Abstract
The in vitro intracellular effect of clarithromycin, amoxicillin, metronidazole, lansoprazole, and rifabutin, tested at concentrations corresponding to one times the MIC, two times the MIC, and four times the MIC, was evaluated against an invasive Helicobacter pylori strain. At four times the MIC, clarithromycin showed an early bactericidal effect within 4 h of incubation and, in determining the complete killing within a 16 h-incubation period, lansoprazole and rifabutin showed comparable activity, yielding bactericidal activities within 4 and 8 h of incubation, respectively. Amoxicillin and metronidazole showed bacteriostatic activity only.
Helicobacter pylori is a gram-negative, microaerophilic spiral bacterium that has been undisputably implicated in the etiology of chronic active gastritis, peptic ulceration disease, and gastric carcinoma in humans worldwide. H. pylori in vivo is commonly found adhering to gastric epithelial cells, and it is not considered as an invasive pathogen. However, observations both in vitro and in vivo have shown that H. pylori is capable of invading epithelial cells (4, 18). Some electron microscopic studies have also shown that H. pylori is often observed at the intercellular junctions between gastric epithelial cells or within intercellular spaces and gastric mucosal cells (11, 12, 18).
Recently, we have shown that H. pylori can survive intracellularly for at least 48 h in vitro and that the intracellular bacteria can be killed by clarithromycin (15); these data suggest that treatment failures may be due, at least in part, to the existence of an ecological niche for the organism that allows it to survive both the hostile gastric environment and antibacterial therapy. Rifabutin, a spiropiperidyl-rifamycin derived from rifamycin S, has a broad spectrum of antimicrobial activity, including activity against mycobacteria (8). Its stability over a wide pH range suggests that it is a sensible alternative to study for activity against H. pylori. The in vitro activities of amoxicillin, clarithromycin, metronidazole, lansoprazole, and rifabutin were examined against one intracellular H. pylori clinical strain.
The clinical strain H. pylori UdA203 was grown onto modified chocolate agar (14) under microaerophilic conditions (5% O2, 10% CO2, 85% N2; CampyGen; Oxoid/Garbagnate Milanese, Milan, Italy) for 72 h at 37°C. Amoxicillin and metronidazole were purchased from Sigma-Aldrich (Milan, Italy); clarithromycin, rifabutin, and lansoprazole were kindly supplied by Abbott Italy S.p.A. (Campoverde, Latina, Italy), Pharmacia & Upjohn (Milan, Italy), and Takeda Chemical Industries (Osaka, Japan), respectively. Stock solutions were prepared according to the manufacturers' recommendations on the day of use. MICs were assessed in duplicate by the agar dilution method according to NCCLS guidelines (9). Twofold dilutions of each drug ranging from 0.008 to 64 μg/ml were tested. The minimal bactericidal concentration (MBC) was established by spreading 0.01 ml from each concentration onto Columbia blood agar plates. The MBC was the lowest antibiotic concentration to result in no growth.
Intracellular activity was studied in a human epithelial cell line: HEp-2 cells (ATCC CCL 23). The cell culture medium contained RPMI 1640 (Gibco — BRL/Life Technology, Paisley, Scotland), 10% fetal calf serum, 20 mM HEPES, 2 mM glutamine, and 0.05% NaHCO3. HEp-2 cells (ATCC CCL 23) were routinely grown in 25-cm2 plastic tissue culture flasks at 35°C and 5% CO2. Confluent HEp-2 cell monolayers in 24-well tissue culture plates (105 cells/well) were exposed to approximately 108 CFU of H. pylori per ml in the RPMI medium. Internalization was allowed to occur for 12 h at 37°C under microaerophilic conditions, and then extracellular bacteria were killed by gentamicin assay (15). Samples were cultured to verify that the extracellular bacteria were eradicated. HEp-2 monolayers were washed three times with phosphate-buffered saline, and then (time zero) each drug was added at concentrations equal to the MIC and at two- and fourfold the MIC for the strain tested. HEp-2 cells infected with H. pylori which had not been exposed to the drug were included as a control. The drug-treated cells, along with the drug-free controls, were incubated at 37°C in an atmosphere of 5% CO2. After fixed times of incubation (0, 2, 4, 8, 24, and 48 h), the cells were washed three times and lysed in deionized water for 10 min. Colonies of viable bacteria were counted by plating 10-fold dilutions of the lysates onto Columbia blood agar supplemented with 5% defibrinated sheep blood and then incubated in a microaerophilic atmosphere for up to 5 days (limit of detection, 10 CFU/ml). Experiments were carried out in triplicate. The bactericidal activity was defined for at least a 3-log10 reduction of the internalized bacteria.
