Abstract
The residual antibiotic concentration of vancomycin (2 mg/ml)- or ceftazidime (2 mg/ml)-heparin solutions instilled in ports in pediatric hematology-oncology patients 1 to 34 days earlier was measured. Antibiotic concentrations of ≥100 μg of either antibiotic per ml persisted for at least 21 days. For treatment of lumenal port infections, antibiotic-heparin dwell times of ≥2 weeks may be appropriate.
Totally implantable venous access devices (“ports”) are surgically placed silicone central venous catheters designed for long-term use that require maintenance with a heparin flush once every 4 weeks when not being accessed. The cure rate of port-related bloodstream infection (PRBI) with systemic antibiotic therapy through the catheter without device removal is as high as 80% (2, 9, 15, 18). Messing et al. (14) developed the antibiotic-lock technique (ALT), a novel approach to treating catheter-related bloodstream infections in which an antibiotic is instilled (“locked”) in the catheter and is replaced daily. ALT has been used alone or as a follow-up to systemic antibiotic therapy to treat infected tunneled catheters of the Broviac-Hickman type (3, 11, 12, 14, 17) or ports (4, 6, 8, 10, 11). Since ports need only a monthly heparin flush rather than the daily flush required for Broviac-Hickman catheters, PRBI can potentially be treated by the ALT leaving the antibiotic intralumenally for days to weeks. In vitro study of the stability of five antibiotic-heparin solutions potentially useful for ALT showed that four of them retained greater than 90% activity after 10 days (1). To determine the feasibility of studying the treatment of PRBI with an antibiotic dwell time of days to weeks, we measured the concentrations of vancomycin and ceftazidime after their installation in ports for prolonged time periods.
Patients aged 2 months to 21 years with functioning ports (for infusing and withdrawing blood) in place for at least 2 weeks were recruited from the pediatric hematology-oncology division at the Schneider Children's Hospital. Informed consent and informed assent (if older than 9 years of age) were obtained from all participants. Exclusion criteria were as follows: (i) patients requiring their next via-port infusion sooner than 48 h; (ii) current port infection or evidence of acute illness such as fever, rash, or systemic symptoms; and (iii) current or anticipated antibiotic therapy. The following data were recorded for each patient: age, sex, underlying disease, date of port placement, and port type.
Standard clinical powder of antibiotic (vancomycin [500 mg; Eli Lilly & Co., Indianapolis, Ind.] or ceftazidime [1 g; Glaxo Wellcome Co., Research Triangle Park, N.C.]) was hydrated and diluted in sterile water and mixed with heparin solution to achieve a final concentration of 2 mg and 100 U of heparin per milliliter of antibiotic and heparin, respectively. Then, 3 ml of the solution was instilled into each accessed port and allowed to dwell for 2 to 34 days. The dwell duration was determined by the clinical need to access the port. The first 0.2 to 1.0 ml of the aspirated fluid from the accessed port was taken for assay of residual antibiotic activity and hemoglobin (for bloody specimens; Abbott Cell-Dyn 1400; Abbott Laboratories, Abbott Park, Ill.). The following data was recorded: the heparin-antibiotic dwell time, the volume of aspirated fluid, the gross appearance of fluid (bloody, blood-tinged [slightly discolored], or clear), and the peripheral blood Hb. Antibiotic assays were performed on the specimens or on an appropriate dilution of the specimens within 12 h. Vancomycin levels were measured by a fluorescence polarization immunoassay (Abbott Laboratories) after sample dilution of up to 1:100 with TDx buffer. The coefficient of variation in our laboratory is <2%. Ceftazidime concentrations were assayed by using a bioassay by a disk diffusion method and a susceptible bacterium (Escherichia coli ATCC 25922) (5) with inoculation of 6-mm-diameter paper disks in triplicate with 0.01 ml of test fluid or ceftazidime (8 to 2,000 μg/ml)-heparin solution. Zones of inhibition were measured with a micrometer on a photocopy of the agar plate, and a standard curve was constructed. The correlation coefficient (r) of the standard curves for 13 trial sets ranged from 0.9907 to 0.9995 (median, 0.9972); the sensitivity was 8 μg/ml. The reproducibility of the interassay zone-of-inhibition measurements demonstrated means ± standard deviation values of 30.6 ± 2.2 mm, 29.0 ± 1.8 mm, and 12.7 ± 1.1 mm for concentrations of 1,000, 500, and 16 μg/ml, respectively.
For bloody samples, a corrected antibiotic concentration was calculated according to the following formula: corrected antibiotic concentration = measured antibiotic concentration/(1 − [Hbsample/Hbperipheral blood]).
