Abstract
Suppressing HIV viral loads to undetectable levels is essential for ending the HIV/AIDS epidemic. We evaluated randomized controlled trials aimed to increase antiretroviral medication adherence and promote undetectable viral loads among people living with HIV through November 22, 2019. We extracted data from 51 eligible interventions and analyzed the results using random effects models to compare intervention effects between groups within each intervention and across interventions. We also evaluated the relation between publication date and treatment effects. Only five interventions increased undetectable viral loads significantly. As a whole, the analyzed interventions were superior to Standard of Care in promoting undetectable viral loads. Interventions published more recently were not more effective in promoting undetectable viral loads. No treatment category consistently produced significant increases in undetectable viral loads. To end the HIV/AIDS epidemic, we should use interventions that can suppress HIV viral loads to undetectable levels.
Keywords: HIV viral load, Undetectable, Adherence intervention, HIV, AIDS
Introduction
Daily adherence to antiretroviral therapy (ART) can suppress the concentration of HIV-1 RNA in an individual’s blood—commonly referred to as “viral load”—to the extent that it is “undetectable” and not transmittable to others through unsafe sex [1]. For people living with HIV, viral suppression is key in living a healthy life and preventing harms associated with HIV and AIDS [2]. On a larger scale, viral suppression is key to reducing HIV transmission and ending the HIV/AIDS epidemic [3]. In 2015, the Joint United Nations Programme on HIV/AIDS (UNAIDS) reported that the HIV/AIDS epidemic could be eradicated by the year 2030 if 73% of all people living with HIV achieve and maintain an undetectable HIV viral load [4]; however, recent estimates suggest that the number of people in the United States and dependent areas who maintain an undetectable HIV viral load is well below this goal (64%) [5]. Because the achievement and maintenance of an undetectable HIV viral load is key to promoting the health of people living with HIV and to eradicating the HIV/AIDS epidemic, the suppression of HIV to undetectable levels should be a primary focus to improve public health.
A review published in 2017 evaluated all studies of interventions designed to promote ART adherence [6]. The review included 85 studies. Of these studies, only 47 reported a measure of viral suppression. The review did not analyze effects of the interventions on suppressing HIV viral loads to undetectable levels. To determine the extent to which available antiretroviral medication adherence interventions promote viral suppression below detectable thresholds, we conducted a systematic review and meta-analysis that updated the 2017 review [6] and included only studies that reported a measure of viral suppression. We included all 47 studies identified in the prior review that reported viral suppression. Using the same search criteria employed in the previous review, we added all relevant studies published through November 22, 2019. Our analyses sought to determine which interventions significantly increased undetectable HIV viral loads and whether interventions in general increased undetectable HIV viral loads. Because of the relatively recent recognition that undetectable viral loads can eliminate some transmission of HIV, we also conducted an analysis to determine if the effectiveness of interventions to promote undetectable viral loads increased over time.
Method
We used the research strategy identified in the prior systematic review and meta-analysis to create an updated search to identify studies for the present review and meta-analysis [6]. First, we included the 47 studies identified by the prior review that aimed to promote ART adherence and contained a measure of HIV viral suppression [6]. Then we used the PRISMA extension [7] to search the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE for reports published through November 22, 2019 with the same search terms as the prior review [6]. To be eligible for the present review, each study must have included a randomized controlled trial, a Standard of Care (SOC) or enhanced Standard of Care (eSOC) control group, an intervention to enhance ART adherence, and reported the following information regarding viral suppression: The number of participants assigned to the intervention and control groups, the number of participant blood samples that were collected from each group at a measurement following the start of an intervention, and the number of collected samples from each group with viral load suppressed below a specific threshold (e.g., “undetectable” = < 200 copies/mL).
Two investigators (FT and AR, MN, or KS) independently extracted data from each study that passed the initial screening stage. Extracted data included: the first author, study year, type of control group, type of treatment group, country, criterion for undetectable HIV viral load, number of participants randomized into each group, timepoint of the primary measurement, number of blood samples collected from each group at the primary measurement, and the number of collected samples from each group at the primary measurement with an undetectable viral load. The two investigators who extracted data for the study also assessed risk-of-bias using the Cochrane Collaboration Tool [8]. Any discrepancies between the data extracted by the two extractors during data extraction or risk-of-bias assessments were resolved by consensus between the extractors through discussion and examination of the study in question.
To facilitate interpretations of the comparisons in the present study, we adopted the same strategy used previously [6] to create ten categories of treatment conditions (See Supplementary Materials A for detailed descriptions): Standard of Care (SOC), enhanced Standard of Care (eSOC), Telephone (TELE), Short Message Service (SMS), Behavioral Skills Training or Medication Adherence Training (BST/MAT), Multimedia (MULTI), Cognitive Behavioral Therapy (CBT), Supporter (SUPP), Incentives (INCENT), and Device Reminders (DEV).
Dependent Measures
The primary outcome was based on the percentage of blood samples submitted by participants in the control (usually SOC) and treatment groups that contained an undetectable viral load (as judged by criteria set within each study) at the primary measurement. The primary measurement was the assessment of viral load in blood samples that followed the start of an intervention and coincided most closely with the end of the intervention. If there was at least one assessment of viral load in blood samples during the intervention, then the assessment that occurred closest to the end of the intervention was treated as the primary measurement. If no assessments occurred during the intervention, then the first assessment following completion of the intervention was treated as the primary measurement.
