Abstract
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by the hyperproliferation of vascular cells, including smooth muscle and endothelial cells. Hyperproliferative cells eventually obstruct the lung vasculature, leading to irreversible lesions that collectively drive pulmonary pressure to life-threatening levels. Although the primary cause of PAH is not fully understood, several studies have indicated it results from chronic pulmonary inflammation, such as observed in response to pathogens’ infection. Curiously, infection by the intravascular parasite Schistosoma mansoni recapitulates several aspects of the widespread pulmonary inflammation that leads to development of chronic PAH. Globally, more than 200 million people are currently infected by Schistosoma spp., with about 5% developing PAH (Sch-PAH) in response to the parasite egg-induced obliteration and remodeling of the lung vasculature. Prior to their settling into the lungs, Schistosoma eggs are released inside the mesenteric veins, where they either cross the intestinal wall and disturb the gut microbiome or migrate to other organs, including the lungs and liver, increasing pressure. Spontaneous or surgical liver bypass via collateral circulation alleviates the pressure in the portal system; however, it also allows the translocation of pathogens, toxins, and antigens into the lungs, ultimately causing PAH. This brief review provides an overview of the gut-mesentery-lung axis during PAH, with a particular focus on Sch-PAH, and attempts to delineate the mechanism by which pathogen translocation might contribute to the onset of chronic pulmonary vascular diseases.
Keywords: Pulmonary Arterial Hypertension, Schistosomiasis, Gut-Lung axis, Mesentery, Endothelial Cell
GRAPHICAL ABSTRACT
Schematic showing the gut-mesentery-lung axis as a crossroad for pathogen translocation, human microbiome dysbiosis, and the development of inflammatory pulmonary diseases, such as pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a life-threatening and multifactorial disease characterized by a mean pulmonary arterial pressure (mPAP) of >20 mmHg and elevated pulmonary vascular resistance (≥3 Wood units) in response to vasoconstriction and progressive remodeling of the lung precapillary vasculature.1,2 Although the primary cause of chronic pulmonary vascular remodeling observed during PAH remains poorly understood, the hyperproliferation of vascular cells, including smooth muscle cells (SMCs) and endothelial cells (ECs), is a predominant factor throughout the course of the disease.1,3,4 The uncontrolled proliferation of vascular cells, in combination with infiltrating and resident immune cells lead to the obstruction of the pulmonary vasculature and the development of irreversible inflammatory vascular lesions, which elevate the mPAP, often leading to right ventricular hypertrophy and death.1,3,5
PAH, which is classified as group 1 pulmonary hypertension (PH),6–8 emerges from a variety of pre-existing conditions, including heritable genetic mutations (HPAH),9 connective tissue diseases,10 systemic sclerosis,11 portal hypertension,12 and congenital heart disease.13 Furthermore, the development of PAH has been largely associated with certain drugs and toxins, including amphetamines and methamphetamines,14 and as a consequence of infection by at least two genetically distinct pathogens, the human immunodeficiency virus (HIV) and the parasite Schistosoma spp. (Sch-PAH).2,6,13 Although all of these factors can lead to the establishment of chronic vascular inflammation observed during the disease, in a significant percentage of patients, the cause of PAH is idiopathic (IPAH),15 indicating an unclear biological mechanism may substantially contribute for the progression of the disease.
The multifactorial nature of PAH suggests that the disease may not result from a unique biological or environmental trigger. However, it is unmistakably clear that distinct endothelium-associated biological processes, including apoptosis-resistant cell growth, clonal expansion, and endothelial-to-mesenchymal transition (EndoMT), play a pivotal role in the onset and development of PAH.4,5,16–19 Interestingly, the involvement of all these processes converge to the idea that chronically injured pulmonary vascular endothelium allows for the survival of an abnormal and hyperproliferative EC phenotype,4,5 which might orchestrate lung vasculature remodeling over time. Consistent with these observations, infection with Schistosoma spp. recapitulates several aspects of widespread pulmonary inflammation that leads to chronic PAH, including the formation of severe inflammatory vascular lesions.20–22 Despite more than 20 species comprising the genus Schistosoma are found worldwide, primarily through Africa, South and Central America, only S. japonicum, S. haematobium, and S. mansoni have been reported to promote lung disease.
Of note, chronically S. mansoni-infected individuals host adult parasites in their mesenteric vasculature, where a single pair can lead to the accumulation of approximately 300 eggs per day.23,24 Half of the eggs usually cross the gut wall and are released with feces, whereas the other half migrate to other host organs, including the liver and lungs, where they cause a significant inflammatory response.24,25 In response to egg transmigration through the gastrointestinal (GI) wall, the gut and mesentery become inflamed and permeable, favoring the disturbance of their microbiome composition.26,27 In fact, gut microbiome disruption has been highly associated with damage to the GI barrier, leading to pathogen translocation into the systemic circulation via the mesenteric blood vessels and lymphatics.28 Besides Sch-PAH, recent findings have also indicated the occurrence of gut microbiome disruption in other forms of PAH, including HPAH, IPAH, HIV-associated PAH, and drug-associated PAH30; however, the specific mechanism of pathogen translocation remains elusive. Once translocated, pathogen-associated molecular patterns (PAMPs) initiate an inflammatory response by activating pathogen recognition receptors (PRRs). During this process, although immune cells are the frontline of systemic immune surveillance, ECs can actively contribute to the immune response in local environments, but eventually can also become targets of pathogens and inflammatory mediators.31,32 Accordingly, disturbance of important signaling pathways for EC function, such as those mediated by transforming growth factor-β (TGF-β), endothelial nitric oxide synthase (eNOS), BMPR2, PPARγ, and PHD2, are crucial for the expansion of an abnormal EC phenotype within the lung vasculature, leading to severe pulmonary vasculopathy.22,33–35 Besides recapitulating recent findings on the gut microbiome and its implications for lung health, this brief review provides key insights into the poorly explored gut-mesentery-lung axis and the mechanism by which pathogen translocation affects pulmonary vascular homeostasis, leading to EC death, abnormal survival, and the development of chronic pulmonary inflammatory diseases, such as observed in PAH.
GUT MICROBIOME AND IMPLICATIONS FOR LUNG HEALTH
General aspects of gut microbiome
The human GI tract is colonized by various microorganisms, including fungi, viruses, and bacteria, primarily belonging to the phyla Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Cyanobacteria.36 Being in a symbiotic relationship with host cells, these microorganisms significantly contribute to the maintenance of the GI tract as well as whole-body homeostasis, whereas disruptions of these communities (i.e., dysbiosis) has been linked to the development of local and systemic inflammatory diseases, including autoimmune disorders, cancer, and pulmonary pathologies.37–42 Overall, the fine-tuned interaction among gut microbiota, environmental antigens, and human cells is essential for protecting the host against invading pathogens, contributes to the production of energy and vitamins, and shapes the immunity of the host.40,42 In line with these observations, studies have demonstrated that the composition and equilibrium of the microbiome are not only essential for intestinal homeostasis but are also critical for overall human health.40,43
Several factors influence the composition of the human gut microbiome, including host genetic and developmental characteristics such as ethnicity, gender, and age40,44, as well as nutritional habits and exposure to pharmacological treatments, including antibiotics45 and anticancer drugs.46 In addition, substance abuse, such as methamphetamine, has also been linked to an altered gut microbiome composition, leading to systemic inflammation and the development of pathological conditions in other tissues and organs, including neurological alterations, such as psychotic syndrome and cognitive impairment.47,48 Drug abuse and therapeutic approaches can also affect the human gut microbiome. For instance, hormonal contraceptive methods can cause a minor dysbiosis within the gut microbiome of young females,49 uncovering gender-associated features of inflammatory diseases such as PAH. Infection is another factor contributing to microbiome dysbiosis. For example, viral infection by HIV can lead to gut mucosal damage, depletion of CD4+ T-cells, and persistent immune activation as a result of the systemic translocation of pathogens. In addition, parasite infection by S. mansoni or S. japonicum and anthelmintic treatment have been reported to disturb gut microbiome homeostasis, favoring the progression of inflammatory disorders,26,27,50,51 including lung pathologies.
