Abstract
Introduction:
Metformin is a first-line diabetic therapy that improves survival in a wide number of ischemic pathologies. We tested the association of metformin with markers of cardiac and renal injury in diabetic post-arrest patients.
Methods:
We performed a retrospective analysis of clinical outcomes in diabetic cardiac arrest patients with and without metformin therapy at a single academic medical center. We used generalized linear models to test the independent association of metformin, insulin, and other hypoglycemic agents with peak 24-hour serum creatinine and peak 24-hour serum troponin.
Results:
Metformin prescription at the time of SCA was independently associated with lower 24-hour peak serum troponin and lower 24-hour peak serum creatinine when compared to non-metformin patients.
Conclusion:
Metformin pretreatment may offer cardiac and renal protection for diabetic patients during sudden cardiac arrest.
Introduction
Sudden cardiac arrest (SCA) affects over 600,000 patients annually in the United States (1). Patients with return of spontaneous circulation after SCA experience systemic ischemia-reperfusion injury, which may result in end-organ dysfunction including cardiogenic shock, acute renal failure, liver damage, and hypoxic-ischemic brain injury (2–4). Decreased cardiac (5) and kidney function (6,7) are predictors of early post-arrest mortality.
Metformin is an oral antihyperglycemic agent used as the first-line agent for type 2 diabetes (9). Metformin may protect against ischemic injury and significant cardiovascular stress, as demonstrated by improved mortality in the setting of coronary artery disease (9), congestive heart failure (10), acute kidney injury (11), chronic kidney disease (10), septic shock (12,13), and major surgical procedures (14). Pretreatment with metformin demonstrated neurologic protection and improvement in survival in a rat model of SCA (15). Similar studies from our group have found cardiac and renal protection in a murine model of SCA (16).
We explored the association of metformin with post-arrest cardiac and kidney injury in a cohort of diabetic patients resuscitated from SCA.
Methods
Study Design
We performed a retrospective cohort study at a single academic medical center. The University of Pittsburgh Post-Cardiac Arrest Service (PCAS) maintains a registry of in- and out-of-hospital cardiac arrest patients who survive to hospital care. The University of Pittsburgh Human Research Protection Office approved all aspects of this work.
Data Collection
We identified adult patients treated from January 2010 to December 2019 after resuscitation from cardiac arrest. To minimize confounding by indication, we included only patients with a history of type 2 diabetes mellitus. We excluded patients with a history of kidney disease prior to arrest, patients who arrived at our facility over 24 hours after collapse, patients for whom home medications were unknown, patients who rearrested and died before initial blood work could be acquired, and patients resuscitated with extracorporeal support. From our registry, we extracted demographic and arrest characteristics, including patient age, sex, shockable presenting arrest rhythm, witnessed arrest, layperson CPR, arrest duration, number of epinephrine doses administered, cardiac etiology of arrest and Charlson Comorbidity Index. On hospital admission, a pharmacist or nurse compiles a list of each patient’s outpatient medication regimen through a review of the electronic health record, discussion with family and outpatient providers, and verifies this medication history with the patient’s outpatient pharmacy to identify dates at which prescriptions were last filled. From each patient’s admission medication reconciliation, we determined whether patients were prescribed metformin, insulin, or other oral antihyperglycemic medications prior to their arrest, and classified each of these as three independent binary predictors.
Primary outcomes of interest were peak serum creatinine and peak serum troponin I at 24 hours post-arrest. Serum troponin and creatinine were measured as part of routine clinical care, typically at least once daily, which we used to quantify heart and kidney injury, respectively. As post hoc analyses, we evaluated alternative measures of renal (dys)function: urine output in the first 24 hours post-arrest; peak blood urea nitrogen (BUN) 24 hours post-arrest; and among patients surviving at least 72 hours, peak creatinine 72 to 20 hours post-arrest.
