Abstract
Individuals with left temporal lobe epilepsy (TLE) have a higher rate of atypical (i.e., bilateral or right hemisphere) language lateralization compared to healthy controls. In addition, bilinguals have been observed to have a less left-lateralized pattern of language representation. We examined the combined influence of bilingual language experience and side of seizure focus on language lateralization profiles in TLE to determine whether bilingualism promotes re-organization of language networks. Seventy-two monolingual speakers of English (21 left TLE; LTLE, 22 right TLE; RTLE, 29 age-matched healthy controls; HC) and 24 English-dominant bilinguals (6 LTLE, 7 RTLE, 11 HC) completed a lexical-semantic functional MRI task and standardized measures of language in English. Language lateralization was determined using laterality indices based on activations in left vs right homologous perisylvian regions-of-interest (ROIs). In a fronto-temporal ROI, LTLE showed the expected pattern of weaker left language lateralization relative to HC, and monolinguals showed a trend of weaker left language lateralization relative to bilinguals. Importantly, these effects were qualified by a significant group by language status interaction, revealing that bilinguals with LTLE had greater rightward language lateralization relative to monolingual LTLE, with a large effect size particularly in the lateral temporal region. Rightward language lateralization was associated with better language scores in bilingual LTLE. These preliminary findings suggest a combined effect of bilingual language experience and a left hemisphere neurologic insult, which may together increase the likelihood of language re-organization to the right hemisphere. Our data underscore the need to consider bilingualism as an important factor contributing to language laterality in patients with TLE. Bilingualism may be neuroprotective pre-surgically and may mitigate post-surgical language decline following left anterior temporal lobectomy, which will be important to test in larger samples.
Keywords: temporal lobe epilepsy, bilingualism, neuroplasticity, language lateralization, laterality, functional magnetic resonance imaging (fMRI)
1. Introduction
Temporal lobe epilepsy (TLE) is the most common form of focal epilepsy in adults and is successfully treated by surgical intervention in approximately 70% of drug-resistant patients. Language impairment in TLE is a frequent and debilitating co-morbidity both before and after surgery, with deficits most commonly observed in naming and fluency (Hamberger, 2015; Sherman et al., 2011). While the left hemisphere is typically considered dominant for language in 95-99% of neurologically healthy right-handed individuals (Corballis, 2014), a higher proportion of atypical (i.e., bilateral or rightward) language lateralization is observed in TLE (approximately 23-33%) (Adcock et al., 2003; Stewart et al., 2014); especially in those with a left hemisphere seizure focus (Dijkstra and Ferrier, 2013; Möddel et al., 2009) and those who are left-handed (Stewart et al., 2014). This is commonly attributed to early language re-organization—an adaptive process that occurs in response to a neurological insult and may help mitigate language impairment in left TLE (LTLE).
Similar to TLE, bilingualism has been associated with more bilateral language representation compared to monolingualism (Hull and Vaid, 2007 for review; Jasinska and Petitto, 2013; Mergen and Kuruoglu, 2021; Palomar-García et al., 2015; Park et al., 2012; Román et al., 2015; Wang et al., 2011). Explanations for the greater involvement of the right hemisphere and a wider neural network in bilinguals include neuroplasticity within language networks and/or greater computational demands and increased cognitive control associated with processing of two languages, typically modulated by proficiency and age of acquisition (AoA) of the second language (for review see Costa & Sebastian-Galles, 2014; Perani & Abutalebi, 2006). With over 50% of the world’s population communicating in more than one language, bilingualism is extremely common in patients with epilepsy and may influence language profiles. Both bilinguals with early (i.e., before age 6) and late AoA of their second language have been shown to have more bilateral or right-sided language network representation (Jasinska and Petitto, 2013; Navarro et al., 2009; O’Grady et al., 2016; Palomar-García et al., 2015; Park et al., 2012; Román et al., 2015). Together, these findings suggest that bilingualism may influence language laterality at different time points in life.
