Abstract
Objective:
To determine the independent risk factors for diastolic dysfunction (DD) in patients with systemic sclerosis (SSc) and to evaluate the impact of DD on mortality.
Methods:
SSc patients enrolled in the Johns Hopkins Scleroderma Center Cohort between 11/01/2006 through 11/01/2017 with at least one analyzable 2D echocardiogram (2DE) in our system were included, totaling 806 participants. DD risk factors and SSc disease characteristics were prospectively obtained, and presence/absence of DD was determined by the most recent 2DE. Logistic regression models examined associations between clinical risk factors and DD, and Cox proportional hazards models were used to assess survival.
Results:
DD was present in 18.6% of participants. The majority of participants were female (84%) with a median age of 58.4 years (IQR 48.8, 68.1). Older age (OR 1.12, 95%CI 1.09-1.15), coronary artery disease (OR 3.69, 95%CI 1.52-8.97), obesity (4.74, 95%CI 2.57-8.74), longer SSc disease duration (OR 1.04, 95%CI 1.01-1.06), diffusing capacity (DLCO) ≤60% of predicted (OR 2.41, 95%CI 1.40-4.16), and history of scleroderma renal crisis (OR 3.18, 95%CI 1.12-9.07), were all independently associated with an increased risk of DD. Anti-Scl70 positivity (OR 0.49, 95%CI 0.26-0.93) and severe gastrointestinal disease (OR 0.48, 95%CI 0.30-0.79) were associated with a reduced risk of DD. The presence of DD was independently associated with an increase in mortality (HR 1.69, 95%CI 1.07-2.68).
Conclusion:
DD is independently associated with an increased risk of mortality in patients with SSc. Potentially modifiable risk factors, including CAD and obesity, should be addressed in patients with SSc to reduce mortality risk.
INTRODUCTION
Primary cardiac involvement in systemic sclerosis (SSc) accounts for one-third of SSc-related deaths, and traditionally includes conduction blocks, arrhythmias, and non-ischemic cardiomyopathy.1,2 Recent studies report a prevalence of diastolic dysfunction (DD) ranging between 18-62% in patients with SSc,3–6 compared with a prevalence of 1.4-38.1% in similarly aged community dwelling adults without SSc.7,8 The higher prevalence of DD in SSc has been attributed to SSc myocardial involvement. However, the contributing risk factors across SSc and non-SSc conditions have been incompletely evaluated. Given the increased risk of mortality in SSc patients with DD,4,5,9 it is important to improve comprehensive identification of potentially modifiable risks.
DD is caused by progressive left ventricular (LV) stiffness leading to impaired compliance, and is diagnosed by 2D echocardiography (2DE). Over time, atrial enlargement, arrhythmia, and elevated LV filling pressures underlying the clinical syndrome of heart failure with preserved ejection fraction (HFpEF) is observed. Risk factors for DD in the general population include older age, female sex, hypertension (HTN), coronary artery disease (CAD), diabetes mellitus (DM), obesity, chronic obstructive pulmonary disease (COPD), tobacco use, dyslipidemia, and chronic kidney disease (CKD).7,10 In SSc-specific studies, longer disease duration9,11 and presence of pulmonary fibrosis6 have been inconsistently associated with an increased risk for DD.5,12 A major limitation in these studies is the ascertainment of and adjustment for other traditional DD risk factors, which requires a large cohort size.5,6,9 Additionally, the definition of DD has varied widely among studies and has not consistently applied echocardiographic guideline-based criteria, which further limits conclusions and generalizability of results.
In this study, we sought to overcome these limitations by prospectively ascertaining DD risk factors and SSc characteristics in a large, well-characterized SSc cohort. DD was rigorously defined using the most current guidelines from the American Society of Echocardiography (ASE)/European Society of Cardiovascular Imaging (ESCVI).13 The primary goals of this study were to 1) identify DD risk factors in SSc, and 2) determine the independent impact of DD on mortality in SSc by controlling for other SSc specific risk factors and cardiovascular risk factors for mortality.
PATIENTS AND METHODS
Study Population
All SSc patients enrolled in the Institutional Review Board approved Johns Hopkins Scleroderma Center (JHSC) cohort with at least one technically adequate 2DE performed within our institution between 11/01/2006 through 11/01/2017 were reviewed for inclusion.
All participants satisfied at least one of the following classification criteria for SSc: 1) 1980 or 2013 American College of Rheumatology criteria,14,15 2) ≥3 of 5 features of CREST (calcinosis, Raynaud’s phenomenon (RP), esophageal dysmotility, sclerodactyly, telangiectasia) syndrome, or 3) the combination of definite Raynaud’s phenomenon, abnormal nailfold capillaries, and a SSc-specific antibody (anti-centromere, anti-topoisomerase I/Scl70, or anti-RNA polymerase III).
Our Center’s standard practice includes obtaining an annual 2DE to screen for pulmonary hypertension (PH), regardless of clinical symptoms. The most recent 2DE for a participant was included if deemed technically adequate for diastology assessment. 2DEs were excluded for any of the following reasons: 1) 2DE performed in the setting of an acute myocardial infarction, shock, or while patient was in the intensive care unit, 2) presence of moderate or severe aortic or mitral valvulopathy, 3) presence of moderate or severe mitral annular calcification which may underestimate tissue Doppler velocities, 4) presence of a moderate or large pericardial effusion, or 5) presence of a depressed LV ejection fraction of <50%. If the 2DE did not meet inclusion criteria or met exclusion criteria, the prior 2DE (if available) was assessed until the most recent 2DE meeting inclusion/exclusion criteria was identified.
Study Procedures and Measurements
I. Clinical Procedures and Measurements.
All demographic and clinical variables were collected prospectively for cohort participants during routine clinical appointments at approximately 6-month intervals. Patients were classified as having limited or diffuse cutaneous SSc by established criteria.16 Disease duration was defined as time from onset of the first SSc symptom, either RP or non-RP, to time of 2DE. Race was defined by self-report. Measurements of forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) from pulmonary function tests (PFT) were standardized for gender and age.17,18 Organ involvement and clinical severity scores19,20 are defined in the Supplement and included maximum expression/severity between date of cohort enrollment and date of 2DE. Traditional DD risk factors (HTN, CAD, DM, obesity, COPD, ever tobacco use, dyslipidemia, and CKD) were defined by established criteria (see Supplement) and updated every six months.