Data Analysis
The data extracted from the studies included the number of participants assigned to each group, the number in each group who provided a blood sample that was assessed at the primary timepoint, and the number of those assessed blood samples from each group that had an undetectable HIV viral load as judged by the criteria identified in each study. We calculated the percentage of samples with an undetectable viral load using two methods of treating missing samples: with imputation and without imputation. To conduct these analyses, each study must have reported the number of participants assigned to each group, the number of participants from each group who provided blood samples that were assessed at a timepoint following the start of an intervention, and the number of those assessed blood samples that contained an undetectable viral load. In the analysis with imputation, the missing-detectable analysis, missing samples were treated as though they contained a detectable viral load. In the analysis without imputation, the missing-missing analysis, missing samples were ignored and the percentage of participants with an undetectable viral load for each group was based on the number of participants assessed in each group.
After extracting data from the studies included in this review, it became clear that the procedures included within the ten intervention-type categories varied widely [6]. For example, within the INCENT category, some studies arranged incentives to be delivered at regular intervals if viral suppression criteria were met [9], whereas other studies delivered incentives irregularly if participants engaged in aspects of the treatment that were not tied directly to viral suppression, such as attending meetings with a peer supporter [10]. Procedural variability of this kind obscures individual treatment effects in an analysis that aggregates effects of all studies within a treatment category. Therefore, it was determined that comparisons of treatment effects should occur at the level of the individual intervention used in each study rather than across studies that would fall into the same treatment category. The present analysis does not include a statistical comparison of treatment effects across treatment categories.
Statistical Analysis
A logistic regression model was used to examine treatment effects of interventions within each intervention and across interventions. This analysis yielded results that indicate whether treatments used in each study produced significant differences compared to the control group. The magnitude of effect was expressed using risk ratios (RR) with 95% confidence intervals (95%CI), and p-values in relation to an α of 0.05. Stata Statistical Software: Release 15 (College Station, TX; StataCorp LLC) and R software 4.0.0 were used to perform these analyses.
We hypothesized that, because recent findings indicate that undetectable levels of HIV viral load are not transmittable to others even through unsafe sex (undetectable = untransmittable; U = U) and highlight the importance of promoting undetectable HIV viral loads [1, 3, 11], studies conducted more recently may be more likely to measure HIV viral suppression and may be more effective in promoting undetectable viral loads. Therefore, in addition to the statistical analysis to assess the effects of treatment in each study, we used a mixed-effects meta-regression model to analyze whether the effectiveness of studies at promoting undetectable HIV viral load changed as a function of publication year. We also evaluated publication bias using Egger’s regression test checking for funnel plot asymmetry.
Results
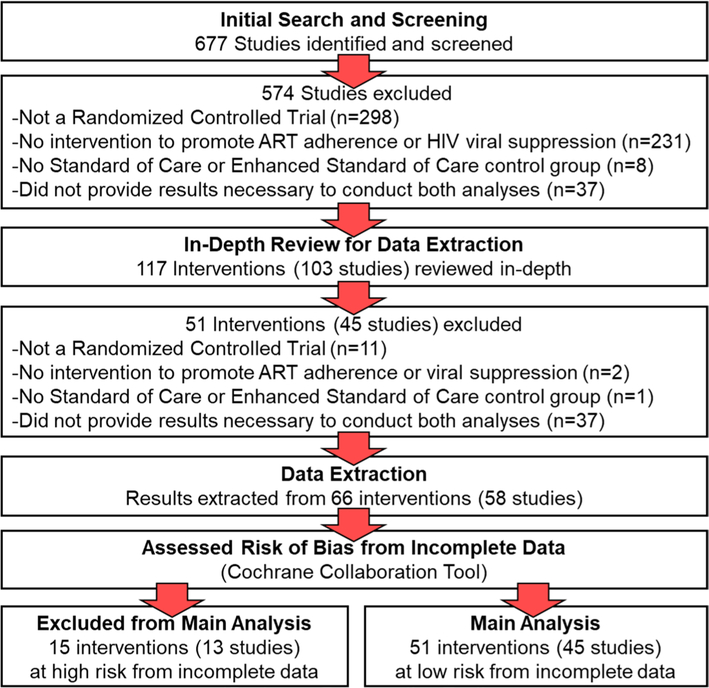
Figure 1 shows a flowchart of the studies and interventions included in the main analysis. Of the 677 studies identified in our search, 574 were excluded in the screening process because they did not meet criteria for inclusion. The studies were excluded because they did not include a randomized controlled trial (n = 298), they did not include an intervention to promote ART adherence or viral suppression (n = 231), they did not include a SOC or eSOC control group (n = 8), or they did not include the information required for the missing-detectable and missing-missing analyses (n = 37). The remaining 117 interventions were from 103 studies and were reviewed in-depth for data extraction.
Fig. 1.
