Abstract
Objective:
We sought to evaluate the effect of 52 weeks of exenatide extended release (XR) on maintenance of meal replacement therapy induced BMI reduction in adolescents with severe obesity.
Methods:
In this randomized, double-blind, placebo-controlled trial, 100 participants aged 12-<18 years with BMI ≥1.2 × 95th percentile were enrolled in a short-term meal replacement therapy run-in phase. Those who achieved ≥5% BMI reduction during the run-in were then randomized to 52 weeks of exenatide XR 2.0 mg vs. placebo. Both groups also received lifestyle therapy. The pre-specified primary endpoint was mean percent change in BMI from randomization (post run-in) to 52 weeks in the intention-to-treat population.
Results:
100 participants were enrolled and 66 (mean age 16±1.5 years; 47% female) achieved ≥5% BMI reduction with meal replacement therapy and were randomized (33 to exenatide XR and 33 to placebo). From randomization (post run-in) to 52-weeks, mean BMI increased 4.6% and 10.1% in the exenatide XR and placebo groups, respectively. The placebo-subtracted exenatide XR treatment effect was −4.1% (95% CI [−8.6 to 0.5], P=0.078).
Conclusions:
Though not achieving statistical significance, exenatide XR vs. placebo may partly mitigate the propensity toward BMI rebound in adolescents who achieved initial weight loss with dietary intervention.
Keywords: weight maintenance, glucagon like peptide-1, exenatide, meal replacement, obesity, adolescent
Introduction
Severe obesity (defined as a body mass index [BMI] ≥ 1.2 × 95th age- and sex-specific percentile or ≥ 35 kg/m2) is a serious, chronic, and relapsing disease that afflicts nearly 8% of adolescents in the United States.1 While the cornerstone of obesity management in youth is lifestyle therapy, only a small percentage of individuals with severe obesity are able to achieve and maintain clinically significant BMI reduction with this intervention alone.2 Indeed, long-term weight loss maintenance is elusive for most individuals with obesity because of numerous biological adaptations such as increased hunger and enhanced perception of food palatability, as well as reduced satiety and resting energy expenditure. To address these counter-regulatory processes and support weight loss maintenance, various anti-obesity medications have been used successfully in adults with obesity.3–5
One of the most promising candidate classes of medications for blunting the physiological adaptations that promote weight regain, and therefore, for supporting weight loss maintenance, is the glucagon-like peptide-1 receptor agonist (GLP-1RA). GLP-1RAs reduce appetite through activation of GLP-1 receptors in the hypothalamus, enhance satiety by slowing gastric emptying and inhibit reward pathways associated with hedonic eating.6–8 Moreover, GLP-1RAs, via weight loss dependent and independent mechanisms, improve many obesity-associated risk factors and complications such as impaired glucose tolerance, insulin resistance, hypertension, vascular dysfunction, and inflammation.9–11
The purpose of this randomized, double-blind, placebo-controlled clinical trial was to evaluate the effect of the GLP-1RA, exenatide extended release (XR), on the maintenance of BMI reduction and cardiometabolic risk factor improvement that was induced by a short-term run-in phase consisting of meal replacement therapy (MRT) among adolescents with severe obesity. We hypothesized that following ≥5% BMI reduction achieved during an MRT run-in phase: 1) those randomized to exenatide XR compared to placebo would demonstrate superior maintenance of MRT-induced BMI reduction at 52 weeks; 2) a higher proportion of those randomized to exenatide XR compared to placebo would maintain the MRT-induced BMI reduction (≥5%) at 52 weeks; and 3) exenatide XR compared to placebo would result in superior maintenance of MRT-induced improvements in body fat, blood pressure, triglyceride/HDL ratio, fasting glucose, fasting insulin, hemoglobin A1c, and quality of life (QOL) at 52 weeks.
Methods
Trial Design
This study, conducted in a single U.S. academic health center, was a double-blind, randomized, placebo-controlled clinical trial designed to examine the effect of 52 weeks of exenatide XR on improving the maintenance of BMI reduction induced by a short-term MRT run-in phase. Participants had up to 8 weeks to achieve ≥5% BMI reduction with MRT during the run-in phase.12 Only those who achieved this benchmark at the 4-, 6-, or 8-week time point were randomized 1:1 to either exenatide XR or placebo for an additional 52 weeks; both treatment arms also received lifestyle therapy for 52 weeks. The study protocol was approved by the University of Minnesota Institutional Review Board and registered at ClinicalTrials.gov (NCT02496611). The only notable protocol change after trial commencement was reducing the maximum duration of the MRT run-in from 12 to 8 weeks because our experience indicated that if the MRT BMI reduction goal was not achieved by 8 weeks, it was very unlikely to be achieved by 12 weeks.
Participants
Participant inclusion criteria included: ages 12 to <18 years and BMI ≥1.2 × 95th percentile (based on CDC-derived age and sex norms) or ≥35 kg/m2, whichever was lower. Key exclusion criteria included: Tanner stage <2; type 1 or 2 diabetes mellitus; previous (within 6 months) or current use of medications used primarily for weight loss; history of bariatric surgery; and dose changes in medications for dyslipidemia, prediabetes, or hypertension within the prior 6 months.