The MIC and MBC values of amoxicillin, clarithromycin, metronidazole, rifabutin, and lansoprazole for H. pylori UdA203 were 0.5 and 2, 0.5 and 1, 8 and 32, 1 and 4, 4 and 8, and 8 and 32 μg/ml, respectively. The MIC of gentamicin was 0.5 μg/ml.
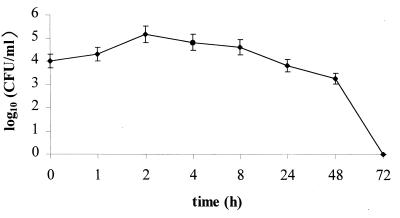
The ability of H. pylori UdA203 to invade HEp-2 cells is shown in Fig. 1. At a cell/bacterium multiplicity of 1:1,000, the uptake of H. pylori by HEp-2 cells was rapid: an intracellular concentration of 9 × 104 CFU/ml (internalization efficacy of 0.09%) was recorded within 2 h of incubation. The recovery of intracellular bacteria decreased with time: a 2-log10 reduction of intracellular H. pylori was achieved after 48 h of incubation. No intracellular bacteria were viable after 72 h of incubation.
FIG. 1.
Number of H. pylori UdA203 organisms internalized by HEp-2 cells as determined by gentamicin survival assay.
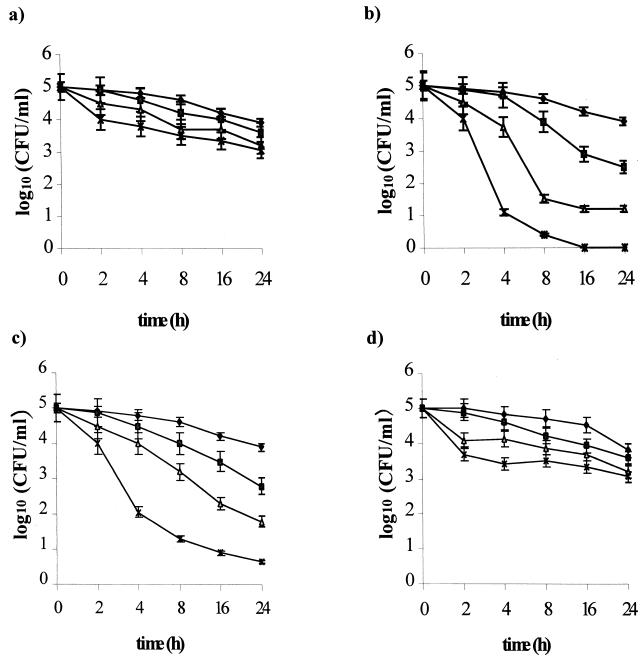
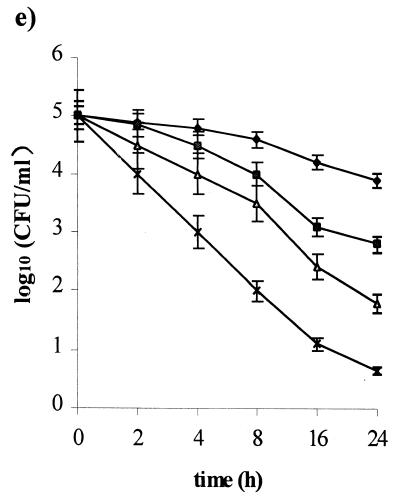
Figure 2 shows the resulting intracellular killing curves based on mean values from three experiments for each drug tested. At the MIC, clarithromycin, lansoprazole, and rifabutin showed comparable intracellular activity, causing a 2-log10 reduction of internalized bacteria within 24 h. Amoxicillin, as well as metronidazole, was intracellularly ineffective. At two times the MIC, clarithromycin, lansoprazole, and rifabutin showed bactericidal activities within 6, 20, and 20 h of incubation, respectively. Amoxicillin and metronidazole are bacteriostatic within 24 h of incubation, causing a 2-log10 reduction of the viable count. At four times the MIC, clarithromycin was the most active, with a 4-log10 reduction in the viable count within 4 h of incubation, and showing complete killing of intracellular H. pylori within 16 h of incubation. Lansoprazole and rifabutin yielded bactericidal activity within 4 and 8 h of incubation, respectively. Amoxicillin and metronidazole showed intracellular activity comparable to that performed at two times the MIC.
