Abstract
The melanocortin system plays an essential role in the regulation of immune activity. The anti-inflammatory microenvironment of the eye is dependent on the expression of the melanocortin-neuropeptide alpha-melanocyte stimulating hormone (α-MSH). In addition, the melanocortin system may have a role in the development and retinal cell survival under conditions of retinal degeneration. We have found that treating experimental autoimmune uveitis (EAU) with α-MSH suppresses retinal inflammation. Also, this augmentation of the melanocortin system promotes immune tolerance and protection of the retinal structure. The benefit of α-MSH-therapy appears to be dependent on different melanocortin receptors. Therefore, we treated EAU mice with α-MSH-analogs with different melanocortin-receptor targets. This approach demonstrated which melanocortin-receptors suppress inflammation, preserve retinal structure, and induce immune tolerance in uveitis. At the chronic stage of EAU the mice were injected twice 1 day apart with 50 μg of α-MSH or an α-MSH-analog. The α-MSH-analogs were a pan-agonist PL8331, PL8177 (potent MC1r-only agonist), PL5000 (a pan-agonist with no MC5r functional activity), MT-II (same as PL5000) and PG901 (MC5r agonist, but also an antagonist to MC3r, and MC4r). Clinical EAU scores were measured until resolution in the α-MSH-treated mice, when the eyes were collected for histology, and spleen cells collected for retinal-antigen-stimulated cytokine production. Significant suppression of EAU was seen with α-MSH or PL8331 treatment. This was accompanied with significant preservation of retinal structure. A similar effect was seen in EAU-mice that were treated with PL8177, except the suppression of EAU was temporary. In EAU mice treated with PL5000, MTII, or PG901, there was no suppression of EAU with a significant loss in whole retina and outer-nuclear layer thickness. There was significant suppression of IL-17 with induction of IL-10 by retinal-antigen stimulated spleen T cells from EAU mice treated with α-MSH, PL8331, PL8177, or PL5000, but not from EAU mice treated with MT-II, or PG901. Our previous studies show the melanocortin system's importance in maintaining ocular immune privilege and that α-MSH-treatment accelerates recovery and induces retinal-antigen-specific regulatory immunity in EAU. Our current results show that this activity is centered around MC1r and MC5r. In addition, the results suggest that a therapeutic potential to target MC1r and MC5r together to suppress uveitis induces regulatory immunity with potentially maintaining a normal retinal structure.
Keywords: Alpha-melanocyte stimulating hormones, Anti-inflammation, Melanocortins, Melanocortin receptor agonists, Melanocortin system, Neuroimmunomodulation, Retina, Uveitis
1. INTRODUCTION
The melanocortin system consists of the pro-opiomelanocortin hormone (POMC) derived neuropeptides and five G-coupled, seven-transmembrane domain receptors (Dores and Baron, 2011; Gantz and Fong, 2003; Schioth et al., 2005; Voisey et al., 2003). The prototypical melanocortin is the neuropeptide alpha-melanocyte stimulating hormone (α-MSH). The neuropeptide α-MSH holds a central role within the ocular microenvironment suppressing inflammation and promoting regulatory immunity (Taylor and Ng, 2018). There is a constitutive expression of α-MSH within the eye with retinal pigment epithelial cells (RPE), a significant source of the neuropeptide (Kawanaka and Taylor, 2011; Taylor et al., 2021; Taylor et al., 1994). This neuropeptide promotes the production of anti-inflammatory cytokines by endotoxin-activated macrophages, induces suppressor cell activity in antigen presenting cells, and converts antigen-specific effector T cells into Treg cells (Bhardwaj et al., 1996; Lau and Taylor, 2009; Lee and Taylor, 2013; Taylor and Lee, 2011). Under conditions of inflammation within the eye, the levels of α-MSH dimnish and treatments that augment α-MSH concentration systemically or locally result in resolution of inflammation (Lee et al., 2009; Lee and Taylor, 2013; Ng et al., 2021). In addition, the α-MSH therapy promotes retinal cell survival, and induction of protective regulatory immunity in the spleen (Lee and Taylor, 2013; Ng et al., 2021). These effects of α-MSH have demonstrated the vital role that the melanocortin system has in governing immune responses.
Through three melanocortin receptors (MC1r, MC3r, and MC5r), α-MSH mediates its anti-inflammatory and regulatory immunity in immune cells (Clemson et al., 2017; Lee and Taylor, 2011; Ng et al., 2021; Spana et al., 2019). Since α-MSH does not bind to MC2r its immunosuppressive actions are independent of cortisol. Adrenocorticoid hormone, also a pro-opiomelanocortin derived peptide, is the only ligand for MC2r, exclusively expressed on the adrenal glands (Yang et al., 2019). Through MC5r α-MSH suppresses effector T cell activity and promotes regulatory T cell activity (Lee et al., 2016; Lee and Taylor, 2011, 2013; Taylor et al., 2006). In addition, through MC5r α-MSH induces expansion in the spleen of a unique population of antigen presenting cells with the capacity of activating and counter-converting effector T cells into Treg cells in an antigen-specific manner (Lee and Taylor, 2013). Through MC1r and MC3r α-MSH suppresses inflammation mediated by activated monocytes, macrophages, dendritic cells, and neutrophils (Getting et al., 2006; Ignar et al., 2003; Lam and Getting, 2004; Li and Taylor, 2008; Montero-Melendez et al., 2011; Taherzadeh et al., 1999).