Participants were recruited via letters mailed to potentially eligible individuals seen in the University of Minnesota, MHealth Fairview health system and Children’s Hospitals and Clinics of Minnesota health system. Additionally, healthcare providers from the Minnesota Pediatric Obesity Consortium recruited potentially eligible participants from their respective clinics. Participants and their parents/legal guardians provided written informed assent and consent, respectively, before enrollment.
Intervention and Procedures
Meal Replacement Therapy Run-in Phase
Upon enrollment, all participants were instructed to adhere to an MRT plan for at least 4 weeks, and up to 8 weeks, with the goal of achieving ≥5% BMI reduction.12 The prescribed eating plan consisted of three liquid shakes, two pre-packaged low calorie frozen entrée meals, and two servings of fruit and three servings of vegetables per day for a total of approximately 1,400 kcals per day. Shakes and frozen meals were provided free of charge to the participants.
Randomization Procedure
Participants who achieved ≥5% BMI reduction after 4–8 weeks of the MRT run-in phase were randomized 1:1 to either exenatide XR plus lifestyle therapy or placebo plus lifestyle therapy for an additional 52 weeks. For those who did not achieve ≥5% BMI reduction by 8 weeks, study participation was terminated. Treatment allocation was blinded to the participants, investigators, coordinators, data collectors, and sponsors throughout the trial. Randomization, using permuted blocks of 2, 4, or 6, was computer-generated and codes were maintained by the University of Minnesota Investigational Drug Service Pharmacy.
Exenatide XR and Matching Placebo Injection
Exenatide XR, a long-acting GLP-1RA, is administered once per week via subcutaneous injection. It was initiated and maintained at a dose of 2.0 mg weekly, the FDA approved dose for treatment of type 2 diabetes mellitus in children ≥10 years of age and adults. Compliance was monitored via review of medication logs and visual inspection of returned injection devices. Exenatide XR and matching placebo devices were donated by Astra Zeneca Pharmaceuticals and distributed from University of Minnesota Investigational Drug Service Pharmacy. An Investigational New Drug license was obtained by the FDA.
Lifestyle Therapy
All participants received the same lifestyle therapy regardless of group assignment. Lifestyle therapy was delivered monthly at each in-person study visit and by telephone for months when there was no in-person visit. The curriculum was adapted from the National Institute of Diabetes and Digestive and Kidney Diseases-sponsored TODAY Study lifestyle therapy materials13 and has been used in previous clinical trials.11,14 Trained study coordinators delivered the therapy, which focused on making small, successive changes in dietary and physical activity behaviors supported by self-monitoring, goal setting, reinforcement for goal achievement, stimulus control, social support, problem solving, and motivational techniques. The intensity of the lifestyle therapy was designed to be practical and feasible in the clinic setting.
Data Collection
Primary and Secondary Outcomes
The pre-specified primary outcome was mean percent change in BMI (weight in kg divided by height in meters squared) from randomization (post-MRT run-in phase) to 52 weeks. Height and weight were measured with a calibrated, wall-mounted stadiometer and an electronic scale, respectively. The pre-specified secondary outcomes were changes from randomization to 52 weeks in the following: body composition (i.e., total body fat and visceral adipose tissue) measured with dual x-ray absorptiometry (DXA) (GE Healthcare, iDXA, Madison, WI, USA); blood pressure; fasting (≥8 hours) lipid profile, glucose, and insulin; hemoglobin A1c; and QOL. All blood assays were performed by the Fairview Diagnostics Laboratories, Fairview-University Medical Center, Minneapolis, MN – a Centers for Disease Control and Prevention certified laboratory. QOL was measured with Impact of Weight on Quality of Life (IWQOL) – Kids questionnaire. Scaled scores of IWQOL range from 0–100, with higher scores representing better quality of life.15 The mean clinically important difference in QOL is 4.8 for the overall total score.16
Safety Assessments
Adverse events were assessed monthly. The following safety measures were assessed at baseline, randomization, 26, and 52 weeks: Tanner stage, ECG, and gastrointestinal symptoms with the PedsQL™ Gastrointestinal Symptoms Scale (where the scores are transformed to a 0–100 scale and higher values indicate lower symptoms).17 Safety labs, heart rate, and blood pressure were assessed at baseline, randomization, 4, 12, 26, 39, and 52 weeks.
Sample Size
Based on our preliminary data using MRT in adolescents with obesity14 and results of two other trials,12 3 we conservatively anticipated that at least 60% of our enrolled sample would achieve the ≥5% BMI reduction goal, a clinically relevant outcome,18 during the MRT run-in phase, and would therefore be randomized. Considering a dropout rate of 20%, we enrolled 100 participants to have at least 60 randomized and complete follow-up data at 52 weeks on at least 48 individuals. Power associated with a placebo-subtracted BMI reduction of 5%, based on an overall sample size of 60 (30 in each treatment arm), was >90% using a standard deviation estimate of 6 from our pilot trials11,19 and a correlation between baseline and follow-up scores of 0.5.
