Abstract
Aim:
To identify pathogenic rare coding Mendelian/high-effect size variant(s) by whole-exome sequencing in familial PCOS patients to elucidate PCOS related pathways.
Methods:
Twenty women and their affected available relatives diagnosed with polycystic ovary syndrome according to Rotterdam Criteria were recruited. Whole-exome sequencing on germ-line DNA from 31 polycystic ovary syndrome probands and their affected relatives were performed. Whole-exome sequencing data was further evaluated by pathway and chemogenomics analyses. In-slico analysis of candidate variants were done by VarCards for functional predictions and VarSite for impact on 3D structures in the candidate proteins.
Results:
Two heterozygous rare FBN3 missense variants in three patients, and one FN1 missense variant in one patient from three different PCOS families were identified.
Conclusions:
We identified three novel FBN3 and FN1 variants for the first time in the literature and linked with polycystic ovary syndrome. Further functional studies may identify causality of these newly discovered PCOS related variants, and their role yet remain to be investigated. Our findings may improve our understanding of the biologic pathways affected and identify new drug targets
Keywords: Extracellular Matrix, Genetics, PCOS, Whole Exome Sequencing
Introduction
Polycystic ovary syndrome (PCOS) is a prevalent and heterogenous endocrine disease affecting 7–10% women of reproductive age with multi-factorial etiology (1). Based on twin studies, it is highly heritable (over 70%) (2) and first-degree female relatives of PCOS patients show increased prevalence (3–14). In addition to oligogenic/polygenic models and environmental effects on the pathogenesis of PCOS, autosomal dominant mode of inheritance has been recognized with familial clustering of cases (5–8, 10, 11, 13, 15, 16).
The criteria for PCOS diagnosis have been revised several times and there is no universally accepted version. The most widely used is the Rotterdam criteria, and PCOS is diagnosed by two or more of its reproductive features of ovulatory dysfunction (oligomenorrhea-amenorrhea), hyperandrogenism, and polycystic morphology of the ovaries on ultrasound exam, along with exclusion of other etiologies (17–19). Approximately two-third of affected individuals experience subfertility, obesity, and metabolic disorders in the PCOS background (20–29). As future consequences of the syndrome such as diabetes and cardiovascular disease risk are well-known, and also extend to first-degree relatives (30–33), further investigation is needed for possible clinical outcomes of the PCOS (34, 35).
Previous genetic approaches to PCOS, consisting mainly of candidate gene, and to a lesser extent genome-wide association studies, have identified variants that account for only a small percent of inherited PCOS risk and remaining are yet to be identified (36).
Whole-exome sequencing (WES) has been successfully identifying rare mutations that have a greater impact on human diseases since most disease-causing mutations are located within protein coding regions (37). Rare variants with large effects in a specific gene may be found in extreme phenotypes, which can provide insights into the underlying pathophysiology of the common disorder, and eventually lead to the development of risk prediction models and therapeutic strategies for patient care (38). Here, we aimed to identify pathogenic rare coding Mendelian/high-effect size variant(s) using WES in familial PCOS patients to elucidate PCOS related pathways.
Methods
Study co-investigators evaluated the patients at their participating institutions and obtained written consent under Gazi University, Medical Faculty, Ethics Committee (Decision#223).
Study Cohort
20 women and their affected available relatives from unrelated families with two or more individuals diagnosed with PCOS were recruited (Supplementary Table 1). PCOS was diagnosed according to the criteria from The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) (20).
All probands met the Rotterdam criteria for diagnosis of PCOS, i.e. they had two of the three following features: 1) oligomenorrhea (defined as menstrual cycles >35 days), 2) clinical (hirsutism defined as a modified Ferriman-Gallwey score (FGS) >6 or acne) and/or biochemical evidence of androgen excess (defined as serum levels of dehydroepiandrosterone sulfate (DHEAS), total (TT) or free testosterone (fT) above ±2SD of controls, the upper ±2SD of androgen levels among controls were 404 μg/dL for DHEAS, 0.61 ng/mL for TT and 2.12 pg/mL for fT, and 3) polycystic ovaries (defined as ovarian volume above 10 ml or ovaries having ≥12 follicles measuring 2–9 mm in at least one ovary).
As the Rotterdam ESHRE/ASRM–Sponsored PCOS consensus workshop group (19) suggests using more narrow diagnostic criteria in familial studies to identify affected individuals, such as the presence of PCO alone, or hyperandrogenemia per se, in this study, sisters were affected; 1. if they had PCOS according to Rotterdam criteria, 2. if they had only PCOM or 3. if they had hyperandrogenemia (clinical or biochemical). Mothers were considered affected if they had one of the features of PCOS or if they had a known history of PCOS or history of oligomenorrhea and/or hirsutism in their reproductive years.