Autoantibodies were defined as positive if ever 1) positive by clinical laboratory evaluation, or 2) moderate(++) or high-positive(+++) by laboratory-based immunoassay (EUROLINE Systemic Sclerosis (Nucleoli) Profile; Euroimmun) (see Supplement).
Mortality data was obtained via medical record review, family notification of patient’s death, and annual review of the United States Social Security Death Index.
II. 2DE Procedures and Measurements.
DD classification and grade of severity was determined based on the 2016 ASE/ESCVI recommendations.13 Diastolic parameters including the peak velocities of early (E) and late (A) filling waves and the E/A ratio, and the deceleration time (DT) of the mitral E-wave velocity were obtained.13 Tissue Doppler mitral annular velocities of the septal and/or lateral annulus (e′ velocity) were obtained and the ratio of early mitral inflow to early tissue Doppler e’ velocities were used to calculate the E/e′ ratio, a noninvasive surrogate of LV end-diastolic filling pressures.13 Left atrial volumes (LAV) were obtained utilizing the modified biplane method from the apical 4- and 2-chamber views and indexed by body surface area (LAVi).21 If both the apical 4- and 2-chamber views were not available or did not have technically adequate quality, LAV was determined based on a single view.13 Peak tricuspid regurgitant (TR) velocity was obtained and using the modified Bernoulli equation, was utilized to calculate right ventricular systolic pressure (RVSP). The diagnosis of DD was defined by the presence of ≥3 of the following abnormal cutoff values for the following 4 variables: septal e′ velocity <7 cm/ sec, septal E/e’ ratio >15, LAVi >34 mL/m2, and peak TR velocity >2.8 m/sec. Diastolic function was classified by how many of these four parameters were normal or abnormal: normal if <50% were abnormal, indeterminate if 50% were abnormal, and DD if >50% were abnormal.13 In the event that one of the four variables were missing, DD classification was based on three instead of four parameters as per recommendations.13 Diastology grade was determined in those with DD by evaluating the ratio of early and late transmitral velocities and deceleration time.13
III. Statistical Analysis.
Differences in demographics, cardiovascular and pulmonary disease, SSc characteristics, and medications were assessed across normal, indeterminate, and DD categories using χ2 tests of proportions for categorical data, ANOVA for parametric data, and Kruskal-Wallis tests for non-parametric data. These analyses were completed in Stata/IC 14.2 (StataCorp, College Station, TX).
Univariable and multivariable logistic regression of DD versus normal diastolic function examined associations between DD and clinical features. We decided a priori to include all known DD risk factors (age, sex, HTN, CAD, obesity, dyslipidemia, CKD, DM, COPD, and ever tobacco use) in multivariable models. Multivariable models were constructed including SSc clinical characteristics which had a 95% confidence interval for the OR which did not pass 1.0 and/or a p-value ≤0.10.22 An ordinal polytomous logistic regression analysis framing indeterminate diastolic function as a step between normal and DD, yielding three levels of diastolic function with increasing dysfunction (normal→indeterminate→dysfunction), was also performed and results are included in supplementary data.
Cox proportional hazards models compared mortality in patients with DD versus patients with normal function.23 We adjusted this analysis for clinical features identified as being related to DD in the literature or in our above analyses, as well as clinical characteristics with known associations with mortality in SSc. Unadjusted and age-adjusted Kaplan-Meier curves evaluated the association between mortality and diastology groups. The time scale was years from 2DE, and patients were censored either at i) one year from last clinic visit date, or ii) date of death.
Multiple imputation analyses were used to address missing clinical data, which was assumed to be missing at random, and are presented as the primary analyses. Missing measurements were imputed using predictive mean matching with ten separate imputations in a chained imputation conditional on other factors of interest.24 Multivariable logistic regression models and Cox proportional hazards models were replicated in patients with complete information, excluding those who were imputed, with results shown in supplementary data for comparison.
All analyses were conducted in R version 3.6.2,25 unless otherwise noted.
RESULTS
Summary of overall cohort characteristics.
A total of 1,405 2DE were screened, and 806 2DE from 806 individual participants met inclusion/exclusion criteria for final analysis. Demographic, clinical, and serological characteristics of 806 SSc participants are presented in Table 1, according to diastolic function groups. A total of 538 (67%) participants had normal diastolic function, 118 had indeterminate diastolic function (15%), and 150 had DD (18%). Of those with DD, most had Grade 2 DD (n=108; 72%). Few patients had Grade 1 DD (n=2; 1.3%) or Grade 3 DD (n=14; 9.3%). Grade of DD was inconclusive in the remaining patients (n=26; 17.3%). The majority of participants were female (84%), Caucasian (70%), and had limited/sine cutaneous disease (63%). The median age at time of 2DE for the entire cohort was 58.4 years (IQR 48.8, 68.1), and the median disease duration was 10.6 years (IQR 5.1, 17.9). At least one traditional DD risk factor was present in 97% (n=146/150) of participants with DD.
Table 1.
Clinical and serological characteristics of subjects with SSc, according to diastolic function groups.