Despite increasing interest in the influence of bilingualism on language profiles, only a few studies have examined functional activations associated with language laterality in bilinguals with epilepsy (Centeno et al., 2014; Cheung et al., 2006). Cheung and colleagues (2006) reported that laterality profiles of Chinese-English bilinguals with TLE were more bilateral for Chinese characters relative to English words, but the direct effect of bilingualism was not tested. Another study reported similar lateralization for both the first (L1) and second (L2) languages across bilingual patients with a broad range of focal epilepsies (Centeno et al., 2014). However, to our knowledge, no studies have directly examined language laterality profiles in a well-characterized sample of bilingual TLE compared to monolingual TLE, as well as bilingual and monolingual controls. Further, previous studies have not evaluated how fMRI language laterality profiles in bilingual patients with TLE relate to performance on the most commonly impaired aspects of language—naming and fluency.
To that end, this study examined the combined influence of bilingualism and epilepsy on language lateralization in a series of patients with LTLE and right TLE (RTLE). We predicted that the combination of bilingualism and a left-sided seizure focus would result in greater atypical language lateralization than in healthy bilinguals or monolinguals with LTLE.
2. Methods
The study was approved by the Institutional Review Boards (IRB) at the University of California, San Diego (UCSD) and University of California, San Francisco (UCSF) under a joint IRB plan. All participants provided consent according to the Declaration of Helsinki.
2.1. Participants
Our final sample consisted of 56 patients with drug-resistant TLE (27 LTLE; 29 right TLE; RTLE) between ages 17-65 and 40 age-matched healthy controls (HC) who had imaging data that passed quality inspection. HC were between the ages of 18-65 with no reported history of neurological or psychiatric disorders. Sixteen TLEs and 14 healthy controls were excluded for excessive motion artifact and/or poor alignment, and several bilingual TLEs were excluded due to unconfirmed bilingual language proficiency (see section 2.2 below). All patients were medically refractory and underwent pre-operative evaluation at UCSD and UCSF Epilepsy Centers between 2013 and 2018. A board-certified neurologist with expertise in epileptology established each patient’s diagnosis using criteria defined by the International League Against Epilepsy and based on video-EEG, seizure semiology, and neuroimaging. MRIs were visually inspected by a board-certified neuroradiologist for detection of mesial temporal sclerosis (MTS). The majority of patients had MTS (n=31; 55%) or were non-lesional (n=21; 38%) on MRI. A subset of patients (n=7) had small temporal lobe lesions (e.g., heterotopia, cavernoma, cyst, etc) and one monolingual LTLE had a suspected left frontal cortical dysplasia—see Supplementary Table 1 for details. All patients had scalp or intracranial EEG evidence of a temporal lobe seizure focus. For the majority of the patients, seizures were localized to the mesial temporal region (n=31) or to both mesial and lateral temporal regions (n=3); for the remainder of the patients, mesial vs lateral onset was not clear (n=20).
2.2. Language status characterization
Bilingualism was established according to the following criteria: participants self-identified as bilingual and reported proficiency or active use of a language other than English. Bilingual patients (B-TLE) reported either learning English as their L2 or simultaneously learning English and another language before age 61, commonly considered as an early AoA of L2. All B-TLEs expressed preference for testing in English and were evaluated by a board-certified neuropsychologist who determined English proficiency (see Table 2 for English vocabulary scores that were within two standard deviations of the population mean). Bilingual status was established prior to participation in the fMRI task. Notably, although the majority of bilinguals learned English as their L2, all reported dominance in English at the time of their evaluation. Previous work has demonstrated that bilinguals are able to accurately self-report their dominant language (Garcia and Gollan, 2021; Gollan et al., 2012), thus mitigating concerns that bilinguals were not tested in their dominant language.
Table 2.