Statistical Analysis
Descriptive summaries were tabulated for participants who initiated MRT, participants who were randomized, and separately by randomized assignment. These included mean and standard deviation for continuous variables and frequency with percentage for categorical variables. Primary endpoint was percent change in BMI from randomization (post-MRT) through 52 weeks. The primary analysis compared mean percent change between exenatide XR and placebo groups using the intention-to-treat (ITT) population, adjusting for randomization BMI for enhanced precision.20,21 BMI values of randomized participants who did not complete the study were imputed using the last observation carried forward (LOCF) approach, whereby the missing 52-week follow-up value was imputed using the most recent study visit for which data were available. Confidence intervals and p-values were obtained using robust variance estimation. Supplemental analyses were conducted using multiple imputation in place of LOCF-imputation. Additionally, results were analyzed using the per-protocol population, which included participants who maintained their assigned treatment, were without major protocol deviations, and completed the 52-week follow-up visit. Per-protocol analysis did not involve data imputation. A secondary endpoint comparing the odds of maintaining the ≥5% BMI reduction attained during the MRT phase through the 52-week follow-up between treatment groups was evaluated using logistic regression, also adjusting for BMI at randomization. Other secondary endpoints were analyzed similarly, with adjustment made for baseline value.
Adverse event data are reported for randomized participants, by treatment group, from randomization through week 52. The number of individuals who experienced at least one event and total number of events are included. Event rate used total number of exposure years, calculated by totaling length of time in the study for each participant to account for dropout. Data were managed using REDCap22 with analyses performed using R, v3.6.3.23
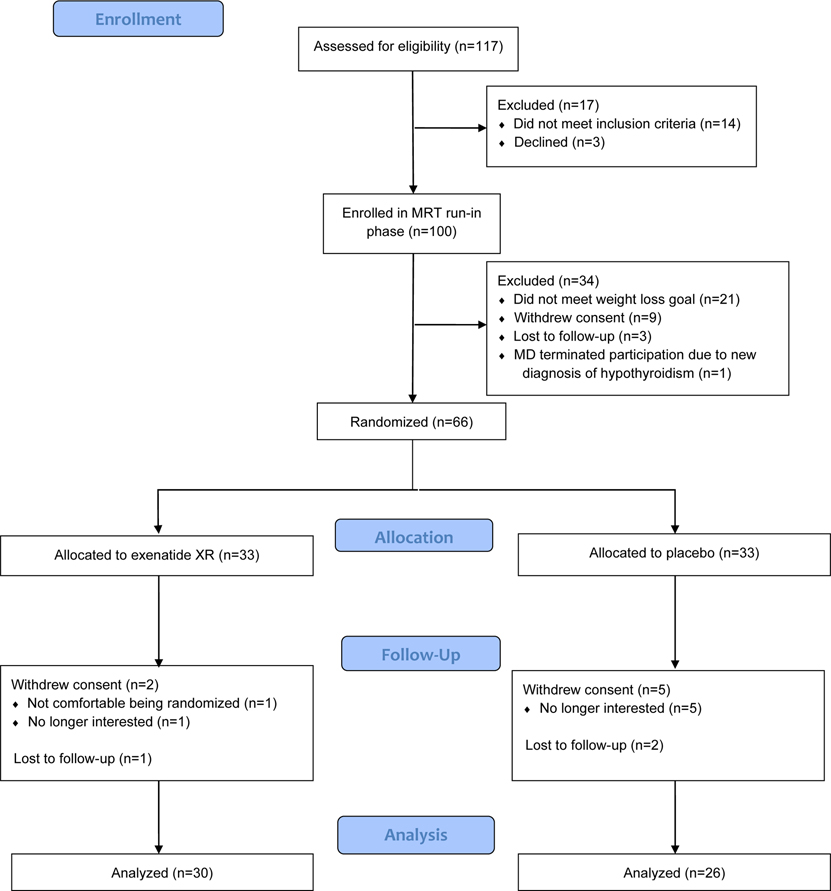
Results
Between December 3, 2015 and June 26, 2019, 100 participants were enrolled in the MRT run-in phase. Of these, 66 achieved the ≥5% BMI reduction goal. Of the 66 participants who achieved the goal, 33 participants were randomized to exenatide XR plus lifestyle therapy and 33 to placebo plus lifestyle therapy (Figure 1). The retention rate at 52 weeks was 91% for the exenatide XR group and 79% for the placebo group. All randomized participants maintained their assignments, and 29 and 25 participants in the exenatide XR and placebo groups, respectively, did not have significant protocol deviations and completed 52 weeks of intervention. For description of changes in anthropometrics, cardiometabolic profile and QOL during the MRT run-in phase, see Table S1.
Figure 1. CONSORT Flow Diagram.