FIG. 2.
Intracellular killing activity of amoxicillin (a), clarithromycin (b), lansoprazole (c), metronidazole (d), and rifabutin (e) against the H. pylori invasive strain UdA203. Bacterial counts were determined at time zero and after 2, 4, 8, 24, and 48 h of incubation with different antibiotic concentrations (⧫, control; ■, one times the MIC; ▴, two times the MIC; ×, four times the MIC). Mean values from triplicate measurements are shown. Bars indicate the standard deviations.
Although H. pylori is commonly considered a noninvasive pathogen, several reports support an intracellular location of the bacterium both in vitro and in vivo (4, 7, 15, 18). The results of the present study showed that H. pylori penetrates rapidly into HEp-2 cells, thus confirming the previous findings: the bacterium is not able to replicate inside the cells, and no intracellular bacteria were viable after 72 h of incubation, a result probably due to the hostile microenvironment it encounters inside the vacuoles created by acidic pH and limited nutrient availability (6). The low internalization efficacy we found (0.09%) suggests that only a subpopulation of H. pylori has the capacity to “switch on” virulence determinant-associated genes that allows intracellular penetration. In this sense, Björkholm et al. (1) observed an increased internalization in a strain producing the vacuolating cytotoxin. The ability of the bacterium to survive inside an epithelial cell could determine the ability to establish persistent infection: internalized H. pylori might survive by avoiding the extracellular antimicrobial action and the humoral cell response, reappearing on the luminal surface upon the reestablishment of favorable conditions. The results of Engstrand et al. (3), evaluating the effect of anti-H. pylori treatment on the organism in vivo by determining urease-associated heat shock protein (hsp60) expression, supported the possibility of reinfection by an H. pylori previously internalized by epithelial cells.
The need for an antibiotic with intracellular activity probably reflects the ability of internalized H. pylori to evade antibacterial treatment. In view of this, the objective of the present study was to evaluate the activities of clarithromycin, metronidazole, amoxicillin, lansoprazole, and rifabutin against intracellular H. pylori. Our data indicated that all drugs, except amoxicillin and metronidazole, showed marked concentration- and incubation time-dependent killing of internalized H. pylori. Clarithromycin was the most active drug against intracellular H. pylori, showing an early bactericidal effect, with complete killing at concentration of four times the MIC (corresponding to 2 μg/ml). Similarly, Hultén et al. (6) found that azithromycin is active against a clinical isolate of H. pylori internalized by HEp-2 cells: however, extracellular concentrations of 200 times the MBC (corresponding to 100 μg/ml) were necessary to achieve intracellular killing. The good intracellular activity of clarithromycin found in the present study may explain the improved efficacy of treatment regimens that contain this macrolide. However, in ca. 10% of the treated patients the pharmacological regimen fails (10), principally because of clarithromycin and metronidazole resistance. Clarithromycin resistance is still low in most communities; however, when present, it has a higher negative impact on treatment outcome than metronidazole resistance (2).
Rifabutin, a spiropiperidyl derivative of rifamycin S, is highly active against strains of H. pylori resistant to metronidazole and clarithromycin (16). This may explain the efficacy of rifabutin in patients who failed two or more courses of proton pump inhibitor-based triple therapies (13). In our study, rifabutin showed good intracellular activity against H. pylori, as did lansoprazole. These results, in combination with the stability of rifabutin over a wide range of pH values, suggest that rifabutin may be considered as an effective component of therapy for H. pylori infection.
Metronidazole and amoxicillin showed poor intracellular activity, as expected since they have been shown to have limited access to the intracellular space of phagocytic cells (17). However, the peak concentration of amoxicillin in serum is reported to vary from 5.17 to 12.20 μg/ml (5). Thus, the concentration used in the present study is lower than the values achieved in the clinical setting. Further studies are warranted to evaluate the in vitro activity of amoxicillin at therapeutically achievable concentrations against intracellular H. pylori.
The real challenge for the future will be the management of patients who do not respond to first-line treatment. The results of the present study may provide additional insight that can be used to design future treatment strategies for treating H. pylori infections. In particular, the efficacy of an anti-H. pylori “rescue” treatment might be highly improved by utilizing new antibiotics, such as rifabutin, that show promise for the treatment of extracellular and intracellular H. pylori infections. Further studies are needed to extend our results to the general population of H. pylori strains.
Acknowledgments
This work was supported by grants from the Abbott Italia S.p.A. (Campoverde, Latina, Italy) and the Biomedical Sciences Department of Chieti University.