Study selection
Following in-depth review of the remaining articles, 51 interventions were excluded because they did not include a randomized controlled trial (n = 11), they did not include an intervention to promote ART adherence or viral suppression (n = 2), they did not include a SOC or eSOC control group (n = 1), or they did not include the information required for the missing-detectable and missing-missing analyses (n = 37; see Supplementary Materials B for more information). Information from 66 interventions were from 58 studies [9, 10, 12–67] and were extracted and assessed using the Cochrane Collaboration Tool (results reported in Supplementary Materials C and D). Most interventions had a high risk of bias from blinding—which resulted naturally from the additional supports provided to participants in the intervention groups—and a low risk of bias from sequence generation, allocation concealment, selective reporting, and other sources of bias. Some interventions (n = 15) assessed less than 70% of the enrolled participants in the primary measurement, which put them at high risk of bias from incomplete data [55–67]. These 15 interventions were from 13 studies and were excluded from the main analysis because of this risk. The remaining 51 interventions were from 45 studies [9, 10, 12–54] and were included in the main analysis.
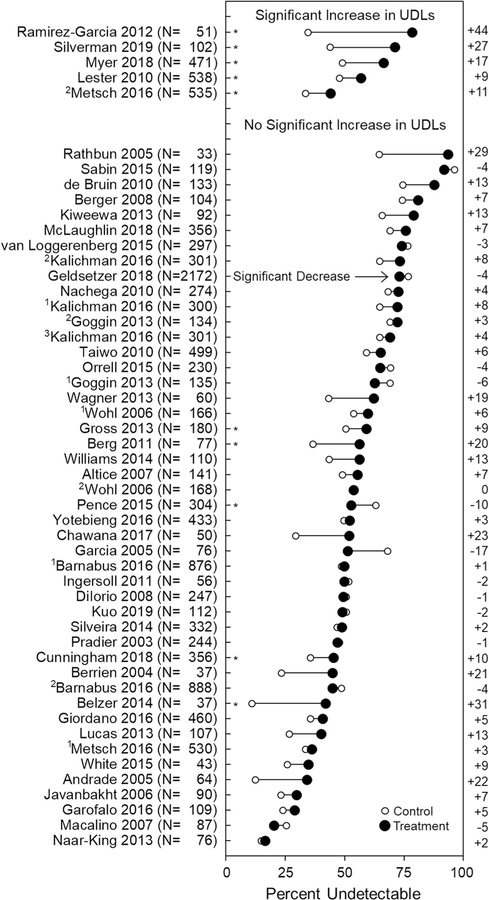
Table 1 and Fig. 2 show the effectiveness of analyzed interventions in suppressing viral load to the undetectable threshold identified in each study in the main analysis, as judged by logistic regression using the missing-detectable analysis (see Supplementary Materials E and F for results for interventions included in the main analysis as judged by the missing-missing analysis and results from both analyses for interventions excluded due to high risk of bias from incomplete data). Overall, the results presented in Table 1 and Fig. 2 show that most studies were not effective in promoting undetectable viral loads. According to logistic regression, only five interventions (10%) [9, 10, 34, 38, 44] significantly increased undetectable viral loads (p < 0.05) as judged by the missing-detectable analysis. These five interventions were also judged significant at the p < 0.05 level by the missing-missing analysis, lending some support to the effects of these treatments. These five interventions included eSOC [44], INCENT [9], SMS [34], SUPP [38], and a combination of INCENT and SUPP [10]. See Supplementary Materials G for information about the number of interventions of each type evaluated in the present analysis and significant results of the interventions as judged by logistic regression using missing-detectable and missing-missing analyses.
Table 1.
Effects of interventions included in the main analysis (n = 51)
| First author year | Treatment type | Criterion copies/mL | % (n) Undetectable viral load |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | RR | 95% CI | ||||||
|
| |||||||||
| Altice 2007 | SUPP | < 400 | 49 | (26) | 56 | (49) | 1.14 | 0.81 | 1.58 |
| Andrade 2005 | DEV | < 50 | 13 | (4) | 34 | (11) | 2.75 | 0.98 | 7.74 |
| 1Barnabus 2016 | eSOC | < 50 | 49 | (214) | 50 | (219) | 1.03 | 0.90 | 1.18 |
| 2Barnabus 2016 | eSOC, SUPP | < 50 | 49 | (214) | 45 | (202) | 0.92 | 0.80 | 1.06 |
| Belzer 2014 | TELE | < 400 | 11 | (2) | 42 | (8) | 3.79 | 0.93 | 15.51 |
| Berg 2011 | SUPP | < 75 | 37 | (14) | 56 | (22) | 1.53 | 0.93 | 2.52 |
| Berger 2008 | BST/MAT | < 50 | 75 | (38) | 81 | (43) | 1.09 | 0.89 | 1.34 |
| Berrien 2004 | BST/MAT | < 2.6log | 24 | (4) | 45 | (9) | 1.91 | 0.71 | 5.12 |
| Chawana 2017 | SUPP | < 1000 | 30 | (8) | 52 | (12) | 1.76 | 0.87 | 3.55 |
| Cunningham 2018 | SUPP | < 75 | 36 | (63) | 46 | (82) | 1.27 | 0.99 | 1.64 |