Statistical Analysis
We used descriptive statistics to summarize baseline population characteristics and outcomes. We used multiple imputation with chained equations to impute missing continuous variables, then used generalized linear models with a gamma distribution and log link to test the independent association of metformin with each outcome. For our primary adjusted analysis, we included covariates based on biological plausibility. As sensitivity analyses, we used several alternative approaches to model, including backward stepwise model, sequentially removing predictors with p<0.1, and complete case analysis. To reduce selection bias, we also repeated modeling excluding patients receiving insulin, who may be fundamentally sicker than those receiving no medications or oral antihyperglycemics only.
Results
Overall, 692 adult post-arrest patients with a history of type 2 diabetes were treated at our facility during the study period (Figure 1). We excluded 268 patients with chronic kidney disease, 20 who were transferred more than 24 hours post-arrest, 56 for whom home medications were unknown, seven who rearrested prior to labs being drawn, and one who was resuscitated with extracorporeal membrane oxygenation, leaving 341 patients in our final analysis (Figure 1). The mean age was 65 ± 13 years and 148 (43%) were female (Table 1). Overall, 140 (41%) patients were prescribed metformin prior to arrest, 153 (45%) were prescribed insulin, and 92 (27%) were prescribed other oral hyperglycemic medications. Median peak troponin in the first 24 hours post-arrest was 1.4 (interquartile range, IQR: 1.0–1.7) and median peak creatinine was 1.4 (IQR: 1.0–2.0).
Figure 1. Summary of clinical inclusion and exclusion data for retrospective analysis of the Pittsburgh Post-Cardiac Arrest Service patient database.
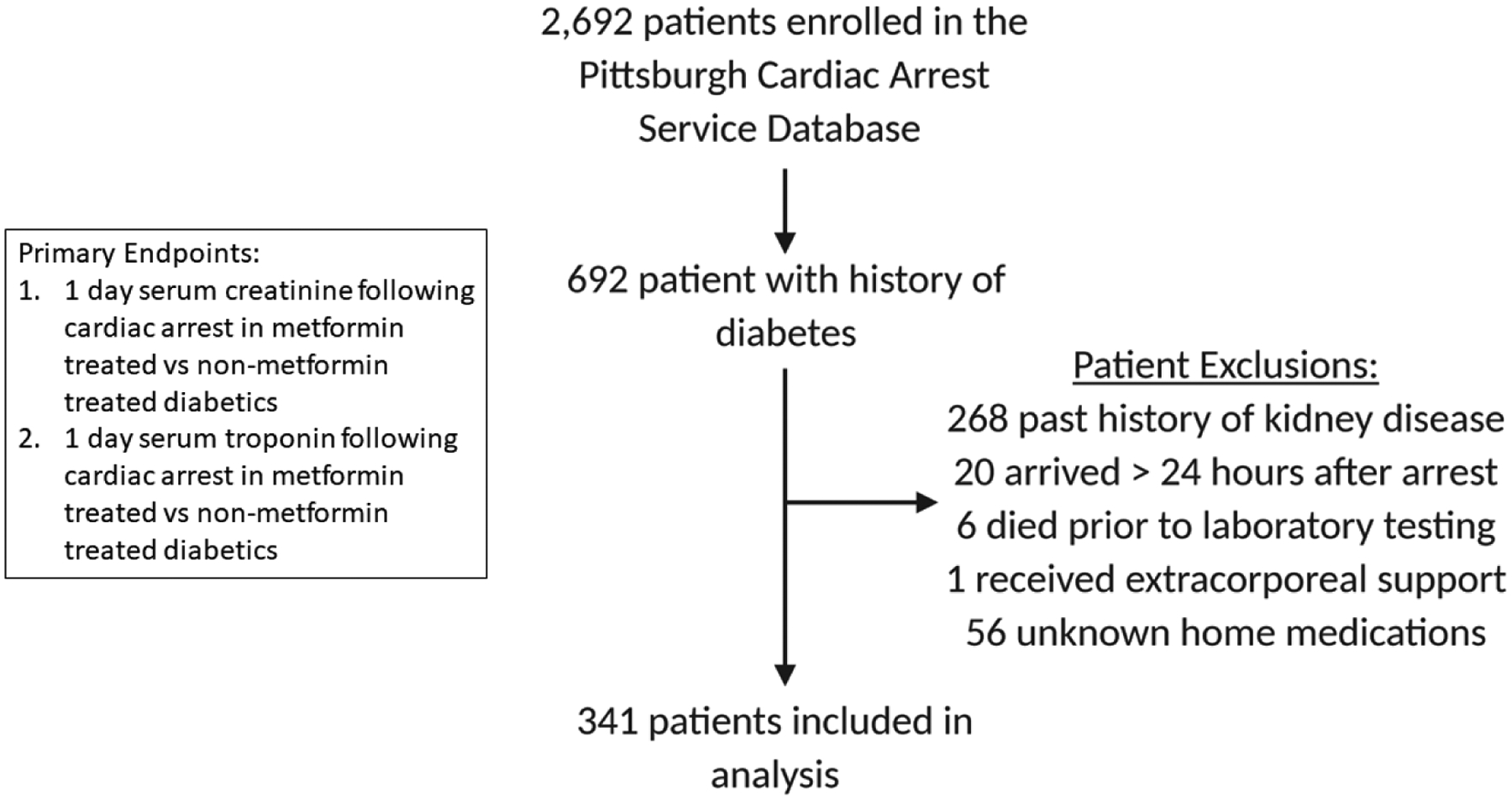
Primary outcomes evaluated included peak serum troponin and peak serum creatinine in diabetic patients with (n=122) and without (n=174) a history of metformin therapy.