Of the 66 participants randomized, mean age at randomization was 16.0±1.5 years, 53% were male, 88% self-identified as non-Hispanic/Latino, 82% as white, and 21% were eligible for free or reduced-cost school lunch (Table 1). Mean BMI at randomization was 36.9±4.4 kg/m2 or 131% of the 95th BMI percentile. Mean systolic and diastolic blood pressure, heart rate, fasting lipids, glucose, and insulin, and hemoglobin A1c at randomization were all within normal ranges. Mean IWQOL-Kids total score at randomization was 82.0±17.7.
Table 1.
Participant demographics, anthropometrics, body composition, cardiometabolic risk factors, and quality of life expressed as mean (SD) or frequency (%) where indicated.
| All Enrolled Participants (pre-MRT) | All Randomized Participants (post-MRT) | Randomized to Exenatide XR | Randomized to Placebo | |
|---|---|---|---|---|
| N = 100 | N = 66 | N = 33 (50%) | N = 33 (50%) | |
| Male | 47 (47%) | 35 (53%) | 15 (45%) | 20 (61%) |
| Age (years) | 15.8 (1.5) | 16 (1.5) | 15.9 (1.6) | 16.1 (1.5) |
| Tanner Stage | ||||
| Stage 2 | 3 (3%) | 2 (3%) | 1 (3%) | 1 (3%) |
| Stage 3 | 8 (8%) | 3 (5%) | 1 (3%) | 2 (6%) |
| Stage 4 | 20 (20%) | 9 (14%) | 4 (12%) | 5 (15%) |
| Stage 5 | 69 (69%) | 49 (74%)1 | 24 (73%) | 25 (76%) |
| Race/Ethnicity | ||||
| Non-Hispanic | 85 (85%) | 58 (88%) | 27 (82%) | 31 (94%) |
| Hispanic2 | 13 (13%) | 7 (11%) | 5 (15%) | 2 (6%) |
| White | 74 (74%) | 54 (82%) | 26 (79%) | 28 (85%) |
| Black | 8 (8%) | 5 (8%) | 3 (9%) | 2 (6%) |
| Asian | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| American Indian | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Race - Multiple | 10 (10%) | 5 (8%) | 3 (9%) | 2 (6%) |
| Race - missing | 4 (4%) | 2 (3%) | 1 (3%) | 1 (3%) |
| Race - Other | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Highest Level of Parent/Guardian Education3 | ||||
| Some High School | 4 (4%) | 2 (3%) | 2 (6%) | 0 (0%) |
| High School/GED | 32 (32%) | 21 (32%) | 7 (21%) | 14 (42%) |
| College Graduate | 52 (52%) | 35 (53%) | 20 (61%) | 15 (45%) |
| Postgraduate | 9 (9%) | 6 (9%) | 4 (12%) | 2 (6%) |
| Eligible for Free or Reduced Lunch4 | 26 (26%) | 14 (21%) | 7 (21%) | 7 (21%) |
| Height (cm) | 169.5 (8.8) | 171.2 (8.2) | 169.7 (8) | 172.7 (8.3) |
| Weight (kg) | 113.5 (20.4) | 108.5 (17.6) | 105.6 (17.7) | 111.4 (17.2) |
| BMI (kg/m2) | 39.4 (4.9) | 36.9 (4.4) | 36.5 (4.3) | 37.3 (4.6) |
| Percent of 95th BMI percentile | 140.4 (16.7) | 131.1 (14.5) | 129.5 (13.2) | 132.6 (15.8) |
| Body Composition5 | ||||
| Total Body Fat Mass (kg) | 53.1 (12.2) | 50.1 (11.1) | 48.8 (10.1) | 51.4 (11.9) |
| Percent Body Fat (%) | 49.1 (5.1) | 47.5 (5.5) | 47.7 (4.8) | 47.4 (6.1) |
| Visceral Adipose Tissue (kg) | 1.49 (0.77) | 1.29 (0.65) | 1.23 (.72) | 1.35 (0.57) |
| Subcutaneous Adipose Tissue (kg) | 51.60 (11.8) | 48.8 (10.6) | 47.5 (9.5) | 50.0 (11.6) |
| Total Lean Muscle Mass (kg) | 55.2 (9.9) | 54.9 (9.6) | 53.5 (10.1) | 56.4 (9.1) |
| SBP (mmHg) | 121(10) | 117(9) | 117(10) | 117(8) |
| DBP (mmHg) | 70 (8) | 66 (9) | 67 (8) | 65 (9) |
| Heart rate (bpm) | 78 (12) | 74 (12) | 73 (10) | 74 (14) |
| Total Cholesterol (mg/dL) | 159.5 (31.1) | 133.6 (24.6) | 130.7 (22.6) | 136.5 (26.5) |
| LDL-Cholesterol (mg/dL) | 95 (28.6) | 78.5 (23.4) | 75.6 (22.2) | 81.3 (24.6) |
| HDL-Cholesterol (mg/dL) | 43.8 (12.4) | 37.9 (14.6) | 37.8 (9.4) | 37.9 (18.6) |
| Triglycerides (mg/dL) | 105.9 (45.7) | 95.7 (33.1) | 90 (28.1) | 101.4 (37) |
| Triglyceride/HDL ratio | 2.7 (1.6) | 2.8 (1.4) | 2.6 (1.3) | 3 (1.5) |
| Glucose (mg/dL) | 78.5 (10.9) | 76.7 (8.9) | 76.7 (8.1) | 76.7 (9.7) |
| Insulin (μIU/mL)6 | 19.6 (11.5) | 11.6 (6.7) | 11.1 (4.8) | 12.1 (8.3) |
| Hemoglobin A1c (%) | 5.5 (0.3) | 5.2 (0.2) | 5.2 (0.2) | 5.2 (0.3) |
| IWQOL-Kids Total | 78.9 (14.5) | 82 (17.7) | 84.3 (11.1) | 79.8 (22.3) |
3 participants did not have Tanner stage readings following meal replacement therapy
2 participants did not indicate their ethnicity
3 participants did not indicate their highest level of parent/guardian education
2 participants did not indicate their eligibility for free or reduced lunch
1 participant did not have body composition readings at enrollment
3 participants did not have insulin readings at enrollment; 4 participants did not have insulin readings following meal replacement therapy.