| de Bruin 2010 | CBT | < 50 | 75 | (50) | 88 | (58) | 1.18 | 1.00 | 1.39 |
| Dilorio 2008 | CBT | < 0.4log | 51 | (62) | 50 | (62) | 0.98 | 0.76 | 1.25 |
| Garcia 2005 | CBT | < 400 | 68 | (28) | 51 | (18) | 0.75 | 0.51 | 1.11 |
| Garofalo 2016 | SMS | < 75 | 24 | (13) | 29 | (16) | 1.21 | 0.64 | 2.26 |
| Geldsetzer 2018 | SUPP | < 1000 | 77 | (777) | 73 | (852) | 0.95 | 0.91 | 1.00 |
| Giordano 2016 | SUPP | < 400 | 36 | (84) | 41 | (92) | 1.14 | 0.91 | 1.44 |
| 1Goggin 2013 | CBT | < 400 | 69 | (45) | 63 | (44) | 0.91 | 0.71 | 1.16 |
| 2Goggin 2013 | CBT, SUPP | < 400 | 69 | (45) | 72 | (50) | 1.05 | 0.84 | 1.30 |
| Gross 2013 | TELE | < 75 | 51 | (45) | 59 | (54) | 1.17 | 0.90 | 1.53 |
| Ingersoll 2011 | MULTI | < 49 | 52 | (14) | 50 | (13) | 0.96 | 0.57 | 1.64 |
| Javanbakht 2006 | INCENT | < 400 | 23 | (10) | 30 | (14) | 1.28 | 0.64 | 2.57 |
| 1Kalichman 2016 | CBT | < 100 | 65 | (98) | 72 | (108) | 1.12 | 0.96 | 1.30 |
| 2Kalichman 2016 | SMS | < 100 | 65 | (98) | 73 | (110) | 1.13 | 0.97 | 1.32 |
| 3Kalichman 2016 | CBT, SMS | < 100 | 65 | (98) | 69 | (104) | 1.07 | 0.91 | 1.25 |
| Kiweewa 2013 | SUPP | < 400 | 66 | (29) | 79 | (38) | 1.20 | 0.97 | 1.32 |
| Kuo 2019 | eSOC, SMS | < 200 | 51 | (28) | 49 | (28) | 0.96 | 0.67 | 1.40 |
| Lester 2010 | SMS | ≤ 400 | 48 | (127) | 57 | (155) | 1.18 | 1.01 | 1.39 |
| Lucas 2013 | SUPP | < 50 | 27 | (14) | 40 | (21) | 1.50 | 0.86 | 2.62 |
| Macalino 2007 | SUPP | < 50 | 26 | (11) | 20 | (9) | 0.80 | 0.37 | 1.73 |
| McLaughlin 2018 | SUPP | < 400 | 69 | (108) | 76 | (152) | 1.10 | 0.96 | 1.25 |
| 1Metsch 2016 | SUPP | < 200 | 34 | (89) | 36 | (97) | 1.08 | 0.86 | 1.36 |
| 2Metsch 2016 | SUPP, INCENT | < 200 | 34 | (89) | 44 | (120) | 1.31 | 1.06 | 1.63 |
| Myer 2018 | SUPP | < 50 | 49 | (117) | 67 | (155) | 1.35 | 1.16 | 1.58 |
| Naar-King 2013 | BST/MAT | NR | 15 | (6) | 17 | (6) | 1.11 | 0.39 | 3.14 |
| Nachega 2010 | SUPP | < 400 | 68 | (93) | 73 | (99) | 1.06 | 0.91 | 1.24 |
| Orrell 2015 | DEV, SMS | < 40 | 70 | (80) | 65 | (75) | 0.94 | 0.78 | 1.12 |
| Pence 2015 | SUPP | < 50 | 63 | (98) | 53 | (79) | 0.84 | 0.69 | 1.02 |
| Pradier 2003 | BST/MAT | < 40 | 48 | (58) | 47 | (58) | 0.98 | 0.76 | 1.28 |
| Ramirez-Garcia 2012 | eSOC | < 50 | 35 | (8) | 79 | (22) | 2.26 | 1.25 | 4.08 |
| Rathbun 2005 | BST/MAT | < 400 | 65 | (11) | 94 | (15) | 1.45 | 1.00 | 2.10 |
| Sabin 2015 | SMS | < 50 | 96 | (54) | 92 | (58) | 0.95 | 0.87 | 1.04 |
| Silveira 2014 | BST/MAT | < 50 | 47 | (78) | 49 | (81) | 1.04 | 0.83 | 1.30 |
| Silverman 2019 | INCENT | < 200 | 44 | (22) | 71 | (37) | 1.62 | 1.13 | 2.31 |
| Taiwo 2010 | SUPP | < 400 | 59 | (149) | 65 | (162) | 1.10 | 0.96 | 1.26 |
| van Loggerenberg 2015 | CBT | < 400 | 77 | (115) | 74 | (109) | 0.97 | 0.85 | 1.10 |
| Wagner 2013 | BST/MAT | < 50 | 43 | (13) | 63 | (15) | 1.44 | 0.86 | 2.41 |
| White 2015 | SUPP | ≤ 400 | 26 | (6) | 35 | (7) | 1.34 | 0.54 | 3.34 |
| Williams 2014 | SUPP | < 400 | 44 | (24) | 56 | (31) | 1.29 | 0.88 | 1.89 |
| 1Wohl 2006 | SUPP | < 400 | 54 | (45) | 60 | (50) | 1.11 | 0.85 | 1.45 |
| 2Wohl 2006 | SUPP | < 400 | 54 | (45) | 54 | (44) | 1.00 | 0.75 | 1.33 |
| Yotebieng 2016 | INCENT | < 40 | 50 | (108) | 52 | (113) | 1.05 | 0.87 | 1.26 |
For all interventions, the control condition was either Standard of Care (SOC) or Enhanced Standard of Care (eSOC). Treatment type is based on classifications listed in Supplementary Materials A. The analysis presented in this table is the missing-detectable analysis which imputed missing samples as containing a detectable viral load. Statistically significant increases in undetectable viral loads at the p < .05 level are shown in bold. Superscript numbers (1, 2, or 3) designate the first, second, or third intervention evaluated within a single study and correspond with the superscript numbers in Fig. 2
Fig. 2.