For all participants medical history, signs of hirsutism and menstrual irregularities were recorded. Weight and height were determined, and body mass index (BMI) was calculated as weight (kg)/height(m2). The presence of hirsutism was defined as a Ferriman-Gallwey score of >6 and acne were noted as present or absent. Blood sampling was performed during the follicular phase from all the subjects for the measurement of serum follicle stimulating hormone (FSH), luteinizing hormone (LH), androstenedione (A), 17-hydroxy progesterone (17-OHP), sex hormone binding globuline (SHBG), total testosterone (TT), dehydroepiandrostenodione sulfate (DHEAS) and anti-mullerian hormone (AMH). Hormone assays of LH, FSH, E2, DHEAS, TT was measured using electro-chemiluminescence immune assay with the Roche e Cobas 601 immunoassay analyzer, using the Roche kit. Androstenedione, SHBG and 17-OHP were measured using the Dia.Metra kit by “Enzyme-linked imunosorbent assay” (ELISA) method manually with μ-Quant Bio-Tek analyzer (μ-Quant Bio-Tek Instruments Inc. USA). Serum AMH was assayed by ELISA using Beckman Coulter AMH Gen 2 kits. Serum levels of total cholesterol, HDL-C, LDL-C, and TG were determined with the use of an AU680 Chemistry System (Beckman– Coulter). On the same day ovarian morphology was evaluated with Siemens Acuson Antares (Mountain View, CA, USA) ultrasound machine equipped with a CH6-2 MHz abdominal or an EC9-4 MHz transvaginal probe.
Whole-Exome Sequencing (WES) and Analysis
After DNA extraction from whole blood using Gentra Puregene Blood Kit (QIAGEN, Hilden, Germany), whole-exome sequencing was performed with targeted enrichment of coding genome with NimbleGen 2.1M human exome array (Roche Nimblegen, Inc.) according to the manufacturer’s protocol with modifications, described previously (37). Sequencing of the prepared libraries was performed on Illumina’s HiSeq2000 using 75bp reads and paired-end chemistry. Base calling was performed with Illumina Casava pipeline version 1.8, and sequencing data were analyzed using BWA for alignment (39), GATK for variant calling and local pipelines for annotation (40). Main steps of bioinformatics pipeline are given in Supplementary Figure 1.
In-slico Analysis of Candidate Variants
Online database PCOSKB (http://www.pcoskb.bicnirrh.res.in), a curated set of genes and phenotype associations, along with biochemical pathways were (41) interrogated for phenotype/genotype correlations and sex specific validations. Chemogenomics analysis was performed with QuartataWeb server (42) and DrugBank (43). We used VarCards for functional predictions (44). Candidate variants were annotated for impact on 3D structures in the Protein Data Bank (PDB) through VarSite (45).
Results
Study Population
Central tendency measures as compared to standard distributions are outlined in Table 1. Mean age of the patients was 32.04 (±11.95) years and mean modified Ferriman Galways Scores were 10.13 (±7.83). High BMI, and increased hip and waist circumference values were observed. Insulin levels and OGTTs were normal. TT, SHBG, DHEASO4, 17OH progesterone, triglycerides, cholesterol, HDL and LDL measurements were all in normal ranges with the exception of high androstenedione levels.
Table 1.
Measures of central tendency [median (min-max values)]
| Variable (metric; normal values) | All patients (n=27) |
|---|---|
| Age (years) | 32.04 (±11.95)a |
| AMH (ng/mL; 0,07–7,35) | 2.4 (0.1 – 8.5)b |
| FGS (Ferriman Gallwey score) | 10.13 (±7.83)a |
| BMI (kg/m2 ; 18,5–24,9) | 28.44 (±7.38)a |
| Waist / hip circumference (cm; <80) | 86.5 (61 – 144)b |
| Hip perimeter (cm; <105) | 107.6 (±15.6)a |
| Insulin (IU/mL; 1,9–23) | 8.49 (1.77– 43.3)b |
| Oral glucose tolerance test 75gr (mmol/L; <100 fasting blood sugar) | 97 (60 – 168)b |
| Oral glucose tolerance test 75gr_2 hours ((mmol/L; 2 hour<153) | 84.22 (±14.52)a |
| Dehydroepiandrosterone sulfate (μg/dL; 23–266) | 173.96 (±8.83)a |
| Sex hormone-binding globulin (nmol/L; 18–144) | 37,31 (6.96 – 153)b |
| Total Testesterone (ng/L; 0.15–0.7) | 0.395 (0.071 – 1.09)b |
| 17-hydroxyprogesterone (ng/L; <8) | 0.96 (0.42 – 2.815)b |
| Androstenedione (ng/dL; 0,3–3,3) | 4.26 (0.66 – 11.41)b |
“Mean” and “standard deviation” values in parentheses were specified for the data that fit the normal distribution.
“Median” and “lowest - highest values” in parentheses were specified for data that do not fit the normal distribution.
Whole Exome Sequencing
31 germ line DNA were processed for WES from twenty unrelated families (2 patients from 11/20 families) (Supplementary Table 1). While 4 out of 20 families had a single affected individual, the remaining 16 (80%) had two or more affected individuals. Across the cohort, an average of 68,176,055 reads were obtained with 46.55% targeting coding (RefSeq) and flanking sequences. Mean target coverage was 65.17 and an average coverage of 10X or greater was achieved for 93.14% of the targeted bases, generating sufficient support to detect dominant and recessive single nucleotide and indel variants (Supplementary Table 2).