To convert cholesterol to mmol/L, multiply values by 0.0259
To convert triglycerides to mmol/L, multiply values by 0.0113
To convert glucose to mmol/L, multiply values by 0.0555
To convert insulin to pmol/L, multiply values by 6.945
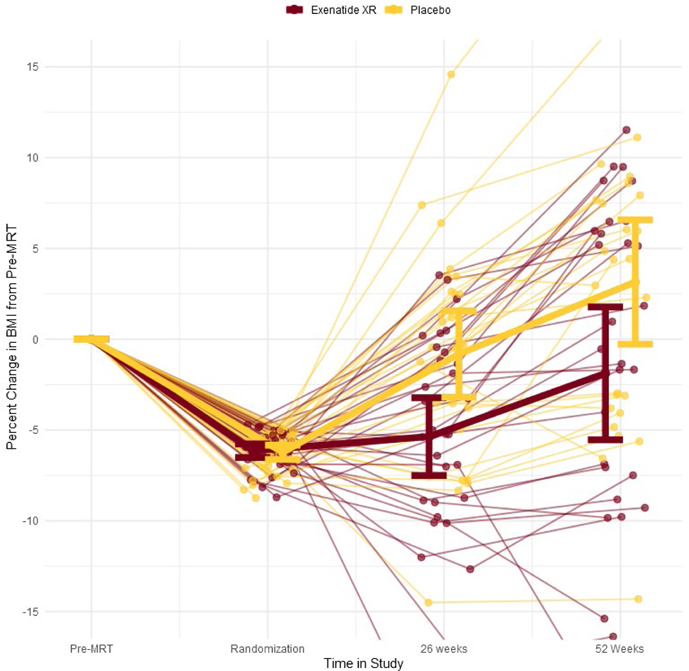
Using the ITT population, mean (SD) percent change in BMI from randomization to 52 weeks for the exenatide XR and placebo groups, respectively, were 4.6±10.5% and 10.1±9.0% (Figure 2). (See Figure S1 for change in weight.) Adjusted estimated treatment difference in percent change in BMI was −4.1 percentage points (95% confidence interval (CI) [−8.6, 0.5], P=0.078) (Table 2). Within the per-protocol population, estimated treatment difference was −5.7 percentage points (95% CI [−10.9, −0.5], P=0.03). Using multiple imputation (supportive analysis), the effect of exenatide XR on mean percent change in BMI from randomization to 52 weeks was estimated to be −4.4% (95% CI [−9.7, 1.0]; P=0.113). The odds of maintaining the MRT-induced ≥5% BMI reduction at 52 weeks was 2.3 times greater (95% CI [0.8, 7.0], P=0.136) with exenatide XR as compared to placebo. (See Figures S2 and S3 for graphs of absolute change in weight and percent change in weight from baseline (pre-MRT) to 52 weeks.) Of the cardiometabolic risk factors, only change in heart rate and TG/HDL-c ratio achieved statistical significance. The exenatide XR group experienced a mean 8 beats/minute increase in heart rate compared to 1 beat/minute increase for the placebo group; adjusted estimated treatment difference was 5.3 beats/minute (95% CI [0.5, 10], P=0.03). For TG/HDL-c ratio, adjusted estimated treatment difference was −0.61 (95% CI [−1.2, 0], P=0.05). The change in IWQOL-Kids total score and all sub-scales were not statistically significant (Table 2).
Figure 2.
Percent change in BMI from start of meal replacement therapy (MRT) run-in phase to 52 weeks among those randomized to exenatide XR and placebo groups with superimposed group means and 95% confidence intervals.
Table 2.