Effects of interventions in the main analysis (n = 51). The number of participants assigned to the compared study groups are shown next to the first author and year. Interventions are grouped by whether interventions produced increases in undetectable viral loads (UDLs) that were significant at the p < .05 level. Full statistical analyses are provided for each study in Table 1. Filled circles represent the percentage of blood samples collected at the primary measurement of the study that contained an undetectable HIV viral load, as defined in the study, for participants in the treatment group; unfilled circles show the percentage of samples with an undetectable viral load for the control group. In this analysis, missing samples were imputed as having a detectable viral load. Asterisks in the left portion of the figure show treatment effects that were judged significant in the analysis without imputation of missing samples. The difference in the percentage undetectable between study groups (Treatment–Control) for each intervention is shown to the right of the figure. Superscript numbers (1, 2, or 3) designate the first, second, or third intervention evaluated within a single study and correspond with the superscript numbers in Table 1
According to the logistic regression model analysis conducted to compare overall effects of treatment across interventions in the main analysis, participants in a treatment group were more likely to achieve an undetectable viral load than participants in a control group using the missing-detectable analysis (RR[95%CI]: 1.08[1.04, 1.13], Z = 3.745, p < 0.05) and the missing-missing analysis (RR[95%CI]: 1.07[1.03, 1.11], p < 0.05). However, it is important to note that this overall effect is influenced by results of individual interventions, of which few produced significant increases in the number of blood samples with an undetectable viral load. See Supplementary Materials H–S for Forest Plots and Funnel Plots that show results of the interventions, evaluations of publication bias, and evaluations of participant attrition for interventions in the main analysis and for all evaluated interventions.
The evaluation of the relation between study publication date and treatment effects was not significant (slope coefficient[SE]: - 0.0005[0.005], p = 0.920). This indicates that, to date, there is no relation between intervention publication date and treatment effects.
The interventions included in the main analysis differed based on the type of treatment that was compared against the Standard of Care control group, the criterion used to determine whether HIV viral load was undetectable, and the country in which the intervention took place (see Table 1 and Supplementary Materials G). The intervention type used most frequently as the sole intervention was supporter (SUPP; n = 19), but across interventions, each type of treatment was used, and some were used in combination. The criterion used to judge whether blood samples contained a detectable or undetectable HIV viral load also varied across studies, but the most frequent criterion used was 400 copies/mL (n = 19; see Supplementary Materials G). Finally, the interventions were conducted in 15 different countries, but most took place in the USA (n = 30).
Discussion
The suppression of HIV viral load below detectable thresholds is key to ending the HIV/AIDS epidemic, but few interventions published to date have effectively increased the percentage of participants who achieve undetectable viral loads. The present review includes the results of interventions published through November 22, 2019 and compares intervention effects with and without imputation of missing samples using a logistic regression model. Results of the statistical analysis revealed that five interventions were associated with significant increases in the percentage of blood samples submitted with an undetectable HIV viral load as judged by both methods of analysis. The 51 evaluated interventions were associated with an overall increase in percentage of blood samples with an undetectable viral load relative to the SOC or eSOC control groups. There was no association between the publication date and the effectiveness of the interventions, suggesting that, at present, studies published more recently were not more effective in suppressing HIV viral load.
A feature of the present systematic review and meta-analysis that distinguishes it from prior reviews is that the focus is solely on the outcome measure of ART medication adherence: Undetectable viral loads. As such, findings from our review may differ from existing reviews. For example, the Centers for Disease Control and Prevention’s (CDC) Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention included 23 studies that earned ratings as “Good” or “Best” evidence-based interventions for promoting medication adherence [68]. Interventions from six of the studies that received ratings as “Good” or “Best” evidence in the CDC’s compendium were included in the main analysis of the present review. Five studies were categorized as “Good” evidence [12, 20, 24, 28, 31] and one was categorized as “Best” evidence [9]. Although these studies received high ratings from the CDC for promoting medication adherence, only one intervention [9] produced a significant increase in undetectable viral loads as judged by both methods of analysis in the present review; three were judged significant by only the missing-missing analysis [9, 20, 28]. The poor correspondence between interventions that produced significant increases in undetectable viral load and ratings from the CDC on medication adherence might result from the different focuses of measuring increases in medication adherence versus the biological outcome of medication adherence (i.e., viral suppression). These results suggest that recommendations based on medication adherence alone may not lead to effective HIV viral suppression. In the future, the CDC might consider developing a method of evaluating interventions that is focused specifically on HIV viral suppression.