Table 1.
Baseline demographics and clinical characteristics.
| Characteristic | Overall cohort (n = 341) | Metformin (n = 140) | No metformin (n = 201) |
|---|---|---|---|
| Age, years | 65 ± 13 | 65 ± 12 | 64 ± 14 |
| Female sex | 148 (43) | 58 (41) | 90 (45) |
| Arrest out-of-hospital | 256 (75) | 108 (77) | 148 (74) |
| Shockable rhythm | 109 (32) | 39 (28) | 70 (35) |
| Witnessed collapse | 160 (47) | 70 (50) | 90 (45) |
| Layperson CPR | 156 (46) | 72 (51) | 84 (42) |
| Epinephrine doses | 2 [1 – 4] | 3 [1 – 4] | 2 [1 – 4] |
| Arrest duration, min | 16 [8 – 27] | 16 [8 – 32] | 16 [8 – 23] |
| Cardiac etiology | 96 (28) | 36 (26) | 60 (30) |
| Charlson Comorbidity index | 2 [2 – 3] | 2 [1 – 3] | 3 [2 – 3] |
| Insulin | 152 (45) | 34 (24) | 118 (59) |
| Other oral diabetic medication | 92 (27) | 47 (34) | 45 (22) |
| Peak 24h troponin | 0.88 [0.19 – 5.7] | 0.97 [0.29 – 4.71] | 0.84 [0.14 – 7.0] |
| Peak 24h creatinine | 1.4 [1.0 – 2.0] | 1.3 [1.0 – 1.7] | 1.6 [1.0 – 2.1] |
Data are presented as mean ± standard deviation, median [interquartile range], or sample number (corresponding percentage).
Overall, 2% of data were missing and we created 10 imputed data sets. Metformin prescription at the time of SCA was associated with lower 24-hour peak serum troponin and lower 24-hour peak serum creatinine (Table 2). In post hoc analyses, metformin prescription was associated with lower 24-hour peak BUN, but not urine output or delayed peak creatinine as well as lower blood urea nitrogen (Supplemental Table 2). Insulin prescription was associated with elevated creatinine but not associated with troponin level. Other oral hypoglycemic agents were not associated with creatinine but were associated with lower troponin levels (Table 2). We observed the same independent associations in our primary adjusted models and most sensitivity analyses (Table 3).
Table 2.