Gut microbiome on lung homeostasis and disease
The gut microbiota has been demonstrated to be vital in lung homeostasis and defense against respiratory infections, including those by the new coronavirus SARS-COV-2 (COVID19 coronavirus), influenza A virus (IAV), and respiratory syncytial virus (RSV).52–54 The elevated innate immune response via the secretion of interferon type I (INF-I) induced by gut-derived microbial metabolites, such as desaminotyrosine (DAT) and short chain fatty acids (SCFAs), in combination with the increased function of CD8+ T-lymphocytes, have been shown to be essential contributions of the gut microbiome to lung homeostasis and immune defense.37 Altered gut microbiome has been observed in patients with acute and chronic inflammatory lung diseases, including acute respiratory distress syndrome (ARDS), PAH, chronic obstructive pulmonary disease (COPD), and asthma, indicating that the GI tract might also shape the microbial community within the lungs. Finally, as gut dysbiosis affects lung homeostasis, the altered lung microbiome might affect other organs, including the gut itself,37 suggesting a bi- or even multidirectional networking of host microbial communities. Hence, understanding the mechanism by which this communication occurs is fundamental for human health and disease prevention.
Uncovering a lung microbial community has led to a fundamental shift in the field of lung immunity and inflammation, negating the long-term conviction that a healthy lung tissue is a sterile environment. Recent studies have shown that a healthy human lung is indeed populated by a vast microbiome, including bacterial communities mainly composed of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria55,56 and fungal communities of Ascomycota and Microsporidia.57 The lung microbiome seems to be formed during development and, similar to the gut microbiome, its composition throughout the years depends significantly on intrinsic and extrinsic host factors, including aging, sex, and infection by pathogenic microorganisms38,40,58 (Figure 1). Consistent with these facts, the bacterial load in murine lungs was demonstrated to be increased during the first 2 weeks of life, with the phyla shifting from Gammaproteobacteria and Firmicutes to Bacteroidetes.58 The relevance of such developmental changes is that alterations in these microbial communities have been associated with the accumulation of immune cells, specifically the infiltration of a regulatory T-cell population that could promote tolerance to a potential subsequent pathogenic challenge.59 These data have strongly suggested that the acquisition of diverse microorganisms and their harmonic interaction is an important early life event that protects the lungs from injurious responses to inhaled or systemic intravascular pathogens and antigens throughout the course of life and might be fundamental for preventing the development of chronic lung diseases. Finally, developmental changes in the gut microbiome composition also contribute to lung homeostasis and microbiome profile. Although the specific mechanism of pathogen translocation between these organs is yet to be fully determined, the mesentery is considered the major route for parasites and opportunistic microorganisms to achieve systemic circulation, colonizing other organs, including the lungs.
FIGURE 1. Intrinsic and extrinsic factors affecting gut and lung microbiome composition.
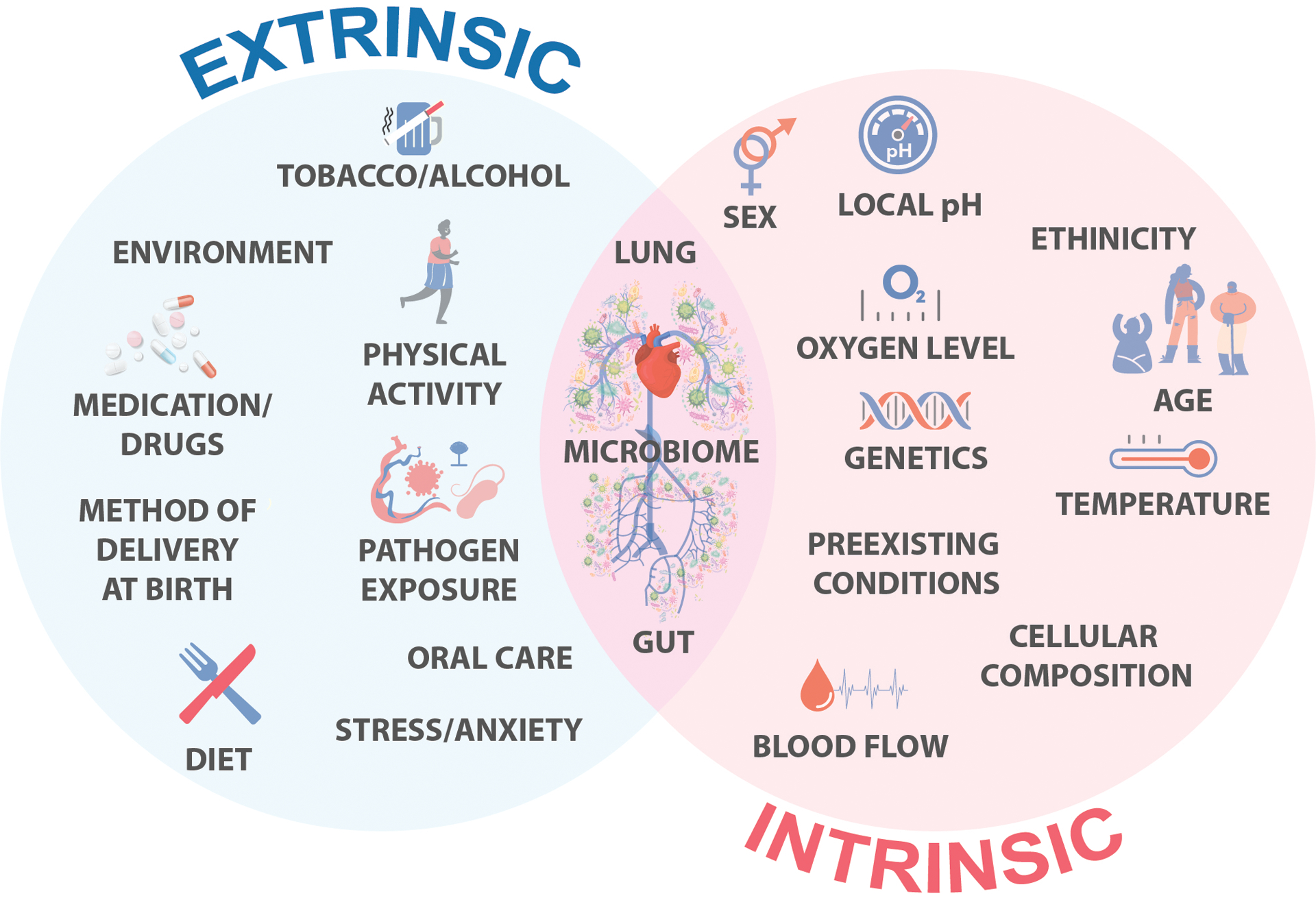
The human gut and lung microbiome (or ecosystem) are formed during development and their compositions through the years dependent significantly on intrinsic and extrinsic host factors, including infection by other pathogens.