The common mouse model of ocular inflammation is experimental autoimmune uveitis (EAU), and α-MSH treatment of the mice with EAU suppresses the intraocular inflammation and induces Treg cells with specificity to retinal-antigen (Lee and Taylor, 2013). In addition, α-MSH-treatment protects the retina from damage by inflammation (Ng et al., 2021). While the uveitis in MC5r knocked-out mice is suppressed by α-MSH, there is no induction of counter-converted Treg cells in the spleen and there is a lack of protection to the retina (Lee and Taylor, 2011; Ng et al., 2021). In models of diabetic retinopathy, it has been shown that expression of MC1r and MC5r is necessary to prevent the death of retinal ganglion cells and photoreceptors (Maisto et al., 2017; Rossi et al., 2016). This suggests that along with the differential effects of α-MSH on immune cells through the different MCrs, there may also be an MCr-dependent signal for retinal cell survival during inflammation.
In this study we used a series of α-MSH analogs with different affinities and activation strengths through the different MCrs (mostly between MC1r and MC5r) to treat EAU in mice. The mice were clinically examined for suppression of uveitis, for the preservation of retinal cells and structure by histology, and for expression of cytokines-produced by retinal antigen-stimulated spleen T cells. This was done to show that MCr-targeted therapy can suppress EAU while promoting retinal cell survival and immune tolerance.
2. Methods:
2.1. α-MSH analogs.
The pan-agonists that stimulate functional activity for all 4 MCrs were the neuropeptide α-MSH obtained from Bachem (Torrance, CA), and PL8331 from Palatin Technologies (Cranbury, NJ) that through MC1r, MC3r, MC5r at 32.9, 7.5 and 20.6-fold greater functional activity than α-MSH (Supplemental Table 1). Also, from Palatin Technologies, PL8177 a highly potent MC1r-only agonist (2-fold greater activity than α-MSH), and PL5000 that has higher(2.3-fold) actions on MC1r than α-MSH and has no affinity to MC5r. The analog MTII was from Bachem and has a slightly higher melanocortin-receptor activating profile as PL5000. The α-MSH-analog PG901 was a gift from Dr. Victor Hruby (University of Arizona, Tucson, AZ), which has affinity to only MC3r, MC4r, and MC5r and is a functional antagonist to MC3r and MC4r, while being a potent MC5r agonist (1430-times higher than α-MSH) (Grieco et al., 2002).
2.2. Animals.
The mice used in these experiments were 6-8 week-old C57BL/6J female mice (Jackson Laboratories, Bar Harbor, ME). The mice were housed in the AAALAC-certified Boston University Animal Science Center. All experimental use of the mice were approved by the Boston University institutional animal care and use committee, and followed the Association For Research in Vision and Ophthalmology (ARVO) statement for the use and care of animals in vision research.
2.3. Induction of experimental autoimmune uveitis, and MCr agonists therapy.
The EAU was induced in the mice as previously described (Lee et al., 2009; Lee and Taylor, 2011, 2015; Wang et al., 2017). Briefly, a synthetic peptide of amino acid residues 1-20 of human interphotoreceptor retinoid binding protein (IRBP, GenScript, Piscataway, NJ) was emulsified in complete Freund’s adjuvant (BD Difco, Sparks, MD). The emulsified antigen preparation was subcutaneously injected followed by an intraperitoneal injection of pertussis toxin (Sigma, St Louis, MO). A second pertussis toxin was given two days later. Microscopic fundus exams of the retina were done every three or four days and the severity of retinal inflammation was scored on a scale of 0-5. As previously described (Agarwal et al., 2012; Kitaichi et al., 2005; Namba et al., 2002; Ng et al., 2021; Taylor and Namba, 2001; Taylor et al., 2000), the eyes were scored 0 for no inflammation; score 1 for only white focal lesions of vessels; score 2 for linear vessel lesions, over less than half of the retina; score 3 for linear vessels lesions, over more than half of the retina; score 4 for severe chorioretinal exudates or retinal hemorrhages in addition to the vasculitis; or score 5 for a subretinal hemorrhage or a retinal detachment. The data presented were from 3-5 independent mouse treatment-cohorts with 5 – 10 mice per treatment-cohort. The lyophilized α-MSH and analogs were reconstituted in sterile PBS (Biowhittaker, Walkersville, MD) to a concentration of 500 μg/ml. When EAU reached its chronic inflammatory plateau, sustained EAU score of 3, 50 μg of the α-MSH or an analog was injected intraperitoneally, and two days later a second 50 μg injection was done. The eyes were scored until the α-MSH-treated mice reached an average EAU score of 1, which was the 10th week after immunization for EAU (Fig.1).