| Normal = 538 N (%) | Indeterminate = 118 N (%) | DD = 150 N (%) | P-value | |
|---|---|---|---|---|
| Female sex | 452 (84) | 96 (81) | 129 (86) | 0.59 |
| Age at echo, years, median (IQR) | 54.3 (45.0, 62.6) | 65.4 (54.7, 72.1) | 67.7 (60.4, 74.2) | 0.0001 |
| Race | ||||
| Caucasian | 382 (71) | 74 (63) | 109 (73) | 0.028 |
| Black | 115 (21) | 38 (32) | 37 (25) | |
| Other‡ | 41 (8) | 6 (5) | 4 (3) | |
| SSc duration at time of echo, from first RP or non-RP symptom, years, median (IQR) | 9.3 (4.6, 16.6) N=536 |
11.5 (5.5, 18.8) N=116 |
13.8 (7.7, 22.8) N=149 |
0.0001 |
| SSc Subtype | ||||
| Limited/Sine | 326 (61) | 74 (63) | 110 (73) | 0.016 |
| Diffuse | 212 (39) | 44 (37) | 40 (27) | |
| Autoantibodies – ever positive | ||||
| ANA | 515/529 (97) | 113/116 (97) | 140/146 (96) | 0.63 |
| Anti-Centromere | 166/531 (31) | 34 (29) | 50/146 (34) | 0.63 |
| Anti-Scl70/Topoisomerase I | 153/531 (29) | 22/116 (19) | 28/145 (19) | 0.014 |
| Anti-RNA Polymerase III | 96/491 (20) | 19/103 (18) | 22/137 (16) | 0.65 |
| PM/Scl | 31/403 (8) | 5/93 (5) | 6/110 (5) | 0.58 |
| Th/To | 12/403 (3) | 4/93 (4) | 2/110 (2) | 0.58 |
| Fibrillarin/U3RNP | 13/403 (3) | 3/93 (3) | 3/110 (3) | 0.96 |
| Ro/SSA | 102/526 (19) | 31/114 (27) | 41/144 (28) | 0.026 |
| Traditional Risk Factors for DD | ||||
| HTN | 207/532 (39) | 77/117 (66) | 90/145 (62) | <0.001 |
| CAD | 17/532 (3) | 13 (11) | 25/146 (17) | <0.001 |
| DM | 25/527 (5) | 8/115 (7) | 14/147 (10) | 0.085 |
| Obesity (BMI ≥ 30kg/m2) | 86 (16) | 27 (23) | 38 (25) | 0.016 |
| COPD | 46/531 (9) | 15/117 (13) | 26/141 (18) | 0.004 |
| CKD | 72/530 (14) | 24/115 (21) | 41/148 (28) | <0.001 |
| Dyslipidemia | 190/505 (38) | 43/110 (39) | 71/137 (52) | 0.010 |
| Former/Current Tobacco Use | 208 (37) | 57 (48) | 80 (53) | 0.002 |
| Clinical Characteristics§ | ||||
| FVC ≤ 70% | 256/528 (48) | 72 (61) | 82/145 (57) | 0.022 |
| DLCO ≤ 60% | 213/517 (41) | 75/114 (66) | 84/138 (61) | <0.001 |
| PH by RHC | ||||
| None | 22/59 (37) | 9/44 (20) | 10/56 (18) | 0.24 |
| Pre-capillary | 30/59 (51) | 28/44 (63) | 38/56 (68) | |
| Post-capillary | 4/59 (7) | 2/44 (5) | 4/56 (7) | |
| Combined pre- and post-capillary | 3/59 (5) | 5/44 (11) | 4/56 (7) | |
| Severe RP | 305/537 (57) | 73 (62) | 86/148 (58) | 0.60 |
| Myopathy (≤ 4/5 MMT) | 123/534 (23) | 24 (20) | 29/148 (20) | 0.60 |
| Severe GI | 287/537 (53) | 69/117 (59) | 77/148 (52) | 0.48 |
| TFR | 98/537 (18) | 16 (14) | 22/149 (15) | 0.35 |
| Synovitis | 116/537 (22) | 25 (21) | 36/149 (24) | 0.78 |
| Telangiectasias | 494/536 (92) | 110 (93) | 141/149 (95) | 0.58 |
| Calcinosis | 182/536 (34) | 48 (41) | 58/149 (39) | 0.27 |
| SRC | 21 (4) | 4/117 (3) | 13 (9) | 0.040 |
| Max mRSS, median (IQR) | 6 (3, 16) | 6.5 (4, 16) | 6 (3, 13.5) | 0.67 |
Includes Asian, Indian, Middle Eastern, Native American, Native Alaskan, Native Hawaiian/Pacific Islander, Unknown/Not reported
Clinical characteristics are reported as the maximum expressed at any timepoint up until time of echo.
Severe RP: ever presence of digital pitting scars, digital tip ulceration, or digital gangrene; Severe GI: high-dose GERD meds, antibiotics for bacterial overgrowth, malabsorption syndrome, episodes of pseudo-obstructions, or TPN requirement; TFR: tendon friction rubs; Myopathy: ≤ 4/5 manual muscle testing (MMT); SRC: scleroderma renal crisis; mRSS: modified Rodnan skin score
Analyses of clinical and serological features according to diastolic function groups.
Median age increased significantly across normal, indeterminate, and DD groups (p=0.0001; Table 1). The frequency of CAD, obesity, COPD, CKD, dyslipidemia, and ever tobacco use was highest in the DD group compared with the indeterminate and normal groups. HTN was more frequent in the indeterminate function (66%) and DD groups (62%) compared with the normal diastolic function group (39%) (p<0.001). Female sex was equally distributed across groups.
Median disease duration increased significantly across normal, indeterminate, and DD groups (p=0.0001; Table 1). Participants with DD had a higher frequency of limited cutaneous disease (p=0.016) and a history of scleroderma renal crisis (SRC) (p=0.040) compared with indeterminate or normal diastolic function. Participants with indeterminate diastology or DD had higher frequencies of a positive Ro/SSA antibody (p=0.026), restrictive lung disease (FVC ≤70%) (p=0.022), DLCO ≤60% (p<0.001), or an elevated RVSP (ever ≥45mmHg) (p<0.001) compared with participants with normal diastolic function. Participants with normal diastolic function had a higher frequency of the Scl70 antibody compared with the indeterminate and DD groups (p=0.014). Other SSc clinical manifestations had no significant associations with diastolic function groups (Table 1).
Prior use of mycophenolate mofetil, methotrexate, or hydroxychloroquine was found more frequently in those who had normal diastolic function (Table 2). The frequency of ever using a cardiovascular medication prior to 2DE, with the exception of calcium channel blockers, was higher in either indeterminate or DD groups compared with the normal diastolic function group (Table 2). The use of a calcium channel blocker was evenly distributed across groups.
Table 2.
Medication use at any time point prior to echocardiogram, according to diastolic function groups.
| Normal= 538 N (%) | Indeterminate = 118 N (%) | DD = 150 N (%) | P-value | |
|---|---|---|---|---|
| Immune Medications (ever use) | ||||
| Mycophenolate mofetil | 160/537 (30) | 27/116 (23) | 20/148 (14) | <0.001 |
| Methotrexate | 96/537 (18) | 9 (8) | 18/148 (12) | 0.010 |
| Hydroxychloroquine | 132/537 (25) | 20 (17) | 22/148 (15) | 0.016 |
| Cyclophosphamide | 38/537 (7) | 8 (7) | 6/148 (4) | 0.41 |
| Azathioprine | 21/536 (4) | 7/117 (6) | 6/148 (4) | 0.60 |
| Prednisone | 168/536 (31) | 53 (45) | 49/148 (33) | 0.018 |
| Cardiovascular Medications (ever use) | ||||
| Beta blocker | 57/461 (12) | 19/103 (18) | 36/118 (31) | <0.001 |
| Calcium channel blocker | 344/537 (64) | 83/118 (70) | 94/148 (64) | 0.40 |
| ACE inhibitor | 93/524 (18) | 32/115 (28) | 46/144 (32) | <0.001 |
| ARB | 58/457 (13) | 21/99 (21) | 27/115 (23) | 0.005 |
| Diuretics | 124/537 (23) | 68 (58) | 82/148 (55) | <0.001 |
| Aspirin | 226/537 (42) | 56 (47) | 79/148 (53) | 0.042 |
| PDE5 inhibitor | 91/536 (17) | 37 (31) | 48/148 (32) | <0.001 |
| Endothelin receptor antagonist | 26/537 (5) | 19 (16) | 24/148 (16) | <0.001 |
| Prostacyclin analog | 17/537 (3) | 7 (6) | 13/148 (9) | 0.012 |
PDE5: phosphodiesterase 5 inhibitor; ERA: endothelin receptor antagonist
A summary of 2DE parameters for each of the diastolic function groups (normal, indeterminate, and DD) is provided in supplemental data (Supplemental Table S1).