Estimated treatment effect of exenatide XR from randomization to week 52 in the intention-to-treat population, using last observation carried forward.
| Mean (SD) Change from Randomization to Week 52 | Estimated Treatment Difference (95% CI) | |||
|---|---|---|---|---|
| Exenatide XR N = 33 (50 %) |
Placebo N = 33 (50 %) |
(Exenatide XR – Placebo) | P-value | |
| BMI (%) | 4.6 (10.5) | 10.1 (9.0) | −4.1 (−8.6, 0.5) | 0.078 |
| Weight (kg) | 6.1 (11.4) | 12.4 (10) | −4.4 (−9.5, 0.6) | 0.087 |
| BMI (kg/m2) | 2.7 (13.2) | 9.6 (11.5) | −4.8 (−10.6, 0.9) | 0.098 |
| Percent of 95th BMI Percentile | 1.8 (3.9) | 3.7 (3.2) | −1.4 (−3.0, 0.2) | 0.096 |
| Total Fat Mass (kg) | 2.4 (8.2) | 6.4 (8) | −3.0 (−6.7, 0.7) | 0.108 |
| Percent Body Fat (%) | −0.7 (3.4) | 0.4 (3.2) | −1.1 (−2.5, 0.3) | 0.126 |
| Visceral Adipose Tissue (kg) | 0.2 (0.5) | 0.3 (0.4) | −0.2 (−0.4, 0.1) | 0.169 |
| Subcutaneous Adipose Tissue (kg) | 2.2 (7.8) | 6.1 (7.6) | −2.8 (−6.3, 0.7) | 0.111 |
| Total Lean Muscle Mass (kg) | 3.2 (4.3) | 5.5 (2.9) | −1.6 (−3.3, 0.2) | 0.082 |
| SBP (mmHg) | 4 (10) | 7 (10) | −3 (−7, 1) | 0.107 |
| DBP (mmHg) | 1 (10) | 3 (11) | −2 (−7, 2) | 0.343 |
| Total Cholesterol (mg/dL) | 19.9 (33.9) | 15.3 (19.3) | 4.4 (−7.3, 16) | 0.462 |
| LDL (mg/dL) | 14.2 (21.3) | 6.9 (18.4) | 5.8 (−2.2, 13.9) | 0.155 |
| HDL (mg/dL) | 9.1 (16.3) | 5.8 (29.7) | 6 (−4.3, 16.2) | 0.254 |
| art Rate (bpm) | 8 (10) | 1 (14) | 5 (0, 10) | 0.030 |
| Triglycerides (mg/dL) | 5.3 (39.6) | 19 (45.7) | −12.3 (−31.6, 7) | 0.213 |
| TG/HDL-c | −0.4 (1.2) | 0.2 (1.7) | −0.6 (−1.2, 0) | 0.050 |
| Glucose (mg/dL) | 4.9 (8.7) | 4.8 (8.9) | 0.5 (−2.6, 3.6) | 0.759 |
| Insulin (μIU/mL) | 9.5 (11.9) | 9.1 (10.1) | 0.9 (−4.4, 6.1) | 0.752 |
| Hemoglobin A1c (%) | 0.2 (0.3) | 0.1 (0.2) | 0 (−0.1, 0.1) | 0.880 |
| IWQOL-Kids – Total | −1.7 (19.4) | 4 (8.8) | −4.2 (−10.7, 2.4) | 0.210 |
To convert cholesterol to mmol/L, multiply values by 0.0259
To convert triglycerides to mmol/L, multiply values by 0.0113
To convert glucose to mmol/L, multiply values by 0.0555
To convert insulin to pmol/L, multiply values by 6.945
Overall, the percentage of randomized participants who experienced any adverse event was similar between groups (Table 3). Nausea, vomiting, and diarrhea were more commonly reported in the exenatide XR group compared to the placebo group. The PedsQL™ Gastrointestinal Symptoms Scale score decreased by 69 points in the exenatide XR group and increased 109 points in the placebo group. There was one serious adverse event in the exenatide XR group: a participant was hospitalized for chemical dependency treatment after a drug (not related to the study) and alcohol overdose.
Table 3.
Adverse events in randomized participants.