Bilingual TLE language profile and epilepsy characteristics
| Case | L1 | L2 | L2 AoA |
Duration of L2 |
Method of L2 Exposure |
Age | Sex | English Fluency (raw, z) |
Side of seizure onset |
English vocab (raw, T) |
Age of seizure onset |
MTS | Hand ednes s |
fMRI Laterality^ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cantonese | English | 9 | 19 | Elementary-College | 34 | F | 15 (−0.37) | Left | 52 (44) | 11 | Y | Left | Right (−.71) |
| 2 | Japanese/English | — | SB* | 28 | Elementary-College | 35 | F | 12 (−.87) | Left | 42 (33) | 18 | Y | Right | Left (1.0) |
| 3 | Spanish | English | 14 | 35 | Middle School-College | 49 | F | 21 (0.64) | Left | 49 (40) | 40 | Y | Left | Right (−.32) |
| 4 | Vietnamese | English | 4 | 24 | Elementary-College | 28 | F | 15 (−0.37) | Left | 49 (56) | 23 | N | Right | Left (.35) |
| 5 | Spanish | English | 5 | 29 | Kindergarten | 34 | F | 18 (0.14) | Left | 47 (39) | 25 | Y | Right | Right (−.25) |
| 6 | Spanish | English | 14 | 34 | College | 48 | M | 16 (−0.20) | Left | 31 (41) | 46 | Y | Right | Bilateral (.03) |
| 7 | Arabic | English | 4 | 22 | Elementary-College | 28 | F | 19 (0.31) | Right | 41 (33) | 21 | Y | Right | Left (.25) |
| 8 | Spanish/ English |
— | SB* | 30 | Parents | 30 | M | 17 (−0.03) | Right | 35 (45) | 25 | N | Right | Left (.44) |
| 9 | Spanish | English | 4 | 18 | Elementary-College | 22 | F | 12 (−0.87) | Right | 50 (43) | 2 | Y | Right | Left (1.0) |
| 10 | Spanish | English | 3 | 28 | Elementary-College | 31 | F | 18 (0.14) | Right | 60 (53) | 17 | Y | Right | Left (.68) |
| 11 | Spanish | English | 16 | 25 | High School/College | 41 | F | 12 (−0.87) | Right | —- | 29 | N | Right | Left (.57) |
| 12 | Italian | English | 11 | 33 | Formal Schooling | 44 | F | 18 (0.14) | Right | 45 (59) | 31 | N | Right | Left (.65) |
| 13 | Spanish/ English |
— | SB* | 24 | Parents/Siblings | 24 | M | 31 (2.32) | Right | 55 (50) | 5 | Y | Right | Left (.32) |
Note. L1 and L2 refer to the order of the languages learned as reported by patients (i.e., L1 = First learned language; L2 = Second learned language)
SB: simultaneous bilingual (i.e., acquired both languages from birth). AoA = Age of acquisition; MTS=mesial temporal sclerosis. English fluency scores are based on animal fluency, and English vocabulary scores are derived from the WASI Vocabulary subtest. English vocabulary score was unavailable for one bilingual RTLE.
fMRI lateralization category with the laterality index in parenthesis; categorization for LI was based on the following: omnibus LI > .2 = left; −.2 < LI < .2 = bilateral; and LI < −.2 = right
Based on these criteria, three additional patients were excluded from the study: one B-RTLE with low English proficiency (vocabulary score > 2 standard deviations below the mean normative sample) and two B-LTLEs who reported learning English as L1 and acquired their L2 later in life. In the final dataset 6 LTLEs were classified as bilingual (B-LTLE) and 21 as monolingual (M-LTLE). Seven RTLEs were classified as bilingual (B-RTLE) and 22 as monolingual (M-RTLE). Eleven HC were classified as bilingual (B-HC) and 29 as monolingual (M-HC). B-TLEs learned English at an average age of 6.46 years (SD=5.70; range=0-16). B-RTLE and B-LTLE did not differ in age of English acquisition (p=.445) or English vocabulary (p=1.0). Importantly, vocabulary scores did not differ between M-LTLE, B-LTLE, M-RTLE, and B-RTLE (one-way ANOVA p=.518); this was confirmed with independent samples t-tests between each pair of patient groups (all ps ≥ .16).
As an additional way of ensuring that English proficiency in B-TLE was comparable to M-TLE, z-scores were computed on a measure of semantic fluency (i.e., animal fluency) based on a large sample of epilepsy patients (see Supplementary Online Material for details). Notably, no B-TLE patient’s score fell more than one standard deviation below the mean fluency score of the TLE sample (Table 2). In addition, no significant differences in animal fluency z-scores emerged when comparing the four TLE groups in our study (p=.551); mean scores of B-RTLE and B-LTLE did not differ from each other (p=.578). Table 2 shows detailed language characteristics and demographic and clinical characteristics for the B-TLE sample.