Association between traditional DD risk factors and DD in SSc.
In univariable analyses of traditional DD risk factors including age, sex, HTN, CAD, obesity, dyslipidemia, CKD, DM, COPD, and tobacco use, all risk factors except female sex were associated with an increased risk of DD (Table 3). On multivariable analysis, only older age (OR 1.11, 95%CI 1.08-1.13), CAD (OR 3.07, 95%CI 1.40-6.74), and obesity (OR 3.31, 95%CI 1.92-5.71) remained independently associated with an increased risk of DD. Logistic regression of complete cases only showed similar results (Supplemental Table S2).
Table 3.
Association between traditional DD risk factors and the presence of DD versus normal diastolic function, by logistic regression (imputed cases).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
|
|
|
|
||
| Age at time of echo (years) | 1.10 (1.08, 1.13) | <0.001 | 1.11 (1.08, 1.13) | <0.001 |
| Female sex | 1.17 (0.70, 1.96) | 0.55 | 1.20 (0.64, 2.25) | 0.57 |
| HTN | 2.46 (1.69, 3.58) | <0.001 | 1.17 (0.75, 1.83) | 0.49 |
| CAD | 5.60 (2.94, 10.67) | <0.001 | 3.07 (1.40, 6.74) | 0.005 |
| Obesity | 1.78 (1.16, 2.75) | 0.009 | 3.31 (1.92, 5.71) | <0.001 |
| Dyslipidemia | 1.79 (1.23, 2.60) | 0.002 | 0.82 (0.52, 1.30) | 0.41 |
| CKD | 2.39 (1.55, 3.70) | <0.001 | 1.68 (0.99, 2.86) | 0.057 |
| DM | 2.06 (1.04, 4.06) | 0.038 | 1.19 (0.54, 2.64) | 0.67 |
| COPD | 2.43 (1.44, 4.08) | 0.001 | 1.29 (0.70, 2.35) | 0.41 |
| Tobacco use (ever vs. never) | 1.81 (1.26, 2.61) | 0.001 | 1.32 (0.85, 2.05) | 0.21 |
An ordinal regression analysis, permitting the inclusion of the indeterminate diastolic function group as an intermediate step between normal and DD, showed similar associations (Supplementary Table S3).
A sex-stratified analysis of traditional DD risk factors and SSc clinical characteristics was also performed, as described in the supplement.
Association between SSc characteristics and DD, after adjustment for traditional DD risk factors.
SSc characteristics were analyzed to evaluate for SSc disease specific risk factors for DD versus normal function (Table 4). Each SSc characteristic was then individually adjusted for traditional DD risk factors including age, female sex, HTN, CAD, obesity, dyslipidemia, CKD, DM, COPD, and tobacco use to evaluate for independent associations. On adjusted univariable analyses, longer SSc disease duration (OR 1.03, 95%CI 1.01-1.05), black race (OR 2.02, 95%CI 1.15-3.53), anti-Ro/SSA positivity (OR 1.91, 95%CI 1.09-3.33), FVC ≤70% (OR 1.72, 95%CI 1.08-2.73) and DLCO ≤60% (OR 2.66, 95%CI 1.65-4.31) were associated with an increased risk of DD (Table 4, Adjusted Univariable). Anti-centromere antibody positivity (OR 0.59, 95% CI 0.36-0.98) was associated with a reduced risk of DD. Given the association between anti-Ro/SSA and DD on univariable analysis, and the possibility that anti-Ro/SSA can occur in conjunction with other SSc autoantibodies, interactions between anti-Ro/SSA and anti-centromere, anti-Scl70, and anti-RNA Polymerase III were modeled. No statistically significant associations between the interaction terms and DD were found.
Table 4.
Association between SSc characteristics and DD, unadjusted and adjusted for DD risk factors (imputed cases).