The present analysis has limitations. Comparisons of interventions are complicated by multiple factors, including the socio-demographic characteristics of the participants in the interventions, the context in which the intervention was implemented (e.g., as a procedure to promote achievement of an undetectable viral load vs maintenance of an undetectable viral load), the criteria used to determine whether HIV viral load was “undetectable,” and the specific procedures used to promote HIV viral suppression across interventions within the same category. The last two limitations warrant additional consideration and will be expanded upon below.
Across interventions, “undetectable” viral load was defined in various ways (see Supplementary Materials G for more information) and, across studies, participants had different histories of prior achievement of undetectable viral load. For example, participants in Cunningham (2018) had all achieved undetectable HIV viral loads (measured as < 75 copies/mL) while they were incarcerated. In this study, the aim of the intervention could be interpreted as the maintenance of undetectable HIV viral loads rather than the initial achievement of undetectable viral loads. This aim contrasts slightly with most of the reviewed interventions, which aimed to promote the initial achievement of undetectable viral loads among individuals with uncontrolled HIV viral loads. The present review was not aimed to address these complications. It simply reports treatment effects by comparing the number of samples that met the undetectable viral load criteria used in each study at the timepoint that aligned most closely with the end of each intervention. Because criteria for determining undetectable viral loads, participants, and participant history varied across studies (e.g., Barnabus 2016 used < 49 copies/mL whereas Chewana 2017 used < 1000), comparisons of treatment effects across interventions are complicated; However, the fact that the same measure (i.e., the percentage of participants with undetectable viral loads) and the same viral suppression criteria were applied to the control and treatment groups within each study ensures that the within-intervention comparisons are sound. Nevertheless, readers should be cautioned about making interpretations based on across-intervention comparisons in this review.
Another limitation is that there was wide procedural variability within the intervention-type categories. These variations affected our decision to conduct a meta-analysis similar to the one conducted in the prior review and meta-analysis [6]. A thorough description of these variations is outside of the scope of the present review, however future research may consider evaluating aspects that differed between interventions of the same type that did and did not produce significant increases in the percentage of blood samples that contained an undetectable viral load, as doing so could lead to improved interventions.
Conclusions
Only five of 51 evaluated interventions (10%) produced significant improvements in undetectable viral loads. Because no treatment category consistently produced significant increases in undetectable viral loads, we cannot identify any specific category of treatments as being effective. Individuals interested in effective treatments will need to examine procedures used in specific interventions. Efforts aimed at ending the HIV/AIDS epidemic should focus on interventions identified in this review that produced significant increases in undetectable viral loads.
Supplementary Material
Acknowledgements
The study was supported by the National Institute on Allergy and Infectious Diseases and the National Institute on Drug Abuse of the National Institutes of Health under Grants R01AI117065 and T32DA07209. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Funding The study was supported by the National Institute on Allergy and Infectious Diseases and the National Institute on Drug Abuse of the National Institutes of Health under Grants R01AI117065 and T32DA07209. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest We declare that there are no conflict of interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10461-021-03534-z.
Data Availability Additional data are reported in Supplementary Materials. Data from any portion of the study can be obtained by contacting the corresponding author.
Code Availability Not applicable.
Declarations
Ethical Approval Not applicable.
Informed Consent Not applicable.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affliations.
References
- 1.Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321(5):451–2. [DOI] [PubMed] [Google Scholar]
- 2.Montaner JS, Wood E, Kerr T, Lima V, Barrios R, Shannon K, et al. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55:S5–9. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. J Am Med Assoc. 2019;321(9):844–5. [DOI] [PubMed] [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS 2016–2021 strategy: on the fast-track to end AIDS. UNAIDS; Geneva. 2015. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and careobjectives by using HIV surveillance data—United States and 6 dependent areas, 2018. HIVSur-veillance Supplemental Report 2020;25(No. 2). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2020. Accessed 10 Feb 2021. [Google Scholar]
- 6.Kanters S, Park JJH, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–40. [DOI] [PubMed] [Google Scholar]
- 7.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman K, Holtyn AF, Rodewald AM, Siliciano RF, Jarvis BP, Subramaniam S, et al. Incentives for viral suppression in people living with HIV: a randomized clinical trial. AIDS Behav. 2019. 10.1007/s10461-019-02592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA. 2016;316(2):156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. An ambitious target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS. 2014.