Data Analysis
WES data set generated 403,773 variants from 31 patients which were initially filtered based on following criteria: (i) GnomAD v.2.1.1 was used to filter out variants with higher than 1% minor allele frequency, (ii) only heterozygous variants with no multiple allelic sites were retained, (iii) variants labelled as frameshift variant, in-frame indel, missense variant, deleterious initiator codon variant, splice acceptor/donor variant or stop gain/lost variant were selected. A resulting set of 25,353 variants were further selected by excluding the variants that are not shared between the family members. These 11,823 variants were distributed across 5,514 genes with 1,827 genes harbored variants in more than one family (Figure 1). 43 common genes from OMIM and PCOSK curations were found to overlap with this set, are given in Table 2. When we manually interrogated the variants related to these 43 genes, we identified two heterozygous FBN3 variants in three patients from two different families. Applying American College of Medical Genetics and Genomics and the Association for Molecular Pathology variant pathogenicity criteria to FBN3:c.4823A>G and FBN3:c.4498G>A variants, we classified these variants as variants of unknown significance with PM2 and PP3 (46) (Supplementary Table 3). These variants are rare, according to publicly available databases comprising multiethnic individuals. FBN3:c.4823A>G is never reported in GnomAD and GME Variome databases and for FBN3:c.4498G>A, minor allele frequency is 2,4×10−5 in GnomAD, and 5×10−4 in GME Variome, respectively, and predicted to be pathogenic by in slico prediction tools. We have observed consensus for pathogenicity across functional prediction tools (FATHMM (47), MutationTaster (48), PolyPhen2 (49) and SIFT (50), VEST3 (51)), for conservation (GERP++, phastCons (52) and PhyloP (53)), eight ensemble methods (CADD (54), DANN (55), Eigen (56), FATHMM-MKL (57), REVEL (58), MetaLR (59), and MetaSVM (59). No other candidate variants were detected for phenotype causality (44, 60). Based on identified variants’ rarities, in slico patogenicity predictions and previous studies implicating FBN3 gene’s role in pathogenesis of PCOS, FBN3 variants in these families may be disease causing. In pathway analysis (STRING (61)), FBN3 first and second neighbors were identified (Figure 1 and Supplementary Table 4) and investigated for variants. However, there was no candidate gene mutation identified among other primary and secondary FBN3 interactors in the study cohort. A heterozygous rare ovarian expressed FN1 gene variant was identified in a familial PCOS patient. Applying same criteria to FN1:c.1802C>T, PM2 and PP3 led again to the classification of variant of unknown significance (Supplementary Table 3) (44, 46). The residue 601 is a proline with a rigid side chain predicted to restrict the conformation of the protein at this point (45). Patient’ variant leads to a leucine replacement with an aliphatic and hydrophobic side chain. While the missense mutation is predicted to have a low ‘disease propensity’ value of 0.95 (45), it is very highly unfavoured in terms of conserved amino acid properties. The FN1 variant is in collagen binding region of Fibronectin type-I 9 domain and expressed ubiquitously including uterus, fallopian tubes, and ovary. Previously FN1 heterozygous disease-causing variants were shown to be associated with glomerulopathy with fibronectin deposits (GFND2; MIM#601894) (62), and the corner fracture type of spondylometaphyseal dysplasia (SMDCF; MIM#184255) (63). Interestingly, in SMDCF reported FN1 mutations affect disulfide bond of Fibrin- and heparin-binding 1 region of FN1 and GFND2 related FN1 variants affect Fibronectin type-III domains 4 and 15.
Figure 1.
