in individuals with RHT than for control groups receiving only usual care. Heated water-based exercise specifically might be beneficial in BP reduction and more studies are needed investigating this therapeutic modality. The overall results suggest that exercise programmes can be used as an effective therapeutic modality in the management of BP in RHT. Due to high heterogeneity of the meta-analyses and low overall study quality, further studies are needed to confirm such findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Thelma Fisher, the subject librarian, for her support and guidance with developing the search strategy and her helpful comments provided during the development of this systematic review.
Declarations
The authors have no relevant financial or non-financial interest to disclose.
Contribution statement
All authors contributed to the study conception and design. Suranga Dassanayake, Gisela Sole and Emily Gary undertook material preparation, data collection and analysis. Suranga Dassanayake wrote the first manuscript draft and all authors commented on versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflicts of interest
No conflicts of interests.
Availability of data and material
Supplementary data will be available in online materials.
Code availability
Not applicable
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
We, the authors, give our consent for publication of this systematic review in the High Blood Pressure and Cardiovascular Prevention Journal.
References
- 1.Cao L, Li X, Yan P, Wang X, Li M, Li R, Shi X, Liu X, Yang K. The effectiveness of aerobic exercise for hypertensive population:a systematic review and meta-analysis. J Clin Hypertens. 2019;00:1–9. doi: 10.1111/jch.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM, American Heart Association Professional Education C. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–26. [DOI] [PubMed]
- 3.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB. Resistant hypertension: detection, evaluation, and management: a scientific statement from the american heart association. Hypertension. 2018;72(5):e53–e90. doi: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster R, Salam A, de Silva HA, Selak V, Stepien S, Rajapakse S, Amarasekara S, Amarasena N, Billot L, de Silva AP, Fernando M, Guggilla R, Jan S, Jayawardena J, Maulik PK, Mendis S, Mendis S, Munasinghe J, Naik N, Prabhakaran D, Ranasinghe G, Thom S, Tisserra N, Senaratne V, Wijekoon S, Wijeyasingam S, Rodgers A, Patel A, Group TS. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018;320(6):566–79. [DOI] [PMC free article] [PubMed]
- 5.Kadziela J, Warchol-Celinska E, Prejbisz A, Januszewicz A, Witkowski A, Tsioufi K. Renal denervation—can we press the "ON" button again? Adv Interv Cardiol. 2018;14(4):321–327. doi: 10.5114/aic.2018.79863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morganti A, Mancia G. Resistant hypertension: renal denervation or intensified medical treatment? Eur J Intern Med. 2018;50:6–11. doi: 10.1016/j.ejim.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Naci H, Salcher-Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, Ioannidis JPA. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53(14):859–869. doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 8.Cai A, Feng Y, Zhou Y. A comprehensive review of an unmet public health issue: resistant hypertension. Clin Exp Hypertens. 2017;39(2):101–107. doi: 10.1080/10641963.2016.1226892. [DOI] [PubMed] [Google Scholar]
- 9.Doroszko A, Janus A, Szahidewicz-Krupska E, Mazur G, Derkacz A. Resistant hypertension. Adv Clin Exp Med. 2016;25(1):173–183. doi: 10.17219/acem/58998. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31(4):639–648. doi: 10.1097/HJH.0b013e32835ca964. [DOI] [PubMed] [Google Scholar]
- 11.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res. 2016;39(2):88–94. doi: 10.1038/hr.2015.111. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT, Pescatello LS. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5(10):1–15. doi: 10.1161/JAHA.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho CJ, Marins JCB, de Lade CG, Castilho PR, Reis HHT, Amorim PRS, Lima LM. Aerobic and resistance exercise in patients with resistant hypertension. Rev Bras de Med do Esporte. 2019;25(2):107–111. doi: 10.1590/1517-869220192502175333. [DOI] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Jr CDE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SCJ, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KAS, Williamson JD, Wright JTJ. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):2199–269.