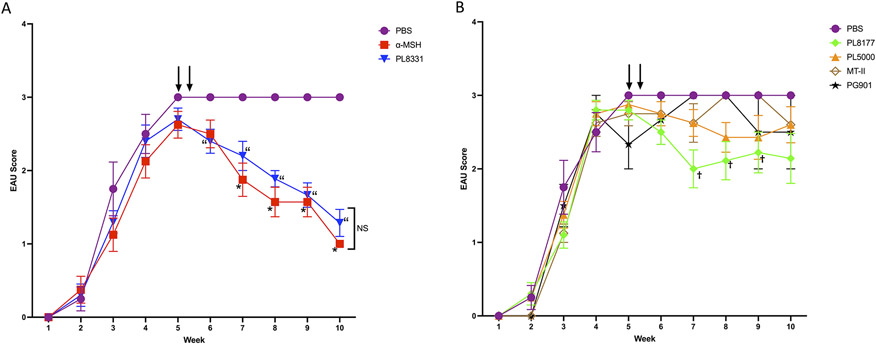
Figure 1. Effects of injecting EAU mice with MCr-agonists.
Wildtype mice were immunized to induce EAU and the retinas were examined by slit-lamp microscopy and scored. The mice were treated with a MCr-agonist on week 5 as indicated by the arrows. Presented are the mean ± SEM of the EAU scores of each treated group (n ≥ 5 mice in each group) over the weeks after immunization to induce EAU. A) EAU scores of PBS, α-MSH, and PL8331-treated EAU mice and B) EAU scores of mice treated with PBS, PL8177, PL5000, MT-II, and PG901. Statistical differences (P ≤ 0.05) of EAU-clinical scores from PBS (vehicle)-treated EAU mice are indicated for α-MSH (*), PL8331 (“), and PL8177 (†). All other values were not statistically different from the PBS-treated EAU-mice.
2.4. Histology.
The eyes from the treated EAU-mice, 10 weeks after immunization for EAU, were fixed in 4% paraformaldehyde for 48 hours, embedding in paraffin, and 5 μm sections were made. The sections were stained with hematoxylin and eosin, and imaged using a CX33 microscope (Olympus Shinjuku City, Tokyo, Japan) and QColor 5 camera system (Olympus). The images were histophathologically scored for EAU using a previously published 0 – 4 scale (Agarwal et al., 2012). The scoring was 0 for no change; 0.5 for mild cellular infiltration with no tissue damage; 1 for infiltration, retinal folds, focal retinal detachments, few small granulomas in choroid and retina, and perivasculitis; 2 for moderate infiltration, retinal folds, detachments, focal photoreceptor cell damage, and small to medium sized granulomas, perivasculitis and vasculitis; 3 for medium to heavy infiltration, extensive retinal folding, detachments, moderate photoreceptor cell damage, medium sized granulomatous lesions, and subretinal neovascularization; or 4 for heavy infiltration, diffuse retinal detachment, serous exudate, subretinal bleeding, extensive photoreceptor cell damage, large granulomatous lesions, and subretinal neovascularization. Also, the images were analyzed using ImageJ software (NIH, Bethesda, MD) to measure thickness of the retina and the outer nuclear layer (ONL). The measurements were made along the full-length of a retinal section centered on the optic nerve, and at 500, 1000, 1500, 2000 μm to the right and left (−500, −1000, −1500, −2000 μm) of the optic nerve head. The images presented are the sections of the retina two optic disk diameters from the center of the optic nerve.
2.5. In Vitro Spleen Cell Cultures.
On the 10th week after immunization for EAU, the spleen cells were made into a single-cell suspension, depleted of red blood cells using RBC lysis buffer (Sigma), washed and suspended in serum-free media (SFM). The SFM was made of RPMI 1640 (Biowhittaker, Walkersville, MD) supplemented with 10 μg/mL gentamicin (Sigma), 10 mM HEPES (Biowhittaker), 1 mM sodium pyruvate (Biowhittaker), and nonessential amino acids (Biowhittaker), 0.2% ITS+1 (Sigma), and 0.1% BSA (Sigma). The spleen cells (4x105 cells/well) were incubated with 50 μg IRBPp at 37°C and 5% CO2 for 48 hours before their culture supernatants were assayed by an ELISA Kit for IL-17 or IL-10 (R&D Systems, Minneapolis, MN).
2.6. Statistical analysis.
The statistical analysis of the EAU clinical and histopathological scores used a two-way ANOVA with non-parametric post-test analysis. The retinal thickness, and outer nuclear layer thickness used a two-way ANOVA with mixed effect analysis post-test. Significant differences in cytokine levels were assayed by ordinary one-way ANOVA post-analysis Dunnett×s multiple comparisons test to compare means between test groups and controls. In all cases differences were considered significant when the p-value was equal to or less than 0.05. Statistical calculations were done using PRISM 9 (GraphPad Software, San Diego, CA).
3. RESULTS
3.1. Effects of MCr-agonists on EAU.
The clinical EAU scores were measured out to 10 weeks after immunization to induce EAU. On week 4 the mice received two consecutive intraperitoneal injections of α-MSH, α-MSH-analog, or the vehicle (PBS). The α-MSH and PL8331 treated EAU mice had significantly suppressed EAU clinical scores after treatment (Fig 1A). There was a significant suppression in the clinical scores of EAU mice treated with PL8117 but was not sustained (Fig 1B). The other α-MSH-analogs PL5000, MT-II, and PG901 had no statistical effect on the EAU clinical scores compared to the vehicle -injected EAU mice (Fig 1B). Therefore, in comparison with α-MSH -treatment, PL8331 was similar in suppressing the clinical scores of EAU.