- 12.Altice FL, Maru DS-R, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45(6):770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade AS, McGruder HF, Wu AW, Celano SA, Skolasky RL Jr, Selnes OA, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41(6):875–82. [DOI] [PubMed] [Google Scholar]
- 14.Barnabas RV, van Rooyen H, Tumwesigye E, Brantley J, Baeten JM, van Heerden A, et al. Uptake of antiretroviral therapy and male circumcision after community-based HIV testing and strategies for linkage to care versus standard clinic referral: a multisite, open-label, randomised controlled trial in South Africa and Uganda. Lancet HIV. 2016;3(5):e212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belzer ME, Naar-King S, Olson J, Sarr M, Thornton S, Kahana SY, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113(2–3):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger S, Schad T, von Wyl V, Ehlert U, Zellweger C, Furrer H, et al. Effects of cognitive behavioral stress management on HIV-1 RNA, CD4 cell counts and psychosocial parameters of HIV-infected persons. AIDS. 2008;22(6):767–75. [DOI] [PubMed] [Google Scholar]
- 18.Berrien VM, Salazar JC, Reynolds E, Mckay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18(6):355–63. [DOI] [PubMed] [Google Scholar]
- 19.Chawana TD, Gandhi M, Nathoo K, Ngara B, Louie A, Horng H, et al. Defining a cutoff for atazanavir in hair samples associated with virological failure among adolescents failing second-line antiretroviral treatment. J Acquir Immune Defic Syndr (1999). 2017;76(1):55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham WE, Weiss RE, Nakazono T, Malek MA, Shoptaw SJ, Ettner SL, et al. Effectiveness of a peer navigation intervention to sustain viral suppression among HIV-positive men and transgender women released from jail: the LINK LA randomized clinical trial. JAMA Intern Med. 2018;178(4):542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bruin M, Hospers HJ, van Breukelen GJ, Kok G, Koevoets WM, Prins JM. Electronic monitoring-based counseling to enhance adherence among HIV-infected patients: a randomized controlled trial. Health Psychol. 2010;29(4):421. [DOI] [PubMed] [Google Scholar]
- 22.DiIorio C, McCarty F, Resnicow K, McDonnell Holstad M, Soet J, Yeager K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia R, Pondé M, Lima M, de Souza AR, de Stolze SMO, Badaró R. Lack of effect of motivation on the adherence of HIV-positive/AIDS patients to antiretroviral treatment. Braz J Infect Dis. 2005;9(6):494–9. [DOI] [PubMed] [Google Scholar]
- 24.Garofalo R, Kuhns LM, Hotton A, Johnson A, Muldoon A, Rice D. A Randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20(5):1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geldsetzer P, Francis JM, Sando D, Asmus G, Lema IA, Mboggo E, et al. Community delivery of antiretroviral drugs: a non-inferiority cluster-randomized pragmatic trial in Dar es Salaam, Tanzania. PLoS Med. 2018;15(9):e1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giordano TP, Cully J, Amico KR, Davila JA, Kallen MA, Hartman C, et al. A randomized trial to test a peer mentor intervention to improve outcomes in persons hospitalized with HIV infection. Clin Infect Dis. 2016;63(5):678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goggin K, Gerkovich MM, Williams KB, Banderas JW, Catley D, Berkley-Patton J, et al. A randomized controlled trial examining the efficacy of motivational counseling with observed therapy for antiretroviral therapy adherence. AIDS Behav. 2013;17(6):1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross R, Bellamy SL, Chapman J, Han X, O’Duor J, Palmer SC, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173(4):300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011;116(1–3):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: results from a randomized trial. J Int Assoc Physicians AIDS Care. 2006;5(4):143–50. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman SC, Kalichman MO, Cherry C, Eaton LA, Cruess D, Schinazi RF. Randomized factorial trial of phone-delivered support counseling and daily text message reminders for HIV treatment adherence. J Acquir Immune Defic Syndr. 2016;73(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiweewa FM, Wabwire D, Nakibuuka J, Mubiru M, Bagenda D, Musoke P, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. J Acquir Immune Defic Syndr. 2013;63(4):e125–32. [DOI] [PubMed] [Google Scholar]
- 33.Kuo I, Liu T, Patrick R, Trezza C, Bazerman L, Uhrig Castonguay BJ, et al. Use of an mHealth intervention to improve engagement in HIV community-based care among persons recently released from a correctional facility in Washington, DC: a pilot study. AIDS Behav. 2019;23(4):1016–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. [DOI] [PubMed] [Google Scholar]
- 35.Lucas GM, Mullen BA, Galai N, Moore RD, Cook K, McCaul ME, et al. Directly administered antiretroviral therapy for HIV-infected individuals in opioid treatment programs: results from a randomized clinical trial. PLoS ONE. 2013;8(7):e68286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macalino GE, Hogan JW, Mitty JA, Bazerman LB, DeLong AK, Loewenthal H, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21(11):1473–7. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin MM, Franke MF, Munoz M, Nelson AK, Saldana O, Cruz JS, et al. Community-based accompaniment with supervised antiretrovirals for HIV-positive adults in Peru: a cluster-randomized trial. AIDS Behav. 2018;22(1):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao NY, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15(3):e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naar-King S, Outlaw AY, Sarr M, Parsons JT, Belzer M, Mac-Donell K, et al. Motivational Enhancement System for Adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38(6):638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nachega JB, Chaisson RE, Goliath R, Efron A, Chaudhary MA, Ram M, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS (London, England). 2010;24(9):1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R. A randomized controlled trial of real-time electronic adherence monitoring with text message dosing reminders in people starting first-line antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70(5):495–502. [DOI] [PubMed] [Google Scholar]
