Summary
Animals with diverse diets must adapt their food priorities to a wide variety of environmental conditions. This diet optimization problem is especially complex for predators that compete with prey for food. Although predator-prey competition is widespread and ecologically critical, it remains difficult to disentangle predatory and competitive motivations for attacking competing prey. Here, we dissect the foraging decisions of the omnivorous nematode Pristionchus pacificus to reveal that its seemingly failed predatory attempts against Caenorhabditis elegans are actually motivated acts of efficacious territorial aggression. While P. pacificus easily kills and eats larval C. elegans with a single bite, adult C. elegans typically survives and escapes bites. However, non-fatal biting can provide competitive benefits by reducing access of adult C. elegans and its progeny to bacterial food that P. pacificus also eats. We show that the costs and benefits of both predatory and territorial outcomes influence how P. pacificus decides which food goal, prey or bacteria, should guide its motivation for biting. These predatory and territorial motivations impose different sets of rules for adjusting willingness to bite in response to changes in bacterial abundance. In addition to biting, predatory and territorial motivations also influence which search tactic P. pacificus uses to increase encounters with C. elegans. When treated with an octopamine receptor antagonist, P. pacificus switches from territorial to predatory motivation for both biting and search. Overall, we demonstrate that P. pacificus assesses alternate outcomes of attacking C. elegans and flexibly reprograms its foraging strategy to prioritize either prey or bacterial food.
Keywords: Pristionchus pacificus, Caenorhabditis elegans, intraguild predation, foraging, territoriality, predation, behavioral flexibility, dopamine D2 receptors, octopamine receptors
Graphical Abstract
eTOC
Quach and Chalasani show that an omnivorous predator can switch between predatory and territorial foraging strategies for biting competing prey. Foraging decisions balance the costs and benefits of alternate outcomes of attacking prey in a specific resource context. Octopamine signaling mediates the switch from territorial to predatory foraging.
Introduction
Animals that exploit diverse food resources are more resilient to suboptimal environmental conditions than animals with specialized diets1,2. To benefit from versatile diets, animals must judge which diet composition maximizes the long-term ratio of energy intake to energy costs. Emphasis on calorie-rich and abundant foods is sufficient when foods are immobile and encountered sequentially3, but foraging contexts are often more complex. For example, hunting effort and travel time should also be considered when foods are mobile and simultaneously encountered4,5,6. In addition to acquiring foods, animals can indirectly prioritize foods by interfering with competitors. However, little is known about the strategies that guide foragers when these factors combine to produce a complex but naturalistic foraging problem: a predator competes with prey for food.
Omnivorous predators often hunt prey that consume another of the predator’s food choices. This predator-prey competition (intraguild predation) is widespread in many food webs7, and its effects on population dynamics and biodiversity are widely researched and debated8,9. Here, prey-killing simultaneously achieves dual food benefits: nutrition from prey corpses and reduced competition for shared resources8,10. Which of these predatory and competitive benefits is the dominant motivation for attacking prey? To rule out predatory motivation, studies report that competitor prey are left uneaten more often than non-competitor prey after killing11,12,13,14. To dismiss competitive motivation, other studies show that predators exhibit aggressive threat displays toward non-prey competitors but not toward competitor prey15. However, non-consumptive prey-killing can indirectly promote predation16, and threat displays are not required for competitive fights17. To resolve these conflicting results and disentangle the motivations that drive a predator to attack a competitor prey, more definitive and positive indicators are needed.
The predatory nematode Pristionchus pacificus (Figure 1A), its competitor prey Caenorhabditis elegans (Figures 1B and 1C), and shared bacterial food comprise a convenient laboratory system for investigating factors that influence an omnivorous predator’s diet decisions18. P. pacificus prefers to eat bacteria but also uses its teeth (Figures S1A and S1B) to attack and eat C. elegans19,20. In contrast, C. elegans lacks teeth (Figure S1C) and exclusively feeds on bacteria. While larval C. elegans are typically killed by a single bite (Figures 1D and 1E, and Video S1), adults are rarely killed and easily escape bites (Figures 1F and 1G, and Video S2). Here, we deconstruct P. pacificus foraging decisions to show that non-fatal adult-targeted bites are not failed predatory attempts, but are instead goal-directed acts of aggression to defend food territory. Overall, we demonstrate that P. pacificus is guided by factors that signal the trade-offs between biting outcomes, and flexibly switches between predatory and territorial strategies for biting C. elegans.
Figure 1. Non-fatal biting compels adult C. elegans to avoid predator-occupied bacteria.
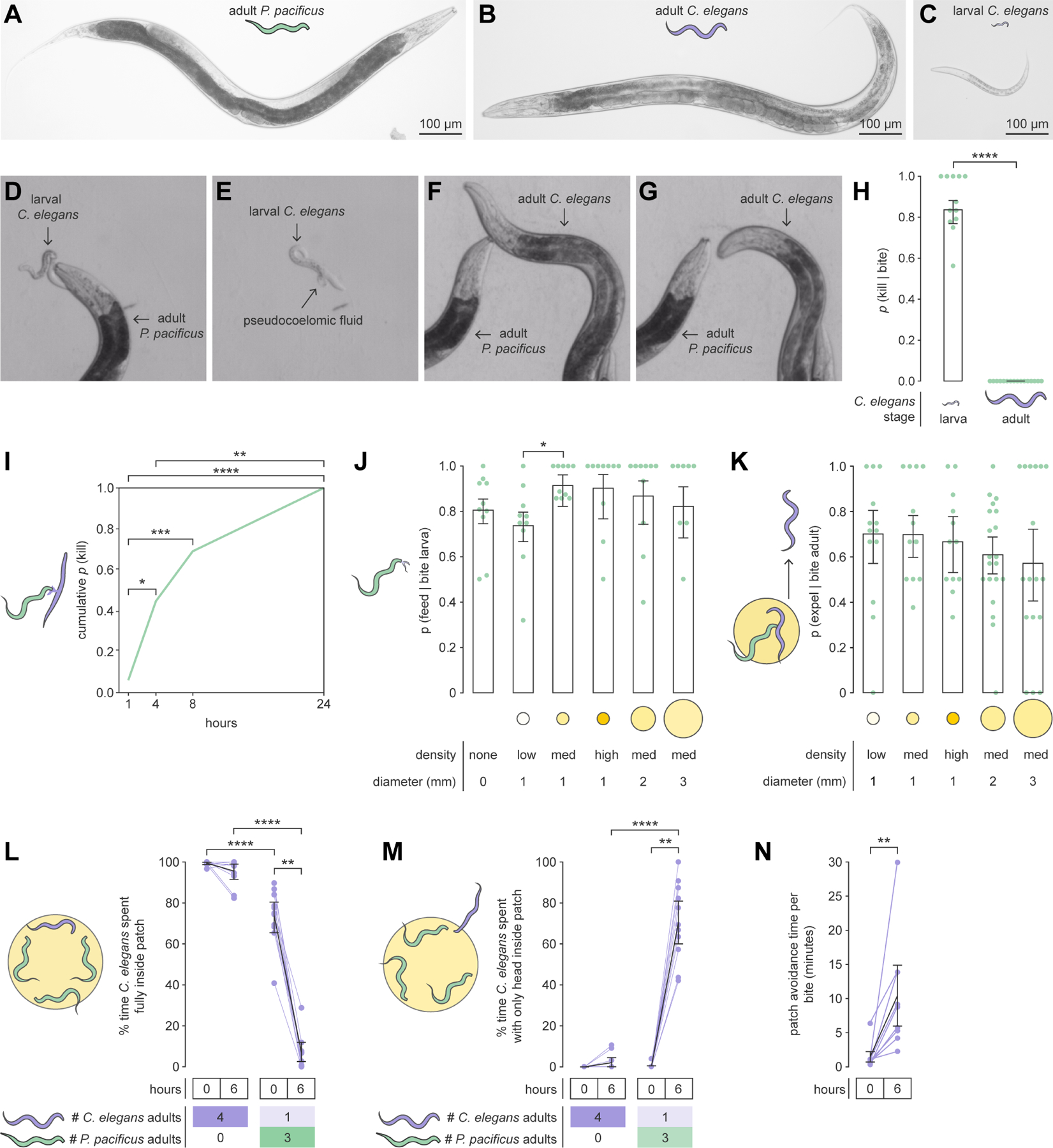
(A) Adult P. pacificus.
(B) Adult C. elegans.
(C) Larval C. elegans.
(D) P. pacificus biting larval C. elegans.
(E) Larval C. elegans after fatal bite.
(F) P. pacificus biting adult C. elegans.
(G) Adult C. elegans escaping non-fatal bite.
(H) Probability of killing during a bite (plotted data: kills/bites for individual P. pacificus) (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 12–16, nbites_per_P.pacificus = 3–39).
(I) Cumulative probability of killing adult prey (with repeated biting) by a certain time (Fisher’s exact test with Benjamini–Hochberg adjustment, nP.pacificus = 16).
(J) Probability of feeding after biting larval prey (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 9–10, nbites_per_P.pacificus = 1–31).
(K) Probability of adult prey exiting a bacterial patch after a bite (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 13–20, nbites_per_P.pacificus = 1–15).
(L–M) Adult prey residence on prey- or predator-inhabited bacterial patches (Wilcoxon’s signed rank test (paired) and Dunn’s test (unpaired) with Benjamini–Hochberg adjustment, nC.elegans = 11).
(N) Mean post-bite avoidance of bacterial patch (Wilcoxon’s signed rank test, nC.elegans = 9).
(H,J,K) Error bars are predicted probabilities and 95% CIs from binomial logistic regression models of data. Other error bars are 95% bootstrap CIs.
Results
Non-fatal biting compels adult C. elegans to avoid predator-occupied bacteria
To identify potential food-related benefits of biting, we first looked at the immediate outcomes of P. pacificus biting larval or adult C. elegans in a small arena. With prey as the only food (no bacteria), most larva-targeted bites were fatal, while adult-targeted bites rarely killed prey (Figures 1H and S1D). Even with all bites focused onto a single target, P. pacificus took ~ 6 hours (Figures 1I and S1E) and ~25 bites (Figure S1F) to kill adult prey. To assess the potential usefulness of biting for defending food, we introduced a small bacterial patch to the arena (Figures S1G to S1L). With or without bacteria, most larva-targeted bites led to prey-feeding (Figures 1J and S1M). Since adult-targeted bites rarely killed, we instead evaluated the efficacy of bites to expel adult prey from bacteria. The majority of bites led to adult prey exiting the bacterial patch (Figures 1K and S1N, and Video S3). Since successful predation also eliminates competition, larva-targeted biting simultaneously achieves both predatory and territorial benefits with relative ease. In adult-targeted biting, predation is rare and labor-intensive, but competitors can be expelled from bacteria without killing.
To explore the long-term effects of non-fatal biting on patch-leaving, we placed adult C. elegans with or without P. pacificus for 6 hours on a small bacterial patch. Without predators, adult prey almost always resided inside the patch (Figure 1L). This decreased upon initial predator exposure, but adult prey still mostly resided inside the patch (Figures 1L and S2A). After 6 hours of predator exposure, adult prey almost completely avoided entering the patch (Figures 1L), with only the head contacting the patch (Figures 1M, S2B, and S2C). Additionally, bites that occurred at 6 hours induced fivefold longer patch avoidance time (Figure 1N), suggesting that adult prey were conditioned to associate the patch with danger. Non-biting encounters were rarely followed by patch-leaving (Figure S2D). Thus, long-term non-fatal biting of adult C. elegans induces persistent bacteria avoidance.
Progeny of predator-exposed adult C. elegans have reduced access to bacteria
For P. pacificus to obtain meaningful territorial benefits from non-fatal biting, its relative fitness must exceed that of C. elegans. We speculated that biting-induced patch avoidance would force adult prey to lay eggs away from food. To test this, we developed an egg distribution assay (Figures 2A, and S2E to S2H) to measure where eggs were laid relative to a small bacterial patch over a 7-hour period (before eggs hatched). Conspecific groups of only predator or only prey adults laid most eggs inside bacteria, indicating absence of within-species territoriality (Figure 2B). In mixed-species groups, however, C. elegans was more likely to lay eggs outside bacteria as predator:prey ratio increased (Figures 2B and 2C), shifting the spatial distribution of prey eggs away from bacteria (LRT on linear mixed-effects model, χ2 = 42.594, df = 3, p = 3.001e-09; Figures 2B and 2D). Meanwhile, P. pacificus egg distribution was unaffected by predator:prey ratio (LRT on linear mixed-effect model, χ2 = 5.1518, df = 3, p = 0.161; Figure 2B). Number of eggs laid per adult remained unchanged (Figure S2I), suggesting that P. pacificus rarely ate eggs. Thus, biting interferes with C. elegans egg-laying on bacteria.
Figure 2. Progeny of predator-exposed adult C. elegans have reduced access to bacteria.