3.2. Effects of MCr-agonists on retinal structure in EAU-mice.
To see if the treatments had an effect on retinal structures, the eyes were collected on Week 10 along with age-matched naive mouse eyes (Fig. 2A) sectioned and stained for histological analysis (Fig. 2). The retinas of vehicle injected EAU-mice (Fig. 2B) had increased infiltration of immune cells in the vitreous and shortened photoreceptor length with disruption of the outer nuclear layer (ONL) (arrow on Fig. 2B). In addition, the retinas showed inflammatory cells in the subretinal space near the disrupted ONL area (asterisk on Fig. 2B). The retinas from α-MSH-treated and PL8331-treated EAU-mice retained almost normal retinal layer structure (Figs. 2C and 2D). The retinas of EAU mice treated with PL8177, which had slightly suppressed EAU (Fig. 1), had only slightly visible thinning of the retinal layers (Fig. 2E). The same was seen in retinas of EAU mice treated with PL5000 (Fig. 2F). In MT-II-treated EAU-mice, the retinas showed disruption of the ONL (Fig. 2G). The retinas of PG901-treated EAU-mice showed heavy immune cell infiltration and marked reduction in photoreceptor outer-segments like the retinas of vehicle injected EAU-mice (Fig. 2H). Compared to the retinas of PBS-injected EAU mice, the histopathological EAU scores were significantly suppression in the α-MSH, PL8331, or PL8177-treated EAU mice and no-statistical change in the scores of retinas from the PL5000, MT-II, or PG-901-treated EAU mice (Fig. 3). This demonstrated a similar beneficial effect of α-MSH, PL8331, and PL8177 treatment as suggested by the clinical EAU scores in Figure 1A.
Figure 2. Retinal images of treated EAU-mice on the Clinical Symptoms of EAU.
Photomicrographs of the histology of retinas from treated-mice 10 weeks after immunization with IRBP and treatment. The retinal structure of a naive mouse (Fig 2A) demonstrated its normal thickness and lengthy photoreceptors. In the retina of the EAU mice treated with PBS (vehicle) had noticeably shortened photoreceptors length and disruption of the ONL (arrow) due to inflammation (Fig 2B). Also, dense inflammatory cells (asterisk) were found in the subretinal space near the disrupted ONL area. The retinal structure in EAU mice treated with α-MSH (Fig 2C), PL8331 (Fig 2D), PL8177 (Fig 2E) and PL5000 (Fig 2F) have a similar appearance as the retinas of naive mice (Fig 2A). There was a small disruption (arrow) of ONL in the retinas of EAU mice treated with MT-II (Fig 2G). The retinas in the PG901 (Fig 2H) treated EAU mice was as damaged as the retinas in the PBS-treated EAU-mice with noticeable photoreceptor shorting and ONL disruption (arrow) with densely packed inflammatory cells (asterisk). Presented micrographs are representative images that corresponds with the mean retinal thickness of each experimental group. The scale bar is 50μm long.
Figure 3. Effects of injecting EAU mice with MCr-agonist on retinal histopathological scores.
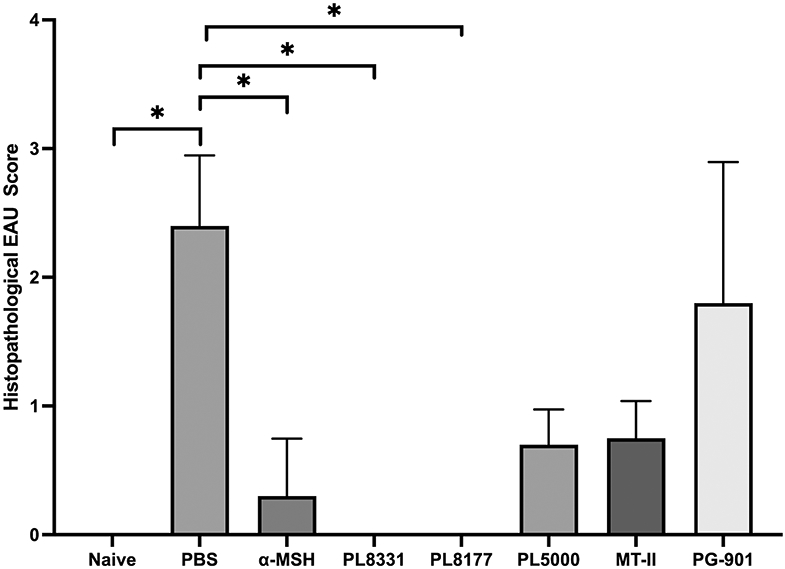
The retinal sections imaged for Figure 2 from mice 10 weeks after immunization with IRBP and treatment were histopathologically scored. Presented are the mean ± SEM of the scores of each treated group (n = 5 mice in each group). *Statistical differences (P ≤ 0.05) of the histopathological scores from PBS (vehicle)-treated EAU mice are indicated for naive mice, α-MSH, PL8331, and PL8177-treated EAU mice. All other values were not statistically different from the PBS-treated EAU-mice.