2.3. Materials and Procedure
2.3.1. Neuropsychological measures
As part of a comprehensive neuropsychological test battery, participants completed the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (WASI), which assesses the breadth of an individual’s expressive English vocabulary. Participants completed the Block Design subtest of the WASI as a measure of perceptual reasoning. Participants completed the Auditory Naming Test (Hamberger and Seidel, 2003)—a measure of auditory definition-to-naming which requires participants to produce the name of an item based on an auditory description (e.g., “A long, yellow fruit with a thick peel”). In addition, they completed two measures of verbal fluency from the Delis-Kaplan Executive Function System (Delis, D.C et al., 2001)—Category Fluency, a measure of semantic fluency (i.e., name as many animals in one minute) and Letter Fluency, a measure of letter/lexical fluency (i.e., name as many words that begin with the letters F, A, and S). For correlational analyses, raw-scores were transformed into z-scores based on the total HC sample (i.e., bilingual and monolingual HC scores)2.
2.3.2. fMRI language task
Participants completed two runs of a semantic judgment task in English that has previously shown robust perisylvian activations at a single-patient level. A detailed description of this task is provided in Supplementary Online Material and elsewhere (Chang et al., 2017, 2018). Stimuli included novel object nouns, false font stimuli (i.e., alphabet-like characters matched in size and number of characters to each novel word stimulus to control for sensory content), and target words (i.e., animal nouns). Participants pressed a button each time an animal word (e.g., ‘sheep’) was presented on the screen, which was included to ensure task engagement. The primary contrast of interest (novel words minus false fonts) isolates linguistic activity including orthographic, phonological, and lexical-semantic processing, and is not contaminated by button presses.
2.3.3. Image acquisition
Imaging data were acquired on a General Electric Discovery MR750 3T scanner using an 8-channel head coil. The sequence of image acquisition included the following: a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR=8.08 ms, TE=3.16 ms, TI=600 ms, flip angle=8°, FOV=256 mm, matrix=256 × 192, slice thickness=1.2 mm), and two functional T2*-sensitive echo-planar imaging (EPI) scans (TR=3000 ms, TE=30 ms, flip angle=90°, FOV=220 mm, matrix=64 × 64, slice thickness=2.5 mm). Scans were acquired for each individual using two different phase encoding directions to correct for geometric distortions in the EPI images (Holland et al., 2010).
2.3.4. Image processing
fMRI data processing was carried out using Analysis of Functional NeuroImages (AFNI (Cox, 1996), SUMA (Saad and Reynolds, 2012) and custom Matlab scripts. Detailed preprocessing steps are outlined in the Supplementary Online Material and elsewhere20,23. Briefly, preprocessing consisted of correction for gradient nonlinearities and B0 magnetic field inhomogeneities, co-registration of functional and structural data, motion correction, time series alignment, re-sampling, smoothing, and scaling. First-level analyses were performed using AFNI’s 3dDeconvolve function. Statistical maps were generated by calculating linear contrasts, which modeled the difference between the mean regression coefficients for the two main conditions of interest (novel words minus false fonts) to identify regions associated with lexical-semantic processing.
2.3.5. Region-of-Interest (ROI) analysis
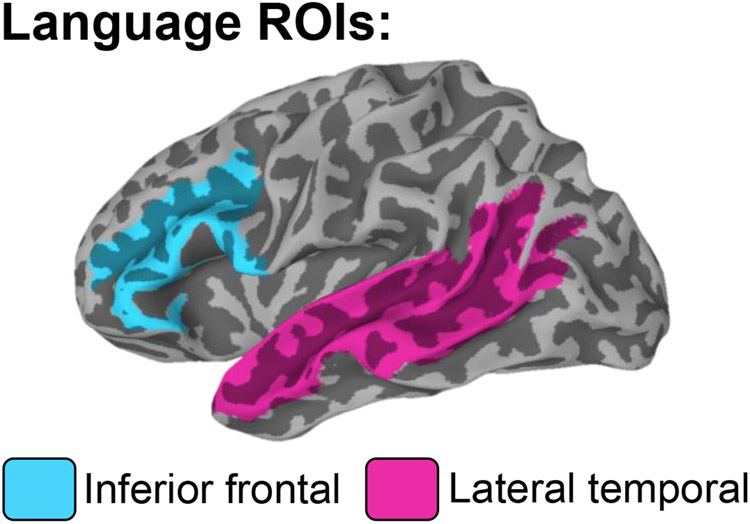
Multiple parcellations from the Destrieux atlas (Destrieux et al., 2010) were combined to create two language ROIs: inferior frontal and lateral temporal; see Figure 1. Selection of these ROIs was guided by previous fMRI studies that identified these regions as critical to different aspects of language processing, including lexical access, semantics, and phonological processing (Chang et al., 2018; Kaestner et al., 2019; McDonald et al., 2010; Thesen et al., 2012) Each participant’s statistical maps were corrected for multiple comparisons using a voxel-wise significance of p < .01 and a cluster size of at least 20 voxels, for a cluster-corrected p of .05 determined by 3dClustSim. The number of significantly activated voxels was counted within each of the ROIs for each hemisphere and used to calculate laterality indices (LI=[L−R]/[L+R]) between the two hemispheres for each of the two ROIs, and for an omnibus language ROI (i.e., sum of all regions in Figure 1) to provide an individual measure of language lateralization. Positive LI indicates a leftward asymmetry in activation (i.e., left-lateralized language), whereas negative LI indicates more rightward asymmetry (i.e., right-lateralized language).