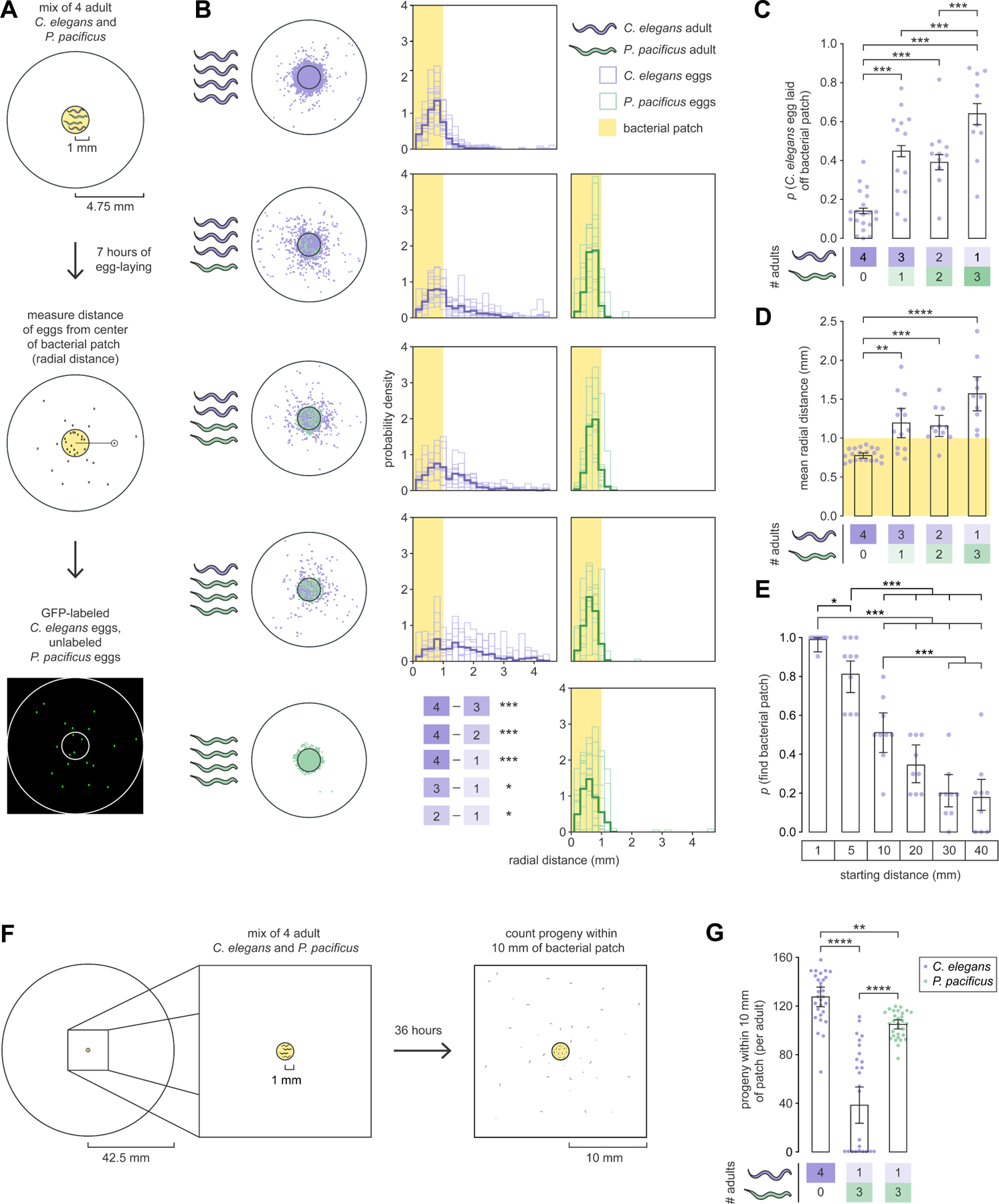
(A) Egg distribution assay.
(B) Egg distributions. Circular plots show actual egg locations (outer circle: arena, inner circle: bacterial patch). Histogram plots represent distributions of egg radial distances (light: individual arenas, dark: pooled across arenas, yellow shading: bacterial patch) (Wald test with single-step adjustment for Tukey contrasts, narena = 10–20).
(C) Probability of C. elegans egg being laid off bacteria (Wald test with single-step adjustment for Tukey contrasts, narena = 10–20, neggs_per_arena = 12–166).
(D) Mean egg radial distance (yellow shading: bacterial patch) (Dunn’s test, narena = 10–20). (E) Probability of newly hatched larva finding a small bacterial patch within 36 hours, from various starting distances (Wald test with single-step adjustment for Tukey contrasts, narena = 9, nlarvae_per_arena = 10–11).
(F) Extended egg distribution assay.
(G) Number of progeny per adult within 10 mm of a small bacterial patch (Dunn’s test, narena = 29–30).
(C,E) Error bars are predicted probabilities and 95% CIs from binomial logistic regression models of data.
Other error bars are 95% bootstrap CIs.
See also Figure S2.
We next asked whether prey larvae would struggle to find food from afar. We gently placed clean, newly hatched larvae at various distances from a small bacterial patch and counted how many found bacteria within 36 hours. Reproductive development arrests if food is not encountered by 36 hours (‘dauer’ state)21. From 10 mm away, larvae had only a ~0.5 probability of finding bacteria, with lower probabilities at farther distances (Figure 2E). To verify that predator exposure induces egg-laying at these unfavorable distances, we spatially and temporally extended the egg distribution assay (100 mm, 36 hours) (Figure 2F). With predators present, prey larvae within 10 mm of the patch reduced to less than half, compared to without predators (Figure 2G). Here, predator progeny outnumbered prey progeny (Figure 2G), despite prey being more prolific (Figure S2I). Furthermore, patch avoidance and egg-laying were similar across predator- and prey-conditioned patches (Figures S2J to S2L, see STAR Methods: Pheromone-conditioned patches). Thus, bite experience, but not predator-secreted pheromones, was responsible for prey behavior during prolonged predator exposure. These results illustrate how non-fatal biting accrues long-term territorial benefits and increases the relative fitness of P. pacificus.
P. pacificus inflicts non-fatal biting to achieve territorial outcomes
Next, we determined whether the territorial benefits of non-fatal adult-targeted biting were goal-directed or a serendipitous result of failed predation. First, we assessed food values by analyzing the long-term net energy yield of various single-food diets. P. pacificus freely fed on excess bacteria (E. coli OP50), larval prey, or adult prey for 6 hours before being stained for fat stores with the lipophilic dye Oil Red O (See STAR Methods: Fat-staining). Bacteria-fed predators displayed the most stained fat, followed by adult-fed and then larva-fed predators (Figures 3A and S3A to S3D). Thus, bacteria-based diets had higher energy yields than prey-based diets. Furthermore, given enough time for successful predation, adult-based diets are more efficient than larva-based diets.
Figure 3. P. pacificus inflicts non-fatal biting to achieve territorial outcomes.
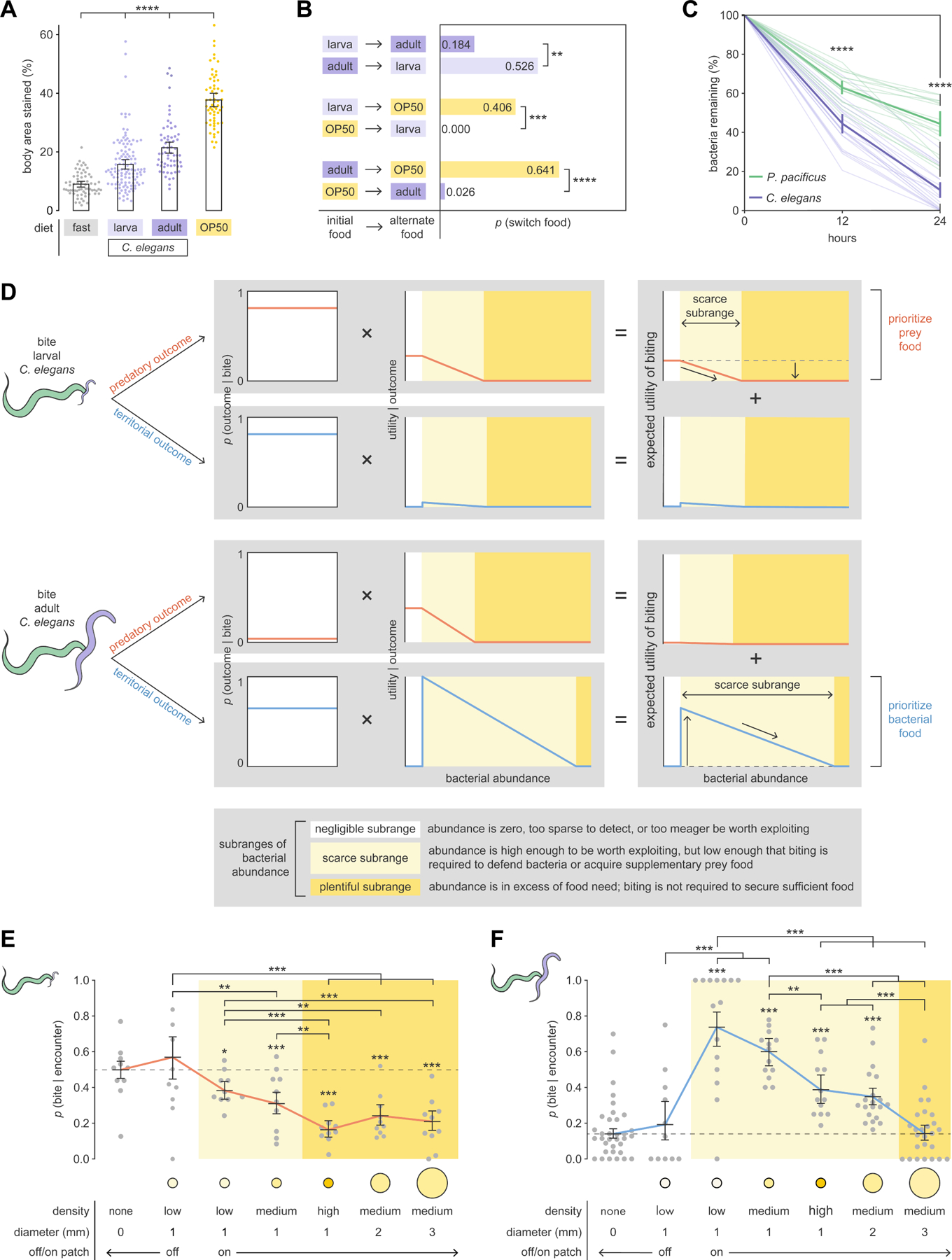
(A) Percent of P. pacificus body stained with Oil Red O after 6 hours on different diets (Dunn’s test with Benjamini–Hochberg adjustment, nP.pacificus = 60–117).
(B) Probability of P. pacificus switching food patches (Fisher’s exact test with Benjamini–Hochberg adjustment, nP.pacificus = 29–39).
(C) Bacteria consumption (Dunn’s test with Benjamini–Hochberg adjustment, nadult = 16–23).
(D) Model of expected utility of biting (dashed lines: expected utility for negligible subrange, vertical arrows: predicted change from negligible subrange, sloped arrows: predicted monotonic decrease). Bacterial abundance and utility are in arbitrary units.
(E) Probability of biting during encounter with larval C. elegans (red line: interpolated) (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 9–10, nencounters_per_P.pacificus = 1–66).
(F) Probability of biting during encounter with adult C. elegans (blue line: interpolated) (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 12–34, nencounters_per_P.pacificus =1–38).
(E,F) Error bars are predicted probabilities and 95% CIs from binomial logistic regression models of data.
Other error bars are 95% bootstrap CIs.
Although adult prey offered more long-term food value than larval prey, P. pacificus may discount delayed rewards22 to avoid food deprivation. To assess short-term food preference, we placed P. pacificus in one of two adjacent plentiful food patches, and then checked an hour later whether P. pacificus had switched patches. Stationary prey patches were created using locomotion-impaired mutants (see STAR Methods: Food switching). By comparing switching probabilities, we found that P. pacificus preferred bacteria over all prey, and larval over adult prey (Figure 3B). Preference for bacteria over larvae matches previous findings20. Contrary to long-term food value (Figure 3A), P. pacificus preferred larval over adult prey (Figure 3B). Notably, inverse switches (a→b, b→a) had combined probabilities less than 1 (Figure 3B), which conforms with studies reporting that nematodes tend to stay in food patches23, and with foraging theory that devalues food by travel time4,5. P. pacificus exhibited very low switching between prey-conditioned and pristine (unconditioned) bacterial patches (Figure S3E), indicating that prey pheromone did not affect bacteria value. Overall, preference for closer and easily consumed foods suggests that P. pacificus prefers immediate over delayed food rewards.
We next explored the potential competitive value of territorial biting outcomes. To compare bacteria consumption rates, we placed an adult C. elegans and an adult P. pacificus onto separate identical patches of GFP-labeled bacteria (OP50-GFP), and then measured fluorescence at 12 and 24 hours. We found that adult C. elegans consumed bacteria ~1.5x faster than adult P. pacificus at both time points (Figure 3C). Eggs laid by adult C. elegans began hatching at 12 hours, with a range of 20 to 62 larvae present by 24 hours (Figures S3F and S3G). However, bacterial consumption rate did not increase between 12 and 24 hours (Figure 3C), and we found no correlation between number of larvae and bacteria consumption (Pearson’s r = 0.2480, p = 0.8066). These results show that adult C. elegans more efficiently exploits bacteria and can outcompete P. pacificus, but larvae pose minimal competitive threat.
To determine the relative contributions of predatory and territorial outcomes toward the overall value of biting C. elegans, we applied neuroeconomic theories of rational decision-making about actions with probabilistic outcomes. In expected utility theory24, the expected utility (overall subjective value) of an action takes into account both the probability that an action will lead to a particular outcome, as well as the utility (subjective value) attained if that outcome occurs. Specifically, expected utility is calculated as the sum of the probability-weighted utilities of each outcome. To model the expected utility of biting larval or adult C. elegans, we estimated the probabilities that biting leads to predatory and territorial outcomes, as well as how the utilities of those outcomes change with bacterial abundance.