LINKING GUT TO LUNGS: PATHOGEN TRASLOCATION AND EC DYSFUNCTION
“Leaky gut” and mesentery as a microbial crossroad
Increased intestinal permeability plays a role in several GI-related diseases, including celiac disease (CD), inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer (CRC).60 The translocation of bacteria, their metabolites, and cell wall components, which normally cannot cross the epithelial intestinal wall, is achieved in a “leaky” gut, allowing their presence within the systemic circulation and disruption of the homeostasis of other organs through injury and inflammation. In response to increased gut permeability, some microbes that translocate more efficiently than other commensal microorganisms, including Escherichia coli,61 also accumulate into the peritoneal cavity, inside the mesenteric lymph nodes, or achieve systemic circulation, causing multiorgan disease, including sepsis and acute lung injury (ALI/ARDS). The lymphatic system significantly accounts for the physiological and pathological translocation of pathogens and their molecules into other organs such as the lungs.62 However, the mesenteric circulation, through its branching into the portal system and subsequently into the cava vein and lung vasculature, provides an essential gut-lung communication route (Figure 2). In fact, increased GI permeability in complex gut diseases such as Crohn’s disease has been associated with inflammation within the mesenteric fat tissue.63–65 Despite the recent classification of the mesentery as an important human organ with circulatory function and fundamental immunological capabilities,66,67 and the reports on pathogen translocation through the mesenteric lymphatic system, the specific role of mesenteric venous circulation in pathogen translocation remains poorly investigated; with schistosomiasis being a well-known example of how the mesenteric circulation connects the gut and lungs leading to disease.
FIGURE 2. Linking the gut to the lungs via the mesentery: a poorly investigated crossroad.
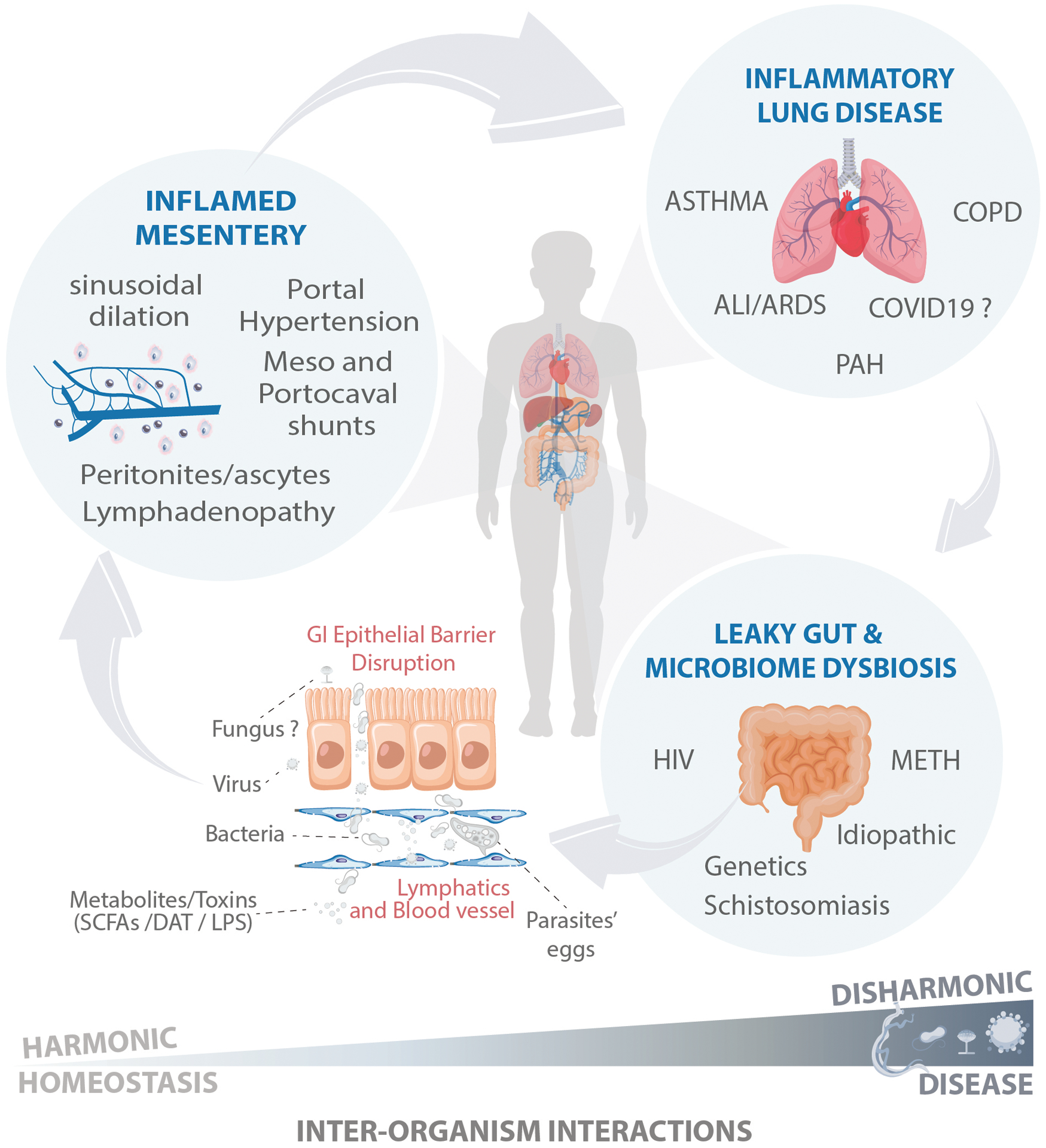
In response to increased gastrointestinal (GI) permeability (“leaky gut”), some opportunistic microbes, which translocate more efficiently than other commensals microorganisms, including Escherichia coli, accumulate into the peritoneal cavity, inside the mesenteric lymph nodes, or achieve systemic circulation, causing multiorgan disease. The lymphatic system significantly accounts for the physiological and pathological translocation of pathogens, its molecules, and toxins into other organs such as the lungs; however, the mesenteric circulation provides an essential gut-lung communication route through its branching into the portal system and subsequently into the cava vein and lung vasculature. Spontaneous or surgical mesovacal shunts can allow a direct gut-lung communication, bypassing hepatic clearance. Tissue homeostasis is preserved under harmonic interorganism interactions. However, following a disharmonic interaction, such as in chronic stages of schistosomiasis, the host succumbs to disease.
During schistosomiasis, the presence of adult parasites and their eggs inside the mesenteric veins stimulates a potent angiogenic response,68 and modulates GI tract inflammation to facilitate pathogen transmission across the gut wall.25,69,70 However, the eggs released inside the mesentery can also transmigrate into the peritoneal cavity or lead to obstruction of the hepatic sinusoids, increasing the pressure in the portal system and causing severe hepatic inflammation and development of portal hypertension. Interestingly, schistosomiasis-dependent or independent portal hypertension triggers the formation of collateral circulation between the mesenteric and portal circulation and the superior cava vein,71 ultimately bringing toxins and pathogenic molecules, including bacteria and endotoxins, into the pulmonary circulation, causing PAH. Simultaneous portopulmonary hypertension is not exclusive to schistosomiasis infection; it can also emerge from cirrhotic and noncirrhotic liver diseases, which are also stimuli for porto/mesocaval shunt formation. However, the specific mechanism by which nonsurgical formation of porto or mesocaval collateral vessels occurs remains to be determined. Thus, a better understanding of the normal mesenteric anatomy, physiology, and alterations under pathological conditions is fundamental for uncovering the contribution of mesenteric circulation in the development of chronic vascular diseases inside and outside of the peritoneal cavity.