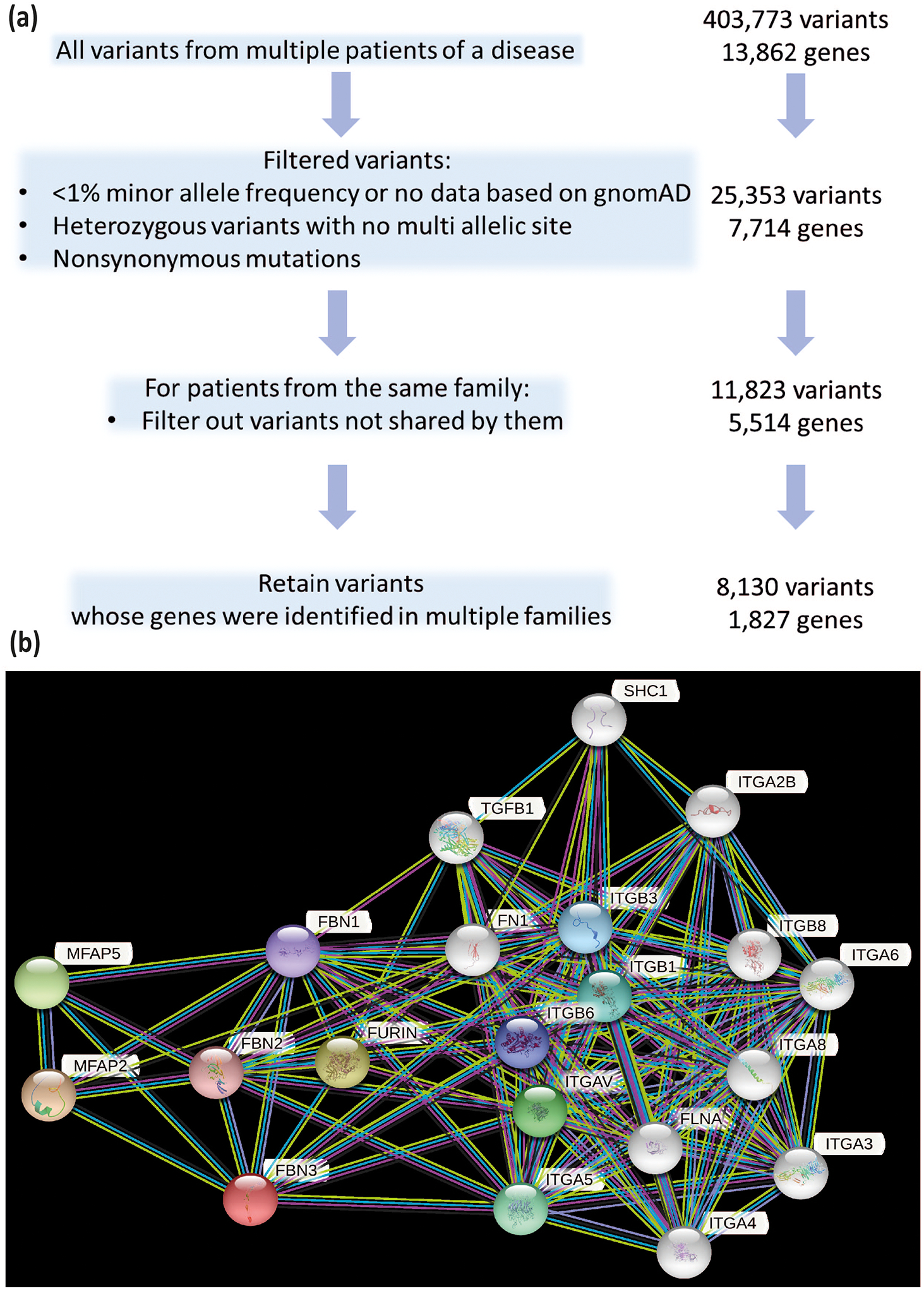
A. Prioritization strategy of identified variants. B. FBN3 gene’ first and second neighbors were shown by STRING database.
Table 2.
PCOS-related genes in the study cohort obtained from OMIM and/or PCOSKB databases
| Gene Symbol | Gene Name |
|---|---|
| ABCA1 | ATP binding cassette subfamily A member 1 |
| ACE | Angiotensin I converting enzyme |
| ADIPOQ | Adiponectin, C1Q and collagen domain containing |
| ALDH1A3 | Aldehyde dehydrogenase 1 family member A3 |
| ANGPTL1 | Angiopoietin like 1 |
| APC | APC regulator of WNT signaling pathway |
| APOB | Apolipoprotein B |
| AR | Androgen receptor |
| ATF4 | Activating transcription factor 4 |
| CAPN10 | Calpain 10 |
| CD14 | CD14 molecule |
| CPZ | Carboxypeptidase Z |
| CR1 | Complement C3b/C4b receptor 1 (Knops blood group) |
| CYP11B2 | Cytochrome P450 family 11 subfamily B member 2 |
| DENND1A | DENN domain containing 1A |
| ESR1 | Estrogen receptor 1 |
| F5 | Coagulation factor V |
| FASN | Fatty acid synthase |
| FBN3 | Fibrillin 3 |
| FGA | Fibrinogen alpha chain |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit |
| HLA-DRB1 | Major histocompatibility complex, class II, DR beta 1 |
| HSD3B2 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 |
| IGF2R | Insulin like growth factor 2 receptor |
| INSR | Insulin receptor |
| LIPE | Lipase E, hormone sensitive type |
| LPA | Lipoprotein(a) |
| MAP3K4 | Mitogen-activated protein kinase kinase kinase 4 |
| NFKB1 | Nuclear factor kappa B subunit 1 |
| NID2 | Nidogen 2 |
| NPPB | Natriuretic peptide B |
| PEPD | Peptidase D |
| PIK3CG | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma |
| PKD1 | Polycystin 1, transient receptor potential channel interacting |
| PLCB3 | Phospholipase C beta 3 |
| PRDX2 | Peroxiredoxin 2 |
| RPS6KA1 | Ribosomal protein S6 kinase A1 |
| SLC2A4 | Solute carrier family 2 member 4 |
| SRA1 | Steroid receptor RNA activator 1 |
| TH | Tyrosine hydroxylase |
| THADA | THADA armadillo repeat containing |
| TLR2 | Toll like receptor 2 |
| VEGFB | Vascular endothelial growth factor B |
FBN3 and FN1 variants detail were given in Supplementary Tables 3 and 5. Although we were not able to check segregation of these variants due to lack of their consents, identified FBN3 and FN1 variants were rare, conserved and predicted pathogenic in almost all prediction tools. We then compared three individuals who have FBN3 or FN1 variant(s) according to their clinical and laboratory findings (Supplementary Table 6). Although classic presentation of PCOS was observed in patients with FBN3 variants, increased level SHBG was detected in the patient with FN1 variant. (Supplementary Table 6).