To model the decision between biting outcomes, rather than between prey types, we contrived food choices such that P. pacificus encountered only larval or only adult C. elegans (Figure 3D). We assigned probabilities to each biting outcome (predator, territorial), for each type of prey (larval, adult) (Figure 3D). For larva-targeted biting, we set both p(predatory outcome|bite) and p(territorial outcome|bite) as equal to the pooled probability that a bite led to prey-feeding (p(feed|bite) = 0.8115; Figure S1M), since feeding also eliminated competitors (Figure 3D). For adult-targeted biting, we assigned p(predatory outcome|bite) a very low probability (see STAR Methods: Expected utility of biting) since bites rarely killed adult prey (Figures 1H, 1I, and S1F), and p(territorial outcome|bite) as equal to the pooled probability that a bite expelled adult prey from a bacterial patch (p(expel|bite) = 0.6483; Figure S1N).
Next, we determined how utility of biting outcomes should change with bacterial abundance. We subdivided bacterial abundance into three behaviorally defined subranges. In the ‘negligible’ subrange, bacteria are absent, too sparse to detect, or too meager to be worth exploiting. In the ‘scarce’ subrange, bacterial abundance is high enough to be worth exploiting, but low enough that biting is required to defend bacteria or acquire supplementary prey food. In the ‘plentiful’ subrange, bacterial abundance exceeds food needs, and biting is unnecessary to secure sufficient food. The bounds of these subranges should shift with prey type and outcome type.
We first outlined the general shape of how utility of predatory biting outcomes should change with bacterial abundance, assuming that the goal of biting is to kill prey for food (Figure 3D). Predatory biting utility should be highest in the negligible subrange, when prey is the only viable food, and then monotonically decrease as preferred food (bacteria) becomes more abundant. This is supported by reports of P. pacificus biting larvae less when bacteria are present20. We used ORO fat-staining of prey-fed predators (Figure 3A) to estimate predatory biting utility in the negligible subrange, and the probabilities of switching from prey to bacteria (Figure 3B) to estimate rate of utility decrease over the scarce subrange (see STAR Methods: Expected utility of biting). Even though predatory biting utility is higher for adult prey than for larval prey over the negligible subrange, P. pacificus should drop adult prey from its diet at a lower bacterial abundance (start of plentiful subrange) than for larval prey due to preference for immediate food rewards.
We then modeled how utility of territorial outcomes should change with bacterial abundance, assuming that the goal of biting is to defend bacteria from competitors (Figure 3D). Territorial biting utility should be zero in the negligible subrange, which lacks bacteria worth defending, and then abruptly peak in the negligible-to-scarce transition, where scarcity-induced competitive pressure is strongest. Using C. elegans bacteria consumption rate (Figure 3C), we estimated peak utility to be high for adult-targeted biting and low for larva-targeted biting (See STAR Methods: Expected utility of biting). Across the scarce subrange, territorial biting utility should monotonically decrease as competitive pressure is alleviated by increasing bacteria (Figure 3D). While predatory biting utility ignores C. elegans bacteria consumption, here, wider scarce subranges account for bacteria lost to competitors, and plentiful subranges begin when bacteria fulfill the food needs of both C. elegans and P. pacificus (See STAR Methods: Expected utility of biting). The overall non-monotonic shape of territorial biting utility resembled energy cost models of feeding-based territoriality25. Notably, predatory and territorial biting utility both monotonically decrease across scarce and plentiful subranges, but differ in the negligible-to-scarce transition.
For each combination of outcome type (predatory or territorial) and prey type (larval or adult), we multiplied the outcome probability, p(outcome|bite), by its corresponding outcome utility function (utility|outcome) (Figure 3D). The expected utility of biting a particular prey type was estimated as the sum of the probability-weighted utilities of both outcome types, such that expected utility consisted of both predatory and territorial components. Therefore, the outcome with the higher probability-weighted utility is the primary contributor towards biting motivation. For larva-targeted biting, the predatory outcome should be prioritized due to low competitive pressure from larvae. For adult-targeted biting, the territorial outcome should be prioritized due to low probability of killing adult prey.
Next, we tested our predictions to determine which outcomes are considered in biting decisions. We placed P. pacificus in an arena with either larval or adult prey, and varied the size or density of a bacterial patch (Figures S1G to S1L). To quantify biting incentive, we calculated the probability of biting during an encounter, p(bite|encounter). Biting encounters were typically shorter than non-biting encounters, and p(bite|encounter) was uncorrelated with non-biting encounter duration or number of encounters (Figures S3H to S3L). Thus, bites were decided early into an encounter, and encounters could be treated as independent events. We then analyzed biting incentive to infer predatory or territorial biting motivation. As predicted, larva-targeted biting incentive monotonically decreased with bacterial abundance (Spearman’s ρ = – 0.58, p < 0.001; Figure 3E), resembling the predatory component of expected utility of larva-targeted biting (Figure 3D). In contrast, adult-targeted biting incentive was low when bacteria were negligible, high in the negligible-to-scarce transition, and monotonically decreased thereafter (Spearman’s ρ = –0.74, p < 0.001) (Figure 3F). This non-monotonic shape (Spearman’s ρ = 0.08, p = 0.374; Hoeffding’s D = 0.03, p < 0.001) conformed with the territorial component of the expected utility of adult-targeted biting (Figure 3D).
In addition to overall shape of biting incentive, other key model predictions were also validated. Adult-targeted biting incentive diminished at a higher bacterial abundance than larva-targeted biting incentive (Figures 3E and 3F), matching the predicted wider scarce subrange for adult-targeted territorial biting utility (Figure 3D). To examine the critical negligible-to-scarce transition, we used a low-density bacterial patch (Figure S1H) that induced patch-staying and patch-leaving with roughly equal probabilities (Figure S4A, and Videos S4 and S5). Using a choice variability approach for probing decision-making26, we segregated encounters into off- and on-patch events to reflect P. pacificus decision to ignore or exploit the patch, respectively. On-patch biting incentive was higher than off-patch biting incentive for adult-targeted bites (Figure 3F), but did not significantly differ for larva-targeted bites (Wald test with single-step adjustment for Tukey contrasts, p = 0.07663; Figure 3E). This vindicates our prediction of an abrupt negligible-to-scarce peak for territorial biting utility. Collectively, these results show that both predatory and territorial biting outcomes influence biting motivation in a context-specific manner, and P. pacificus incorporates both prey and bacterial information to direct predatory attacks against larval prey and territorial aggression against adult prey.
Territorial biting is driven by chemosensation and mechanosensation of bacteria
We next explored how P. pacificus senses bacteria for adjusting territorial biting incentive. Predatory biting incentive was suppressed by increasing density, but not diameter, of the bacterial patch (ANOVA; density: F = 17.84, df = 3, p < 0.0001; diameter: F = 22.19, df = 2, p = 0.168). In contrast, territorial biting was suppressed by increasing either density or diameter (ANOVA; density: F = 11.668, df = 3, p < 0.0001; diameter: F = 1.838, df = 2, p < 0.0001). This suggests that P. pacificus senses at least two bacterial features that are relevant for territorial biting decisions.
To identify a role for chemosensation, we ablated P. pacificus amphid neurons with exposed cilia at the nose (Figure 4A), and then measured p(bite|encounter) with adult prey on a scarce bacterial patch (medium-density, 1 mm). Relative to mock-ablated controls, ablation of AM1 and AM7 neurons decreased p(bite|encounter), while ablation of AM2 neurons increased p(bite|encounter) (Figure 4B). With limited research on these neurons, it is unclear whether ablations affected bacteria-sensing, or other sensory capabilities involved in biting implementation. However, studies of homologous neurons in C. elegans offer clues. AWC (homolog of AM7) triggers local search upon removal from a bacterial patch27, and senses bacteria-related odorants28,29. ASH and ADL (homologs of AM1 and AM2) are involved in avoiding high ambient oxygen30,31 and migrating to the thick boundary of a bacterial patch, where local oxygen concentration is lower due to higher bacterial metabolism27. P. pacificus can similarly distinguish between oxygen levels32 (albeit via different molecular mechanisms33), and mutants with cilia-defective amphid neurons exhibit reduced predation rates in addition to impaired oxygen-sensing34,35.
Figure 4. Territorial biting is driven by chemosensation and mechanosensation of bacteria.
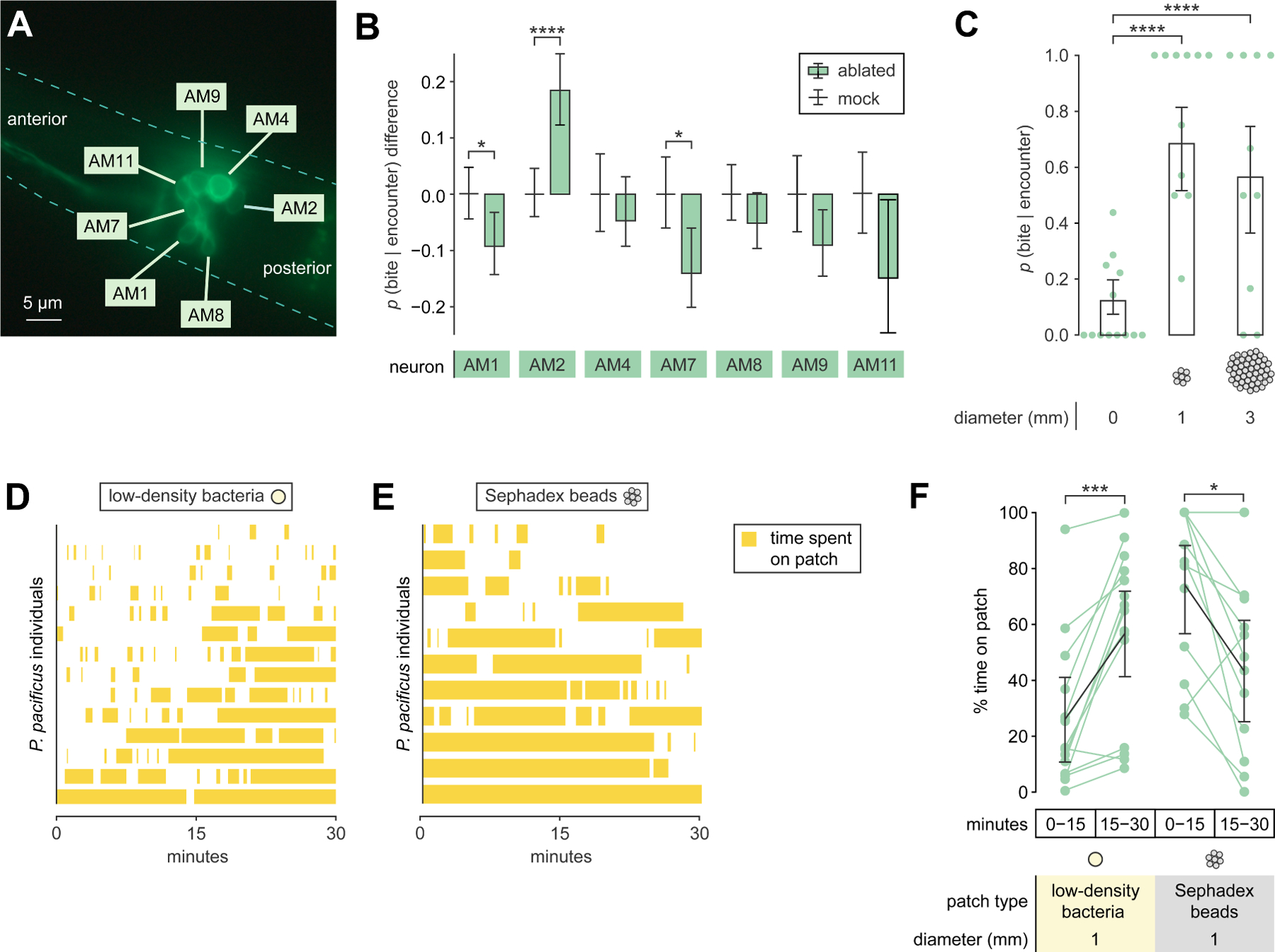
(A) DiO-stained amphid neurons of P. pacificus (dashed line: head silhouette).
(B) Difference in p(bite|encounter) between mock-ablated (centered at zero) and neuron-ablated P. pacificus, with adult C. elegans on a scarce bacterial patch (Wald test with Benjamini-Hochberg adjustment, nP.pacificus = 5–31, nencounters_per_P.pacificus =2–27.
(C) p(bite|encounter) with adult C. elegans on a bead patch (Wald test with single-step adjustment for Tukey contrasts, nP.pacificus = 7–13, nencounters_per_P.pacificus = 1–14).
(D, E) Timecourse of P. pacificus residence on a patch made of (D) low-density bacteria or (E) beads.
(F) Change in P. pacificus patch residence over time (Wilcoxon’s signed rank test with Benjamini–Hochberg adjustment, nP.pacificus = 11–14).
(B,C) Error bars are predicted probabilities and 95% CIs from binomial logistic regression models of data.
Other error bars are 95% bootstrap CIs.
See also Figure S4.
We next probed how mechanosensation modulates territorial biting. We measured adult-targeted biting incentive on patches comprised of Sephadex beads (Figure S4B), whose surfaces elicit mechanosensation similar to that of bacterial surfaces36. Importantly, these beads were inedible and lacked bacterial chemical signatures. As with low-density bacterial patches, P. pacificus resided less on bead patches than on medium- or high-density bacterial patches (Figure S4A). On-patch p(bite|encounter) was high on bead patches (Figure 4C), similar to that for low-density bacterial patches (Figure 3F). Unlike with bacterial patches, increasing the diameter of bead patches did not suppress p(bite|encounter) (Figure 4C). Thus, P. pacificus may sense some minimal ‘bacterial’ abundance in bead patches, or prefer some non-food-related property of beads. However, P. pacificus residence on bead but not bacteria patches decreased over time (Figures 4D to 4F, and S4C), suggesting that eating is required to sustain patch exploitation and territorial defense. Based on existing knowledge, we surmise that P. pacificus uses olfaction to locate bacteria from afar, oxygen sensation to sense bacterial density, and mechanosensation to detect low-density bacteria when odor and oxygen gradients are too low.