Lung and mesenteric ECs as microbiome gatekeepers
As the gatekeepers between blood and tissues, vascular ECs are in direct contact with blood components and intravascular pathogens, which can often lead to EC dysfunction and even cell death. Although broad as a term, EC dysfunction is generally associated with the inability of ECs to produce normal levels of nitric oxide (NO) in response to reduced or uncoupled expression of the enzyme, endothelial nitric oxide synthase (eNOS). Moreover, when persistent, EC dysfunction can result in chronic vascular disease, especially due to the hyperproliferation and mutation of cells that fail to undergo cell death.5,16 This phenomenon is highly evident during PAH, in which dysfunctional lung ECs undergo phenotypic alterations characterized by eNOS uncoupling, oxidative stress, and the depletion of multiple key signaling proteins including the bone morphogenetic protein receptor type 2 (BMPRII), a major antiapoptotic receptor.72 Depletion of BMPRII reduces the levels of SMAD1/5/8 canonical signaling pathway, significantly contributing to EC pathology, including the development of a mesenchymal-like phenotype (EndoMT). The lack of “endotheliality” has not only been linked to reduced expression of BMPRII but also to BMPR2 genetic mutations, which have been implied in the increased EC susceptibility to cell death and overexpansion of dysfunctional EC populations leading to severe vascular remodeling.73–78 In opposition to BMPRII, the effect of the major member of the TGF-β family, on ECs remains contradictory,79 with TGF-β-treated cells either dyeing80 or undergoing EndoMT.19,81–83 In addition to TGF-β and BMPRII-mediated signaling, growth factors cooperate with different signaling pathways, including Notch and Wnt, to maintain EC quiescence, prevent dysfunction, and promote EC survival and vessel stability. Both the autocrine and endocrine production of these factors, especially VEGF and FGF, maintain EC survival through the regulation of autophagy84 and prevention of uncontrolled proliferation.85 However, in response to persistent vascular injury, loss of key EC components including Caveolin-1, BMPRII, PPARγ and PHD2 in association with increased cell death results in DNA fragmentation, membrane blebbing, and release of extracellular vesicles,86 which can culminate in severe vascular remodeling.
The apoptosis-resistant cell phenotype has also been related to metabolic dysfunction via elevated glycolysis, which is known to generate less ATP than mitochondrial respiration. As ATP is also a potent damage-associated molecular pattern (DAMP), reduced extracellular ATP (eATP) prevents the activation of the major purinergic DAMP sensor, P2X7R, protecting cells from ATP-induced cell injury. Long-lasting activation of P2X7R by elevated eATP induces the formation of cell membrane pores, evoking a massive Ca2+ influx,87–89 inflammasome activation, extracellular vesicle shedding, ultimately leading to programmed cell death.90 Previous observations in chronically S. mansoni-infected mice revealed that the function and expression of P2X7R on macrophages and MECs was downregulated,91,92 potentially priming an “apoptosis-resistant” epigenetic memory on these cells, and thus favoring the prolonged survival of the host and better transmissibility of the parasite. During schistosomiasis, ECs are important components of the frontline defense, directly interacting with parasites and their eggs, simultaneously as fighters and targets of the infection.24,93,94 The parasite and its egg size and capillary diameter also facilitate pathogen-host interactions. Indeed, with a size larger than 50 μm,70 S. mansoni eggs easily obstruct small capillaries and induce EC activation, which in the mesentery is known to elevate the expression of adhesion molecules,95 reduce the availability of protective NO levels,92,96 increase leukocyte adhesion,92,97 proliferation,68 and eventually, cell death. Pathological analysis of the lungs of patients with Sch-PAH and animal models has also revealed an increased number of pathological ECs inside plexiform lesions.21,33 Interestingly, no accumulation of soluble egg antigens (SEA) was detected in the lungs of patients with chronic Sch-PAH,35 suggesting the rapid clearance of antigenic molecules in the initial stages of lung pathology. S. mansoni eggs embolized into the lung vasculature promote the significant recruitment of Th2 CD4+ lymphocytes and IL-4/IL-13 secretion, which are pivotal for the activation of macrophage-derived TGF-β, and severe pulmonary vascular remodeling.34,98 Moreover, Th2-derived IL-13 also contributes to the migration of lung ECs with abnormal phenotype,99 indicating that the communication between Th2 cells and the endothelium is also critical for the expansion of pathogenic ECs, as observed in other pulmonary inflammatory diseases.100 Moreover, heterozygous BMPR2 mutation in mice was reported to facilitate the hepatic shunting of S. mansoni eggs into the lungs via sinusoidal dilation, leading to Sch-PAH101, suggesting TGF-β-BMPRII dichotomy may also play a role in Sch-PAH. Further details on the involvement of multiple signaling pathways resulting in lung EC dysfunction and vascular remodeling has been recently revised elsewhere4.
IS S. MANSONI-ASSOCIATED PAH A UNIQUE GUT-MESENTERY-LUNG AXIS MODEL?
Schistosoma spp.-associated PAH
Globally relevant and but often neglected, Sch-PAH is a life-threatening complication of chronic infection by a metazoan parasite of the genus Schistosoma, which can lead to heart failure and premature death. Despite being the leading cause of PAH worldwide, no targeted therapies exist for Sch-PAH to ameliorate morbidity and prevent the mortality associated with secondary diseases.20 Among the 24 identified Schistosoma species, S. mansoni remains the primary cause of Sch-PAH worldwide. However, a few studies have indicated that at least 2 species, S. japonicum and S. haematobium, can cause lung disease. From an evolutionary point of view, the biological success of Schistosoma in infecting its hosts is largely due to its digenetic profile, that is, its life cycle depends on the successful infection and survival in 2 different hosts, an intermediate and a definitive.24 Infection of the host is initiated by the transcutaneous invasion of either the miracidium or cercaria, which are water-dependent life forms of the parasite. After cercarial infection, parasites migrate through the cardiovascular system of the host, interacting with the endothelium in different tissues and organs, including the lungs, heart, mesentery, liver, and gut.24,102,103 While the journey of S. mansoni and S. japonicum stops in the mesenteric circulatory, intestinal, and hepatic circulatory systems, S. haematobium reaches inside the venous plexus of the bladder and rectal venules.104 Inside the mesentery or the bladder circulatory system, adult schistosomes acquire nutrients and copulate, releasing their antigenic eggs,24 which either cross the gut or bladder wall103 being released with the host feces and urine, respectively. Whereas, the remaining eggs obliterate small capillaries in different organs, including the liver and lungs, where they lead to the development of portal105 or pulmonary hypertension.20,33
During infection with S. mansoni and S. japonicum, formation of porto or mesocaval shunts can lead to liver bypass, alleviating the parasite-induced pressure in the portal system, but also allowing the translocation of eggs, antigens, and toxins into the lungs, leading to obliteration, inflammation, and severe remodeling of the pulmonary vasculature.22,33 Moreover, release of S. mansoni and S. japonicum eggs into the mesenteric veins induces a Th2-like inflammatory response, with the formation of a complex granulomatous reaction. Inflammatory cell recruitment contributes to egg translocation into the gut lumen, but can also damage the intestinal wall,70,103,106,107 thus allowing the translocation of pathogens and toxins into the peritoneal cavity and the mesenteric bloodstream. In fact, gut microbiome and microbial metabolites were significantly altered in mice infected with S. japonicum, exhibiting elevated expansion of pathogenic bacteria and reduced SCFA production, which has been associated with damage to the GI wall.27 S. japonicum eggs cause severe GI inflammation, including jejunal ulceration,108 leading to focal intestinal lesions similar to those produced by infection with S. haematobium, but different from the widespread and diffuse lesions produced by S. mansoni eggs, potentially accounting for differences in the inflammatory profile of the disease and the mechanism by which eggs and other pathogenic molecules translocate into the lungs (Figure 3). Finally, urogenital schistosomiasis caused by S. haematobium can also increase the risk of HIV infection, especially in women,109 which can ultimately evolve for PAH.
FIGURE 3. Gut-mesentery-lung axis in Schistosomiasis-associated PAH (Sch-PAH).