Later, chemogenomics analyses of drug targets for the possible treatment of FBN3 and FN1 linked PCOS resulted with 3 known interactions (drugs) with FN1 gene including Zn (42) (Supplementary Table 7). Indeed, zinc and its role in female reproductive system has recently been studied (64). A meta-analysis investigating PCOS and Zinc relationship showed lower zinc levels in patients with PCOS than healthy controls and this association should be further investigated (65). Further, PCOS related diseases such as type 2 diabetes (T2DM), and cardiovascular disease (CVD) prevalence increased in patients with zinc deficiency (66). Lastly, we performed enrichment analysis to of 1,827 variant-carrying genes that were identified in multiple families and mostly extracellular matrix related molecules and pathways were emerged. Since extracellular matrix provides infrastructure for specific ovarian cells, our findings highlight detected variants importance (Supplementary Table 8).
Discussion
We identified two heterozygous rare FBN3 missense variants in three patients, and one FN1 missense variant in one patient from three different PCOS families. FBN3 encodes an extracellular matrix (ECM) protein (67) and the variants are in TB 6 and EGF-like 25 calcium-binding domains. Previous linkage and immunohistochemical analyses strongly suggest a role for FBN3 in the pathogenesis of PCOS (68–75). The FBN3 expression was found in perifollicular stroma of follicles (71, 72, 76, 77), and several changes were reported in the ovarian ECM in PCOS patients including thickening of the tunica albuginea, ovarian stromal hyperplasia, stromal cell luteinization, and large cystic antral follicles (78–80).
Fibronectins (FNs) are multi-domain glycoproteins which allow cells to interact with other ECM proteins (81), and play important roles during follicle development (82). Ambekar et. al. (2015) found downregulated levels of Fibronectin by comparing the follicular fluid protein repertoire of PCOS with healthy women (83). Similarly, Hassani et al. (2019) found that downregulation of FN1 levels in the cumulus cells seemed to be related to PCOS (84).
FN1 heterozygous mutations are responsible for glomerulopathy with fibronectin deposits (GFND2; MIM#601894) (62) and the corner fracture type of spondylometaphyseal dysplasia (SMDCF; MIM#184255) (63). It has been noted that GFND2-associated mutations tend to cluster in more C-terminally located regions, whereas the SMDCF-associated mutations are more N-terminally located. We identified a heterozygous, rare, missense variant predicted to be deleterious and located in collagen binding region of Fibronectin type-I 9 domain. Interestingly, the patient with FN1 variant has increased level of SHBG (Supplementary Table 5). Of note, since PCOS patients are expected to have low serum SHBG levels, and we thought this finding might be a coincidental finding.
In spite of researchers’ best endeavors, the etiology (or etiologies) of PCOS remain unknown (85). Efforts have been made to which genes are involved the PCOS pathogenesis via candidate gene approaches (86–89), genome-wide association studies (GWAS) from different populations with replication studies (1, 90–100) and more recently next generation sequencing (NGS)(87, 101). These studies are clearly bringing important novel information with limitations due to background genetic heterogeneity (102), phenotype heterogeneity of the PCOS, limited power and replication, along with limited understanding of disease pathophysiology to guide more informed candidate gene/targeted approaches (89, 103–105).
A wide array of genes have previously been associated with PCOS including those genes related to the biosynthesis and the action of androgens, metabolism and inflammatory cytokines (38, 106), however, it is yet to be elucidated how these genes/variants contribute to PCOS phenotype, and further exploration is warranted (38).
The small number of participants is the main limitation of the study. Technical limitations of whole exome sequencing should be also taken into considerations.
We prioritizated and focused on variants in genes implicated in PCOS pathogenesis including androgen, insulin and lipid metabolism, folliculogenesis, oxidative stress and inflammation and hemostasis. We found three novel FBN3 and FN1 variants and their role yet remain to be investigated. Further functional studies may identify causality of these newly discovered PCOS related variants and may improve our understanding of the biologic pathways affected and identify new drug targets.
The list of PCOS-related candidate genes is long and still open to new entries. Remaining cases without previously implicated presumptive candidate genes must also be investigated for genomic structural variations and epigenetic factors.
Supplementary Material
Supplementary Table 1. Study cohort consisting of families including one to four affected individuals.