Predatory and territorial biting are associated with different search tactics
To understand how biting motivation guides other foraging behaviors, we compared how P. pacificus searches for larval and adult C. elegans on bacteria. First, we contemplated how biting motivation should influence search speed. Under predatory motivation, P. pacificus should minimize search costs since prey are inferior food. Rather than increase speed, P. pacificus should graze normally on bacteria and opportunistically attack prey during chance encounters. Under territorial motivation, P. pacificus should swiftly seek and expel intruders to halt rapid loss of preferred food. To test these predictions, we tracked the location of P. pacificus mouth location on a scarce bacterial patch with larval C. elegans, adult C. elegans, or P. pacificus cohabitants. All cohabitants preferred the thick boundary of a bacterial patch37,32 (Figures S5A and S5B), so we assessed active search by calculating patrol speed: the arc component of forward distance traveled on the patch boundary, divided by total time (Figure 5A and Video S3, see STAR Methods: Patrol speed). To disregard stationary bouts during prey-feeding, we normalized patrol speed by translational speed (Figure 5A, see STAR Methods: Patrol speed). Since bacteria are immobile and P. pacificus do not bite each other, active search for biting targets should entail faster normalized patrol speed than with P. pacificus cohabitants.
Figure 5. Predatory and territorial biting are associated with different search tactics.
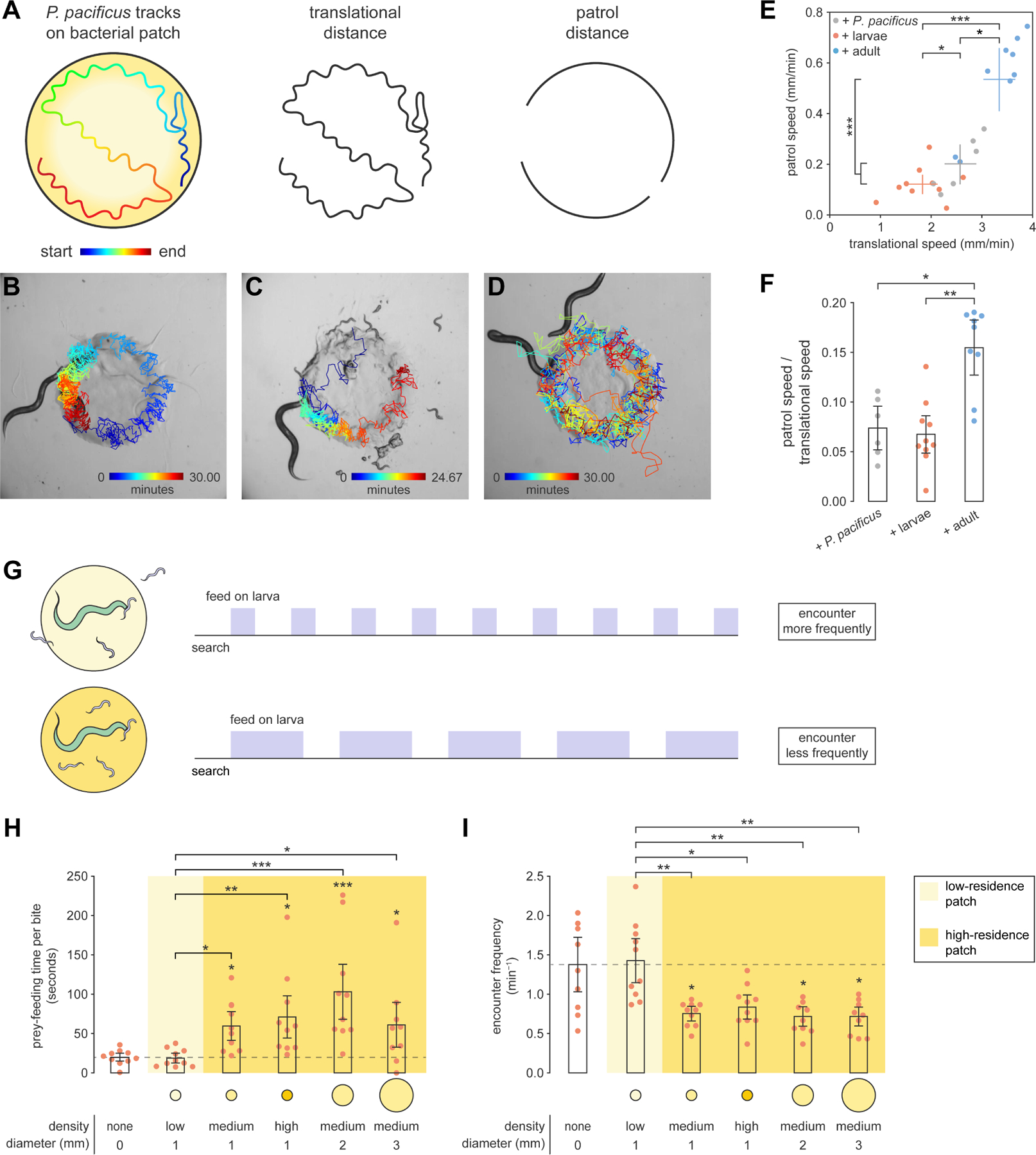
(A) Translational and patrol distances for calculating speeds (see STAR Methods: Patrol speed).
(B-D) P. pacificus mouth tracks with (B) P. pacificus, (C) larval C. elegans, and (D) adult C. elegans cohabitants.
(E) Translational and patrol speeds (Tukey’s test, nP.pacificus = 6–10).
(F) Normalized patrol speed (Dunn’s test with Benjamini–Hochberg adjustment, nP.pacificus = 6–10).
(G) Expected prey-feeding time on bacteria-poor (top) and bacteria-rich patches (bottom).
(H) Prey-feeding time per bite (Dunn’s test with Benjamini–Hochberg adjustment, nadult = 10).
(I) Frequency of encountering larval prey (Dunn’s test with Benjamini–Hochberg adjustment, nP.pacificus = 10).
Error bars are 95% bootstrap CIs.
We found that P. pacificus exhibited faster patrol and translational speeds with adult C. elegans than with other cohabitants (Figure 5B to 5E). Translational speed was slowest with larval C. elegans (Figure 5E), likely due to stationary prey-feeding. Correcting for non-movement, normalized patrol speed was highest with adult C. elegans, signifying that P. pacificus locomotion shifted toward patrolling when adult C. elegans was present (Figure 5F). Normalized patrol speed did not differ across larval C. elegans and P. pacificus cohabitants (Figure 5F), or across C. elegans- and P. pacificus-conditioned patches (Figures S5C and S5D), suggesting that bacteria-grazing motivated locomotion in these conditions. Furthermore, patrolling did not decrease when adult C. elegans was an unc-18 mutant, which barely moved on bacteria but exited the patch when bitten (Figures S5E and S5F). Thus, increased patrolling required physical presence, but not locomotion, of adult C. elegans on the patch. Altogether, territorial but not predatory motivation increases patrolling speed, reflecting an active and energy-intensive search tactic that is commensurate with protecting energy-rich bacterial food.
We next looked for alternate methods of searching for larval prey. Prey-feeding must cease to resume search for other prey, so one way to increase encounters without increasing speed is to reduce prey-feeding time (Figure 5G). When bacterial abundance is too low, prey quickly disperse and thus should be killed first and eaten later. To test this, we placed P. pacificus with ~100 larval prey in an arena with various bacterial conditions, and then measured prey-feeding time per bite. Prey-feeding times resembled a step function: short across absent or low-density bacteria, and high across more abundant bacteria (Figure 5H). This matched P. pacificus’s own patch-leaving behavior (Figure S4A), which may be used to heuristically judge other nematodes’ patch-leaving. Furthermore, conditions that induced low prey-feeding times also had more frequent encounters (Figure 5I). With enough time, P. pacificus returned to feed on previously killed corpses (Figure S5G). Thus, predatory motivation reduces prey-feeding to increase encounters with dispersing prey, which is a passive and energy-efficient way to search for energy-poor food. Overall, by using biting motivation to coordinate search tactics, P. pacificus ensures that efforts are unified into a cohesive predatory or territorial foraging strategy.
Blocking dopamine D2 or octopamine receptors modulates territoriality
We investigated signaling mechanisms for regulating territorial biting and search behavior. With limited P. pacificus information, we consulted known pathways in C. elegans. During C. elegans starvation, absence of bacteria attenuates D2-like receptor signaling, which in turn disinhibits octopamine (invertebrate homolog of norepinephrine) release38. We hypothesized that a similar pathway in P. pacificus may detect bacterial scarcity and modulate territorial behavior. Using a pharmacological approach, we exogenously treated P. pacificus for two hours (see STAR Methods: Drug treatment), moved treated P. pacificus to an arena with adult C. elegans and various bacterial conditions, and then measured p(bite|encounter). We found that amisulpride, a dopamine D2 receptor antagonist, increased p(bite|encounter) on scarce bacteria but had no effect on absent or plentiful bacteria (Figure 6A). Thus, blocking D2 receptors did not affect biting generally, but was context-specific to competitive conditions. In contrast, epinastine, an octopamine receptor antagonist39, affected p(bite|encounter) across all bacterial conditions, but in opposite ways: p(bite|encounter) increased when bacteria were absent, and decreased when bacteria were present (Figure 6B). The overall result was that p(bite|encounter) monotonically decreased with bacterial abundance (Figure 6B), indicative of predatory biting motivation (see Figure 3D). Moreover, epinastine decreased patrolling on a scarce bacterial patch, thereby suppressing territorial search behavior (Figures 6C and 6D). Treatment with a D2 receptor agonist or octopamine did not affect behavior (Figure S6). Collectively, blocking dopamine D2-like receptors enhanced territorial biting, while blocking octopamine receptors switched foraging strategy from territorial to predatory.
Figure 6. Blocking dopamine D2 or octopamine receptors modulates territorial biting.
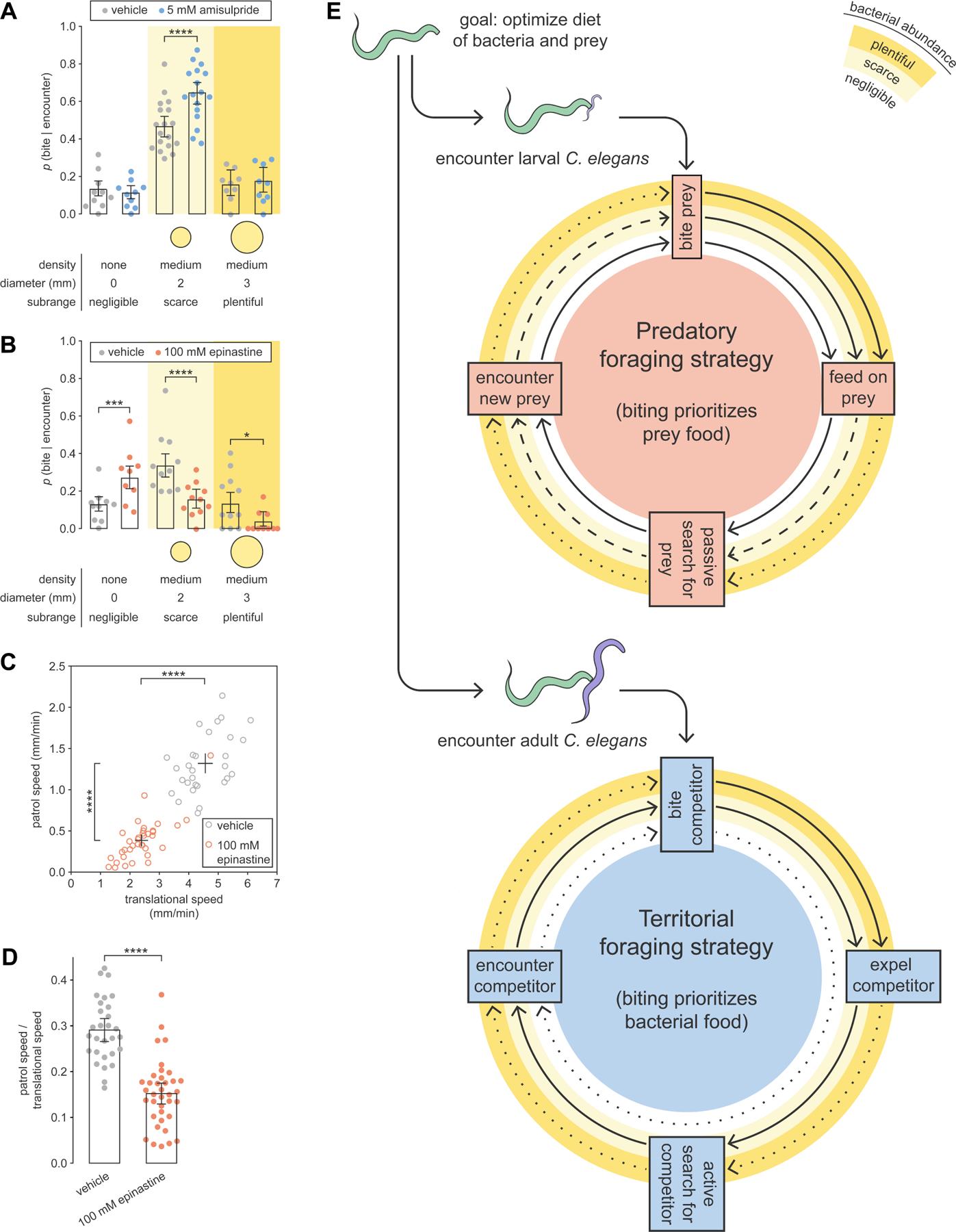
(A–B) p(bite|encounter) with adult C. elegans and P. pacificus treated with (A) amisulpride (Wald test with Benjamini–Hochberg adjustment, nP.pacificus = 9–18, nencounters_per_P.pacificus = 24–42), and (B) epinastine (Wald test with Benjamini–Hochberg adjustment, nP.pacificus = 9–11, nencounters_per_P.pacificus = 9–38).