| Exenatide XR (N = 33) | Placebo (N = 33) | |||||
|---|---|---|---|---|---|---|
| Event | Number of Participants (%) | Number of Events | Events/Exposure-years | Number of participants (%) | Number of Events | Events/Exposure-years |
| Any Adverse Event | 32 (96.97) | 179 | 5.55 | 30 (90.91) | 141 | 4.918 |
| Serious Adverse Event | 1 (3.03) | 1 | 0.03 | 0 (0) | 0 | 0 |
| Nausea | 13 (39.39) | 15 | 0.47 | 7 (21.21) | 7 | 0.244 |
| Vomiting | 8 (24.24) | 10 | 0.31 | 2 (6.06) | 3 | 0.105 |
| Diarrhea | 11 (33.33) | 14 | 0.43 | 6 (18.18) | 6 | 0.209 |
| Constipation | 6 (18.18) | 6 | 0.19 | 6 (18.18) | 9 | 0.314 |
| Dyspepsia | 5 (15.15) | 5 | 0.16 | 4 (12.12) | 5 | 0.174 |
| Gastrointestinal disorders; other-abdominal pain | 3 (9.09) | 4 | 0.12 | 3 (9.09) | 3 | 0.105 |
| Dizziness | 4 (12.12) | 4 | 0.12 | 3 (9.09) | 3 | 0.105 |
| Flu Like Symptoms | 3 (9.09) | 3 | 0.09 | 4 (12.12) | 4 | 0.14 |
| Headache | 19 (57.58) | 20 | 0.62 | 14 (42.42) | 17 | 0.593 |
| Injection Site Reaction | 26 (78.79) | 32 | 0.99 | 24 (72.73) | 29 | 1.012 |
| Upper Respiratory Infection | 7 (21.21) | 7 | 0.22 | 13 (39.39) | 16 | 0.558 |
Discussion
The primary finding of the current trial is that exenatide XR improved the maintenance of BMI reduction achieved with a dietary intervention among adolescents with severe obesity, though contrary to our hypothesis, the treatment effect did not achieve statistical significance. Both the exenatide XR group and the placebo group on average demonstrated a rebound in BMI following the initial MRT run-in phase, underscoring the difficulty that many adolescents face in maintaining weight loss. Yet, though not statistically significant, the degree of BMI rebound after MRT was less than half with exenatide XR as compared to placebo at 52 weeks, with an estimated treatment difference exceeding 4% favoring exenatide XR. Further, and again not statistically significant, the odds of participants maintaining the MRT-induced ≥5% BMI reduction at 52 weeks was 2.3 times greater for the exenatide XR group compared to the placebo group.
To our knowledge, this is one of the first studies to examine a pharmacological intervention that specifically targets weight loss maintenance, as opposed to weight loss, in adolescents with obesity. This paradigm, however, has been studied in adults. Wadden et al. randomized 422 adults with obesity who initially lost ≥5% of their weight with a low-calorie diet to liraglutide 3.0 mg per day or placebo for 56 weeks.3 The liraglutide group lost an additional 6.2% of body weight while the placebo group maintained the initial 5% weight loss at the end of the intervention. The stark contrast in the experience of the adolescents in our trial compared to the adults in the Wadden et al. study is notable, especially for the placebo group in which the adolescents were not only unable to maintain their initial BMI reduction with lifestyle therapy, but experienced a significant BMI rebound exceeding the baseline value. The reasons for the differential experiences are unclear and require further study. It is possible that there may be developmental aspects unique to adolescence, such as relatively poor executive functioning,24 that make weight loss maintenance particularly challenging.
Regarding body composition, cardiometabolic risk factors, and QOL, we observed relatively modest changes, which did not achieve statistical significance, favoring exenatide XR over placebo in total body fat, visceral adipose tissue, blood pressure, and triglyceride/HDL ratio. Noteworthy, however, is that baseline mean values for most cardiometabolic risk factors and QOL were within normal ranges and these values remained in the normal ranges at the end of the MRT run-in phase. As with BMI, placing the changes in these secondary outcomes in the context of previous studies is challenging given that the objective of this trial was to examine an intervention for weight loss maintenance, not eliciting initial weight reduction. Yet, it is worth noting that the largest randomized, placebo-controlled trial of a GLP-1RA (liraglutide 3 mg) for adolescent obesity similarly showed only modest, non-statistically significant differences between the liraglutide and placebo arms in cardiometabolic variables and weight related QOL after 56 weeks of intervention.25
The overall adverse event rate was similar between the exenatide XR and placebo groups. A higher proportion of participants in the exenatide XR group experienced gastrointestinal symptoms, which is consistent with the mechanism of action of this class of medications. Additionally, compared to placebo, mean heart rate increased with exenatide XR, which is another well-documented phenomenon of GLP-1RAs. The single serious adverse event in the trial, a hospitalization related to chemical dependency, occurred in a participant in the exenatide XR group, and was deemed by the blinded medical safety officer to not be related to the study.
Strengths of this study included the explicit objective of examining a pharmacological intervention for weight loss maintenance in the pediatric population, which to our knowledge has not been studied. Additional strengths included the randomized and placebo-controlled design, the relatively large sample size for a pediatric obesity pharmacotherapy clinical trial, the high retention rate of participants after randomization (~85%), and the comprehensive assessments of total/regional body fat and cardiometabolic risk. Limitations of the trial included a lack of precision in the estimated treatment effect such that the confidence interval included both the null and meaningful differences between groups. The design of future studies would benefit from using this updated data on the degree of variability of this endpoint. Additionally, low representation of non-white participants places limits on the generalizability of the findings. Finally, there was no quantitative measure of adherence to the intervention, i.e. drug levels.
In conclusion, weight loss maintenance, particularly in adolescents, is a common and vexing problem and continues to be an understudied area of research. The current trial demonstrated the potentially useful role of GLP-1RAs to enhance weight loss maintenance in the management of adolescent severe obesity, though we did not demonstrate a statistically significant result. A majority of the participants were able to reduce their BMI by at least 5% through engagement in short-term MRT and those subsequently treated with exenatide XR may be less likely to experience significant weight regain as compared to placebo. It is possible that more potent GLP-1RAs would have more robust results given the data on weight loss in adults.26 Furthermore, the high degree of heterogeneity in treatment effect highlights the need for identifying predictors of response to pharmacotherapy for weight loss maintenance.