The effects of the melanocortin-receptor agonist treatments on retinal structure were quantified by measuring along the retina the whole and ONL thickness (Figs. 4 and 5). Only EAU mice treated with α-MSH, PL8331, or PL8177 had no significant change in retinal thickness compared to naive mice and were significantly different in retinal thickness compared to retinas of vehicle injected EAU-mice (Figs. 4A, 4B, and 4C). The other agonists PL5000, MT-II and PG901 had significantly thinner retinas compared to naive mice. While the thinning of retinas of PL5000 or MT-II-treated EAU mice was not as much as the retinas from vehicle injected EAU-mice (Figs. 4D, and 4E) there was no statistical difference in the thinning of retinas from PG901-treated EAU-mice to vehicle injected EAU-mice (Fig. 4F). The ONL thickness was statistically unchanged in the retinas of EAU-mice treated with α-MSH, PL8331, or PL8177 (Figs. 5A, 5B, 5C). There was statistically measured thinning of the ONL in retinas of EAU mice treated with PL5000, MT-II, and PG901 (Figs. 5D, 5E, 5F). However, their ONLs were statistically thicker than retinas from vehicle injected EAU-mice. These results demonstrate a beneficial effect on the retinal structure, and photoreceptor length following melanocortin-receptor agonist treatment of EAU-mice. While the most significant protection of retinal structure was seen in EAU-mice treated with α-MSH, PL8331, or PL8177, all of the MCr-agonists at least supported some survival of photoreceptor cells.
Figure 4. Comparison of the thickness of the retina.
From the retinal images of MCr-agonist treated EAU-mice after 10 weeks, the whole retinal thickness was measured. The thickness of retinas from each treated EAU mouse group (n ≥ 5 eyes per group), α-MSH (A), PL8331 (B), PL8177 (C), PL5000 (D), MT-II (E), and PG901 (F) was compared to the whole retinal thickness of naive and PBS-treated EAU mouse eyes at 0.5 μm intervals on both sides of the optic nerve head (mean (mm) thickness ± SEM). *Significant differences (P ≤ 0.05) were determined between the measured retinal thickness of treated EAU-mouse retinas and the measurements of naive mouse retinas and PBS-treated EAU mouse retinas. The same pooled measurements of the PBS-injected and normal retinas were used for comparison to each treatment group. The retinal thickness of EAU mice treated with α-MSH, PL8311, and PL8177 was not statistically different (ns) from the retinal thickness of naive mouse eyes.
Figure 5. Comparison of the thickness of the Outer Nuclear Layer (ONL).
From the same retinal images used in Figure 3, the ONL thickness was measured. The thickness of the ONL from each treated EAU-mouse group (n ≥ 5 eyes per group), α-MSH (A), PL8331 (B), PL8177 (C), PL5000 (D), MT-II (E), and PG901 (F) was compared to the ONL thickness of naive and PBS-treated EAU mouse eyes at 0.5 μm intervals on both sides of the optic nerve head (mean (mm) thickness ± SEM). *Significant differences (P ≤ 0.05) were determined between the measured ONL thickness of treated EAU-mouse retinas and the measurements of naive mouse retinas and PBS-treated EAU-mouse retinas. The same pooled measurements of the PBS-injected and normal retinas were used for comparison to each treatment group. The ONL thickness of EAU mice treated with α-MSH, PL8311, or PL8177 was not statistically different (ns) from the ONL thickness of naive mouse eyes.
3.3. Effects of the MCr-agonists on immune activity.
In the spleens of EAU-mice treated with α-MSH, the T cell response to IRBP-peptide (the antigen used to induce EAU) shifts from an effector Th17 response to a Treg response (Kitaichi et al., 2005; Lee and Taylor, 2015). The spleen cells of the MCr-agonist treated EAU-mice treated were assayed for antigen-stimulated T cell production of IL-17 and IL-10. After the 10th week of EAU, the spleen cells of the treated mice were collected and assayed in vitro for their recall response to IRBP-peptide. Except for MT-II and PG901-treated EAU-mice, the EAU-mice treated with the MCr-agonists were significantly suppressed in IL-17 production while expressing significantly higher levels of IL-10 in comparison to the IRBP-peptide-stimulated spleen cells of vehicle-treated EAU-mice (Fig. 6).
Figure 6: Effects of injecting EAU mice with MCr-agonists on retinal-antigen immune response.

Spleens were collected from MCr-agonist-treated mice 10 weeks after immunization to induce EAU (n ≥ 5 mice per group). The splenocytes were stimulated with addition of IRPB-peptide into the cultures, and 48 hrs later the culture media were assayed by ELISA for (A) IL-17 and (B) IL-10. Statistical differences between the mean (pg/ml) ± SEM of the reactivated splenocytes from treated EAU mice with splenocytes from the PBS-treated EAU mice were calculated; (ns) not significantly different, (*)P ≤ 0.05, (**)P≤0.01, (****)P≤.001. There was a significant difference (P ≤ 0.001) between splenocytes from naive and EAU mice in IL-17 levels, but not significantly different in IL-10 levels.