(C,D) Effect of epinastine on patrolling, with adult C. elegans on a scarce bacterial patch (Dunn’s test, nP.pacificus = 29–37).
(E) Predatory and territorial foraging strategies. Solid, dashed, and dotted arrows indicate maximal, intermediate, and minimal probabilities, respectively, of achieving the next foraging stage.
(A–D) Drug concentrations refer to the solution applied to a bacterial patch (See STAR Methods: Drug treatment).
(A,B) Error bars are predicted probabilities and 95% CIs from binomial logistic regression models of data. Other error bars are 95% bootstrap CIs.
See also Figure S6.
Discussion
We present a model of two distinct, flexible, and coordinated foraging strategies that P. pacificus uses for biting C. elegans (Figures 6E). The predatory foraging strategy (Figure 6E) is engaged against larval C. elegans, which is easily killed and poses minimal competitive threat. Here, biting is used to kill and eat prey, so P. pacificus bites most when bacteria is negligible, and bites less as bacterial abundance increases. Following a bite, prey-feeding can be cut short to passively search for and immobilize dispersing larvae. Alternatively, the territorial foraging strategy (Figure 6E) is deployed against adult C. elegans, which is difficult to kill and rapidly consumes bacteria. Here, biting serves to protect bacterial food. Accordingly, P. pacificus bites most when bacteria are scarce, and bites least when bacteria are negligible or plentiful. Non-fatal bites effectively expel competitors from bacterial territory and induce patch avoidance. To actively search for intruders, P. pacificus increases speed to patrol bacterial territory. Altogether, we illustrate how P. pacificus weighs costs and benefits of biting outcomes, flexibly reprograms biting motivation to prioritize prey or bacterial food, and orchestrates foraging strategies that are energetically commensurate with its food choice.
C. elegans mobility was key for predicting differences in predatory and territorial responses, echoing prior findings that foraging theory often failed to predict behavior when prey mobility was insufficiently accounted for6. While C. elegans escape from a bite is a failure by predatory standards, escape can be leveraged for territorial benefit if directed away from bacteria. Territorial benefit was amplified when non-fatal bites conditioned C. elegans to seek prey refuge, which typically have less food but minimize predator danger40. Prey mobility was also critical for interpreting prey-feeding, which has typically been associated with predatory motivation. Lack of prey-feeding was previously used to implicate potentially competitive motivation for intraguild predation11, as well as for P. pacificus surplus-killing of C. elegans larvae in the absence of bacteria20,41. However, we predicted and confirmed that contexts with high predatory incentive were associated with reduced prey-feeding and high prey dispersal, contrary to classic foraging models that predict lower prey utilization when prey densities are high42.
We demonstrate that a nematode with ~300 neurons43 can solve complex foraging problems in which relevant factors have multiple potential roles: C. elegans as prey and competitor, bacteria as food and habitat, and bites with predatory and territorial outcomes. Our deconstruction of predator-prey interactions disentangled these dualities, but with some limitations. For example, biting was not fully suppressed when it was predicted to have zero utility, potentially because biting is sometimes used to test beliefs about biting outcomes, or biting is stochastic (or appears stochastic due to overlooked behavioral variables44). Additionally, further work is needed to confirm the sensory mechanisms that we proposed for sensory neurons involved in territorial biting. It remains uncertain how P. pacificus distinguishes between larval and adult C. elegans, although small peptide-mediated recognition of self and non-self may be involved41. Mutations of receptors and biosynthesis would further elaborate on the function of dopamine and octopamine signaling in foraging decisions, and can be reasonably pursued as CRISPR/Cas9 in P. pacificus is well-established45,46. Our ultimate goal for future work is to identify the neuronal bases of foraging decisions, and especially to discern which calculations are hard-wired and which are plastic.
Our investigation of predator motivations contributes to a multiscale understanding of an ecologically critical phenomenon, intraguild predation10. While intraguild predation is typically considered as the killing and sometimes eating of competitor prey, we describe a more versatile variant that achieves competitive benefits without killing. While intraguild predators often selectively kill younger stages of prey while leaving adults to freely compete for resources10,47, our study shows that non-fatal attacks can effectively deter competitors. The deterrent benefits of non-fatal attacks are consistent with population-level studies that observe fear-driven avoidance of predator niches after a predator population is introduced to competitor prey populations9,48. Here, our work presents a complementary perspective of how predators consider this avoidance behavior in planning attacks against competitor prey.
By introducing a model system for investigating predator-prey competition between two nematode model organisms with well-developed functional tools, we offer a powerful approach for investigating molecular and neural mechanisms underlying both predator and prey behaviors. Compared to field studies, our laboratory system offers the ability to finely adjust many parameters for granular, multivariate behavioral analyses. We explored only a very small set of potential parameters, leaving many alternative parameters to be tested in future work. Importantly, modification of alternate parameters may change the outcomes of the experiments we presented. We only explored energy value of food, but foraging choices are also constrained by nutrients and toxins3. In addition to bacterial abundance, prey abundance is likely to also affect biting decisions. Biting variability across P. pacificus individuals should be investigated to understand why some individuals are consistently more aggressive. Besides predator behavior, there is immense opportunity to investigate prey foraging strategies. Our work represents only a small fraction of the potential well-controlled experiments that can be performed.
Taken together, our use of neuroeconomics, foraging theory, and fine-grained experimental manipulations illustrate that a careful accounting of decision-making context is required to attribute particular motivational states to observed behavior. Our study supports a resurgent effort to reaffirm the importance of behavioral interrogation for understanding cognitive processes49, with emphasis on how an animal’s responses are relevant to its natural life50.
STAR Methods
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sreekanth Chalasani (schalasani@salk.edu)
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the Key Resources Table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Escherichia coli: OP50 | Caenorhabditis Genetics Center | WBStrain00041969 |
| Escherichia coli: OP50-GFP | Caenorhabditis Genetics Center | WBStrain00041972 |
| Chemicals, peptides, and recombinant proteins | ||
| Oil Red O | Alfa Aesar | Cat#A12989 |
| Fast DiO | ThermoFisher | Cat#D3898 |
| Sephadex G-100 | Sigma-Aldrich | Cat#G10050 |
| Amisulpride | Sigma-Aldrich | Cat#A2729 |
| Epinastine hydrochloride | Sigma-Aldrich | Cat#E5156 |
| Sumanirole maleate | Tocris | Cat#2773 |
| (±)-Octopamine hydrochloride | Supelco | Cat#68631 |
| Experimental models: Organisms/strains | ||
| Pristionchus pacificus: RS5194: wild isolate | Sommer Lab (Max Planck Campus Tübingen)53,54 | N/A |
| Pristionchus pacificus: RS5275: wild isolate | Sommer Lab (Max Planck Campus Tübingen) 53,54 | N/A |
| Pristionchus pacificus: PS312: wild isolate | Sommer Lab (Max Planck Campus Tübingen) 51 | WBStrain00047433 |
| Caenorhabditis elegans: N2: wild isolate | Caenorhabditis Genetics Center52 | WBStrain00000001 |
| Caenorhabditis elegans: CX7389: [kyIs392 [Pstr-2::GFP::rab-3; Pttx-3::lin-10::dsRed; Pelt-2::GFP] | Bargmann Lab (Rockefeller), this paper | N/A |
| Caenorhabditis elegans : IV95 : [ueEx46 [gcy-7-sl2-mCherry; Punc-122::RFP]; gvIs246 [ida-1::GFP+ pRF4 rol-6(su1006)], Caenorhabditis elegans strain | this paper | N/A |
| Caenorhabditis elegans: CB81: [unc-18(e81) X], | Caenorhabditis Genetics Center55 | WBStrain00004094 |
| Software and algorithms | ||
| ImageJ, Fiji | 58 | https://imagej.net/software/fiji/ |
| Colour Deconvolution plugin, Fiji | 61 | https://imagej.net/plugins/colour-deconvolution |
| R 4.0.2 | 67 | https://www.r-project.org/ |
| lme4 | 68 | https://cran.r-project.org/web/packages/lme4/ |
| multcomp | 69 | https://cran.r-project.org/web/packages/multcomp/ |
| MATLAB 2017b | MathWorks | https://www.mathworks.com/help/releases/R2017b/ |
| code for analyzing egg distribution | this paper | DOI: 10.5281/zenodo.5976742 |
| code for analyzing patrol speed | this paper | DOI: 10.5281/zenodo.5976742 |
Experimental model and subject details
Nematodes
P. pacificus and C. elegans were grown on E. coli OP50 bacteria and maintained under standard conditions at 20°C51,52. Hermaphrodites were used for all experiments. For simplicity, we use ‘adult’ to refer to the young adult (day 1) stage of both nematode species, and ‘larval’ to refer to the L1 stage of C. elegans. All P. pacificus animals used for behavior were confirmed to have the dual-toothed eurystomatous mouth form (Figures S1A and S1B), which more efficiently kills larval C. elegans compared to single-toothed stenostomatous mouth form20.
The P. pacificus wild isolate RS519453,54 and the standard C. elegans N2 strain52 were used, unless otherwise stated. Other P. pacificus wild isolates, PS31251 and RS527553,54, were tested during the process of selecting the strain that was most effective at harming C. elegans. The C. elegans strain CX7389: kyIs392 [Pstr-2::GFP::rab-3; Pttx-3::lin-10::dsRed; Pelt2::GFP]) was used to label eggs with GFP. The C. elegans strain IV95: ueEx46 [gcy-7-sl2-mCherry; Punc-122::RFP]; gvIs246 [ida-1::GFP+ pRF4 rol-6(su1006)] was used to produce stationary adult prey patches. The C. elegans strain CB81: unc-18(e81) X55 was used produce stationary larval prey patches, and to limit adult C. elegans movement on bacteria. All P. pacificus and C. elegans strains used in this study are listed in the Key Resources Table.
We limited the number of P. pacificus to 1–4 animals in behavioral experiments to focus on decision-making on an individual level and minimize social interactions that may emerge from a larger number of P. pacificus. To minimize pressure on animals to leave a bacterial patch due to crowding, we limited the total number of adult nematodes to allow enough room for all adults to occupy the patch simultaneously. We found that 2 adult nematodes fit comfortably on 1 mm diameter patch, and 4 adults on a 2 mm diameter patch. We also used 4 adults on a 3 mm diameter patch since we could not confidently distinguish individual worms when more than four happened to gather together. We did not observe any changes in P. pacificus behavior when 1 or 3 were together in the same arena. We only used 1 C. elegans in each arena to maintain the same abundance of C. elegans across all bacterial conditions.
For all experiments, data was excluded if any P. pacificus or C. elegans escaped the arena, typically due to improper application of the corral onto the agar surface. Unless otherwise indicated, all replicates included matched controls and were conducted across multiple days.
Method details
Behavioral recordings
Behavioral video recordings were acquired using an optiMOS sCMOS camera (QImaging) and Streampix software. Copper corral arenas were used to keep animals within the field-of-view.
Bacterial patches
Stock liquid cultures of E. coli OP50 were prepared by inoculating LB broth, adjusting concentration to OD600 = 0.4, and then storing at 4°C. To produce working liquid cultures the stock culture was either diluted with LB broth, or concentrated by centrifugation (1 ml at 845 rcf for 5 min) and the removal of supernatant. ‘Low’, ‘medium’, and ‘high’ density patches were seeded using working liquid culture concentrations of OD600 = {0.01, 0.30, and 1.00}, respectively (Figures S1H to S1J). 1 mm diameter bacteria patches were created by dipping a 10 μl pipette tip into liquid culture and then gently contacting the tip onto the surface of 3% agar NGM plates56 (Figures S1H to S1J). 2 mm (Figure S1K) and 3 mm (Figure S1L) diameter lawns were created by dispensing 0.3 μl and 1 μl, respectively, of liquid culture onto the agar surface. The total number of bacteria pipetted for a high-density, 1 mm patch was less than for a medium-density, 2 mm patch. The bacteria patches were then grown for 20 hours at 20°C. Fully grown patches were stored at 4°C and then allowed to come to room temperature for 1 hour before immediate used for behavior. Bacterial patches were inspected for roundness, size, and features associated with different density lawns (low-density is patchy and has minimal boundary, medium-density is not patchy and has a raised boundary that is distinct from the interior, high-density has a thick wide boundary that transitions smoothly with the interior.
Pheromone-conditioned patches
To condition bacterial patches with pheromones secreted by P. pacificus or C. elegans, clean adult nematodes (1 for 1 mm diameter patches, 3 for 2 mm diameter patches) were placed on the side of the bacterial patch and allowed to dwell on the patch for 6 hours (the length of predator exposure that induces C. elegans to persistently avoid the bacterial patch). To minimize experimenter-induced disturbance of the patch, adult nematodes were removed by gently tapping an eyelash onto the top of a nematode until it left the patch, and then removed. Conditioned patches were then immediately used for behavioral experiments. Patches conditioned with the same species as the tested animal were used as controls.
Identification of encounters and bites
Our criteria for determining encounters and bites were slightly modified from those used by Serobyan and colleagues19 and Wilecki and colleagues20.