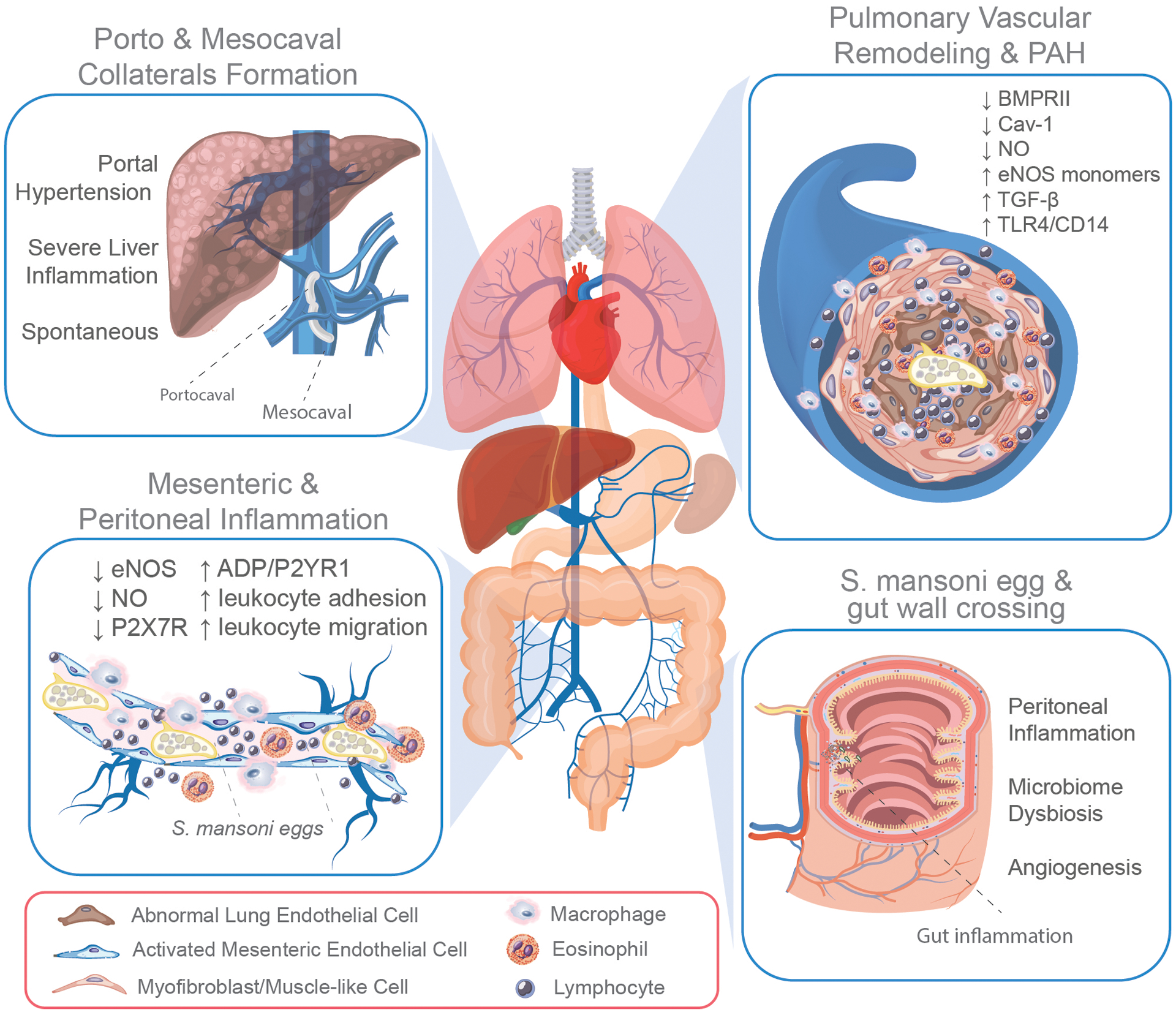
Chronically infected individuals host in their mesenteric system adult schistosomes that lay thousands of eggs. The interaction of adult S. mansoni and eggs with mesenteric endothelial cells contributes to endothelial dysfunction by reducing the expression and function of the endothelial expression of nitric oxide (eNOS) and purinergic receptor P2X7R, and by increasing the ADP-induced activation of P2YR1 and leukocyte adhesion and transmigration into the peritoneal cavity (bottom right box). Once released, half of the eggs actively cross the intestinal wall causing a significant inflammatory response in the gut wall and peritoneal cavity, contributing to microbiome dysbiosis and angiogenesis (bottom left box). The remaining eggs allocate to other organs, including the liver, where they can obstruct the sinusoid vasculature leading to severe inflammation and the development of portal hypertension. In response to elevated portal pressure, the formation of porto and mesocaval collateral circulation not only bypasses the liver but also allows the translocation of S. mansoni eggs, antigens, and toxins into the lungs. Although rare, the formation of spontaneous collateral circulation can also occur (top left box). The translocation of eggs from the mesentery into the lung leads to obliteration, inflammatory remodeling of the pulmonary vasculature, and development of Sch-PAH, which exhibits abnormal endothelial proliferation owing to their transformation into myofibroblasts or mesenchymal-like cells, associated with the reduced expression of bone morphogenetic protein receptor 2 (BMPRII), caveolin-1 (Cav-1), and nitric oxide (NO) and the elevated activation of TGF-β and TLR4/CD14-mediated signaling pathway (top right box).
HIV-associated PAH
Besides schistosomiasis, HIV infection can also disrupt the gut epithelial barrier, altering its microbiome composition,110 an effect that persists despite successful antiretroviral therapy (ART).111 Altered microbiome composition in patients with chronic HIV is marked by an abundance of bacterial taxa belonging to the gram-negative class Negativicutes, which has been positively correlated with elevated levels of plasma IFN-γ and IL-1β. The disruption of the gut epithelial barrier and resulting gut leakage due to HIV infection leads to elevated bacterial translocation into the circulation, potentially accounting for a further increase in IFN-γ and LPS-associated inflammatory responses. A possible route for HIV-associated pathogen translocation is the mesentery, as nonviral presence of severe mesenteric lymphadenopathy, which might result from opportunistic infections, is commonly observed in patients with HIV.112
Patients with HIV, especially those receiving ART, can also display noncirrhotic portal hypertension.113,114 As previously described, portal hypertension can result in spontaneous or surgical formation of mesocaval shunts, enabling the direct translocation of pathogens and toxins from the mesentery into the lungs. Moreover, HIV infection can directly affect the lung microbiome composition, reducing the alveolar microbiome richness and diversity within individuals, whereas increasing beta diversity,115 potentially contributing to susceptibility toward development of chronic lung diseases.
Idiopathic and heritable PAH
In addition to direct evidence of pathogen-associated PAH, a few studies have investigated the role of the gut-lung axis in the development of other forms of PAH. For example, a recent study by Kim et al.41 using genomic wide sequencing showed that a small cohort of patients with PAH had a distinct gut microbiome composition, characterized by an elevated presence of bacterial communities associated with trimethylamine/trimethylamine N-oxide and purine metabolism. These observations were in line with previous animal studies using a rat model of PH (SUGEN/hypoxia-induced PH116 and monocrotaline-treated rats117), in which an altered gut microbial community and metabolome profile was detected, with at least a 3-fold increase in the Firmicutes-to-Bacteroidetes ratio compared with that in control animals,116 which might have contributed to the presence of opportunistic bacteria and their pathogenic products into the bloodstream. In fact, the translocation of bacteria from the disrupted gut microbiota seems to play a role in the development of other forms of PAH, including human IPAH.41,118,119 Moreover, elevated levels of soluble CD14 in the plasma and lungs have been observed in patients with IPAH and heritable disease concurrently with significant dysbiosis within their gut microbiome.30 Regarding heritable diseases, the translocation of pathogens might be a relevant mechanism for determining the reduced penetrance of BMPR2 mutations associated with the disease. Finally, although lung inflammatory diseases appear to alter the microbiome,38,58 the specific mechanism that leads to the disruption of the lung ecosystem or identification of biomarkers that could predict susceptibility to chronic lung diseases are lacking.