| Univariable | Adjusted† Univariable | Adjusted† Multivariable | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | P | OR (95% CI) | P | |
|
|
|
|
|
|||
| SSc duration from 1st symptoms ever (per one year) | 1.05 (1.03, 1.07) | <0.001 | 1.03 (1.01, 1.05) | 0.013 | 1.04 (1.01, 1.06) | 0.004 |
| Race | ||||||
| Black vs. Caucasian | 1.13 (0.74, 1.73) | 0.58 | 2.02 (1.15, 3.53) | 0.014 | 1.35 (0.73, 2.50) | 0.33 |
| Other‡ vs. Caucasian | 0.34 (0.12, 0.98) | 0.045 | 1.21 (0.37, 3.97) | 0.76 | 1.24 (0.36, 4.21) | 0.73 |
| Diffuse SSc Subtype | 0.56 (0.37, 0.84) | 0.005 | 0.82 (0.49, 1.35) | 0.43 | 0.95 (0.52, 1.73) | 0.87 |
| Autoantibodies – Combined | ||||||
| Euroimmun/Clinical | ||||||
| Centromere | 1.17 (0.79, 1.72) | 0.43 | 0.59 (0.36, 0.98) | 0.041 | 0.48 (0.23, 1.00) | 0.051 |
| Topo I/Scl70 | 0.58 (0.37, 0.91) | 0.018 | 0.76 (0.44, 1.33) | 0.34 | 0.49 (0.26, 0.93) | 0.030 |
| RNA Pol III | 0.80 (0.48, 1.34) | 0.40 | 0.53 (0.28, 1.00) | 0.050 | 0.51 (0.25, 1.03) | 0.060 |
| PM-Scl | 0.72 (0.29, 1.78) | 0.48 | 0.48 (0.16, 1.42) | 0.19 | ||
| Th/To | 0.62 (0.13, 2.85) | 0.54 | 0.22 (0.04, 1.15) | 0.073 | 0.21 (0.04, 1.06) | 0.060 |
| Fibrillarin/U3-RNP | 0.89 (0.29, 2.78) | 0.85 | 1.78 (0.41, 7.71) | 0.44 | ||
| Ro/SSA | 1.68 (1.07, 2.63) | 0.026 | 1.91 (1.09, 3.33) | 0.024 | 1.51 (0.70, 3.25) | 0.30 |
| Autoantibody Interactions | ||||||
| Ro*Centromere | 1.85 (0.94, 3.65) | 0.079 | 1.18 (0.54, 2.57) | 0.69 | 0.95 (0.27, 3.31) | 0.94 |
| Ro*Topo I/Scl70 | 0.71 (0.24, 2.13) | 0.55 | 0.75 (0.19, 2.86) | 0.67 | ||
| Ro*RNA Pol III | 1.91 (0.85, 3.65) | 0.12 | 1.66 (0.61, 4.52) | 0.32 | ||
| Ro*ThTo | 1.71 (0.35, 8.42) | 0.51 | 0.79 (0.13, 4.77) | 0.80 | ||
| Clinical Characteristics | ||||||
| Severe RP | 1.06 (0.73, 1.53) | 0.76 | 1.21 (0.77, 1.91) | 0.42 | ||
| FVC ≤ 70 | 1.39 (0.96, 2.01) | 0.082 | 1.72 (1.08, 2.73) | 0.023 | 1.22 (0.69, 2.16) | 0.49 |
| DLCO ≤ 60 | 2.20 (1.51, 3.21) | <0.001 | 2.66 (1.65, 4.31) | <0.001 | 2.41 (1.40, 4.16) | 0.002 |
| Myopathy (MMT≤4) | 0.81 (0.51, 1.27) | 0.35 | 1.12 (0.64, 1.94) | 0.70 | ||
| Severe GI | 0.94 (0.65, 1.36) | 0.75 | 0.68 (0.44, 1.05) | 0.084 | 0.48 (0.30, 0.79) | 0.004 |
| TFR | 0.77 (0.47, 1.28) | 0.31 | 0.84 (0.44, 1.61) | 0.61 | ||
| Synovitis | 1.15 (0.75, 1.76) | 0.53 | 0.94 (0.55, 1.59) | 0.81 | ||
| Telangiectasias | 1.51 (0.69, 3.29) | 0.30 | 0.91 (0.32, 2.61) | 0.86 | ||
| Calcinosis | 1.24 (0.85, 1.80) | 0.26 | 1.24 (0.78, 1.96) | 0.36 | ||
| SRC | 2.34 (1.14, 4.78) | 0.021 | 2.13 (0.75, 6.08) | 0.16 | 3.18 (1.12, 9.07) | 0.031 |
| Max mRSS | 0.99 (0.98, 1.01) | 0.37 | 0.99 (0.97, 1.02) | 0.61 | ||
Adjusted for all DD risk factors including age, sex, HTN, CAD, obesity, dyslipidemia, CKD, diabetes, COPD, and tobacco use.
Includes Asian, Indian, Middle Eastern, Native American, Native Alaskan, Native Hawaiian/Pacific Islander, Unknown/Not reported
Multivariable model constructed based on significant variables on unadjusted or adjusted univariable analysis (95% CI that do not cross 1.00, or p<0.10).
A multivariable model was then constructed with all covariates that were associated with DD on univariable or adjusted univariable models. The multivariable model was adjusted for all cardiovascular and pulmonary risk factors listed in Table 3. Longer SSc disease duration (OR 1.04, 95%CI 1.01-1.06) and a reduced DLCO ≤60% (OR 2.41, 95%CI 1.40-4.16) remained independently associated with DD. Additionally, a history of SRC, which was significant on univariable but not adjusted univariable analysis, associated with an increased risk of DD (OR 3.18, 95%CI 1.12-9.07). Race, diffuse subtype, anti-centromere, anti-RNA polymerase III, anti-Ro/SSA, and FVC ≤70% were no longer statistically significantly associated with an increased or decreased risk of DD in the final multivariable adjusted model. A positive anti-Scl70 was associated with a 51% reduction in odds of DD (OR 0.49, 95%CI 0.26-0.93). Additionally, a history of severe GI disease was associated with a 52% reduction in risk of DD (OR 0.48, 95%CI 0.30-0.79).
Only the traditional DD risk factors of older age (OR 1.12, 95%CI 1.09-1.15), CAD (OR 3.69, 95%CI 1.52-8.97), and obesity (OR 4.74, 95%CI 2.57-8.74) were independently associated with DD on final multivariable model (data not shown).
Analyses of complete cases only are presented in Supplementary Table S4.
An ordinal regression including indeterminate diastolic cases showed similar trends in risk factor associations as seen in the primary logistic regression model (Supplementary Table S5).
Association between medication exposure and the presence of DD on univariable and multivariable regression.
On univariable analyses, ever exposure to mycophenolate mofetil or hydroxychloroquine was associated with a 63% (OR 0.37, 95%CI 0.22-0.61) and 47% (OR 0.53, 95%CI 0.33-0.88) reduction in risk of DD, respectively (Supplementary Table S6). These associations were no longer present after adjustment for traditional DD risk factors or in adjusted multivariable model. These medication analyses were considered hypothesis generating and not included in the final model, as dose and duration were not available and would be hypothesized to impact associations. Associations were seen between most cardiovascular medications (with the exception of calcium channel blockers and nitrates) and DD in univariable models, which were mildly attenuated when adjusting for DD risk factors (Supplementary Table S6).
Impact of DD on survival in SSc.
There were 135 deaths among 672 SSc subjects with either DD or normal function. SSc subjects with DD had an almost 3-fold increase in risk of mortality in univariable Cox proportional hazards analysis (HR 2.86, 95%CI 2.03-4.01)(Table 5). After adjusting for traditional DD risk factors, DD conferred a >2-fold increase in risk of mortality (HR 2.38, 95%CI 1.56-3.61), with CKD (HR 1.64, 95%CI 1.11-2.42) and COPD (HR 1.93, 95%CI 1.09-2.96) also associated with an increased risk of mortality. Female sex was protective (OR 0.59, 95%CI 0.39-0.91).
Table 5.