Supplementary Material
Study Importance:
What is already known?
Maintenance of BMI reduction with lifestyle therapy alone is elusive for most adolescents with severe obesity.
What does this study add?
Exenatide XR compared to placebo may partly mitigate weight regain after an initial diet-induced weight loss in adolescents with severe obesity.
How might these results change the focus of research:
More potent anti-obesity pharmacotherapy should be examined for enhancing weight loss maintenance in adolescents with severe obesity.
Acknowledgements
The authors would like to thank the participants and families for their time and effort and acknowledge the expert study coordination and data collection support provided by Cameron Naughton, Andrea Metzig, Annie Mathews, Kristin Flores, Rebecca Hollister, and Neyva Deolarte.
Funding:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases through award number 1R01DK105953 (PI: Kelly) and in part by NCATS award number UL1TR002494. Study medication (exenatide XR and matching placebo) was generously provided by AstraZeneca which did not have any no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Data sharing: Individual participant data that underlie the results reported in this article, after de-identification, will be available beginning 12 months and ending 36 months following article publication. Researchers who provide methodologically sound proposals should direct inquiries to kelly105@umn.edu.
Disclosures: Dr. Fox receives research support from Novo Nordisk and Rhythm Pharmaceuticals. Dr. Ryder receives a donation of drug and placebo from Boehringer Ingelheim. Dr. Gross received research support from Rhythm Pharmaceuticals. Dr. Bensignor receives research support from Vivus Pharmaceuticals. Drs. Sunni, Nathan, Dengel, and Rudser and Mr. Clark have no disclosures. Dr. Kelly currently serves as an unpaid consultant for Novo Nordisk, Vivus, Eli Lilly, and Boehringer Ingelheim; he has served as an unpaid consultant for WW; he has received donated drug and placebo from Astra Zeneca for the current project; he is receiving donated drug and placebo from Vivus for an ongoing NIDDK-funded clinical trial.
Clinical trial registration: ClinicalTrials.gov NCT02496611
References
- 1.Ogden CL, Fryar CD, Martin CB, et al. Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018. Jama. 2020;324(12):1208–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Archives of Pediatrics & Adolescent Medicine. 2012;166(12):1103–1108. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. International journal of obesity (2005). 2013;37(11):1443–1451. [DOI] [PubMed] [Google Scholar]
- 4.Astrup A, Caterson I, Zelissen P, et al. Topiramate: long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obesity research. 2004;12(10):1658–1669. [DOI] [PubMed] [Google Scholar]
- 5.Early JL, Apovian CM, Aronne LJ, et al. Sibutramine plus meal replacement therapy for body weight loss and maintenance in obese patients. Obesity (Silver Spring). 2007;15(6):1464–1472. [DOI] [PubMed] [Google Scholar]
- 6.Bojanowska E Physiology and pathophysiology of glucagon-like peptide-1 (GLP-1): the role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Medical science monitor : international medical journal of experimental and clinical research. 2005;11(8):Ra271–278. [PubMed] [Google Scholar]
- 7.Burcelin R, Gourdy P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(1):86–98. [DOI] [PubMed] [Google Scholar]
- 8.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(4):R465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. American journal of hypertension. 2010;23(3):334–339. [DOI] [PubMed] [Google Scholar]
- 10.Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes care. 2010;33(5):1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167(4):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz RI, Wadden TA, Gehrman CA, et al. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring, Md). 2011;19(6):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GROUP TS. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International journal of obesity (2005). 2010;34(2):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox CK, Kaizer AM, Rudser KD, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity (Silver Spring). 2016;24(12):2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolotkin RL, Zeller M, Modi AC, et al. Assessing weight-related quality of life in adolescents. Obesity (Silver Spring, Md). 2006;14(3):448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi AC, Zeller MH. The IWQOL-Kids(©): establishing minimal clinically important difference scores and test-retest reliability. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(2–2):e94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varni JW, Bendo CB, Denham J, et al. PedsQL gastrointestinal symptoms module: feasibility, reliability, and validity. Journal of pediatric gastroenterology and nutrition. 2014;59(3):347–355. [DOI] [PubMed] [Google Scholar]
- 18.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. International journal of obesity (2005). 2016;40(7):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly AS, Metzig AM, Rudser KD, et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity (Silver Spring). 2012;20(2):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med. 1992;11(13):1685–1704. [DOI] [PubMed] [Google Scholar]
- 21.Senn S Change from baseline and analysis of covariance revisited. Stat Med. 2006;25(24):4334–4344. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2020. [Google Scholar]
- 24.Mamrot P, Hanć T. The association of the executive functions with overweight and obesity indicators in children and adolescents: A literature review. Neurosci Biobehav Rev. 2019;107:59–68. [DOI] [PubMed] [Google Scholar]
- 25.Kelly AS, Auerbach P, Barrientos-Perez M, et al. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. The New England journal of medicine. 2020;382(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 26.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet (London, England). 2018;392(10148):637–649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.