Figure 1. Selected regions of interest.
Two language regions of interest (ROIs) selected for fMRI analysis using the Destrieux atlas. The inferior frontal region consists of the opercular, orbital, and triangular parts of the inferior frontal gyrus and the inferior frontal sulcus. The lateral temporal region consists of the middle temporal gyrus, superior temporal gyrus, and the superior temporal sulcus. These two regions were combined to produce an omnibus language LI which was used as the main outcome variable in analyses.
2.4. Statistical analyses
Fisher’s exact tests (for categorical variables) and one-way analysis of variance (ANOVAs) or Kruskal-Wallis H tests (for continuous variables) with Bonferroni-adjustments for pair-wise comparisons were used to examine differences in demographic and clinical variables. Group (RTLE, LTLE, HC) by language status (bilingual, monolingual) ANOVA was used to test for group differences in LIs. P-values for post-hoc tests were Bonferroni-adjusted. Simple main effects using the pooled variance were performed for significant interactions. Main assumptions of ANOVA were met such that within each group, the main outcome variable (omnibus LI) was normally distributed (Komogorov-Smirnov test ps ≥.087) and there was equality of variances (Levene’s test p=.293), which is important for unbalanced sample sizes. Kurtosis and skewness were considered within normal limits (< +/− 2). Q-plots were inspected and determined to be acceptable. Two-tailed Fisher’s exact tests were conducted to examine rates of typical (i.e., left hemisphere; LI > .20) versus atypical (i.e., right hemisphere: LI < −.20 or bilateral: LI between .20 and −.20) lateralization between bilingual and monolingual groups based on the omnibus LI.
3. Results
3.1. Demographic and epilepsy-related variables
Table 1 shows descriptive statistics for group differences in demographic and epilepsy-related variables for the whole sample. Groups did not significantly differ in age. M-HC were more educated than M-LTLE (p=.004) and M-RTLE (p=.002). B-HC were more educated than M-LTLE (p=.001) and M-RTLE (p=.001). M-HC had higher vocabulary scores than all four patient groups (ps ≤ .046). B-HC had higher vocabulary scores than B-LTLE (p=.012) and M-RTLE (p=.048). M-HC had higher Block Design scores than M-LTLE (p=.048) and M-RTLE (p<.001). B-HC had higher Block Design scores than M-RTLE (p=.017). There were no significant differences in the distribution of sex or handedness. B-RTLE had a higher proportion of patients that identified as Hispanic. No group differences arose in the age of seizure onset, duration of epilepsy, presence of MTS, and number of anti-seizure medications. Direct comparisons using independent samples t-tests revealed no differences in demographic or clinical variables for B-LTLE versus M-LTLE (all ps ≥ .07), or for B-HC versus M-HC (all ps ≥ .07). B-RTLEs were more educated than M-RTLEs (p = .003), but no other group differences reached significance (ps ≥ .16). Fisher’s exact tests revealed that a higher proportion of B-RTLE patients were non-White and Hispanic compared to M-RTLE (p = .009), and a higher proportion of B-LTLE patients were non-White compared to M-LTLE (p = .013); no other group differences were significant (ps ≥ .18).