Cox Proportional Hazards Models Evaluating the Association between Mortality and SSc/DD risk factors in 672 subjects (imputed cases).
| Univariable | Multivariable DD Risk Factors | Multivariable DD & SSc Risk Factors | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | P | HR (95% CI) | P | |
|
|
|
|||||
| DD versus normal function | 2.86 (2.03, 4.01) | <0.001 | 2.38 (1.56, 3.61) | <0.001 | 1.69 (1.07, 2.68) | 0.027 |
| Age at time of echocardiogram | 1.01 (0.99, 1.03) | 0.26 | 1.02 (1.00, 1.04) | 0.037 | ||
| Female sex | 0.59 (0.39, 0.91) | 0.018 | 0.70 (0.44, 1.11) | 0.13 | ||
| HTN | 1.30 (0.89, 1.89) | 0.18 | 1.35 (0.90, 2.03) | 0.15 | ||
| CAD | 0.65 (0.35, 1.18) | 0.16 | 0.62 (0.32, 1.21) | 0.16 | ||
| Obesity | 0.63 (0.38, 1.04) | 0.071 | 0.68 (0.40, 1.16) | 0.16 | ||
| Dyslipidemia | 0.91 (0.63, 1.31) | 0.62 | 0.88 (0.60, 1.31) | 0.54 | ||
| CKD | 1.64 (1.11, 2.42) | 0.015 | 1.42 (0.91, 2.20) | 0.13 | ||
| DM | 1.73 (0.99, 3.02) | 0.058 | 1.16 (0.64, 2.10) | 0.63 | ||
| COPD | 1.93 (1.09,2.96) | 0.025 | 1.56 (0.90, 2.69) | 0.12 | ||
| Tobacco (ever vs. never) | 1.21 (0.85, 1.73) | 0.28 | 1.18 (0.81, 1.72) | 0.39 | ||
| Race | ||||||
| Black vs. Caucasion | 1.02 (0.64, 1.62) | 0.94 | ||||
| Other‡ vs. Caucasion | 1.35 (0.59, 3.07) | 0.48 | ||||
| SSc duration from 1st symptoms ever (years) | 0.99 (0.97, 1.01) | 0.23 | ||||
| Diffuse SSc subtype | 0.95 (0.61, 1.48) | 0.83 | ||||
| Autoantibodies – Combined Euroimmun/Clinical | ||||||
| Centromere | 1.32 (0.74, 2.36) | 0.34 | ||||
| Topo I/Scl70 | 1.09 (0.69, 1.71) | 0.72 | ||||
| RNA Pol III | 1.24 (0.73, 2.11) | 0.43 | ||||
| Th/To | 0.65 (0.24, 1.74) | 0.39 | ||||
| Ro/SSA | 1.28 (0.75, 2.20) | 0.37 | ||||
| Ro*Centromere | 0.85 (0.35, 2.05) | 0.72 | ||||
| Clinical Characteristics | ||||||
| FVC ≤ 70% | 1.34 (0.83, 2.15) | 0.24 | ||||
| DLCO ≤ 60% | 3.24 (2.00, 5.25) | <0.001 | ||||
| Severe GI disease | 1.10 (0.74, 1.65) | 0.63 | ||||
| SRC | 1.78 (0.90, 3.50) | 0.10 | ||||
Includes Asian, Indian, Middle Eastern, Native American, Native Alaskan, Native Hawaiian/Pacific Islander, Unknown/Not reported
In the final multivariable model, black race, diffuse disease, anti-centromere, anti-Scl70, anti-RNA polymerase III, anti-Th/To, FVC ≤70%, DLCO ≤60%, and SRC were all included given known positive or negative associations with mortality in SSc.1,2 Anti-Ro/SSA, anti-Ro/SSA*anti-centromere interaction, and severe GI disease were also included in the final model given demonstrated associations with DD (p≤0.10 on logistic regression analyses in Table 4). In the final multivariable model, DD remained independently associated with an increase in risk of mortality in imputed cases (Table 5, HR 1.69, 95%CI 1.07, 2.68). Only age (HR 1.02, 95%CI 1.00-1.04) and DLCO ≤60% (HR 3.24, 95%CI 2.00-5.25) were independently associated with mortality on multivariable model. The point estimate for the HR of DD was similar (HR 1.78, 95%CI 0.98-3.24) for complete (non-imputed) cases (Supplementary Table S8).
Unadjusted (Figure 1a) and age-adjusted (Figure 1b) Kaplan Meier curves are shown for DD and normal diastolic function groups. The unadjusted median survival for DD from time of 2DE was 2.76 years, and unable to be calculated for normal function due to survival over 50% during the time under observation. In participants with DD, the age-adjusted 5- and 10-year survival was 40.8% and 30.5%, respectively. In participants with normal diastolic function, the age-adjusted 5- and 10-year survival was 68.9% and 61.1%, respectively. Kaplan Meier graphs including the indeterminate function group, which showed similar survival as the DD group, are included in supplementary text (Supplementary Figure S1).
FIGURE 1. Association between normal diastolic function and DD with mortality in 672 SSc subjects on unadjusted (A) and age-adjusted (B) Kaplan Meier analyses.
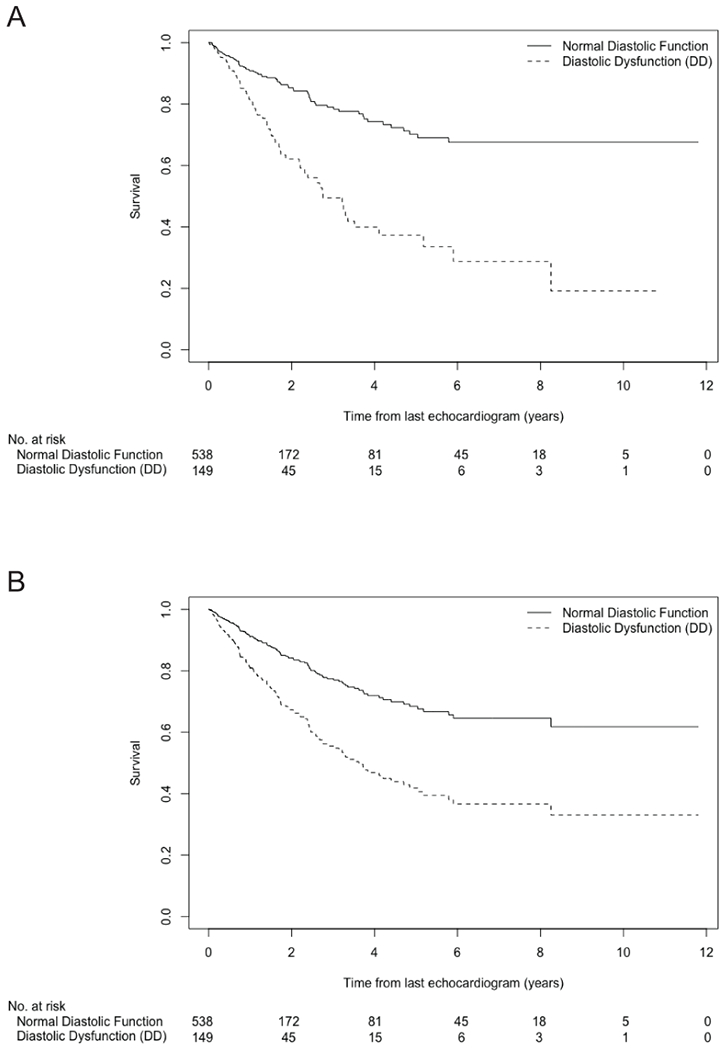
Age adjusted 5- and 10-year survival was 68.9% and 61.1%, respectively, for normal function. Age adjusted 5- and 10-year survival was 40.8% and 30.5%, respectively, for DD.