We defined an encounter to be a contact between P. pacificus mouth and C. elegans body during which P. pacificus detects C. elegans and has a feasible opportunity to bite. It is important to note that not all contacts between the P. pacificus mouth and the C. elegans body were considered encounters. Individual encounters were counted when: 1) the P. pacificus mouth fully contacted the C. elegans body, and 2) P. pacificus interrupted it normal locomotion by slowing down as it approached C. elegans or contorting its head toward C. elegans, thereby positively indicating detection of C. elegans. Since adult C. elegans can move faster than P. pacificus, pacificus slowing or contorting towards C. elegans does not typically occur when adult C. elegans is moving at high speeds.
Biting incentive was calculated by dividing the number of bites by the number of encounters, p(bite|encounter). Biting incentive was measured using the same behavioral setup for assaying the immediate consequences of biting (arena 3.2 mm in diameter, 30 minutes, 1 adult P. pacificus with ~100 larval C. elegans or 1 adult C. elegans). p(bite|encounter) was pooled across 3 P. pacificus individuals for larger bacterial patches (see STAR Methods: Animals) to acquire enough encounters events for meaningful probability estimates within the same time frame.
The criteria for identifying bites depended on the level of attachment of the P. pacificus teeth onto the C. elegans body. Poorly attached bites were identified by the coincidence of: 1) concurrent P. pacificus head shortening and stiffening associated with biting (Figures 1D, 1F, and 1G), and 2) C. elegans escape response typical of receiving a hard touch57. Strongly attached bites were identified by disrupted normal locomotion in either nematode caused by the P. pacificus mouth being fastened to the C. elegans body. This manifested as C. elegans thrashing in place while anchored by a P. pacificus bite, or dragging of the P. pacificus mouth as an adult C. elegans attempts to escape from the bite (Videos S1 and S2). Kills were indicated by a breached cuticle and visible leaking of pseudocoelomic fluid (Figure 1E), ultimately leading to an unresponsive corpse.
To blind the scorer to experimental group, videos were named with numeric IDs and did not have any indicator of experimental group. For all analyses involving encounters, videos in which no encounters occurred were excluded from analyses.
Killing ability and outcomes of biting
Short-term killing ability was assayed using a modified version of the biting assay described by Wilecki and colleagues20. A single adult P. pacificus was placed in a copper-corralled arena (3.2 mm in diameter) with either 8 adult C. elegans or ~100 larval C. elegans. Biting behavior was recorded for 30 minutes and subsequently scored for bites and kills. Videos with no bites were excluded from analysis.
Biting outcomes (prey-feeding, prey exiting patch) were observed using a similar behavioral setup, but with multiple types of bacterial patches. Videos with no bites were excluded from analysis.
Long-term killing ability success was assayed by placing a single adult P. pacificus with a single adult C. elegans for 24 hours in a copper-corralled arena 3.2 mm in diameter. The presence of a killed adult C. elegans was checked at 1, 4, 8, and 24 hours.
Patch avoidance
To provide ample space for avoiding a bacterial patch, we used a larger arena (9.5 mm in diameter) with a 2 mm patch (medium density) in the center. A single adult C. elegans and 3 adult P. pacificus were placed into the arena and recorded for 30 minutes at 0 and again at 6 hours (same animals). The time that C. elegans spent fully inside the patch, with only its head in the patch, and fully outside the patch were manually scored by someone blind to the experimental group.
Egg distribution
The egg distribution assay used the same behavioral setup as the patch avoidance assay (9.5 mm diameter, medium-density 2 mm patch). A variable 4-nematode mixture of adult P. pacificus and/or adult C. elegans were placed into the arena and removed 7 hours later. The C. elegans strain CX7389 with integrated GFP reporter that expresses in eggs (Pelt-2::GFP) was used to visually distinguish C. elegans eggs from non-fluorescent P. pacificus eggs. Egg plates were incubated at room temperature for one hour and then at 4°C for 2 days to allow GFP expression to increase while preventing hatching. Arenas were then imaged under bright-field and fluorescence microscopy using a Zeiss Axio Zoom.V16 microscope. The distances of eggs from the center of the patch were measured using MATLAB (see Key Resources Table).
The extended egg distributions assay was conducted on a 100 mm 3% agar plate (no corral) with a medium-density 2 mm patch centered on the plate. 4 adult nematodes (4 prey, or 3 predator:1 prey) were placed near the patch, and the progeny within 10 mm of the patch were checked 36 hours later. Plates were stored at 4°C for 2 days to allow GFP expression to increase while preventing hatching. To minimize larval movement, plates were imaged immediately after being removed from 4°C. A 22 mm square area centered on the bacterial patch was imaged under bright-field and fluorescence microscopy using a Zeiss Axio Zoom.V16 microscope. Progeny were counted using the multi-point tool in Fiji58 (see Key Resources Table).
Patch-finding
The patch-finding assay was conducted on the same setup as the extended egg distribution, on a 100 mm agar plate (no corral) with a medium-density 2 mm patch centered on the plate. Mature CX7389 (Pelt-2::GFP) eggs were transferred from a bacteria-depleted plate to one side of a clean 3% agar NGM plate. Ten newly hatched L1 larvae found on the opposite side of the plate were transferred to a specific radius from the center of the patch. Cylindrical plugs excised from a clean 3% agar plate were used to gently transfer larvae. After transfer, larval health was assessed by checking for normal, vigorous locomotion. Plates were checked on a Zeiss Axio Zoom.V16 microscope 36 hours later for the presence of fluorescent larvae inside the patch.
Fat-staining
The caloric values of various diets were assessed by feeding ~300 adult P. pacificus diets comprised of excess E. coli OP50, adult C. elegans, or larval C. elegans for 6 hours. As a control, P. pacificus was food-deprived for 6 hours. Oil Red O (ORO) lipid staining59 was carried out as described by Escorcia and colleagues60. P. pacificus worms (and any food that were on the same plate) were then washed with PBST, centrifuged at 560 x g for 1 minute, and supernatant removed three times. Worms were next incubated in 40% isopropanol for 3 minutes, centrifuged at 560 x g for 1 minute, and supernatant removed. Worms were then incubated in a filtered solution of 7.34 mM ORO (Alfa Aesar A12989) in 60% isopropanol, while rotating at 30 rpm, for 2 hours. Worms were centrifuged at 560 x g for 1 minute, supernatant removed, resuspended in PBST, centrifuged at 560 x g for 1 minute, and supernatant removed except for 50 µl. 5 µl of resuspended worms were then placed on a microscope slide for imaging. Stained P. pacificus animals were imaged on a Zeiss Axio Imager M2 microscope with a Hamamatsu color CCD camera. To minimize bias, all intact P. pacificus animals on each slide were imaged, with no staining-based exclusion criteria. ORO, background, and unstained body colors were separated in Fiji58 using the Colour Deconvolution plugin61. ORO pixels were quantified as a percentage of worm body area.
Food switching
The food switching assay was adapted from the leaving assay described by Shtonda and colleagues23. Pairs of different food patches were placed 2 mm apart on a 35 mm NGM plate. E. coli OP50 spots were made by seeding 0.3 μl of liquid culture (OD600 = 0.4) and grown for 2 days. To produce C. elegans food patches, we used strains with locomotion phenotypes in order to restrict movement without use of anesthetics, which would also affect P. pacificus and prevent free movement between food spots. Adult C. elegans spots consisted of ~20 animals with roller locomotion phenotype (IV95: ueEx46 [gcy-7-sl2-mCherry; Punc-122::RFP]; gvIs246 [ida-1::GFP+ pRF4 rol-6(su1006)]). Larval C. elegans spots consisted of ~500 animals with kinky locomotion phenotype (CB81: unc-18(e81) X). unc-18 mutant adults were not used because they moved considerably when bacteria were absent, even though they barely moved when they were on bacteria. Food preference was assayed by placing a single adult P. pacificus in one food patch and checking 1 hour later to see if it had switched to the nearby alternate food spot. Switching probability was calculated as the number of P. pacificus that switched divided by the total number of P. pacificus animals. Food preference was determined by using the transitive property of inequalities: if p(a → b) < p(c → b), then P. pacificus prefers food a over food c.
Bacteria consumption and progeny proliferation
Initial bacterial supply was created by seeding 0.3 μl of OP50-GFP liquid culture (OD600 = 0.7) on 3% agar NGM 35 mm plates (with peptone omitted to minimize bacterial growth). Patches were allowed to saturate growth for 2 days. Initial bacterial levels were measured by imaging the OP50-GFP patches under fluorescence with consistent excitation and exposure parameters on a Zeiss Axio Zoom.V16 microscope and measuring GFP luminance in Fiji58. A single adult P. pacificus or adult C. elegans was placed by itself on a patch and imaged at 12 and 24 hours. GFP fluorescence, number of eggs, and number of hatched larvae were recorded.
Expected utility of biting
For a well-fed (with bacteria) P. pacificus individual presented with a particular C. elegans target and bacterial condition, the overall value of biting was estimated by calculating the expected utilities24 of biting outcomes. We calculated the expected utility of each outcome,
where pi,j and ui,j are the probability and utility (subjective value), respectively, of an outcome i (predatory, territorial) given an individual bite against a target j (larval C. elegans, adult C. elegans). Predatory outcomes were defined as feeding on the target, whereas territorial outcomes were defined as removing competitors from the bacterial territory.
First, we estimated pi,j using empirically obtained probabilities. For the probabilities associated with the predatory and territorial outcomes of a larva-targeted bite, pP,L and pP,T, we used the empirically estimated probability that P. pacificus feeds on prey given a larva-targeted bite (Figure S1M),
We equated pP,L to pT,L since killing and feeding on larvae simultaneously eliminates competitors. For the probability of a predatory outcome of an-adult targeted bite, pT,A, we estimated the probability that an adult C. elegans exits a bacterial patch given a bite received while on the patch (Figure S1N).
Since the objective probabilities used for estimating pP,L, pT,L, and pT,A were similar across bacterial abundance (Figures 1J and 1K), we assumed that pP,L, pT,L, and pT,A were constants and pooled data across bacterial conditions. Finally, for the probability of a predatory outcome of an-adult targeted bite, pP,A, we measured the number of bites that a single P. pacificus inflicted on a single adult C. elegans in a bacteria-free arena (3.2 mm diameter) until it successfully killed and fed on the prey (Figure S1F). Since each successive bite may have contributed cumulative harm in a way that killed C. elegans by attrition, the bite events were not independent of each other. Therefore, the true pP,A should be a cumulative probability that is very low during the first bite and very high at ~25 bites. However, treating bites as cumulative or independent resulted in the same long-term incidence of killed prey, so we treated each bite as independent for simplicity of prediction,
Next, we described outcome utility as a function of bacterial abundance, u(a). We divided bacteria abundance into three behaviorally defined subranges: negligible, scarce, and plentiful. The ‘negligible’ subranges encompassed the physical absence of bacteria, as well as bacterial abundance levels that are too small for P. pacificus to detect or care to exploit. We take the negligible subrange to be determined by sensory ability and internal state (hunger, satiety), and therefore consistent across outcome-target pairings when P. pacificus animals have been well-fed on OP50. The ‘scarce’ subrange included the minimum bacterial abundance that P. pacificus is willing to exploit, as well as other low levels of bacteria that induce P. pacificus to use biting as a means to secure additional food. Finally, the ‘plentiful’ subrange referred to excess bacterial abundance levels in which P. pacificus does not need to bite and focuses only on grazing on bacteria. Importantly, the scarce and plentiful subranges varied depending on the outcome and target being considered.
Based on P. pacificus’s preference for bacteria food over prey (Figure 3B), we generally defined predatory utility functions as having a constant maximal value over the negligible subrange where prey is the only acceptable food option, then monotonically decreasing over the scarce subrange, until it bottoms out to zero utility over the plentiful range. We reasoned that predatory utility over the negligible subrange should reflect the relative long-term net energy gain of eating prey when it is the only food option. Instead of calculating energy intake and dividing by food handling time, we approximated long-term net energy gain using ORO staining of fat stores (see STAR Methods: Fat-staining), a proxy indicator of excess energy intake (Figure 3A). With excess food and assumed lack of satiety (OP50, the highest quality food, does not induce satiety62, we assumed that P. pacificus spent the entire time (6 hours) feeding and handling food (search time is assumed to be zero). Using the relative ORO-stained area in prey-fed P. pacificus compared to bacteria-fed P. pacificus (taken to be 1), we estimated predatory utility of biting larval and adult targets over the negligible subrange,
For predatory utility over the scarce subrange, we used the probability that P. pacificus switches from a prey patch to a bacterial patch (Figure 3B) to linearly approximate how much prey P. pacificus foregoes with each increase in bacterial abundance,
Compared to predatory value functions, we set territorial utility functions to be non-monotonic to reflect the multi-faceted dependence of bite utility on both bacterial abundance and on the more abstract property of bacterial territory. We reasoned that territorial utility over the scarce subrange should be zero, since there is no bacteria territory present or worth defending. At the transition between negligible and scarce subranges, territorial utility should jump suddenly to a maximal utility, since this is where scarcity-induced competitive pressure is highest. Like predatory utility functions, territorial utility should also decrease monotonically over the scarce subrange. To estimate the maximal territorial utility, we used the bacterial consumption rate of C. elegans relative to that of P. pacificus (Figure 3C). Adult C. elegans consumed bacteria 1.5x faster than P. pacificus, but we found that the addition of L1 larvae (range 20–62) alongside an adult C. elegans did not increase bacterial consumption rate (Figures 3C, S3E, and S3F). This finding differed considerably from previous reports that L1-L2 stage C. elegans consumes ~25% the rate of an adult C. elegans63. This discrepancy may be due to that study’s use of liquid bacterial culture rather than a viscous patch, or due to our indirect measure of larval bacterial consumption (we did not measure larvae by themselves). To acquire a conservative estimate of larval bacterial consumption rate, we set adult C. elegans consumption to zero and assumed staggered hatching of larvae, and obtained a rate that is 1/20th the rate of adult C. elegans. To alleviate competitive pressure to defend territory, we reasoned that there should be additional bacterial allocated for C. elegans in addition to the amount that would be considered plentiful without C. elegans competition. To approximate this latter amount, we used the length of the scarce subrange for uP,L. Altogether, we defined the territorial value over the scarce subrange for larval and adult C. elegans,
Finally, expected utility was calculated by multiplying the corresponding probability and utility function for each target-outcome pair, and then comparing within-target to predict which outcome is more lucrative for a particular C. elegans target,
It is important to note that the purpose of this bite choice model was to predict the shape of expected utility functions across the behaviorally defined bacterial abundance subranges, rather than to precisely predict p(bite|encounter) values. It is unclear how characteristics of bacterial patches such as diameter and density would map onto the one-dimensional bacterial abundance x-axis in the model, so we could not assign predicted p(bite|encounter) values to particular bacterial patches. Instead, to test the model, we manipulated either only diameter or density, with one patch (medium-density, 1 mm diameter) that was common to both sets of tested patches. Then we assessed monotonicity, which was not affected by the scaling (of bacterial abundance) between bacterial patches that are ordered from lowest abundance to highest abundance.