Supplementary Table 2. WES Quality Metrics of 31 probands from 20 unrelated families.
Supplementary Table 3. Candidate list of variants of unknow significance were identified in the study population by whole exome sequencing in FBN3 and FN1 genes.
Supplementary Table 4. First and second neighbours of FBN3 according to STRING database.
Supplementary Table 5. Bioinformatic results for FBN3 and FN1 amino acid substitutions.
Supplementary Table 6. Clinical and laboratory features of patients with variants of unknow significance in FBN3 and FN1 genes.
Supplementary Table 7. Fibronectin related 3 known interactions (drugs) according to QuartataWeb Server and DrugBank Database.
Supplementary Table 8. Enrichment analysis of 1827 variant-carrying genes that were identified in multiple families
Supplementary Figure 1. Main steps of in-house bioinformatics pipeline.
Acknowledgements
We would like to thank Yusuf Ziya Varli for his assistance during statistical evaluation of the patients’ laboratory findings. This work was supported by the Yale Center for Mendelian Genomics. The Yale Center for Mendelian Genomics (UM1HG006504) is funded by the National Human Genome Research Institute.
Footnotes
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–4. [DOI] [PubMed] [Google Scholar]
- 3.Cooper HE, Spellacy WN, Prem KA, Cohen WD. Hereditary factors in the Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968;100(3):371–87. [DOI] [PubMed] [Google Scholar]
- 4.Wilroy RS Jr., Givens JR, Wiser WL, Coleman SA, Andersen RN, Summitt RL. Hyperthecosis: an inheritable form of polycystic ovarian disease. Birth Defects Orig Artic Ser. 1975;11(4):81–5. [PubMed] [Google Scholar]
- 5.Ferriman D, Purdie AW. The inheritance of polycystic ovarian disease and a possible relationship to premature balding. Clin Endocrinol (Oxf). 1979;11(3):291–300. [DOI] [PubMed] [Google Scholar]
- 6.Hague WM, Adams J, Reeders ST, Peto TE, Jacobs HS. Familial polycystic ovaries: a genetic disease? Clin Endocrinol (Oxf). 1988;29(6):593–605. [DOI] [PubMed] [Google Scholar]
- 7.Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol (Oxf). 1993;38(6):653–8. [DOI] [PubMed] [Google Scholar]
- 8.Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28(1):23–30. [DOI] [PubMed] [Google Scholar]
- 9.Norman RJ, Masters S, Hague W. Hyperinsulinemia is common in family members of women with polycystic ovary syndrome. Fertil Steril. 1996;66(6):942–7. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95(25):14956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84(1):38–43. [DOI] [PubMed] [Google Scholar]
- 12.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75(1):53–8. [DOI] [PubMed] [Google Scholar]
- 13.Kashar-Miller M, Azziz R. Heritability and the risk of developing androgen excess. J Steroid Biochem Mol Biol. 1999;69(1–6):261–8. [DOI] [PubMed] [Google Scholar]
- 14.Franks S, Webber LJ, Goh M, Valentine A, White DM, Conway GS, et al. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93(9):3396–402. [DOI] [PubMed] [Google Scholar]
- 15.Kahsar-Miller M, Azziz R. The development of the polycystic ovary syndrome: family history as a risk factor. Trends Endocrinol Metab. 1998;9(2):55–8. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DN, Clayton JF. DNA polymorphism and the study of disease associations. Hum Genet. 1988;78(4):299–312. [DOI] [PubMed] [Google Scholar]
- 17.Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 18.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 19.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism: clinical and experimental. 2003;52(7):908–15. [DOI] [PubMed] [Google Scholar]
- 21.Obesity Hoeger K. and weight loss in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28(1):85–97, vi–vii. [DOI] [PubMed] [Google Scholar]
- 22.Cotrozzi G, Matteini M, Relli P, Lazzari T. Hyperinsulinism and insulin resistance in polycystic ovarian syndrome: a verification using oral glucose, I.V. Glucose and tolbutamide. Acta Diabetol Lat. 1983;20(2):135–42. [DOI] [PubMed] [Google Scholar]
- 23.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–9. [DOI] [PubMed] [Google Scholar]
- 24.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20(11):2414–21. [DOI] [PubMed] [Google Scholar]
- 25.Azziz R PCOS in 2015: New insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol. 2016;12(2):74–5. [DOI] [PubMed] [Google Scholar]
- 26.Corbett S, Morin-Papunen L. The Polycystic Ovary Syndrome and recent human evolution. Mol Cell Endocrinol. 2013;373(1–2):39–50. [DOI] [PubMed] [Google Scholar]
- 27.Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2008;23(9):2134–9. [DOI] [PubMed] [Google Scholar]
- 28.Hirschberg AL. Polycystic ovary syndrome, obesity and reproductive implications. Womens Health (Lond). 2009;5(5):529–40; quiz 41–2. [DOI] [PubMed] [Google Scholar]
- 29.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–61. [DOI] [PubMed] [Google Scholar]
- 30.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87(5):2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN, Group PCTS. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):66–71. [DOI] [PubMed] [Google Scholar]
- 32.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. [DOI] [PubMed] [Google Scholar]
- 33.Kulshreshtha B, Singh S, Arora A. Family background of Diabetes Mellitus, obesity and hypertension affects the phenotype and first symptom of patients with PCOS. Gynecol Endocrinol. 2013;29(12):1040–4. [DOI] [PubMed] [Google Scholar]