CONCLUSION AND FUTURE PERSPECTIVES
More than 35 years ago, Cohen, et al.. reported an unusual case of PAH associated with the formation of portosystemic collateral shunts without pre-existing liver disease, leading the authors to the conclusion that “the nature of the underlying vasotoxic component remains to be identified”.120 Nowadays, the advent of novel animal models and advanced molecular and imaging techniques have allowed the identification of a possible microbial communication network among lungs and other organs. Except from the clear involvement of S. mansoni in opening the gut-mesentery axis for pathogen translocation as an unconventional route for lung infection, the mechanism by which the microbiome shifts through organs remains unclear. However, the field has been moving towards a clearer definition of specific mechanisms governing the gut-lung axis, and the development of improved approaches will contribute to determining the contribution of the pathophysiology of the mesenteric circulation to local and systemic inflammation, such as that observed during Sch-PAH.
Supplementary Material
MANUSCRIPT HIGHLIGHTS.
Pathogen translocation and chronic lung inflammatory diseases
Effect of gut dysbiosis and inflamed mesentery on PAH
Schistosomiasis as a model to investigate pathogen translocation and lung microbiome dysbiosis
Insights on pathogen-induced abnormal EC survival and proliferation
Acknowledgements
I thank my career development team, which includes Dr. Richard D. Minshall and Sarah E. Lutz (University of Illinois at Chicago), Serpil Erzurum (Cleveland Clinic Foundation), Claudia L. M. Silva (Federal University of Rio de Janeiro), Marcelo G. Bonini (Northwestern University), David L. Williams (Rush University), and Nicholas Morrell (University of Cambridge) for their incredible support and mentorship. I also thank the graphic designer Pedro Casanova H. Simões for the outstanding graphic work in the Graphical abstract and in Figures 1, 2, and 3.
Sources of Funding
This work was supported by a postdoctoral award from the American Heart Association and Circle of Service Foundation (AHA 18POST34020037), a catalyst award from the American Lung Association (ALA 697907), and an K01 award from the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI 1K01 HL159037-01).
Nonstandard Abbreviations and Acronyms
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BMPR2
bone morphogenic protein receptor 2
- CD14
cluster of differentiation 14
- DAMP
damage-associated molecular pattern
- EC
endothelial cell
- EndoMT
endothelial-to-mesenchymal transition
- FGF
fibroblast growth factor
- eNOS
endothelial nitric oxide synthase
- GI
gatrointestinal
- MEC
mesenteric endothelial cell
- mPAP
mean pulmonary arterial pressure
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PAMP
pathogen-associated molecular pattern
- PRR
pathogen recognition receptors
- P2X7R
purinergic receptor X7
- Sch-PAH
schistosomiasis-associated PAH
- TGF-ß
transforming growth factor beta
- TH
T helper type
- TLR4
toll-like receptor 4
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Disclosure
None to disclosure.
References
- 1.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur. Respir. J 2012;40,1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J 2019;53,1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tude RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am. J. Pathol 1994;144,275–285. [PMC free article] [PubMed] [Google Scholar]
- 4.Evans CE, Cober ND, Dai Z, Stewart DJ, Zhao YY. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J 2021;58,2003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cool CD, Kuebler WM, Bogaard HJ, Spiekerkoetter E, Nicolls MR, Voelkel NF. The hallmarks of severe pulmonary arterial hypertension: the cancer hypothesis—ten years later. Am. J. Physiol. Cell. Mol. Physiol 2020;318,L1115–L1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prins KW, Thenappan T. World Health Organization Group I Pulmonary Hypertension. Cardiol. Clin 2026;34,363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins KW, Thenappan T. WHO Group I Pulmonary Hypertension: Epidemiology and Pathophysiology. Cardiol. Clin 2016;34,363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon DF, Nickel NP, Anderson R, Mirza S, de Jesus Perez VA. The 6th World Symposium on Pulmonary Hypertension: what’s old is new. F1000Research 2019;8,888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Southgate L, Machado RD, Gräf S, Morrell NW. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol 2020;17,85–95. [DOI] [PubMed] [Google Scholar]
- 10.Mathai SC, Hassoun PM. Pulmonary Arterial Hypertension in Connective Tissue Diseases. Heart Fail. Clin 2012;8,413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaisson NF, Hassoun PM. Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Chest 2013;144,1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleemi S Portopulmonary hypertension. Ann. Thorac. Med 2010;5,5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol 2013;62,D34–D41. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez RL, Pienkos SM, de Jesus Perez V, Zamanian RT. Pulmonary Arterial Hypertension Secondary to Drugs and Toxins. Clin. Chest Med 2021;42,19–38. [DOI] [PubMed] [Google Scholar]
- 15.Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech 2010;273,268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voelkel NF, Cool C, Taraceviene-Stewart L, Geraci MW, Yeager M, Bull T, Kasper M, Tuder RM. Janus face of vascular endothelial growth factor: the obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit. Care Med 2002;30,S251–256. [DOI] [PubMed] [Google Scholar]
- 17.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15,427–438. [DOI] [PubMed] [Google Scholar]
- 18.Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol 2011;179,2660–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira SDS, Castellon M, Chen J, Bonini MG, Gu X, Elliott MH, Machado RF, Minshall RD. Inflammation-induced caveolin-1 and BMPRII depletion promotes endothelial dysfunction and TGF-β-driven pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol 2017;312, L760–L771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knafl D, Gerges C, King CH, Humbert M, Bustinduy AL. Schistosomiasis-associated pulmonary arterial hypertension: a systematic review. Eur. Respir. Rev 2020;29,190089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papamatheakis DG, Mocumbi AOH, Kim NH, Mandel J. Schistosomiasis-associated pulmonary hypertension. Pulm. Circ 2014;4,596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham BB, Kumar R. Schistosomiasis and the pulmonary vasculature (2013 Grover Conference series). Pulm. Circ 2014;4,353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheever EA, Macedonia JG, Mosimann JE, Cheever AW. Kinetics of Egg Production and Egg Excretion by Schistosoma mansoni and S. japonicum in Mice Infected with a Single Pair of Worms. Am. J. Trop. Med. Hyg 1994;50,281–295. [DOI] [PubMed] [Google Scholar]
- 24.Costain AH, MacDonald AS, Smits HH. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front. Immunol 2018;9,3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz C, Fallon PG. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front. Immunol 2018;9,2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floudas A, Aviello G, Schwartz C, Jeffery IB, O’Toole PW, Fallon PG. Schistosoma mansoni Worm Infection Regulates the Intestinal Microbiota and Susceptibility to Colitis. Infect. Immun 2019;87,e00275–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Chen J, Xu Y, Zhou H, Huang P, Ma Y, Gao M, Cheng S, Zhou H, Lv Z. Alterations of Gut Microbiome and Metabolite Profiling in Mice Infected by Schistosoma japonicum. Front. Immunol 2020;11,569727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg RD. Bacterial Translocation from the Gastrointestinal Tract. Adv Exp Med Biol 1999;473:11–30. [DOI] [PubMed] [Google Scholar]
- 29.Kapur S, Paik E, Rezaei A, Vu DN. Where There Is Blood, There Is a Way: Unusual Collateral Vessels in Superior and Inferior Vena Cava Obstruction. RadioGraphics 2010;30,67–78. [DOI] [PubMed] [Google Scholar]
- 30.Ranchoux B, Bigorgne A, Hautefort A, Girerd B, Sitbon O, Montani D, Humbert M, Tcherakian C, Perros F. Gut-Lung Connection in Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol 2017;56,402–405. [DOI] [PubMed] [Google Scholar]