DISCUSSION
We present the largest study to date examining risk factors for DD and impact of DD on mortality in a well-characterized cohort of patients with SSc. Traditional DD risk factors were ascertained prospectively, and CAD and obesity emerged as significant risk factors for DD in patients with SSc. We confirmed others’ findings that age,5,9 SSc disease duration9 and DLCO ≤60%6,9 are associated with an increased risk of DD, and we are the first to identify an association between a history of SRC and increased risk of DD. Interestingly, we also found that severe GI disease and anti-Scl70 antibodies were associated with a decreased risk of DD. Importantly, DD independently conferred a 69% increase in mortality in SSc. Therefore, interventions targeting modifiable risk factors for DD, including CAD and obesity, may help reduce mortality in SSc.
A major strength of our study was the rigorous application of the most recent societal recommendations for the classification of diastolic function, which combines four 2DE-derived parameters to classify diastology. The aim of the 2016 ASE/ESCVI guidelines were to simplify the approach to diastology determination from the more complex, difficult to apply, and irregularly used 2009 guidelines, by selecting the most reproducible and feasible 2DE-measurements.13 The use of these simplified, yet rigorous, guidelines improves generalizability of our study results and minimizes potential misclassification that occurs when using single parameters.
With application of these guidelines, we found a DD prevalence of 18.6% in our SSc cohort, which falls in the lower range of previous estimates ranging between 18-62%.4,6,12,26 These prior studies, however, utilized older guidelines to classify diastology or utilized a single 2DE parameter which may lead to misclassification if used in isolation. Our prevalence estimate is similar, but slightly lower, than a prevalence of 29% reported by Tennoe et al. who utilized the same 2016 ASE/ESCVI criteria to classify diastolic function.5 However, in contrast to Tennoe et al., we excluded patients with moderate or severe valvular heart disease as we were focused on primary myocardial disease, which may account for this difference. Our prevalence of 18.6% is higher than a prevalence of 1.4% in a similarly aged population-based cohort of 1,000 non-SSc subjects in which the 2016 ASE/ESCVI were applied,8 which may suggest the existence of SSc specific risk factors for DD. However, two other referral-based non-SSc cohorts (i.e., patients referred specifically for a 2DE) have reported a prevalence between 9.4-30%, which may indicate underlying cardiovascular and pulmonary risks are most influential on DD risk.27,28 Further studies in which SSc patients and non-SSc patients are derived from the same population, and have similar demographics and comorbidities, is essential to evaluate for the the potential impact of SSc pathophysiology on DD risk.
With the potential increase in DD prevalence in SSc cohorts compared with non-SSc cohorts, we sought to identify SSc-specific risk factors for DD which may provide further mechanistic insight into DD pathogenesis in this population. To robustly determine independent associations between SSc-specific risk factors and DD, ten traditional DD risk factors were examined. While all of these traditional DD risk factors (with the exception of sex) were significantly associated with DD on univariable analyses, only three of the ten DD risk factors (age, CAD, obesity) were independently associated with DD in our final multivariable model. This is similar to the results by Hinchcliff and colleagues who reported an independent association of age and CAD with tissue Doppler lateral e’ velocity; obesity, however, was not examined.9 Impressively, obesity was independently associated with an over 4.5-fold increase in risk of DD in our population. Interestingly, we did not find an independent association between HTN and DD, which is similar to results presented by Maione and colleagues11 but differs from findings by Hinchcliff et al.9 Given the known association between HTN and obesity, as well as HTN and CAD, it is possible that the effect of HTN on DD presence is mediated through obesity and/or CAD which may explain the lack of association between HTN and DD in the final multivariable model. We did not find an association between sex and DD, which may be due to differences in prevalence of traditional DD risk factors in males compared with females (e.g. males had an increase in frequency of CAD, CKD, and DLCO ≤60%), which we accounted for in our models. Further study is required with general population controls to examine these sex-based differences.
We performed an in-depth examination of SSc-specific risk factors, adjusting for the ten traditional DD risk factors. We found an independent association between SSc disease duration and DD, as previously noted in some studies6,9,26 but not others.5,11 This association between SSc disease duration and DD may suggest a relationship between SSc disease pathophysiology and DD pathophysiology, perhaps mediated by progressive endothelial dysfunction. We also identified a 2.4-fold increased risk of DD in participants who had a DLCO ≤60%, even after controlling for restrictive lung disease, which suggests that the low DLCO may represent concomitant pulmonary vascular disease. In the 159 participants who underwent a clinically indicated RHC, the frequency of pre- and post-capillary PH was evenly distributed among diastolic function groups (Table 1). It is possible that further provocative testing with exercise or fluid challenge may have identified elevated filling pressures in the groups with a DLCO ≤60% and DD, however, this analysis was beyond the scope of this study. Other studies have also reported a lower DLCO in SSc patients with DD.6,9 We additionally report an independent association between SRC and DD in our final model, which adjusted for potential mediators including HTN and CKD. An increased risk of DD in patients with a history of SRC is biologically plausible, as activation of the renin-angiotensin-aldosterone system underlies the pathophysiology of SRC29 and is also known to promote myocardial fibrosis and stiffness.30 It is important to recognize that SRC may increase DD risk, highlighting the importance of addressing potentially modifiable DD risk factors such as CAD and obesity in SSc patients with a prior history of SRC.