- 34.Fauser BC, Bouchard P. Uncertainty remains in women with PCOS regarding the increased incidence of cardiovascular disease later in life, despite the indisputable presence of multiple cardiovascular risk factors at a young age. J Clin Endocrinol Metab. 2011;96(12):3675–7. [DOI] [PubMed] [Google Scholar]
- 35.de Melo AS, Dias SV, Cavalli Rde C, Cardoso VC, Bettiol H, Barbieri MA, et al. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150(1):R11–24. [DOI] [PubMed] [Google Scholar]
- 36.Dapas M, Dunaif A. The contribution of rare genetic variants to the pathogenesis of polycystic ovary syndrome. Curr Opin Endocr Metab Res. 2020;12:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra-Gorur K, Caglayan AO, Schaffer AE, Chabu C, Henegariu O, Vonhoff F, et al. Mutations in KATNB1 cause complex cerebral malformations by disrupting asymmetrically dividing neural progenitors. Neuron. 2014;84(6):1226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Lv Y, Li L, Chen ZJ. Genetic Studies on Polycystic Ovary Syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:56–65. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph S, Barai RS, Bhujbalrao R, Idicula-Thomas S. PCOSKB: A KnowledgeBase on genes, diseases, ontology terms and biochemical pathways associated with PolyCystic Ovary Syndrome. Nucleic Acids Res. 2016;44(D1):D1032–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Pei F, Taylor DL, Bahar I. QuartataWeb: Integrated Chemical-Protein-Pathway Mapping for Polypharmacology and Chemogenomics. Bioinformatics. 2020;36(12):3935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Shi L, Zhang K, Zhang Y, Hu S, Zhao T, et al. VarCards: an integrated genetic and clinical database for coding variants in the human genome. Nucleic Acids Res. 2018;46(D1):D1039–D48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laskowski RA, Stephenson JD, Sillitoe I, Orengo CA, Thornton JM. VarSite: Disease variants and protein structure. Protein Sci. 2020;29(1):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–2. [DOI] [PubMed] [Google Scholar]
- 49.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81. [DOI] [PubMed] [Google Scholar]
- 51.Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying Mendelian disease genes with the variant effect scoring tool. BMC Genomics. 2013;14 Suppl 3:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15(8):1034–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20(1):110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31(5):761–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ionita-Laza I, McCallum K, Xu B, Buxbaum JD. A spectral approach integrating functional genomic annotations for coding and noncoding variants. Nat Genet. 2016;48(2):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shihab HA, Gough J, Mort M, Cooper DN, Day IN, Gaunt TR. Ranking non-synonymous single nucleotide polymorphisms based on disease concepts. Hum Genomics. 2014;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am J Hum Genet. 2016;99(4):877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015;24(8):2125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35(11):1978–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castelletti F, Donadelli R, Banterla F, Hildebrandt F, Zipfel PF, Bresin E, et al. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci U S A. 2008;105(7):2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CS, Fu H, Baratang N, Rousseau J, Kumra H, Sutton VR, et al. Mutations in Fibronectin Cause a Subtype of Spondylometaphyseal Dysplasia with “Corner Fractures”. Am J Hum Genet. 2017;101(5):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasiadek M, Stragierowicz J, Klimczak M, Kilanowicz A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients. 2020;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abedini M, Ghaedi E, Hadi A, Mohammadi H, Amani R. Zinc status and polycystic ovarian syndrome: A systematic review and meta-analysis. J Trace Elem Med Biol. 2019;52:216–21. [DOI] [PubMed] [Google Scholar]
- 66.Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care. 2007;30(3):523–8. [DOI] [PubMed] [Google Scholar]
- 67.Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss JF 3rd, et al. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90(12):6623–9. [DOI] [PubMed] [Google Scholar]
- 69.Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, et al. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91(10):4112–7. [DOI] [PubMed] [Google Scholar]
- 70.Raja-Khan N, Kunselman AR, Demers LM, Ewens KG, Spielman RS, Legro RS. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94(7):2916–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58(10):903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, Zhao ZZ, Painter JN, Hickey TE, et al. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod. 2009;15(12):829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96(15):8573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92(11):4191–8. [DOI] [PubMed] [Google Scholar]
- 75.Tucci S, Futterweit W, Concepcion ES, Greenberg DA, Villanueva R, Davies TF, et al. Evidence for association of polycystic ovary syndrome in caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab. 2001;86(1):446–9. [DOI] [PubMed] [Google Scholar]
- 76.Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83(3):461–72. [DOI] [PubMed] [Google Scholar]