Ten patients (five males) with a median age of 5 years (range, 2.5 to 9 years), all with a double lumen port of the same brand (Life Port; Horizon Medical Products, Manchester, Ga.) in place for a median duration of 10 months (range, 3 to 20 months), participated in the study. Eight of the patients had acute lymphoblastic leukemia, one had an optic glioma, and one had sickle cell disease. Of 72 instilled samples, 40 (56%) were analyzed (22 [65%] of the 34 vancomycin samples and 18 [47%] of 38 ceftazidime samples). A number of samples were not analyzed because samples (i) could not be withdrawn from the port (15 total), (ii) were obtained at an inconvenient time (weekends or vacation times) (8 total), or (iii) were inadvertently discarded (9 total). A total of 29 (70%) samples appeared bloody (Hb range, 1.2 to 9.5 g/dl; median, 6.4 g/dl), 5 were blood tinged, and 6 were clear. Sample volumes ranged from 0.2 to 1.0 ml (median, 0.4 ml). In two cases (both with vancomycin), the samples appeared bloody, but their volume was insufficient to measure Hb concentration and correct the concentration for dilution by blood.
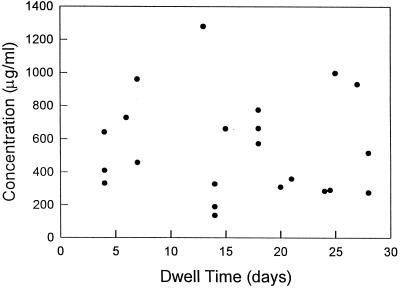
The dwell time of vancomycin ranged from 4 to 28 days, with a median of 17 days. Vancomycin concentrations ranged from 85 to 919 μg/ml (median, 253 μg/ml), and corrected sample concentrations ranged from 136 to 1,280 μg/ml (median, 488 μg/ml; Fig. 1). Thus, all corrected vancomycin samples had a concentration of >130 μg/ml. There was no correlation between the vancomycin dwell time and the vancomycin concentration (r = −0.05, P = 0.83). In two additional patients, vancomycin was assayed in specimens withdrawn from each of the two lumens 3 to 15 min after installation. The mean vancomycin concentration was 60% lower (individual specimen results were 27, 28, 84, and 85% lower) than the instilled vancomycin concentration.
FIG. 1.
Corrected intralumenal vancomycin concentrations after various dwell times in ports.
The ceftazidime dwell time ranged from 2 to 34 days, with a median of 17 days. The measured concentrations and corrected concentrations ranged from <8 to 1,116 μg/ml, and the median concentrations were 92.5 and 196.5 μg/ml, respectively (Fig. 2). There was a significant inverse correlation between the ceftazidime dwell time and the ceftazidime concentration (r = −0.87, P < 0.0001). All samples with dwell times of ≤15 and ≤21 days had corrected antibiotic concentrations of at least 234 and 110 μg/ml, respectively. No adverse reactions were noted during the antibiotic dwell with either antibiotic.
FIG. 2.
Corrected intralumenal ceftazidime concentrations after various dwell times in ports.
In seven instances, samples were simultaneously aspirated from both lumens of the same port. For each pair, the percent differences in the antibiotic concentrations were 0, 2, 2, 4, 19, 26, and 26%.
We found that after the installation of vancomycin at an initial concentration of 2,000 μg/ml, a concentration of at least 130 μg/ml was maintained for up to 28 days. Although the residual vancomycin concentrations varied over an ∼10-fold range, there was no significant decrease over time. In addition, a substantial decrease in vancomycin concentration was seen in samples obtained ≤15 min after instillation. These observations support the concept that the lower residual vancomycin concentration is not due to vancomycin inactivation but may be due to mechanical factors relating to obtaining the specimen or vancomycin loss into the bloodstream or by adherence to the catheter wall or reservoir inner surface. This interpretation is supported by an in vitro study of antibiotic stability that found vancomycin stability to be ≥93% for at least 10 days at 37°C (1). The coefficient of variation of <6% in the assay is unlikely to account for the variability in vancomycin concentration over time.
In contrast to vancomycin, the residual intraluminal ceftazidime activity was found to significantly decrease with time. This is likely to reflect a loss of ceftazidime activity due to limited drug stability. This observation correlated with in vitro studies of ceftazidime stability in which ceftazidime activity decreased by 40% after 10 days at 37°C (1).
The duration of continuing daily antibiotic lock in successfully treated patients has ranged from 7 to 21 days, with an instilled antibiotic concentration generally 25 to 5,000 times the MIC at which 90% of the isolates tested are inhibited (MIC90) for the pathogen (3, 11, 13, 14, 17); only Krzywda et al. used much higher concentrations (12). The concept underlying successful use of ALT in treating tunneled catheter-related infection is that a high intralumenal antibiotic concentration delivered to the inner surface of the bacterial-colonized catheter (the apparent source of the bloodstream infection) for a long contact time can kill the bacteria (13). Both vancomycin and ceftazidime exhibit bacterial killing in a time-dependent manner, with the cure of infection correlating with bacterial exposure to an antibiotic concentration greater than the MIC for over 40 to 50% of the time (7). Vancomycin activity is maintained for at least 21 days at a concentration of >100 times the MIC90 for susceptible organisms. The MIC of most susceptible gram-negative bacilli to ceftazidime is ≤8 μg/ml. Hence, ceftazidime activity was maintained for 15 days at a concentration that is at least 29 times the MIC90 for those organisms (16). It is possible that an antibiotic concentration manyfold higher than the MIC90 may be required for sufficient antibiotic to penetrate the slime layer or other biomaterial on the catheter surface to achieve an antibiotic concentration exceeding the MIC90 for bacteria deep in the biofilm.