Amphid neuron ablation
DiO staining of amphid neurons was adapted from published staining of P. pacificus64. Larval J2 P. pacificus were stained for 2 hours on a nutator in a solution of 15 ng/ml Fast DiO (ThermoFisher D3898) and then de-stained on an empty NGM plate for 1 hour. A 3% agar plug was used to gently transfer stained J2 animals onto a 2% agarose pad (melted in M9) with 20 mM sodium azide paralytic. Pairs of amphid neurons were ablated using an Andor Micropoint focused laser microbeam system. Cell identification was based on the identities described by Hong and colleagues65. Cell death was confirmed by identifying a morphological change within the cell, and by re-staining after behavior was recorded. Each ablated J2 was transferred onto its own bacterial patch to recover before being used 2 days later to measure biting incentive.
Bead patches
Sephadex bead patches were prepared similarly to Sawin and colleagues (2000)36. 30 mg/ml of superfine Sephadex beads (Sigma-Aldrich G10050) in M9 were autoclaved for 45 minutes and allowed to rehydrate overnight at 4°C. Rehydrated beads were then stored at 4°C. To prepare bead patches, the rehydrated bead mix was vortexed well, and then pipetted onto the agar plate. 0.28 µl was used to make 1 mm diameter patches (Figure S4B), and 1 µl was used to make 3 mm diameter patches. The beads were transparent and allowed for encounters and bites to be mostly visible. Encounters were excluded from analysis if encounters or bites were unclear due to bead distortion.
Patrol speed
Since the P. pacificus mouth is engaged in both feeding on bacteria and biting C. elegans, we tracked mouth location instead of the body’s center of mass. Mouth location on a bacterial patch (medium density, 1 mm in diameter) was manually tracked using MATLAB (see Key Resources Table). To focus on deliberate on-patch exploration patterns, we restricted analysis to the longest continuous video segment (≥ 10 minutes of a 30-minute recording) during which P. pacificus did not leave the patch. To measure total movement, we calculated translational speed as the sum of the Euclidean distances between each recorded mouth location, divided by total time. We used MATLAB (see Key Resources Table) to measure patrolling around the circular patch boundary (Video S3). We first measured the widest arc of the patch circumference that P. pacificus traversed (excluding back-and-forth movements or traveling along a chord to another location on the patch circumference that do not contribute to forward progress) in between changing directions (clockwise ↔ counterclockwise). Then, we summed all arc lengths (angle × radius) and divided by total time to arrive at what we call patrol speed.
The ratio of patrol speed to translational speed was used to compare differences in how much movement is dedicated to patrolling. To discount stationary bouts of feeding on larvae, during which time P. pacificus is not searching, we normalized patrol speed by the corresponding translational speed. Both these patrol speed and translational speeds are calculated over the same period of time, so time cancels out and normalized patrol speed becomes a ratio of patrol and translational distances. Since stationary bouts of feeding do not contribute distances to either patrol or translational speed, they do not affect normalized patrol speed.
Drug treatment
The bacterial patch used for treatment was formed by seeding 0.5 µl of liquid E. coli OP50 cultures (OD600 = 0.4) on a 35 mm NGM plate and growing for 2 days at room temperature. 2 µl of a working drug solution (5 mM amisulpride, 10 mM sumanirole, 100 mM octopamine, 100 mM epinastine) was dispensed onto the patch, 1 µl at a time and allowed to dry in between. As soon as the patch was visibly dry, P. pacificus young adults were placed on the treated patch for 2 hours. P. pacificus was then allowed crawl three body lengths on a clean area of an agar plate to remove surface drug residue, and then immediately used for behavior. See Key Resources Table for more information on drugs.
Quantification and statistical analysis
Statistical test parameters and outcomes are indicated in figure legends.
For datasets in which all measurements are independent results, assumptions for statistical tests were assessed to select an appropriate parametric or non-parametric test for comparing samples. The Shapiro–Wilk test was used to test for normality within each sample, and the Levene’s test was used to test for homogeneity of variances across samples. Student’s t-test was used to compare two normally distributed samples with equal variances, while Welch’s t-test was used to compare two non-normally distributed samples with unequal variances. Wilcoxon’s rank sum test was used to compare two non-normally distributed samples with equal variances. Dunn’s test was used to compare non-normally distributed samples with unequal variances. For paired comparisons, the paired t-test was used compare samples with normally distributed differences, while Wilcoxon’s signed rank test was used to compare samples with non-normally distributed differences. One-or two-way ANOVAs were used to compare three or more normally distributed groups. For post-hoc tests after an ANOVA, Dunnett’s test was used to conduct simultaneous multiple comparisons in which samples are compared to a control, and Tukey’s HSD test was used to conduct simultaneous multiples comparisons between all pairs. As a parametric alternative to ANOVA, the Kruskall-Wallis test was used to compare three or more non-normally distributed groups, with Dunn’s test as the post-hoc test for simultaneous comparisons of all pairs. To avoid making assumptions of normality in error bar representation, we performed bootstrap resampling to calculate 95% confidence intervals around the mean.
For datasets in which both independent and dependent variables were categorical, we assembled data into a contingency table and conducted Fisher’s exact test.
For datasets with multiple measurements per independent result, we built statistical models to compare estimated means across categories. Binomial logistic regression66 was used to model data in which independent results consist of a variable number of trials with two possible outcomes (Figures 1H, 1J, 1K, 2C, 2E, 3E, 3F, 4C, 6A, 6B, S1D, S6A, S6B). The primary benefit of binomial logistic regression models is to give more weight to independent results with more trials. All figures with y-axes starting with “p(name of event)” (not including Figure 1I) feature sample probabilities and confidence intervals predicted by binomial logistic regression model. Linear mixed-effects (LME) models were used to model hierarchical egg distribution data (Figure 2B), which has non-independence in the data at one level (egg distances within an arena) and independence at a higher level (arenas). To model the effect of mix of adult nematodes on egg distances (Figure 2B), separate models were fitted for each egg species to model. Convergence of LME models was assessed by fitting models with all available optimizers and checking that all optimizers converge to values that are practically equivalent. For all binomial logistic regression models and LME models, likelihood ratio tests (LRT) were used to assess goodness of fit by comparing full models to null models (Table S1). To compare between multiple levels of a category, Wald tests with single-step p-value adjustment were used to test linear hypotheses and limit issues related to multiple comparisons.
Benjamini–Hochberg correction was used to adjust p-value for all comparisons involving multiple independent tests.
To measure associations between variables, we used different coefficients or combinations of coefficients, depending on the type of association we wanted to describe. To measure linear correlation between two variables, we used Pearson’s r. To measure how well a monotonic function describes the relationship between two variables, we used Spearman’s ρ. To measure the non-linear and non-monotonic relationship between two variables, we first checked for a very low value for Spearman’s ρ (indicative of non-monotocity), and then used Hoeffding’s D.
All statistical analyses were carried out with the R statistical software67. The additional package lme468 was used to conduct linear mixed-effects models, and the additional package multcomp69 to conduct linear hypotheses with single-step adjustment for multiple comparisons.
Supplementary Material
Video S1. P. pacificus easily kills larval C. elegans, related to Figure 1
P. pacificus bites, kills, and feeds on a C. elegans larva (L1 stage).
Video S2. Adult C. elegans escapes P. pacificus after being bit, related to Figure 1
P. pacificus (initially horizontal) bites a young adult C. elegans (initially vertical) in the absence of bacteria, followed by C. elegans escaping from the bite.
Video S3. Adult C. elegans exits bacterial patch after being bitten, related to Figure 1 and Figure 5
P. pacificus (initially outside of patch) enters and patrols the border of a bacterial patch, then bites adult C. elegans (initially inside of patch).
Video S4. P. pacificus decides to exploit low-density bacterial patch, related to Figure 3
P. pacificus (initially on left) decides to stay on a 1 mm, low-density bacteria patch and bites adult C. elegans (initially on top). Adult C. elegans exits the patch after being bitten.
Video S5. P. pacificus decides to not exploit low-density bacterial patch, related to Figure 3
P. pacificus (initially on bottom right) encounters and decides to leave a 1 mm, low-density bacteria patch.
Highlights.
Non-fatal biting by the predator P. pacificus deters competing prey from food.
Non-fatal biting of adult prey is territorial aggression, not failed predation.
Territorial and predatory biting are associated with different prey search tactics.
P. pacificus switches between predatory and territorial foraging strategies.
Acknowledgments
We thank Ralf Sommer, Ray Hong, and Cori Bargmann for strains; Karina Kono, Cassidy Pham, Shw Lew, and Lou Tames for their support roles; Kevin Curran and Suneer Verma for precursor work on P. pacificus biting; Ray Hong and Jagan Srinivasan for their expertise in P. pacificus; Mike Rieger for statistical advice; and Jing Wang, Jagan Srinivasan, Corinne Lee-Kubli, Adam Calhoun, Kenta Asahina, Robert Luallen, David O’Keefe, and members of the lab for their critical reading of the manuscript. This work was supported by National Institutes of Health 5R01MH113905 (S.H.C.), W.M. Keck Foundation (S.H.C.), National Science Foundation (K.T.Q.), Salk Women & Science (K.T.Q.), and Paul F. Glenn Foundation Post-doctoral fellowship (K.T.Q.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests
References
- 1.Richmond CE, Breitburg DL, and Rose KA (2005). The role of environmental generalist species in ecosystem function. Ecological modelling 188, 279–295. 10.1016/j.ecolmodel.2005.03.002. [DOI] [Google Scholar]
- 2.Clavel J, Julliard R, and Devictor V (2011). Worldwide decline of specialist species: toward a global functional homogenization?. Front. Ecol. Environ 9, 222–228. 10.1890/080216. [DOI] [Google Scholar]
- 3.Stephens DW, and Krebs JR (1986). Foraging Theory (Princeton Univ. Press; ). [Google Scholar]
- 4.Waddington KD, and Holden LR (1979). Optimal foraging: on flower selection by bees. Am. Nat 114, 179–196. 10.1086/283467. [DOI] [Google Scholar]
- 5.Stephens DW, Lynch JF, Sorensen AE, and Gordon C (1986). Preference and profitability: theory and experiment. Am. Nat 127, 533–553. 10.1086/284501. [DOI] [Google Scholar]
- 6.Sih A, and Christensen B (2001). Optimal diet theory: when does it work, and when and why does it fail?. Animal behaviour 61, 379–390. 10.1006/anbe.2000.1592. [DOI] [Google Scholar]
- 7.Arim M, and Marquet PA (2004). Intraguild predation: a widespread interaction related to species biology. Ecol. Lett 7, 557–564. 10.1111/j.1461-0248.2004.00613.x. [DOI] [Google Scholar]
- 8.Holt RD, and Polis GA (1997). A theoretical framework for intraguild predation. The Am. Nat 149, 745–764. 10.1086/286018. [DOI] [Google Scholar]
- 9.Pringle RM, Kartzinel TR, Palmer TM, Thurman TJ, Fox-Dobbs K, Xu CC, Hutchinson MC, Coverdale TC, Daskin JH, Evangelista DA et al. (2019). Predator-induced collapse of niche structure and species coexistence. Nature 570, 58–64. 10.1038/s41586-019-1264-6. [DOI] [PubMed] [Google Scholar]
- 10.Polis GA, Myers CA, and Holt RD (1989). The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst 20, 297–330. 10.1146/annurev.es.20.110189.001501. [DOI] [Google Scholar]
- 11.Sunde P, Overskaug K, and Kvam T (1999). Intraguild predation of lynxes on foxes: evidence of interference competition?. Ecography 22, 521–523. 10.1111/j.1600-0587.1999.tb01281.x. [DOI] [Google Scholar]
- 12.Desisto MJ, and Huston JP (1970). Effect of territory on frog-killing by rats. The Journal of general psychology 83, 179–184. [DOI] [PubMed] [Google Scholar]
- 13.Bandler RJ Jr (1970). Cholinergic synapses in the lateral hypothalamus for the control of predatory aggression in the rat. Brain Research 20, 409–424. 10.1016/0006-8993(70)90171-X. [DOI] [PubMed] [Google Scholar]
- 14.Kemble ED, Flannelly KJ, Salley H, and Blanchard RJ (1985). Mouse killing, insect predation, and conspecific attack by rats with differing prior aggressive experience. Physiology & behavior 34, 645–648. 10.1016/0031-9384(85)90063-0. [DOI] [PubMed] [Google Scholar]
- 15.Bridgman LJ, Innes J, Gillies C, Fitzgerald NB, Miller S, and King CM (2013) Do ship rats display predatory behaviour towards house mice?. Animal Behaviour 86, 257–268. 10.1016/j.anbehav.2013.05.013. [DOI] [Google Scholar]