- 31.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells – conditional innate immune cells. J. Hematol. Oncol 2013;6,61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razakandrainibe R, Pelleau S, Grau GE, Jambou R. Antigen presentation by endothelial cells: What role in the pathophysiology of malaria? Trends Parasitol 2012;28,151–160. [DOI] [PubMed] [Google Scholar]
- 33.Crosby A, Jones FM, Southwood M, Stewart S, Schermuly R, Butrous G, Dunne DW, Morrell NW. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am. J. Respir. Crit. Care Med 2010;181,279–288. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun 2017;8,15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham BB, Chabon J, Bandeira A, Espinheira L, Butrous G, Tuder RM. Significant Intrapulmonary Schistosoma Egg Antigens are not Present in Schistosomiasis-Associated Pulmonary Hypertension. Pulm. Circ 2011;1,456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019;7,14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sencio V, Machado MG, Trottein F. The lung–gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol 2021;14,296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chioma OS, Hesse LE, Chapman A, Drake WP. Role of the Microbiome in Interstitial Lung Diseases. Front. Med 2021;8,595522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, Xu L, Zeng Y, Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol 2021;91,107272. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert JA, laser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat. Med 2018;24,392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, Raizada MK. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension 2020;75,1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol 2020;2020,1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol 2021;19,55–71. [DOI] [PubMed] [Google Scholar]
- 44.Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun 2021;22,289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol 2020;10,572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei L, Wen XS, Xian CJ. Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways. Int. J. Mol. Sci 2021;22,9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Yu X, Liu X, Liu G, Zeng K, Wang G. Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci. Rep 2021;11,18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng D, Su H, Song Y, Chen T, Sun Q, Jiang H, Zhao M. Altered Fecal Microbiota Correlated With Systemic Inflammation in Male Subjects With Methamphetamine Use Disorder. Front. Cell. Infect. Microbiol 2021;11,783917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihajlovic J, Leutner M, Hausmann B, Kohl G, Schwarz J, Röver H, Stimakovits N, Wolf P, Maruszczak K, Bastian Ml. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ. Microbiol 2021;23,3037–3047. [DOI] [PubMed] [Google Scholar]
- 50.Vujkovic-Cvijin I, Sortino O, Verheij E, Sklar J, Wit FW, Kootstra NA, Sellers B, Brenchley JM, Ananworanich J, Loeff MSV, Belkaid Y, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun 2020;11,2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crakes KR, Jiang G. Gut Microbiome Alterations During HIV/SIV Infection: Implications for HIV Cure. Front. Microbiol 2019;10,1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, Wack A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep 2019;28,245–256.e4. [DOI] [PubMed] [Google Scholar]
- 53.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci 2011;108,5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70,698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathieu E, Escribano-Vazquez U, Descamps D, Cherbuy C, Langella P, Riffault S, Remot A, Thomas M. Paradigms of Lung Microbiota Functions in Health and Disease, Particularly, in Asthma. Front. Physiol 2028;9,1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am. J. Respir. Crit. Care Med 2011;184,957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandenborght LE, Enaud R, Coron N, Denning D, Delhaes L. From culturomics to metagenomics: the mycobiome in chronic respiratory diseases. in The Lung Microbiome 88–118 (European Respiratory Society, 2019). doi: 10.1183/2312508X.10015918. [DOI] [Google Scholar]
- 58.O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol 2016;196,4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gollwitzer ES, aglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, Marsland BJ. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med 2014;20,642–647. [DOI] [PubMed] [Google Scholar]
- 60.de Waal GM, de Villiers WJS, Forgan T, Roberts T, Pretorius E. Colorectal cancer is associated with increased circulating lipopolysaccharide, inflammation and hypercoagulability. Sci. Rep 2020;10,8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macutkiewicz C, Carlson G, Clark E, Dobrindt U, Roberts I, Warhurst G. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect 2008;10,424–431. [DOI] [PubMed] [Google Scholar]
- 62.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol 2020;10,9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertin B, Desreumaux P, Dubuquoy L. Obesity, visceral fat and Crohnʼs disease. Curr. Opin. Clin. Nutr. Metab. Care 2010;13,574–580. [DOI] [PubMed] [Google Scholar]
- 64.Gambero A, Maróstica M, Saad MJA, Pedrazzoli J. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm. Bowel Dis 2007;13,1357–1364. [DOI] [PubMed] [Google Scholar]
- 65.Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS One 2012;7,e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coffey JC, Walsh D, Byrnes KG, Hohenberger W, Heald RJ. Mesentery — a ‘New’ organ. Emerg. Top. Life Sci 2020;4,191–206. [DOI] [PubMed] [Google Scholar]
- 67.Zimmerman P, Huseynova K, Pillai L. Anatomy and Physiology of the Mesenteric Circulation. in Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set 1014–1026 (Elsevier, 2019). doi: 10.1016/B978-0-323-40232-3.00086-8. [DOI] [Google Scholar]
- 68.Loeffler DA, Lundy SK, Singh KP, Gerard HC, Hudson AP, Boros DL. Soluble egg antigens from Schistosoma mansoni induce angiogenesis-related processes by up-regulating vascular endothelial growth factor in human endothelial cells. J. Infect. Dis 2002;185,1650–1656. [DOI] [PubMed] [Google Scholar]
- 69.Webster JP, Shrivastava J, Johnson PJ, Blair L. Is host-schistosome coevolution going anywhere? BMC Evol. Biol 2007;7,91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takaki KK, Rinaldi G, Berriman M, Pagán AJ, Ramakrishnan L. Schistosoma mansoni Eggs Modulate the Timing of Granuloma Formation to Promote Transmission. Cell Host Microbe 2021;29,58–67.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014;383,2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira SDS, Castellon M, Chen J, Bonini MG, Gu X, Elliott MH, Machado RF, Minshall RD. Inflammation-induced caveolin-1 caveolin-1 and BMPRII depletion and BMPRII depletion promotes endothelial dysfunction and TGF-β-driven pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol 2017;312,L760–L771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrell NW. Pulmonary Hypertension Due to BMPR2 Mutation: A New Paradigm for Tissue Remodeling? Proc. Am. Thorac. Soc 2006;3,680–686. [DOI] [PubMed] [Google Scholar]
- 74.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun 2017;8,14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat. Med 2015;21,777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation 2016;133,1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, et al. BMPR2 Preserves Mitochondrial Function and DNA during Reoxygenation to Promote Endothelial Cell Survival and Reverse Pulmonary Hypertension. Cell Metab 2015;21,596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian W, Jiang X, Sung YK, Shuffle E, Wu TH, Kao PN, Tu AB, Dorfmüller P, Cao A, Wang L, et al. Phenotypically Silent Bone Morphogenetic Protein Receptor 2 Mutations Predispose Rats to Inflammation-Induced Pulmonary Arterial Hypertension by Enhancing the Risk for Neointimal Transformation. Circulation 2019;140,1409–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Alexander PB, Wang XF. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol 2017;9,a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okumura N, Hashimoto K, Kitahara M, Okuda H, Ueda E, Watanabe K, Nakahara M, Sato T, Kinoshita S, Tourtas T, et al. Activation of TGF-β signaling induces cell death via the unfolded protein response in Fuchs endothelial corneal dystrophy. Sci. Rep 2017;7,6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am. J. Pathol 2011;179,1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliveira SDS, Chen J, Castellon M, Mao M, Raj JU, Comhair S, Erzurum S, Silva CLM, Machado RF, Bonini MG, et al. Injury-Induced Shedding of Extracellular Vesicles Depletes Endothelial Cells of Cav-1 (Caveolin-1) and Enables TGF-β (Transforming Growth Factor-β)–Dependent Pulmonary Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol 2019;39,1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma J, Sanchez-Duffhues G, Goumans MJ, ten Dijke P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol 2020;8,260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol 2010;221,3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orriols M, Gomez-Puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell. Mol. Life Sci 2017;74,1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elmore S Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol 2007;35,495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Browne LE, Compan V, Bragg L, North RA. P2X7 Receptor Channels Allow Direct Permeation of Nanometer-Sized Dyes. J. Neurosci 2013;33,3557–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC. The role of the P2X7 receptor in infectious diseases. PLoS Pathog 2011;7,e1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schachter J, Motta AP, de Souza Zamorano A, da Silva-Souza HA, Guimarães MZ, Persechini PM. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. J. Cell Sci 2008;121,3261–3270. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q P2X7 receptor-mediated apoptosis of human cervical epithelial cells. AJP Cell Physiol 2004;287,C1349–C1358. [DOI] [PubMed] [Google Scholar]
- 91.Oliveira SDS, Coutinho-Silva R, Silva CLM. Endothelial P2X7 receptors’ expression is reduced by schistosomiasis. Purinergic Signal 2013;9,81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oliveira SDS, Quintas LE, Amaral LS, Noël F, Farsky SH, Silva CL. Increased endothelial cell-leukocyte interaction in murine schistosomiasis: Possible priming of endothelial cells by the disease. PLoS One 2011;6,e23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trottein F, Descamps L, Nutten S, Dehouck MP, Angeli V, Capron A, Cecchelli R, Capron M. Schistosoma mansoni activates host microvascular endothelial cells to acquire an anti-inflammatory phenotype. Infect. Immun 1999;67,3403–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oswald IP, Eltoum I, Wynn TA, Schwartz B, Caspar P, Paulin D, Sher A, James SL. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc. Natl. Acad. Sci 1994;91,999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figliuolo da Paz VR, Figueiredo-Vanzan D, dos Santos Pyrrho A. Interaction and involvement of cellular adhesion molecules in the pathogenesis of Schistosomiasis mansoni. Immunol. Lett 2019;206,11–18. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira SDA, Coutinho-Silva R, Silva CL. Endothelial P2X7 receptors’ expression is reduced by schistosomiasis. Purinergic Signal 2013;9,81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oliveira SDS, Oliveira NF, Meyer-Fernandes JR, Savio LE, Ornelas FG, Ferreira ZS, Coutinho-Silva R, Silva CL. Increased expression of NTPDases 2 and 3 in mesenteric endothelial cells during schistosomiasis favors leukocyte adhesion through P2Y1 receptors. Vascul. Pharmacol 2016;82,66–72. [DOI] [PubMed] [Google Scholar]
- 98.Kumar R, Mickael C, Kassa B, Sanders L, Koyanagi D, Hernandez-Saavedra D, Freeman S, Morales-Cano D, Cogolludo A, McKee AS, et al. Th2 CD4 + T Cells Are Necessary and Sufficient for Schistosoma Pulmonary Hypertension. J. Am. Heart Assoc 2019;8,e013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takagi K, Yamakuchi M, Matsuyama T, Kondo K, Uchida A, Misono S, Hashiguchi T, Inoue H. IL-13 enhances mesenchymal transition of pulmonary artery endothelial cells via down-regulation of miR-424/503 in vitro. Cell. Signal 2018;42,270–280. [DOI] [PubMed] [Google Scholar]
- 100.Asosingh K, Cheng G, Xu W, Savasky BM, Aronica MA, Li X, Erzurum SC. Nascent Endothelium Initiates Th2 Polarization of Asthma. J. Immunol 2013;190,3458–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crosby A, Soon E, Jones FM, Southwood MR, Haghighat L, Toshner MR, Raine T, Horan I, Yang P, Moore S, et al. Hepatic Shunting of Eggs and Pulmonary Vascular Remodeling in Bmpr2 +/− Mice with Schistosomiasis. Am. J. Respir. Crit. Care Med 2015;192,1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grabe K, Haas W. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int. J. Parasitol 2004;34,927–934. [DOI] [PubMed] [Google Scholar]
- 103.Turner JD, Narang P, Coles MC, Mountford AP. Blood Flukes Exploit Peyer’s Patch Lymphoid Tissue to Facilitate Transmission from the Mammalian Host. PLoS Pathog 2012;8,e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Graham BB, Bandeira AP, Morrell NW, Butrous G, Tuder RM. Schistosomiasis-Associated Pulmonary Hypertension. Chest 2010;137,20S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol. Rev 2004;201,156–167. [DOI] [PubMed] [Google Scholar]
- 106.Holzscheiter M, Layland LE, Loffredo-Verde E, Mair K, Vogelmann R, Langer R, Wagner H, Prazeres da Costa C. Lack of host gut microbiota alters immune responses and intestinal granuloma formation during schistosomiasis. Clin. Exp. Immunol 2014;175,246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol 2005;27,265–270. [DOI] [PubMed] [Google Scholar]
- 108.Rivadeneira DJ, Luo H. Jejunal Ulcer Caused by Schistosoma japonicum. Case Rep. Gastrointest. Med 2019;2019,1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feldmeier H, Krantz I, Poggensee G. Female Genital Schistosomiasis as a Risk-Factor for the Transmission of HIV. Int. J. STD AIDS 1994;5,368–372. [DOI] [PubMed] [Google Scholar]
- 110.Jenkins TP, Peachey LE, Ajami NJ, MacDonald AS, Hsieh MH, Brindley PJ, Cantacessi C, Rinaldi G. Schistosoma mansoni infection is associated with quantitative and qualitative modifications of the mammalian intestinal microbiota. Sci. Rep 2018;8,12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishizaka A, Koga M, Mizutani T, Parbie PK, Prawisuda D, Yusa N, Sedohara A, Kikuchi T, Ikeuchi K, Adachi E, et al. Unique Gut Microbiome in HIV Patients on Antiretroviral Therapy (ART) Suggests Association with Chronic Inflammation. Microbiol. Spectr 2021;9,e0070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric Lymph Nodes Seen at Imaging: Causes and Significance. RadioGraphics 2005;25,351–365. [DOI] [PubMed] [Google Scholar]
- 113.Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, Barreiro P, Mura MS, Babudieri S, et al. Severe Liver Disease Associated With Prolonged Exposure to Antiretroviral Drugs. JAIDS J. Acquir. Immune Defic. Syndr 2006;42,177–182. [DOI] [PubMed] [Google Scholar]
- 114.Scourfield A, Waters L, Holmes P, Panos G, Randell P, Jackson A, Mandalia S, Gazzard B, Nelson M. Non-cirrhotic portal hypertension in HIV-infected individuals. Int. J. STD AIDS 2011;22,324–328. [DOI] [PubMed] [Google Scholar]
- 115.Twigg HL, Weinstock GM, Knox KS. Lung microbiome in human immunodeficiency virus infection. Transl. Res 2017;179,97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callejo M, Mondejar-Parreño G, Barreira B, Izquierdo-Garcia JL, Morales-Cano D, Esquivel-Ruiz S, Moreno L, Cogolludo Á, Duarte J, Perez-Vizcaino F. Pulmonary Arterial Hypertension Affects the Rat Gut Microbiome. Sci. Rep 2018;8,9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong W, Mo Q, Wang L, Peng F, Zhou Y, Zou W, Sun R, Liang C, Zheng M, Li H, et al. Changes in the gut microbiome and metabolome in a rat model of pulmonary arterial hypertension. Bioengineered 2021;12,5173–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ranchoux B, Bigorgne A, Hautefort A, Girerd B, Sitbon O, Montani D, Humbert M, Tcherakian C, Perros F. Gut–Lung Connection in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol 2017;56,402–405. [DOI] [PubMed] [Google Scholar]
- 119.Thenappan T, Khoruts A, Chen Y, Weir EK. Can intestinal microbiota and circulating microbial products contribute to pulmonary arterial hypertension? Am. J. Physiol. Circ. Physiol 2019;317,H1093–H1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cohen MD, Rubin LJ, Taylor WE, Cuthbert JA. Primary pulmonary hypertension: an unusual case associated with extrahepatic portal hypertension. Hepatology 1983;3,588–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