Univariable associations between insulin, metformin and other oral hypoglycemic use and peak serum creatinine and troponin.
| Predictor | Endpoint | Coefficient (95% CI) | P value |
|---|---|---|---|
| Insulin | Creatinine | 0.131 (0.018 to 0.243) | 0.023 |
| Metformin | −0.142 (−0.257 to −0.026) | 0.016 | |
| Other oral hypoglycemic | 0.032 (−0.100 to 0.163) | 0.637 | |
| Insulin | Troponin | 0.094 (−0.390 to 0.578) | 0.703 |
| Metformin | −0.740 (−1.233 to −0.025) | 0.003 | |
| Other oral hypoglycemic | −0.789 (−1.360 to −0.218) | 0.007 |
Table 3:
Adjusted model results.
| Model | Coefficient (95% CI) | P-value |
|---|---|---|
| Creatinine outcome | ||
| Main adjusted model* | −0.19 (−0.30 to −0.08) | 0.001 |
| Backward stepwise+ | −0.16 (−0.28 to −0.06) | 0.002 |
| Complete case* | −0.18 (−0.31 to −0.06) | 0.004 |
| Excluding patients on insulin* | −0.20 (−0.37 to −0.04) | 0.016 |
| Troponin outcome | ||
| Main adjusted model* | −0.68 (−1.30 to −0.07) | 0.028 |
| Backward stepwise+ | −0.96 (−1.52 to −0.41) | 0.001 |
| Complete case* | −0.58 (−1.18 to 0.01) | 0.054 |
| Excluding patients on insulin* | −0.54 (−1.34 to 0.25) | 0.179 |
Model adjusting for age, sex, arrest location (in- vs out-of-hospital), witnessed collapse, layperson cardiopulmonary resuscitation, presenting rhythm, arrest duration, number of epinephrine administered, cardiac etiology of arrest, Charlson Comorbidity index, metformin, insulin, and other oral hypoglycemic medications.
Model adjusting for sex, arrest location, witnessed collapse, number of epinephrine administered, metformin, insulin and other oral hypoglycemics.
Discussion
SCA is common and current treatments are inadequate. Metformin, a first-line diabetes treatment (8), reduces all-cause mortality in diabetic patients with coronary artery disease (9) and CKD (10). We found a significant association between metformin pretreatment and decreased peak serum troponin and serum creatinine levels within 24 hours post-arrest. This suggests that metformin pre-treatment may protect cardiac and renal function independently of other baseline characteristics. These findings are consistent with preclinical studies in SCA, which have shown that metformin improves survival and markers of neurologic, cardiac, and renal function in non-diabetic SCA animals (15,16).
Only diabetic patients were included in this study, so it is not clear whether metformin’s benefits are related to its anti-diabetic effects or whether metformin protects against ischemia-reperfusion injury more broadly. Clinical studies suggest that metformin’s protective effects extend beyond its role in controlling blood glucose levels (17,18). Preclinical studies of metformin in ischemia-reperfusion injury in non-diabetic animals have demonstrated cardiac (19) and renal protection (20), and a large body of data has explored molecular pathways linked to metformin therapy that may drive protection against ischemia-reperfusion injury independent of diabetic status. These mechanisms include protection against oxidative stress, inhibition of apoptosis, and preservation of mitochondrial bioenergetics in both the heart and kidney (18–24). Metformin’s activity is exerted, at least in part, by activation of AMP-activated protein kinase (AMPK), which is a major regulator of fatty acid utilization and glucose metabolism (25,26). AMPK activation, either by metformin therapy or direct activation, is known to be protective against ischemia-reperfusion injury in a variety of tissues (27–29), and may suggest a protective benefit in the absence of diabetes.