- 77.Hatzirodos N, Bayne RA, Irving-Rodgers HF, Hummitzsch K, Sabatier L, Lee S, et al. Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25(7):2256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oksjoki S, Soderstrom M, Inki P, Vuorio E, Anttila L. Molecular profiling of polycystic ovaries for markers of cell invasion and matrix turnover. Fertil Steril. 2005;83(4):937–44. [DOI] [PubMed] [Google Scholar]
- 79.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59–77. [DOI] [PubMed] [Google Scholar]
- 80.Kinnear HM, Tomaszewski CE, Chang FL, Moravek MB, Xu M, Padmanabhan V, et al. The ovarian stroma as a new frontier. Reproduction. 2020;160(3):R25–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kornblihtt AR, Gutman A. Molecular biology of the extracellular matrix proteins. Biol Rev Camb Philos Soc. 1988;63(4):465–507. [DOI] [PubMed] [Google Scholar]
- 82.Monniaux D, Huet-Calderwood C, Le Bellego F, Fabre S, Monget P, Calderwood DA. Integrins in the ovary. Semin Reprod Med. 2006;24(4):251–61. [DOI] [PubMed] [Google Scholar]
- 83.Ambekar AS, Kelkar DS, Pinto SM, Sharma R, Hinduja I, Zaveri K, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100(2):744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hassani F, Oryan S, Eftekhari-Yazdi P, Bazrgar M, Moini A, Nasiri N, et al. Downregulation of Extracellular Matrix and Cell Adhesion Molecules in Cumulus Cells of Infertile Polycystic Ovary Syndrome Women with and without Insulin Resistance. Cell J. 2019;21(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunaif A Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity. J Clin Endocrinol Metab. 2016;101(3):759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urbanek M The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(2):103–11. [DOI] [PubMed] [Google Scholar]
- 87.Gorsic LK, Kosova G, Werstein B, Sisk R, Legro RS, Hayes MG, et al. Pathogenic Anti-Mullerian Hormone Variants in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102(8):2862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional Genetic Variation in the Anti-Mullerian Hormone Pathway in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104(7):2855–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mykhalchenko K, Lizneva D, Trofimova T, Walker W, Suturina L, Diamond MP, et al. Genetics of polycystic ovary syndrome. Expert Rev Mol Diagn. 2017;17(7):723–33. [DOI] [PubMed] [Google Scholar]
- 90.Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–9. [DOI] [PubMed] [Google Scholar]
- 91.Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49(2):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–5. [DOI] [PubMed] [Google Scholar]
- 93.Lerchbaum E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21, and 9q33.3 in a cohort of Caucasian women. Horm Metab Res. 2011;43(11):743–7. [DOI] [PubMed] [Google Scholar]
- 94.Louwers YV, Stolk L, Uitterlinden AG, Laven JS. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(12):E2006–12. [DOI] [PubMed] [Google Scholar]
- 95.Lee H, Oh JY, Sung YA, Chung H, Kim HL, Kim GS, et al. Genome-wide association study identified new susceptibility loci for polycystic ovary syndrome. Hum Reprod. 2015;30(3):723–31. [DOI] [PubMed] [Google Scholar]
- 96.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones MR, Brower MA, Xu N, Cui J, Mengesha E, Chen YD, et al. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS Genet. 2015;11(8):e1005455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Correction: Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2019;15(12):e1008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strauss JF 3rd, McAllister JM, Urbanek M. Persistence pays off for PCOS gene prospectors. J Clin Endocrinol Metab. 2012;97(7):2286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Casarini L, Brigante G. The polycystic ovary syndrome evolutionary paradox: a genome-wide association studies-based, in silico, evolutionary explanation. J Clin Endocrinol Metab. 2014;99(11):E2412–20. [DOI] [PubMed] [Google Scholar]
- 103.Barber TM, Franks S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013;40:28–39. [DOI] [PubMed] [Google Scholar]
- 104.Goodarzi MO. Looking for polycystic ovary syndrome genes: rational and best strategy. Semin Reprod Med. 2008;26(1):5–13. [DOI] [PubMed] [Google Scholar]
- 105.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Escobar-Morreale HF, Luque-Ramirez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26(2):251–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Study cohort consisting of families including one to four affected individuals.
Supplementary Table 2. WES Quality Metrics of 31 probands from 20 unrelated families.
Supplementary Table 3. Candidate list of variants of unknow significance were identified in the study population by whole exome sequencing in FBN3 and FN1 genes.
Supplementary Table 4. First and second neighbours of FBN3 according to STRING database.
Supplementary Table 5. Bioinformatic results for FBN3 and FN1 amino acid substitutions.
Supplementary Table 6. Clinical and laboratory features of patients with variants of unknow significance in FBN3 and FN1 genes.
Supplementary Table 7. Fibronectin related 3 known interactions (drugs) according to QuartataWeb Server and DrugBank Database.
Supplementary Table 8. Enrichment analysis of 1827 variant-carrying genes that were identified in multiple families
Supplementary Figure 1. Main steps of in-house bioinformatics pipeline.