3.4. Cumulative Effects of MCr-Agonists on EAU.
The cumulative effects of this study are presented in Table 1 for comparison between the MCr-agonists. The full benefit of suppressed EAU, preserved retinal structure, and induced regulatory immunity was seen when both MC1r and MC5r were targeted by α-MSH and PL-8331. The noted exceptions are in EAU-mice treated with PL8177 and PL5000. While both were able to promote regulatory immunity, PL8177 protected the retina from damage but only transiently suppressed EAU. There was little benefit seen in EAU-mice injected with MT-II or PG-901. Therefore, treatment with pan-specific MCr-agonists, such as α-MSH and PL8331, suppress the inflammation of uveitis while protecting the retina from inflammation-mediated damage and shifts autoimmunity towards suppression.
Table 1.
Cumulative Effects of MCr-Agonists on EAU
| Maintained Thickness‡ | IRBP-Stimulated Spleen Cells |
|||||
|---|---|---|---|---|---|---|
| Agonist | Receptors | Suppression of EAU |
Whole Retina |
ONL | IL-17 | IL-10 |
| None | n/a | − | − | − | + | − |
| α-MSH | 1,3,4,5 | + | + | + | − | + |
| PL8331 | 1,3,4,5 | + | + | + | − | + |
| PL8177 | 1 | +† | + | + | − | + |
| PL5000 | 1,3,4 | − | − | − | − | + |
| MT-II | 1,3,4 | − | − | − | + | − |
| PG-901 | 3*,4*,5 | − | − | − | + | − |
Antagonist
Not sustained
The same as normal and significantly different from EAU.
4. DISCUSSION
The results demonstrated the efficacy of MCr-agonists in the treatment of EAU. The targeting of MC1r and MC5r suppressed inflammation and provided protection from retinal cell loss and with induction of regulatory immune activity. The results demonstrated that depending on the agonist there are differential effects on the inflammation of EAU, preservation of retinal cells, and induction of regulatory immunity, as previous works suggest (Lee and Taylor, 2011; Ng et al., 2021; Spana et al., 2019). The literature suggests that the stimulation of the MC1r and MC3r is linked to suppression of inflammation, with induction of regulatory immunity linked with the expression of MC5r (Clemson et al., 2017; Lee and Taylor, 2011; Ng et al., 2021; Spana et al., 2019). Recent publications have suggested the importance of MC1r and MC5r in preserving the retinal structure and cell survival in models of diabetic retinopathy (Maisto et al., 2017; Rossi et al., 2016).
Our results demonstrated that at least MC1r and MC5r need to be stimulated to obtain suppressed EAU with preserved retinal structure and induced regulatory immunity. This conclusion was supported by treating the EAU mice with α-MSH or PL8331. While PL8177-treatment protected the retinal structure and induced T cell IL-10 production, its suppression of EAU was not sustained. The observed temporal suppression may be related to the strength of PL8177 having only affinity to MC1r that the results were from the strength of PL8177 anti-inflammatory-signal through this one receptor. Since we did not do a dose curve, higher concentrations of PL8177 may yet provide the full benefit of MCr-agonist therapy. The α-MSH-analogs were used at the same molar concentration as α-MSH and have higher affinities to the MCrs (Supplemental Table 1); however, the α-MSH-analogs, PL5000, MT-II and PG-901 suggest that targeting only MC1r or MC5r without the other is not effective in suppressing EAU that preserves retinal structure.
In mice with MC5r-knocked out, α-MSH suppresses EAU but does not induce regulatory immunity in the spleen (Lee and Taylor, 2011). In addition, without MC5r expression there is severe retinal damage due to EAU (Ng et al., 2021). In mice with diabetic retinopathy, α-MSH treatment provided protection and survival of cells in the retina, and that this is mediated through MC1r and MC5r (Maisto et al., 2017; Rossi et al., 2016). All the MCr-agonists protected at some level the retinal structure. As with α-MSH-treatment, EAU-mice treated with PL8331 or PL8177 also maintained a normal retinal structure. While treating the EAU-mice with one of the other MCr-agonists did not preserve a normal retinal structure, the treatment did provide significantly better protection from retinal-damage compared to vehicle-treated EAU-mice. The differences may well be related to the ability of the MCr-agonists to mediate the resolution of clinical symptoms of EAU as seen with α-MSH, PL8331, and to some extent PL8177. The diabetic retinopathy reports of beneficial melanocortin-based therapy were done with the MCr-agonists injected intravitreally, suggesting the potential for localized action of the melanocortins on retinal cells (Rossi et al., 2016). It is to be seen whether systemically injected α-MSH-analogs, as was done in this study, penetrate the retina and suppress inflammation locally, or if they activate peripheral anti-inflammatory immune cells that enter the retina to suppress inflammation.