It is likely that metformin therapy was discontinued after cardiac arrest in this study population, as metformin is routinely held during inpatient admission given concerns about metformin precipitating lactic acidosis in the presence of kidney injury (30). The effects of metformin as a continued therapy after SCA remain to be studied. Further, the effects of acute administration of metformin, either during or after resuscitation, remain unknown. Preclinical studies in cardiac ischemia have supported metformin’s protective effects when given at the time of reperfusion, which is largely attributed to prevention of mitochondrial permeability transition pore opening and cell death (19,21,31) or decreased inflammation(32). Similar protection, however, was not observed in a pig model of myocardial infarction when metformin was delivered directly through the coronaries at the time of reperfusion (33). Metformin’s effects when delivered after reperfusion has occurred are largely unexplored. However, given the dearth of treatment options for CA, such studies are further supported by this hypothesis-generating study.
Limitations
This is a single center observational study, and thus generalizability is limited. As an observational study, there are many unmeasured confounders that may affect cardiac and renal function, such as pressor support, post-arrest surgical or procedural interventions, or medication changes. Our primary outcomes were acute biomarkers of cardiac and renal damage, though future studies on long term cardiac and renal changes may be more informative. We found no association with metformin at peak creatinine measured between 72 and 120 hours after arrest (Supplemental Table 2). We were unable to find reliable echocardiography data at comparable time-points for this cohort. Our analysis was not powered to look for survival benefits in human subjects, but such a study is warranted. Although missing variables and imputation could create error, our results were robust across multiple sensitivity analyses.
Conclusion
We provide evidence in a retrospective clinical study that metformin pretreatment is associated with cardiac and renal protection after SCA.
Supplementary Material
Acknowledgements
All authors made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, or drafting of the manuscript. All authors have reviewed and approved the final version of the manuscript.
Research reported in this manuscript was supported by: NIH 5T32HL129964-02 to CR, American Heart Association 18TPA34230048 to BK, and NIH 5K23NS097629 to JE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None of the authors have financial disclosures or conflicts of interest
Credit Author Statement
Cody Rutledge: Conceptualization, Methodology, Investigation, Formal Analysis, Data Curation, Writing
Brett Kaufman: Conceptualization, Resources, Writing, Supervision
Cameron Dezfulian: Conceptualization, Formal Analysis, Writing
Jonathan Elmer: Formal Analysis, Methodology, Data Curation, Writing, Supervision
References
- 1.Aparicio HJ, Benjamin EJ, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association.; 2021. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed]
- 2.Mongardon N, Dumas F, Ricome S, et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011;1(45):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication a consensus statement from the International Liaison Committee on Resuscitation. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652 [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Bailén M, Aguayo De Hoyos E, Ruiz-Navarro S, et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Chang WT, Ma MHM, Chien KL, et al. Postresuscitation myocardial dysfunction: Correlated factors and prognostic implications. Intensive Care Med. 2007;33(1):88–95. doi: 10.1007/s00134-006-0442-9 [DOI] [PubMed] [Google Scholar]
- 6.Park YS, Choi YH, Oh JH, et al. Recovery from acute kidney injury as a potent predictor of survival and good neurological outcome at discharge after out-of-hospital cardiac arrest. Crit Care. 2019;23(1):1–11. doi: 10.1186/s13054-019-2535-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm C, Krannich A, Schachtner T, et al. Impact of acute kidney injury on neurological outcome and long-term survival after cardiac arrest – A 10 year observational follow up. J Crit Care. 2018;47:254–259. doi: 10.1016/j.jcrc.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 8.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-cuervo C. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650 [DOI] [PubMed] [Google Scholar]