- 15.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 16.Hess NC, Carlson DJ, Inder DJ, Jesulola E, McFarlane JR, Smart NA. Clinically meaningful blood pressure reductions with low intensity isometric handgrip exercise: a randomized trial. Physiol Res. 2016;65:461–468. doi: 10.33549/physiolres.933120. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro F, Almeida N, Ferreira R, Oliveira N, Oliveira J, Alves AJ, Mesquita-Bastos J. Central and peripheral blood pressure response to a single bout of an exercise session in patients with resistant hypertension. Hypertens Res. 2018;42:114–116. doi: 10.1038/s41440-018-0100-y. [DOI] [PubMed] [Google Scholar]
- 18.Santos LP, Moraes RS, Vieira PJC, Ash GI, Waclawovsky G, Pescatello LS, Umpierre D. Effects of aerobic exercise intensity on ambulatory blood pressure and vascular responses in resistant hypertension: a crossover trial. J Hypertens. 2016;34(7):1317–1324. doi: 10.1097/HJH.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 19.Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60(3):653–658. doi: 10.1161/HYPERTENSIONAHA.112.197780. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes GV, Cruz LGD, Fernandes-Silva MM, Dorea EL, Bocchi EA. Heated water-based exercise training reduces 24-hour ambulatory blood pressure levels in resistant hypertensive patients: a randomized controlled trial (HEx trial) Int J Cardiol. 2014;172(2):434–441. doi: 10.1016/j.ijcard.2014.01.100. [DOI] [PubMed] [Google Scholar]
- 21.Guimaraes GV, Fernandes-Silva MM, Drager LF, de Barros Cruz LG, Castro RE, Ciolac EG, Bocchi EA. Hypotensive effect of heated water-based exercise persists after 12-week cessation of training in patients with resistant hypertension. Can J Cardiol. 2018;34(12):1641–1647. doi: 10.1016/j.cjca.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA, Andrews GR. The effectiveness of exercise training in lowering blood pressure: a meta-analysis of randomised controlled trials of 4 weeks or longer. J Hum Hypertens. 1997;11:641–649. doi: 10.1038/sj.jhh.1000509. [DOI] [PubMed] [Google Scholar]
- 23.Sosner P, Guiraud T, Gremeaux V, Arvisais D, Herpin D, Bosquet L. The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta-analysis of selected moderators. Scand J Med Sci Sports. 2017;27(3):327–341. doi: 10.1111/sms.12661. [DOI] [PubMed] [Google Scholar]
- 24.Semlitsch T, Jeitler K, Hemkens LG, Horvath K, Nagele E, Schuermann C, Pignitter N, Herrmann KH, Waffenschmidt S, Siebenhofer A. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43(10):1009–1023. doi: 10.1007/s40279-013-0065-6. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferd reporting items for systematic review and meta analysis protocols (PRISM-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed]
- 26.Dassanayake S, Sole G, Wilkins G, Skinner M. Effect of exercise and physical activity on blood pressure in adults with resistant hypertension: a protocol for a systematic review. Phys Ther Rev. 2020;2020:1–7. [Google Scholar]
- 27.The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. [Internet]. Copenhagen: The Nordic Cochrane Centre. 2019. https://documentation.cochrane.org/revman-kb/.
- 28.Armitage P, Berry G. Statistical Methods in Medical Research (3rd ed.). London: Blackwell Science Ltd; 1994. p. 108–9.
- 29.Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, Moe-Byrne T, Higgins JPT, Sowden A, Stewart G. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3:82. doi: 10.1186/2046-4053-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V. Cochrane handbook for systematic reviews of interventions, version 6.0 [Internet]: Cochrane. 2019. www.training.cochrane.org/handbook. Accessed 5 Aug 2019.