When EAU mice were treated with α-MSH, antigen presenting cells (APC) emerge in the spleen that counter-converts the retinal antigen-specific Th17 cells into inducible Treg cells (Lee and Taylor, 2013, 2015). Also, this was seen when the mice were left to resolve EAU on their own without therapeutic intervention. The emergence of this regulatory immunity is dependent on the expression of MC5r on the APC, and is mediated by MC5r-induced expression of ATP ectoenzymes by the APC to generate adenosine. While the results of treating the EAU mice with α-MSH and PL8331 can be ascribed to their MC5r-agonist activity, it is interesting that treatment with PL8177 or PL5000, which have no MC5r-agonist activity were similar to α-MSH-treatment. While this suggests that MC1r-agonism is sufficient to induce anti-inflammatory activity, MC1r-agonism cannot be supported by the lack of a similar response with MT-II-treatment. There is little reported about the effects of these MCr-agonists on immune cell activity. Our own studies found that MT-II and another MC3r-agonist D-Trp8-γ-MSH had no impact on LPS-stimulated macrophages (Li and Taylor, 2008). Also, the effect of antagonizing MC3r and MC4r by PG901 is not apparent on immune responses while stimulating MC5r. While the MCrs are collectively called Gs-protein coupled receptors, they are linked to several intracellular pathways involving NF-κB, ERK1/2, JNK phosphorylation, IRAK-M, PI3K, and PTEN (Cao et al., 2013; Carniglia et al., 2016; Lam et al., 2006; Li and Taylor, 2008; Taylor, 2005; Wolf Horrell et al., 2017; Xu et al., 2020). There is some evidence in the literature that some of these pathways regulating cytokine production and cell survival are favored by different MCrs (Carniglia et al., 2016; Ignar et al., 2003; Lam et al., 2006; Li and Taylor, 2008; Xu et al., 2020). In addition, some of the results may be related to the half-life and the penetrance of the analogs in the eye; however, the most unstable neuropeptide is α-MSH itself and it provides a full range of immunosuppression with the protection of the retinal structure.
5. CONCLUSIONS.
In this study we investigated the effects of MCr-agonists in suppressing EAU. Our previous studies showed the importance of the melanocortins in the maintenance of ocular immune privilege and that α-MSH-treatment accelerated recovery and induced retinal-antigen-specific regulatory immunity. Our current results demonstrated MCr-agonists that target MC1r and MC5r have a therapeutic potential to suppress uveitis and induce regulatory immunity. Moreover, they can maintain normal retinal structure and prevent retinal cell loss.
Supplementary Material
Highlights.
Treatment with α-MSH-analogs targeting MC1r with MC5r suppresses experimental autoimmune uveitis with induction of regulatory immunity.
Normal retinal structure was maintained, and retinal cell survival was promoted during experimental autoimmune uveitis when the melanocortin-system was therapeutically targeted.
Augmenting the melanocortin-system is potentially a potent therapy for uveitis.
6. Acknowledgements:
We thank David Yee for his technical support, and Dr. Victor Hruby at the University of Arizona, Tucson, AZ for generously providing us PG901.
7. Funding:
This work was supported in part by a Sponsored Program Project from Palatin Technologies Inc., Massachusetts Lions Eye Research Foundation, and a PHS grant from the NEI EY025961.
Abbreviations:
- α-MSH
alpha-melanocyte stimulating hormone
- EAU
Experimental Autoimmune Uveitis
- MC(1, 2, 3, 4, 5)r
Melanocortin (1, 2, 3, 4, 5 ) receptor
- SFM
Serum Free Media
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declared Conflicts of Interests:
AWT: Scientific Advisor, Palatin Technologies Inc.
TFN: None
KD: None
References:
- Agarwal RK, Silver PB, Caspi RR, 2012. Rodent models of experimental autoimmune uveitis. Methods Mol Biol 900, 443–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RS, Schwarz A, Becher E, Mahnke K, Aragane Y, Schwarz T, Luger TA, 1996. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. Journal of immunology 156, 2517–2521. [PubMed] [Google Scholar]
- Cao J, Wan L, Hacker E, Dai X, Lenna S, Jimenez-Cervantes C, Wang Y, Leslie NR, Xu GX, Widlund HR, Ryu B, Alani RM, Dutton-Regester K, Goding CR, Hayward NK, Wei W, Cui R, 2013. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol Cell 51, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniglia L, Ramirez D, Durand D, Saba J, Caruso C, Lasaga M, 2016. [Nle4, D-Phe7]-alpha-MSH Inhibits Toll-Like Receptor (TLR)2- and TLR4-Induced Microglial Activation and Promotes a M2-Like Phenotype. PLoS One 11, e0158564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson CM, Yost J, Taylor AW, 2017. The Role of Alpha-MSH as a Modulator of Ocular Immunobiology Exemplifies Mechanistic Differences between Melanocortins and Steroids. Ocul Immunol Inflamm 25, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores RM, Baron AJ, 2011. Evolution of POMC: origin, phylogeny, posttranslational processing, and the melanocortins. Ann N Y Acad Sci 1220, 34–48. [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong TM, 2003. The melanocortin system. Am J Physiol Endocrinol Metab 284, E468–474. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M, 2006. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J 20, 2234–2241. [DOI] [PubMed] [Google Scholar]
- Grieco P, Han G, Weinberg D, Van der Ploeg LH, Hruby VJ, 2002. Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochem Biophys Res Commun 292, 1075–1080. [DOI] [PubMed] [Google Scholar]
- Ignar DM, Andrews JL, Jansen M, Eilert MM, Pink HM, Lin P, Sherrill RG, Szewczyk JR, Conway JG, 2003. Regulation of TNF-alpha secretion by a specific melanocortin-1 receptor peptide agonist. Peptides 24, 709–716. [DOI] [PubMed] [Google Scholar]