Anti-Scl70 was associated with a reduced risk of DD in our final model. Hinchcliff et. al. reported a reduction in presence of DD in anti-Scl70 positive patients and increase in those with anti-centromere antibodies, but these associations were not significant after controlling for age.9 A decrease in the frequency of anti-Scl70 in patients with DD was also observed by Tennoe et. al, though results did not reach significance.5
The association between severe GI disease and decreased risk of DD has not been previously examined. We hypothesize that this association may be mediated by a decrease in adipose tissue, with a resulting decrease in inflammatory mediators. Increase in adipose tissue is associated with an increase in inflammatory mediators which can lead to the endothelial dysfunction that underlies DD pathogenesis.31,32 The decreased risk of DD in those with severe GI disease may also be related to a decrease in left ventricular mass, as nutritional status has been shown to correlate with left ventricular mass in SSc.33 It is also possible that use of high-dose GERD medications in patients with severe GI disease may have protected against aspiration induced lung damage with reduced DLCO, thus decreasing risk.
Importantly, we show that DD is an independent risk factor for mortality in SSc, even after adjusting for cardiovascular, pulmonary, and SSc-specific risk factors for mortality. In fully adjusted models, only age and a DLCO ≤60%, likely reflecting pulmonary vascular disease, were also independently associated with mortality. These findings agree with others, who have noted a similar association between DD and mortality in SSc in smaller cohorts.5,9
Our study is limited by examination of a single 2DE per participant. Therefore, while important associations can be made regarding risk factors for prevalent DD, a cause and effect relationship should not be extrapolated. Strengths of this study are the utilization of classification criteria for DD, rigorous ascertainment of DD risk factors and SSc disease characteristics prospectively, and the large cohort size permitting robust multivariable analyses of risk factors.
CONCLUSION
We present the largest cohort study examining risk factors for DD in SSc, and impact of DD on mortality. CAD and obesity are significant comorbidities associated with DD in SSc, which are also potentially modifiable risk factors. Given the strong association between DD and mortality, interventions targeting reduction in risk of CAD and obesity may also decrease mortality in SSc through a reduction in DD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIH) T32AR048522 (A.M.H.), R01AR073208 (A.A.S), P30-AR070254 (J.P.); the Martha McCrory Endowed Professorship (F.M.W.); the Scleroderma Foundation (M.M.); the Johns Hopkins Clinician Scientist Award (M.M.); the Chresanthe Staurulakis Memorial Discovery Fund; the Donald B. and Dorothy L. Stabler Foundation; the Johns Hopkins inHealth Precision Medicine Initiative; and the Scleroderma Research Foundation.
REFERENCES
- 1.Sampaio-Barros PD, Bortoluzzo AB, Marangoni RG, et al. Survival, causes of death, and prognostic factors in systemic sclerosis: analysis of 947 Brazilian patients. J Rheumatol 2012;39:1971–8. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Codina A, Simeon-Aznar CP, Pinal-Fernandez I, et al. Cardiac involvement in systemic sclerosis: differences between clinical subsets and influence on survival. Rheumatol Int 2017;37:75–84. [DOI] [PubMed] [Google Scholar]
- 4.Faludi R, Kolto G, Bartos B, Csima G, Czirjak L, Komocsi A. Five-year follow-up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum 2014;44:220–7. [DOI] [PubMed] [Google Scholar]
- 5.Tennoe AH, Murbraech K, Andreassen JC, et al. Left Ventricular Diastolic Dysfunction Predicts Mortality in Patients With Systemic Sclerosis. J Am Coll Cardiol 2018;72:1804–13. [DOI] [PubMed] [Google Scholar]
- 6.Vemulapalli S, Cohen L, Hsu V. Prevalence and risk factors for left ventricular diastolic dysfunction in a scleroderma cohort. Scand J Rheumatol 2017;46:281–7. [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 8.Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018;19:380–6. [DOI] [PubMed] [Google Scholar]
- 9.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 2012;30:S30–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol 2014;63:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maione S, Cuomo G, Giunta A, et al. Echocardiographic alterations in systemic sclerosis: a longitudinal study. Semin Arthritis Rheum 2005;34:721–7. [DOI] [PubMed] [Google Scholar]
- 12.de Groote P, Gressin V, Hachulla E, et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008;67:31–6. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 14.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 15.Masi AT, Committee SFSCotARADaTC. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 16.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 18.Crapo RO, Jensen RL, Wanger JS. Single-breath carbon monoxide diffusing capacity. Clin Chest Med 2001;22:637–49. [DOI] [PubMed] [Google Scholar]
- 19.Medsger TA Jr., Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003;21:S42–6. [PubMed] [Google Scholar]
- 20.Medsger TA Jr., Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol 1999;26:2159–67. [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 22.Agresti A Categorical Data. Second ed: Wiley; 2002. [Google Scholar]
- 23.Andersen P, Gill R. Cox’s regression model for counting processes, a large sample study. Annals of Statistics 1982;10:1100–20. [Google Scholar]
- 24.van Buuren S, Brand JP, Groothuis-Oudshoorn CG, Rubin DB. Fully conditional specification in multivariate imputation. Journal of Statistical Computation and Simulation 2006;76:1049–64. [Google Scholar]
- 25.R-Core-Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 26.Roque MCF, Sampaio-Barros PD, Arruda AL, et al. Evaluation of Left Ventricular Diastolic Function by Echocardiography with Tissue Doppler in Systemic Sclerosis. Arq Bras Cardiol 2017;109:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorrentino R, Esposito R, Santoro C, et al. Practical Impact of New Diastolic Recommendations on Noninvasive Estimation of Left Ventricular Diastolic Function and Filling Pressures. J Am Soc Echocardiogr 2020;33:171–81. [DOI] [PubMed] [Google Scholar]
- 28.S SK, Desai N, Gona OJ, K VK, B M. Impact of Updated 2016 ASE/EACVI VIS-A-VIS 2009 ASE Recommendation on the Prevalence of Diastolic Dysfunction and LV Filling Pressures in Patients with Preserved Ejection Fraction. J Cardiovasc Imaging 2021;29:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denton CP, Hudson M. Renal Crisis and Other Renal Manifestations of Scleroderma. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, eds. Scleroderma: From Pathogenesis to Comprehensive Management. Second ed. New York: Springer; 2017. [Google Scholar]
- 30.Jia G, Aroor AR, Hill MA, Sowers JR. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018;72:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 32.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosato E, Gigante A, Gasperini ML, et al. Nutritional status measured by BMI is impaired and correlates with left ventricular mass in patients with systemic sclerosis. Nutrition 2014;30:204–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