- 31.Cruz LGD, Bocchi EA, Grassi G, Guimaraes GV. Neurohumoral and endothelial responses to heated water-based exercise in resistant hypertensive patients. Circ J. 2017;81(3):339–345. doi: 10.1253/circj.CJ-16-0870. [DOI] [PubMed] [Google Scholar]
- 32.Kruk PJ, Nowicki M. Effect of the physical activity program on the treatment of resistant hypertension in primary care. Prim Health Care Res Dev. 2018;2018:1–9. doi: 10.1017/S1463423618000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P. Effects of endurance training on ambulatory blood pressure and exercise capacity in refractory hypertension patients. J Shenyang Sport Univ. 2013;32(5):89. [Google Scholar]
- 34.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. Exercise and hypertension: American College of Sports medicine position stand. Med Sci Sports Exerc. 2004;36(3):533–553. doi: 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- 36.Corso LM, Macdonald HV, Johnson BT, Farinatti P, Livingston J, Zaleski AL, Blanchard A, Pescatello LS. Is concurrent training efficacious antihypertensive therapy? A meta-analysis. Med Sci Sports Exerc. 2016;48(12):2398–2406. doi: 10.1249/MSS.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 37.Bento VF, Albino FB, Moura KF, Maftum GJ, Santos Mde C, Guarita-Souza LC, Faria Neto JR, Baena CP. Impact of physical activity interventions on blood pressure in Brazilian populations. Arq Bras Cardiol. 2015;105(3):301–308. doi: 10.5935/abc.20150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruk PJ, Nowicki M. Effects of regular physical activity on pain, anxiety, and depression in patients with treatment resistant arterial hypertension. Fam Med Prim Care Rev. 2016;18(3):268–273. doi: 10.5114/fmpcr/63060. [DOI] [Google Scholar]
- 39.Ngomane AY, Abreu RM, Ciolac EG. Effects of heated water-based exercise on blood pressure: a systematic review. Fisioter Mov. 2018;31:10. doi: 10.1590/1980-5918.031.ao05. [DOI] [Google Scholar]
- 40.Thomas KN, van Rij AM, Lucas SJ, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol. 2017;312(3):R281–R291. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 41.Sugawara J, Tomoto T. Acute effects of short-term warm water immersion on arterial stiffness and central hemodynamics. Front Physiol. 2021;12:620201. doi: 10.3389/fphys.2021.620201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro RE, Guimaraes GV, Da Silva JMR, Bocchi EA, Ciolac EG. Post-exercise hypotension after heart transplant: water- versus land-based exercise. Med Sci Sports Exerc. 2016;48(5):804–810. doi: 10.1249/MSS.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 43.Kruk P, Nowicki M. Intensification of regular physical activity in patients with resistant hypertension. Pediatr Med Rodz. 2017;13(3):368–376. doi: 10.15557/PiMR.2017.0039. [DOI] [Google Scholar]
- 44.Ihalainen JK, Inglis A, Makinen T, Newton RU, Kainulainen H, Kyrolainen H, Walker S. Strength training improves metabolic health markers in older individual regardless of training frequency. Front Physiol. 2019;10:32. doi: 10.3389/fphys.2019.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev. 2018;19(5):654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 46.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011;58(5):950–958. doi: 10.1161/HYPERTENSIONAHA.111.177071. [DOI] [PubMed] [Google Scholar]
- 47.Dobrosielski DA, Papandreou C, Patil SP, Salas-Salvado J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev. 2017;26(144):12. doi: 10.1183/16000617.0110-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Boros S. The effect of physical activity on sleep quality: a systematic review. Eur J Physiother. 2019;2019:1–8. [Google Scholar]
- 49.Burkard T, Mayr M, Winterhalder C, Leonardi L, Eckstein J, Vischer AS. Reliability of single office blood pressure measurements. Heart. 2018;104(14):1173–1179. doi: 10.1136/heartjnl-2017-312523. [DOI] [PubMed] [Google Scholar]
- 50.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.