We thank Cinzia Scarci for technical assistance.
REFERENCES
- 1.Björkholm B, Zhukhovitsky V, Löfman C, Hultén K, Enroth H, Block M, Rigo R, Falk P, Engstrand L. Helicobacter pylori entry into human gastric epithelial cells: a potential for determinant of virulence, persistence, and treatment failures. Helicobacter. 2000;5:148–154. doi: 10.1046/j.1523-5378.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 2.Dore M P, Leandro G, Realdi G, Sepulveda A R, Graham D Y. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45:68–76. doi: 10.1023/a:1005457226341. [DOI] [PubMed] [Google Scholar]
- 3.Engstrand L, Graham D Y, Scheynius A, Genta R M, El-Zaatari F. Is the sanctuary where Helicobacter pylori avoids antibacterial treatment intracellular? Microbiol Infect Dis. 1997;108:504–509. doi: 10.1093/ajcp/108.5.504. [DOI] [PubMed] [Google Scholar]
- 4.Evans D G, Evans D J, Jr, Graham D Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 5.Gordon R C, Regamey C, Kirby W M M. Comparative clinical pharmacology of amoxicillin and ampicillin administred orally. Antimicrob Agents Chemother. 1972;1:504–507. doi: 10.1128/aac.1.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultén K, Cars O, Hjelm E, Engstrand L. In vitro activity of azithromycin against intracellular Helicobacter pylori. J Antimicrob Chemother. 1996;37:483–489. doi: 10.1093/jac/37.3.483. [DOI] [PubMed] [Google Scholar]
- 7.Ko G H, Kang S M, Kim Y K, Lee J H, Park C K, Youn H S, Baik S C, Cho M J, Lee W K, Rhee K H. Invasiveness of Helicobacter pylori into human gastric mucosa. Helicobacter. 1999;4:77–81. doi: 10.1046/j.1523-5378.1999.98690.x. [DOI] [PubMed] [Google Scholar]
- 8.Kunin C M. Antimicrobial activity of rifabutin. Clin Infect Dis. 1996;22(Suppl. 1):S3–S14. doi: 10.1093/clinids/22.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Approved standard M7–A5. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 5th ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 10.National Institutes of Health Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 11.Neri M, Susi D, Bovani I, Laterza F, Mezzetti A, Cuccurullo F. Bacterial mucosal infiltration in Helicobacter pylori-associated gastritis: histological and clinical consequences. Am J Gastroenterol. 1994;89:1801–1805. [PubMed] [Google Scholar]
- 12.Noach L A, Rolf T M, Tytgat G N J. Electron microscopy study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perri F, Festa V, Clemente R, Quitadamo M, Andriulli A. Rifabutin-based “rescue-therapy” for Helicobacter pylori-infected patients after failure of standard regimens. Alim Pharmacol Ther. 2000;14:311–316. doi: 10.1046/j.1365-2036.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 14.Piccolomini R, Di Bonaventura G, Festi D, Catamo G, Laterza F, Neri M. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens. J Clin Microbiol. 1997;35:1541–1544. doi: 10.1128/jcm.35.6.1541-1544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccolomini R, Di Bonaventura G, Neri M. In vitro activity of clarithromycin against intracellular Helicobacter pylori. In: Zinner S H, Young L S, Acar J F, Ortiz-Neu C, editors. New considerations for macrolides, azalides, streptogramins, and ketolides. New York, N. Y: Marcel Dekker, Inc; 1998. pp. 487–491. [Google Scholar]
- 16.Pilotto A, Franceschi M, Rassu M, Furlan F, Scagnelli M. In vitro activity of rifabutin against strains of Helicobacter pylori resistant to metronidazole and clarithromycin. Am J Gastroenterol. 2000;95:834–835. doi: 10.1111/j.1572-0241.2000.01900.x. [DOI] [PubMed] [Google Scholar]
- 17.Scaglione F, Demartini G, Dugnani S, Fraschini F. A new model examining intracellular and extracellular activity of amoxicillin, azithromycin, and clarithromycin in infected cells. Chemotherapy. 1993;39:416–423. doi: 10.1159/000238987. [DOI] [PubMed] [Google Scholar]
- 18.Wyle F A, Tarnawski A, Schulman D, Dabros W. Evidence for gastric mucosal cell invasion by Campylobacter pylori: an ultrastructural study. J Clin Gastroenterol. 1990;12(Suppl. 1):S92–S98. doi: 10.1097/00004836-199001001-00016. [DOI] [PubMed] [Google Scholar]