Although one study showed clinical success with antibiotic concentrations in the range of thousands of times the MIC (3), it was unknown if such concentrations persist for more than a few hours and if lower antibiotic concentrations would suffice for successful therapy. Our study found that intralumenal vancomycin and ceftazidime concentrations many times the MIC90 for most susceptible organisms persist for at least 2 weeks establishes that substantial concentrations persist in vivo for the typical duration of treatment of catheter infection. The findings provide the rationale for clinical studies to evaluate the efficacy of intralumenal antibiotic dwell times of 2 to 3 weeks for the treatment of PRBI using ALT and to monitor the safety of this treatment and the potential emergence of antibiotic-resistant bacteria.
Acknowledgments
We thank Kristin Mahoney and the other pediatric hematology-oncology nurses for their participation in this study.
REFERENCES
- 1.Anthony T U, Rubin L G. Stability of antibiotics used for “antibiotic-lock treatment” of infections of implantable venous devices (ports) Antimicrob Agents Chemother. 1999;43:2074–2076. doi: 10.1128/aac.43.8.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becton D L, Kletzel M, Golladay E S, Hathaway G, Berry D H. An experience with an implanted port system in 66 children with cancer. Cancer. 1988;61:376–378. doi: 10.1002/1097-0142(19880115)61:2<376::aid-cncr2820610229>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Benoit J L, Carandag G, Sitrin M, Arnow P M. Intra-lumenal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin Infect Dis. 1995;21:1286–1288. doi: 10.1093/clinids/21.5.1286. [DOI] [PubMed] [Google Scholar]
- 4.Bregenzer T, Widmer A F. Bloodstream infection from a Port-A-Cath: successful treatment with antibiotic-lock technique. Infect Control Hosp Epidemiol. 1996;17:772. doi: 10.1086/647231. [DOI] [PubMed] [Google Scholar]
- 5.Chapin-Robertson K, Eldberg S C. Measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 295–304. [Google Scholar]
- 6.Cowan C E. Antibiotic lock technique. J Intravenous Nurs. 1992;5:283–287. [PubMed] [Google Scholar]
- 7.Craig A W. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 8.Domingo P, Fontanet A, Sanchez F, Allende L, Vazquez G. Morbidity associated with long-term totally implantable ports in patients with AIDS. Clin Infect Dis. 1999;29:346–351. doi: 10.1086/520213. [DOI] [PubMed] [Google Scholar]
- 9.Groeger J S, Lucas A B, Thaler H T, Freidlander-Klar H, Brown A E, Kiehn T E, Armstrong D. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med. 1993;119:1168–1174. doi: 10.7326/0003-4819-119-12-199312150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hellard M, Fuller A, Spelman D, Spicer W J, Pickles R, Jenney A. Totally implantable venous access device infections in patients with AIDS. AIDS. 1997;11:697–698. [PubMed] [Google Scholar]
- 11.Johnson D C, Johnson F L, Goldman S. Preliminary results of treating persistent central venous catheter infections with the antibiotic-lock technique in pediatric patients. Pediatr Infect Dis J. 1994;13:930–931. doi: 10.1097/00006454-199410000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Krzywda E A, Andris D A, Edmiston C E, Quebbeman E J. Treatment of Hickman catheter sepsis using antibiotic lock technique. Infect Contr Hosp Epidemiol. 1995;16:596–598. doi: 10.1086/647015. [DOI] [PubMed] [Google Scholar]
- 13.Messing B. Catheter sepsis during home parenteral nutrition: use of the antibiotic-lock technique. Nutrition. 1998;14:466–468. doi: 10.1016/s0899-9007(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 14.Messing B, Peitra-Cohen S, Debure A, Beliah M, Bernier J J. Antibiotic-lock technique: A new approach to optimal therapy for catheter-related sepsis in home-parenteral nutritional patients. J Parenteral Nutr. 1988;12:185–189. doi: 10.1177/0148607188012002185. [DOI] [PubMed] [Google Scholar]
- 15.Mueller B U, Skeleton J, Callender D P, Marshall D, Gress J, Longo D, Rubin M, Venzo D, Pizzo P A. A prospective randomized trial comparing the infectious and noninfectious complications of an externalized catheter versus a subcutaneously implanted device in cancer patients. J Clin Oncol. 1992;10:1943–1948. doi: 10.1200/JCO.1992.10.12.1943. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.Rao J S, O'Meara A, Harvey T, Bretnach F. A new approach to the management of Broviac catheter infection. J Hosp Infect. 1992;22:109–111. doi: 10.1016/0195-6701(92)90094-3. [DOI] [PubMed] [Google Scholar]
- 18.Rubin L G, Shih S, Shende A, Karayalcin G, Lanzkowsky P. Cure of implantable venous port-associated bloodstream infections in pediatric oncology patients without catheter removal. Clin Infect Dis. 1999;29:102–105. doi: 10.1086/520135. [DOI] [PubMed] [Google Scholar]