- 9.Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18(96):1–16. doi: 10.1186/s12933-019-0900-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley MJ, Diamantidis CJ, Mcduffie JR, et al. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease. Ann Intern Med. 2017;166:191–200. doi: 10.7326/M16-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell S, Farran B, Mcgurnaghan S, et al. Risk of acute kidney injury and survival in patients treated with Metformin: an observational cohort study. BMC Nephrol. 2017;18(163):1–8. doi: 10.1186/s12882-017-0579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan FI, Sc M, Didari T, et al. A Review on The Protective Effects of Metformin in Sepsis-Induced Organ Failure. 2020;21(4). doi: 10.22074/cellj.2020.6286.Introduction [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jochmans S, Alphonsine JE, Chelly J, et al. Does metformin exposure before ICU stay have any impact on patients’ outcome? A retrospective cohort study of diabetic patients. Ann Intensive Care. 2017;7(116):1–9. doi: 10.1186/s13613-017-0336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitz K, Marroquin O, Zenati M, et al. Association Between Preoperative Metformin Exposure and Postoperative Outcomes in Adults With Type 2 Diabetes. JAMA Surg. 2020;155(6). doi: 10.1001/jamasurg.2020.0416.Association [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Liu K, Huang K, et al. Metformin improves neurologic outcome Via AMP-activated protein kinase-mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc. 2018;7(12). doi: 10.1161/JAHA.117.008389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutledge CA, Chiba T, Redding K, et al. Metformin Confers Cardiac and Renal Protection in Sudden Cardiac Arrest. Circulation. 2020;124(Suppl_4):A360. [Google Scholar]
- 17.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 18.Cameron AR, Morrison VL, Levin D, et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins L, Palee S, Chattipakorn SC, Chattipakorn N. Effects of metformin on the heart with ischaemia-reperfusion injury: Evidence of its benefits from in vitro, in vivo and clinical reports. Eur J Pharmacol. 2019;858(April):172489. doi: 10.1016/j.ejphar.2019.172489 [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Weng X, Guo J, Chen Z, Jiang G, Liu X. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats. Ren Fail. 2016;38(4):614–621. doi: 10.3109/0886022X.2016.1149770 [DOI] [PubMed] [Google Scholar]
- 21.Mohsin AA, Chen Q, Quan N, et al. Mitochondrial complex I inhibition by metformin limits reperfusion injury. J Pharmacol Exp Ther. 2019;369(2):282–290. doi: 10.1124/jpet.118.254300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu Rev Pharmacol Toxicol. 2017;57:535–565. doi: 10.1146/annurev-pharmtox-010715-103335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Gui Y, Ren J, et al. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci Rep. 2016;6(April):1–11. doi: 10.1038/srep23975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corremans R, Vervaet BA, D’haese PC, Neven E, Verhulst A. Metformin: A candidate drug for renal diseases. Int J Mol Sci. 2019;20(1):1–15. doi: 10.3390/ijms20010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou G, Goodyear LJ, Moller DE, et al. Role of AMP-activated protein kinase in mechanism of metformin action Find the latest version : Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI200113505.Introduction [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundewar S, Calvert, John W, Jha S, et al. Activation of AMPK by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ Res. 2009;454(1):42–54. doi: 10.1161/CIRCRESAHA.108.190918.Activation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Lesnefsky EJ. Metformin and myocardial ischemia and reperfusion injury: Moving toward “prime time” human use? Transl Res. 2020;229:1–4. doi: 10.1016/j.trsl.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 28.Declèves AE, Sharma K, Satriano J. Beneficial effects of AMP-activated protein kinase agonists in kidney ischemia-reperfusion: Autophagy and cellular stress markers. Nephron - Exp Nephrol. 2014;128:98–110. doi: 10.1159/000368932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Z, Lau K, Eby B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60(6):1770–1778. doi: 10.2337/db10-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalau JD, Arnouts P, Sharif A, De Broe ME. Metformin and other antidiabetic agents in renal failure patients. Kidney Int. 2015;87(2):308–322. doi: 10.1038/ki.2014.19 [DOI] [PubMed] [Google Scholar]
- 31.Calvert JW, Gundewar S, Jha S, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS- mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Huang L, Shi X, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020;12(23):24270–24287. doi: 10.18632/aging.202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Techiryan G, Weil BR, Palka BA, Canty JM. Effect of intracoronary metformin on myocardial infarct size in Swine. Circ Res. 2018;123(8):986–995. doi: 10.1161/CIRCRESAHA.118.313341 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