- 16.Kruuk H (1972). Surplus killing by carnivores. Journal of Zoology 166, 233–244. 10.1111/j.1469-7998.1972.tb04087.x [DOI] [Google Scholar]
- 17.Adams ES, and Mesterton-Gibbons M (1995). The cost of threat displays and the stability of deceptive communication. Journal of Theoretical Biology 175, 405–421. 10.1006/jtbi.1995.0151. [DOI] [Google Scholar]
- 18.Quach KT, and Chalasani SH (2020). Intraguild predation between Pristionchus pacificus and Caenorhabditis elegans: a complex interaction with the potential for aggressive behaviour. J. Neurogenetics 34, 404–419. 10.1080/01677063.2020.1833004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serobyan V, Ragsdale EJ, and Sommer RJ (2014). Adaptive value of a predatory mouth-form in a dimorphic nematode. Proc. R. Soc. B 281, 20141334. 10.1098/rspb.2014.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilecki M, Lightfoot JW, Susoy V, and Sommer RJ (2015). Predatory feeding behaviour in Pristionchus nematodes is dependent on phenotypic plasticity and induced by serotonin. J. Exp. Biol 218, 1306–1313. 10.1242/jeb.118620. [DOI] [PubMed] [Google Scholar]
- 21.Cassada RC, and Russell RL (1975). The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol 46, 326–342. 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 22.Frederick S, Loewenstein G, and O’Donoghue T (2002). Time discounting and time preference: a critical review. J. Econ. Lit 40, 351–401. 10.1257/002205102320161311. [DOI] [Google Scholar]
- 23.Shtonda BB, and Avery L (2006). Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol 209, 89–102. 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernoulli D (1738). Specimen theoriae novae de mensura sortis. Commentarii Academiae Scientarum Imperialis Petropolitanae 5, 175–192. [Google Scholar]
- 25.Carpenter FL, and MacMillen RE (1976). Threshold model of feeding territoriality and test with a Hawaiian honeycreeper. Science 194, 639–642. 10.1126/science.194.4265.639. [DOI] [PubMed] [Google Scholar]
- 26.Briggman KL, Abarbanel HD, and Kristan WB Jr. (2005). Optical imaging of neuronal populations during decision-making. Science 307, 896–901. 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]
- 27.Gray JM, Hill JJ, and Bargmann CI (2005). A circuit for navigation in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 102, 3184–3191. 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong RL, and Sommer RJ (2006). Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr. Biol 16, 2359–2365. 10.1016/j.cub.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Bargmann CI, Hartwieg E, and Horvitz HR (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. 10.1016/0092-8674(93)80053-H. [DOI] [PubMed] [Google Scholar]
- 30.Rogers C, Persson A, Cheung B, and de Bono M (2006). Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol 16, 649–659. 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 31.de Bono M, and Bargmann CI (1998). Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94, 679–689. 10.1016/S0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 32.Moreno E, McGaughran A, Rödelsperger C, Zimmer M, and Sommer RJ (2016). Oxygen-induced social behaviours in Pristionchus pacificus have a distinct evolutionary history and genetic regulation from Caenorhabditis elegans. Proc. R. Soc. B 283, 20152263. 10.1098/rspb.2015.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno E, Sieriebriennikov B, Witte H, Rödelsperger C, Lightfoot JW, and Sommer RJ (2017). Regulation of hyperoxia-induced social behaviour in Pristionchus pacificus nematodes requires a novel cilia-mediated environmental input. Sci. Rep 7, 17550. 10.1038/s41598-017-18019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno E, Lenuzzi M, Rödelsperger C, Prabh N, Witte H, Roeseler W, Riebesell M and Sommer RJ (2018). DAF-19/RFX controls ciliogenesis and influences oxygen-induced social behaviors in Pristionchus pacificus. Evol. Dev 20, 233–243. 10.1111/ede.12271. [DOI] [PubMed] [Google Scholar]
- 35.Moreno E, Lightfoot JW, Lenuzzi M, and Sommer RJ (2019). Cilia drive developmental plasticity and are essential for efficient prey detection in predatory nematodes. Proceedings of the Royal Society B 286, 20191089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawin ER, Ranganathan R, and Horvitz HR (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631. 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 37.Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA and Bargmann CI (2004). Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322. 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 38.Suo S, Culotti JG, and Van Tol HH (2009). Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J 28, 2437–2448. 10.1038/emboj.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roeder T, Degen J, and Gewecke M (1998). Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur. J. Pharmacol 349, 171–177. 10.1016/S0014-2999(98)00192-7. [DOI] [PubMed] [Google Scholar]
- 40.Sih A (1987). Prey refuges and predator-prey stability. Theoretical Population Biology 31, 1–12. 10.1016/0040-5809(87)90019-0. [DOI] [Google Scholar]
- 41.Lightfoot JW, Wilecki M, Rödelsperger C, Moreno E, Susoy V, Witte H, and Sommer RJ (2019). Small peptide-mediated self-recognition prevents cannibalism in predatory nematodes. Science 364, 86–89. 10.1126/science.aav9856. [DOI] [PubMed] [Google Scholar]
- 42.Sih A (1980). Optimal foraging: partial consumption of prey. Am. Nat 116, 281–290. 10.1086/283626. [DOI] [Google Scholar]
- 43.Sommer RJ, ed. (2015). Pristionchus pacificus: a nematode model for comparative and evolutionary biology (Brill)
- 44.Stephens GJ, Johnson-Kerner B, Bialek W, and Ryu WS (2008). Dimensionality and dynamics in the behavior of C. elegans. PLoS Comput. Biol 4, e1000028. 10.1371/journal.pcbi.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witte H, Moreno E, Rödelsperger C, Kim J, Kim JS, Streit A, and Sommer RJ (2015). Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Dev Genes Evol 225, 55–62. [DOI] [PubMed] [Google Scholar]
- 46.Han Z, Lo WS, Lightfoot JW, Witte H, Sun S, and Sommer RJ (2020). Improving transgenesis efficiency and CRISPR-associated tools through codon optimization and native intron addition in Pristionchus nematodes. Genetics 216, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wissinger SA (1992). Niche overlap and the potential for competition and intraguild predation between size‐ structured populations. Ecology 73, 1431–1444. 10.2307/1940688. [DOI] [Google Scholar]
- 48.Sommers P, and Chesson P (2019). Effects of predator avoidance behavior on the coexistence of competing prey. Am. Nat 193, E132–E148. 10.1086/701780. [DOI] [PubMed] [Google Scholar]
- 49.Niv Y (2020). The primacy of behavioral research for understanding the brain. PsyArXiv, 10.31234/osf.io/y8mxe. [DOI] [PubMed]
- 50.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, and Poeppel D (2017). Neuroscience needs behavior: correcting a reductionist bias. Neuron 93, 480–490. 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Sommer RJ, Carta LK, Kim S-Y, and Sternberg PW (1996). Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda: Neodiplogastridae). Fundam. Appl. Nematol 19, 511–521. [Google Scholar]
- 52.Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Click A, Savaliya CH, Kienle S, Herrmann M, and Pires-daSilva A (2009). Natural variation of outcrossing in the hermaphroditic nematode Pristionchus pacificus. BMC Evol. Biol 9, 75. 10.1186/1471-2148-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrmann M, Kienle S, Rochat J, Mayer WE, and Sommer RJ (2010). Haplotype diversity of the nematode Pristionchus pacificus on Réunion in the Indian Ocean suggests multiple independent invasions. Biol. J. Linnean Soc 100, 170–179. 10.1111/j.1095-8312.2010.01410.x. [DOI] [Google Scholar]
- 55.Sassa T, Harada SI, Ogawa H, Rand JB, Maruyama IN, and Hosono R (1999). Regulation of the UNC-18–Caenorhabditis elegans Syntaxin Complex by UNC-13. J Neurosci 19, 4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stiernagle T (1999) Maintenance of C. elegans. In C. elegans, Hope IA, ed. (Oxford University Press; ), pp. 51–67. [Google Scholar]
- 57.Pirri JK, and Alkema MJ (2012). The neuroethology of C. elegans escape. Curr. Opin. Neurobiol 22, 187–93. 10.1016/j.conb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Rourke EJ, Soukas AA, Carr CE, and Ruvkun G (2009). C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab 10, 430–435. 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Escorcia W, Ruter DL, Nhan J, and Curran SP (2018). Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. JoVE 133, e57352. 10.3791/57352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruifrok AC, and Johnston DA (2001). Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol 23, 291–299. [PubMed] [Google Scholar]
- 62.You YJ, Kim J, Raizen DM, and Avery L (2008). Insulin, cGMP, and TGF-β signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell metabolism 7, 249–257. 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Amaro RL, Valentine ER, Carretero M, LeBoeuf SE, Rangaraju S, Broaddus CD, Solis GM, Williamson JR, and Petrascheck M (2015). Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 200, 443–454. 10.1534/genetics.115.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivasan J, Durak O, and Sternberg PW (2008). Evolution of a polymodal sensory response network. BMC biol 6, 52. 10.1186/1741-7007-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong RL, Riebesell M, Bumbarger DJ, Cook SJ, Carstensen HR, Sarpolaki T, Cochella L, Castrejon J, Moreno E, Sieriebriennikov B, et al. (2019). Evolution of neuronal anatomy and circuitry in two highly divergent nematode species. eLife 8, e47155. 10.7554/eLife.47155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hosmer D, and Lemeshow S (2000). Applied Logistic Regression, Second Edition (New York: Wiley; ). [Google Scholar]
- 67.R Development Core Team (2017). R: a language and environment for statistical computing (R Foundation for Statistical Computing; ). https://www.R-project.org/ [Google Scholar]
- 68.Bates D, Maechler M, Bolker B, and Walker S (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw 67, 1–48. arXiv:1406.5823. [Google Scholar]
- 69.Hothorn T, Bretz F, and Westfall P (2008). Simultaneous inference in general parametric models. Biom J 50, 346–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. P. pacificus easily kills larval C. elegans, related to Figure 1
P. pacificus bites, kills, and feeds on a C. elegans larva (L1 stage).
Video S2. Adult C. elegans escapes P. pacificus after being bit, related to Figure 1
P. pacificus (initially horizontal) bites a young adult C. elegans (initially vertical) in the absence of bacteria, followed by C. elegans escaping from the bite.
Video S3. Adult C. elegans exits bacterial patch after being bitten, related to Figure 1 and Figure 5
P. pacificus (initially outside of patch) enters and patrols the border of a bacterial patch, then bites adult C. elegans (initially inside of patch).
Video S4. P. pacificus decides to exploit low-density bacterial patch, related to Figure 3
P. pacificus (initially on left) decides to stay on a 1 mm, low-density bacteria patch and bites adult C. elegans (initially on top). Adult C. elegans exits the patch after being bitten.
Video S5. P. pacificus decides to not exploit low-density bacterial patch, related to Figure 3
P. pacificus (initially on bottom right) encounters and decides to leave a 1 mm, low-density bacteria patch.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the Key Resources Table. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Escherichia coli: OP50 | Caenorhabditis Genetics Center | WBStrain00041969 |
| Escherichia coli: OP50-GFP | Caenorhabditis Genetics Center | WBStrain00041972 |
| Chemicals, peptides, and recombinant proteins | ||
| Oil Red O | Alfa Aesar | Cat#A12989 |
| Fast DiO | ThermoFisher | Cat#D3898 |
| Sephadex G-100 | Sigma-Aldrich | Cat#G10050 |
| Amisulpride | Sigma-Aldrich | Cat#A2729 |
| Epinastine hydrochloride | Sigma-Aldrich | Cat#E5156 |
| Sumanirole maleate | Tocris | Cat#2773 |
| (±)-Octopamine hydrochloride | Supelco | Cat#68631 |
| Experimental models: Organisms/strains | ||
| Pristionchus pacificus: RS5194: wild isolate | Sommer Lab (Max Planck Campus Tübingen)53,54 | N/A |
| Pristionchus pacificus: RS5275: wild isolate | Sommer Lab (Max Planck Campus Tübingen) 53,54 | N/A |
| Pristionchus pacificus: PS312: wild isolate | Sommer Lab (Max Planck Campus Tübingen) 51 | WBStrain00047433 |
| Caenorhabditis elegans: N2: wild isolate | Caenorhabditis Genetics Center52 | WBStrain00000001 |
| Caenorhabditis elegans: CX7389: [kyIs392 [Pstr-2::GFP::rab-3; Pttx-3::lin-10::dsRed; Pelt-2::GFP] | Bargmann Lab (Rockefeller), this paper | N/A |
| Caenorhabditis elegans : IV95 : [ueEx46 [gcy-7-sl2-mCherry; Punc-122::RFP]; gvIs246 [ida-1::GFP+ pRF4 rol-6(su1006)], Caenorhabditis elegans strain | this paper | N/A |
| Caenorhabditis elegans: CB81: [unc-18(e81) X], | Caenorhabditis Genetics Center55 | WBStrain00004094 |
| Software and algorithms | ||
| ImageJ, Fiji | 58 | https://imagej.net/software/fiji/ |
| Colour Deconvolution plugin, Fiji | 61 | https://imagej.net/plugins/colour-deconvolution |
| R 4.0.2 | 67 | https://www.r-project.org/ |
| lme4 | 68 | https://cran.r-project.org/web/packages/lme4/ |
| multcomp | 69 | https://cran.r-project.org/web/packages/multcomp/ |
| MATLAB 2017b | MathWorks | https://www.mathworks.com/help/releases/R2017b/ |
| code for analyzing egg distribution | this paper | DOI: 10.5281/zenodo.5976742 |
| code for analyzing patrol speed | this paper | DOI: 10.5281/zenodo.5976742 |