- Kawanaka N, Taylor AW, 2011. Localized retinal neuropeptide regulation of macrophage and microglial cell functionality. J Neuroimmunol 232, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi N, Namba K, Taylor AW, 2005. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J Leukoc Biol 77, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, Getting SJ, 2004. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy 3, 311–315. [DOI] [PubMed] [Google Scholar]
- Lam CW, Perretti M, Getting SJ, 2006. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides 27, 404–412. [DOI] [PubMed] [Google Scholar]
- Lau CH, Taylor AW, 2009. The Immune Privileged Retina Mediates an Alternative Activation of J774A.1 Cells. Ocul Immunol Inflamm 17, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Biros DJ, Taylor AW, 2009. Injection of an alpha-melanocyte stimulating hormone expression plasmid is effective in suppressing experimental autoimmune uveitis. Int Immunopharmacol 9, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Preble J, Lee S, Foster CS, Taylor AW, 2016. MC5r and A2Ar Deficiencies During Experimental Autoimmune Uveitis Identifies Distinct T cell Polarization Programs and a Biphasic Regulatory Response. Sci Rep 6, 37790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Taylor AW, 2011. Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Invest Ophthalmol Vis Sci 52, 8862–8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Taylor AW, 2013. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. Journal of immunology 191, 4103–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Taylor AW, 2015. Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J Leukoc Biol 97, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Taylor AW, 2008. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol 84, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto R, Gesualdo C, Trotta MC, Grieco P, Testa F, Simonelli F, Barcia JM, D×Amico M, Di Filippo C, Rossi S, 2017. Melanocortin receptor agonists MCR1–5 protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture. J Cell Mol Med 21, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M, 2011. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol 179, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K, Kitaichi N, Nishida T, Taylor AW, 2002. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta 2. J Leukocyte Biol 72, 946–952. [PubMed] [Google Scholar]
- Ng TF, Manhapra A, Cluckey D, Choe Y, Vajram S, Taylor AW, 2021. Melanocortin 5 Receptor Expression and Recovery of Ocular Immune Privilege after Uveitis. Ocul Immunol Inflamm, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Maisto R, Gesualdo C, Trotta MC, Ferraraccio F, Kaneva MK, Getting SJ, Surace E, Testa F, Simonelli F, Grieco P, Merlino F, Perretti M, D×Amico M, Di Filippo C, 2016. Activation of Melanocortin Receptors MC 1 and MC 5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy. Mediators Inflamm 2016, 7368389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Haitina T, Ling MK, Ringholm A, Fredriksson R, Cerda-Reverter JM, Klovins J, 2005. Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides 26, 1886–1900. [DOI] [PubMed] [Google Scholar]
- Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J, 2019. Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases. Frontiers in Pharmacology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherzadeh S, Sharma S, Chhajlani V, Gantz I, Rajora N, Demitri MT, Kelly L, Zhao H, Ichiyama T, Catania A, Lipton JM, 1999. alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am J Physiol 276, R1289–1294. [DOI] [PubMed] [Google Scholar]
- Taylor A, Namba K, 2001. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol Cell Biol 79, 358–367. [DOI] [PubMed] [Google Scholar]
- Taylor AW, 2005. The immunomodulating neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J Neuroimmunol 162, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Hsu S, Ng TF, 2021. The Role of Retinal Pigment Epithelial Cells in Regulation of Macrophages/Microglial Cells in Retinal Immunobiology. Front Immunol 12, 724601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Kitaichi N, Biros D, 2006. Melanocortin 5 receptor and ocular immunity. Cellular and molecular biology (Noisy-le-Grand, France) 52, 53–59. [PubMed] [Google Scholar]
- Taylor AW, Lee DJ, 2011. The alpha-melanocyte stimulating hormone induces conversion of effector T cells into treg cells. J Transplant 2011, 246856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Ng TF, 2018. Negative regulators that mediate ocular immune privilege. J Leukoc Biol 103, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW, 1994. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation 1, 188–194. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Yee DG, Nishida T, Namba K, 2000. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH). Ann N Y Acad Sci 917, 239–247. [DOI] [PubMed] [Google Scholar]
- Voisey J, Carroll L, van Daal A, 2003. Melanocortins and their receptors and antagonists. Curr Drug Targets 4, 586–597. [DOI] [PubMed] [Google Scholar]
- Wang E, Choe Y, Ng TF, Taylor AW, 2017. Retinal Pigment Epithelial Cells Suppress Phagolysosome Activation in Macrophages. Invest Ophthalmol Vis Sci 58, 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Horrell EM, Jarrett SG, Carter KM, D×Orazio JA, 2017. Divergence of cAMP signalling pathways mediating augmented nucleotide excision repair and pigment induction in melanocytes. Exp Dermatol 26, 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Guan X, Zhou R, Gong R, 2020. Melanocortin 5 receptor signaling pathway in health and disease. Cell Mol Life Sci 77, 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen M, Ventro G, Harmon CM, 2019. Amino acid residue L112 in the ACTH receptor plays a key role in ACTH or alpha-MSH selectivity. Mol Cell Endocrinol 482, 11–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.