- 42.Pence BW, Gaynes BN, Adams JL, Thielman NM, Heine AD, Mugavero MJ, et al. The effect of antidepressant treatment on HIV and depression outcomes: results from a randomized trial. AIDS (London, England). 2015;29(15):1975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradier C, Bentz L, Spire B, Tourette-Turgis C, Morin M, Souville M, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4(2):121–31. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez-Garcia P, Côté J. An individualized intervention to foster optimal antiretroviral treatment-taking behavior among persons living with HIV: a pilot randomized controlled trial. J Assoc Nurses AIDS Care. 2012;23(3):220–32. [DOI] [PubMed] [Google Scholar]
- 45.Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;27(2):199–209. [DOI] [PubMed] [Google Scholar]
- 46.Sabin LL, Bachman DeSilva M, Gill CJ, Zhong L, Vian T, Xie W, et al. Improving adherence to antiretroviral therapy with triggered real-time text message reminders: the China adherence through technology study. J Acquir Immune Defic Syndr. 2015;69(5):551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silveira MPT, Guttier MC, Page K, Moreira LB. Randomized controlled trial to evaluate the impact of pharmaceutical care on therapeutic success in HIV-infected patients in Southern Brazil. AIDS Behav. 2014;18(1):75–84. [DOI] [PubMed] [Google Scholar]
- 48.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54(1):85–92. [DOI] [PubMed] [Google Scholar]
- 49.van Loggerenberg F, Grant AD, Naidoo K, Murrman M, Gengiah S, Gengiah TN, et al. Individualised motivational counselling to enhance adherence to antiretroviral therapy is not superior to didactic counselling in South African patients: findings of the CAPRISA 058 randomised controlled trial. AIDS Behav. 2015;19(1):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner GJ, Lovely P, Schneider S. Pilot controlled trial of the adherence readiness program: an intervention to assess and sustain HIV antiretroviral adherence readiness. AIDS Behav. 2013;17(9):3059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White BL, Golin CE, Grodensky CA, Kiziah CN, Richardson A, Hudgens MG, et al. Effect of directly observed antiretroviral therapy compared to self-administered antiretroviral therapy on adherence and virological outcomes among HIV-infected prisoners: a randomized controlled pilot study. AIDS Behav. 2015;19(1):128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams AB, Wang H, Li X, Chen J, Li L, Fennie K. Efficacy of an evidence-based ARV adherence intervention in China. AIDS Patient Care STDS. 2014;28(8):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wohl AR, Garland WH, Valencia R, Squires K, Witt MD, Kovacs A, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42(11):1619–27. [DOI] [PubMed] [Google Scholar]
- 54.Yotebieng M, Thirumurthy H, Moracco KE, Edmonds A, Tabala M, Kawende B, et al. Conditional cash transfers to increase retention in PMTCT care, antiretroviral adherence, and postpartum virological suppression: a randomized controlled trial. J Acquir Immune Defic Syndr. 2016;72(Suppl 2):S124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abas M, Nyamayaro P, Bere T, Saruchera E, Mothobi N, Simms V, et al. Feasibility and acceptability of a task-shifted intervention to enhance adherence to HIV medication and improve depression in people living with HIV in Zimbabwe, a low income country in sub-Saharan Africa. AIDS Behav. 2018;22(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhargava A, Booysen FLR, Walsh CM. Health status, food insecurity, and time allocation patterns of patients with AIDS receiving antiretroviral treatment in South Africa. AIDS Care. 2018;30(3):361–8. [DOI] [PubMed] [Google Scholar]
- 57.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS ONE. 2010;5(6):e10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cowan FM, Davey C, Fearon E, Mushati P, Dirawo J, Chabata S, et al. Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): a cluster-randomised trial. Lancet HIV. 2018;5(8):e417–26. [DOI] [PubMed] [Google Scholar]
- 59.Fox MP, Pascoe SJS, Huber AN, Murphy J, Phokojoe M, Gorgens M, et al. Effectiveness of interventions for unstable patients on antiretroviral therapy in South Africa: results of a cluster-randomised evaluation. Trop Med Int Health. 2018;23(12):1314–25. [DOI] [PubMed] [Google Scholar]
- 60.Hosseinipour M, Nelson JAE, Trapence C, Rutstein SE, Kasende F, Kayoyo V, et al. Viral suppression and HIV drug resistance at 6 months among women in Malawi’s option B+ program: results from the PURE Malawi Study. J Acquir Immune Defic Syndr (1999). 1999;2017(75 Suppl 2):S149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNairy ML, Lamb MR, Gachuhi AB, Nuwagaba-Biribonwoha H, Burke S, Mazibuko S, et al. Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: the Link4Health cluster randomized trial. PLoS Med. 2017;14(11):e1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rawlings MK, Thompson MA, Farthing CF, Brown LS, Racine J, Scott RC, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr (1999). 2003;34(2):174–83. [DOI] [PubMed] [Google Scholar]
- 63.Rosen MI, Dieckhaus K, McMahon TJ, Valdes B, Petry NM, Cramer J, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21(1):30–40. [DOI] [PubMed] [Google Scholar]
- 64.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50(2):187–99. [DOI] [PubMed] [Google Scholar]
- 65.Tuldrà A, Fumaz CR, Ferrer MJ, Bayés R, Arnó A, Balagué M, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr (1999). 2000;25(3):221–8. [DOI] [PubMed] [Google Scholar]
- 66.Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, et al. Medication diaries do not improve outcomes with highly active antiretroviral therapy in Kenyan children: a randomized clinical trial. J Int AIDS Soc. 2009;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wohl DA, Golin CE, Knight K, Gould M, Carda-Auten J, Groves JS, et al. Randomized controlled trial of an intervention to maintain suppression of HIV viremia after prison release: the imPACT trial. J Acquir Immune Defic Syndr (1999). 2017;75(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.HIV/AIDS Prevention Research Synthesis Project. Compendium of evidence-based interventions and best practices for HIV prevention. Centers for Disease Control and Prevention. https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html. Date last updated: March 2, 2021. Date Accessed 9 Mar 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.