Table 1.
Participant demographics and epilepsy-related characteristics
| Bilinguals | Monolinguals | ||||||
|---|---|---|---|---|---|---|---|
| B-LTLE (n=6) |
B-RTLE (n=7) |
B-HC (n=11) |
M-LTLE (n=21) |
M-RTLE (n=22) |
M-HC (n=29) |
Statistical Test * | |
| Age | 38.00 (8.51) | 31.43 (8.24) | 35.91 (14.86) | 34.43 (13.37) | 33.27 (12.17) | 37.59 (13.45) | H (5,96) = 3.56; p = .614 |
| Education | 13.33 (1.75) | 16.00 (2.65) | 16.91 (2.47) | 13.29 a,b (1.55) | 13.14 a,b (1.86) | 15.55 (1.92) | H (5,96) = 33.58; p < .001 |
| Sex (F/M) | 5/1 | 5/2 | 7/4 | 10/11 | 12/10 | 16/13 | Fisher’s exact = 3.17; p = .695 |
| Handedness (R/L/A) | 4/2/0 | 7/0/0 | 11/0/0 | 19/2/0 | 20/1/1 | 27/2/0 | Fisher’s exact = 4.81; p = .364 |
| Ethnicity (Non-Hispanic/Hispanic) | 4/2 | 2/5 | 11/0 | 17/4 | 18/4 | 27/2 | Fisher’s exact = 15.83; p = .003 |
| Race (White/ >1/ Black/Asian/NH/Unknown |
1/2/0/3/0/0 | 2/4/0/0/1/0 | 9/0/0/2/0/0 | 16/3/1/1/0/0 | 16/1/3/1/1/0 | 23/0/2/4/0/0 | — |
| WASI Block Design1 | 51.50 (8.46) | 50.29 (6.73) | 56.22 (10.73) | 50.42 a(8.36) | 44.17 a,b (10.24) | 58.46 (7.69) | F (5,85) = 6.29; p < .001 |
| WASI Vocabulary 2 | 42.17 a,b (7.68) | 47.17a,b (9.00) | 59.89 (11.71) | 48.55 a,b (9.79) | 48.19 a,b (9.52) | 60.48 (9.48) |
F (5,89) = 7.95; p < .001
|
| Age of seizure onset | 27.17 (13.32) | 17.00 (12.58) | — | 19.05 (13.68) | 21.41 (12.48) | — | H (3,56) = 1.89; p = .596 |
| Duration of epilepsy | 10.83 (7.81) | 14.43 (7.57) | — | 15.38 (14.81) | 11.86 (12.50) | — | H (3,56) = 1.70; p = .637 |
| Number of ASMs | 1.50 (0.55) | 2.29 (0.76) | — | 2.24 (0.89) | 2.32 (1.09) |
— | H (3,56) = 4.19; p = .242 |
| MTS (Y/N) | 5/1 | 4/3 | — | 12/9 | 10/12 | — | Fisher’s exact = 2.71; p = .457 |
Note. Mean (standard deviation) shown for continuous variables. LTLE = left TLE; RTLE = right TLE; WASI = Wechsler Abbreviated Intelligence Scale; ASM = anti-seizure medication; MTS = mesial temporal sclerosis; F = female; M = male; R = right; L = left; A = ambidextrous; >1 = more than one race, NH = Native Hawaiian/Pacific Islander
Block design score expressed as T-scores: missing for 2 B-HC, 3 M-HC, 4 M-RTLEs, and 2 M-LTLEs.
Vocabulary score expressed as T-scores: missing for 2 B-HC, 2 M-HC, 1 M-RTLE, 1 B-RTLE, and 1 M-LTLE.
Statistics based on a one-way ANOVA for normally distributed- or Kruskal-Wallis H-test for non-normally distributed continuous variables with follow-up pairwise comparisons, and Fisher’s exact tests for categorical variables. Significant effects are bolded.
Denotes that this mean/median is significantly different (p<.05; Bonferroni-adjusted) from M-HC in pairwise comparisons.
Denotes that this mean/median is significantly different (p<.05; Bonferroni-adjusted) from B-HC in pairwise comparisons.
3.2. Whole-brain activation maps
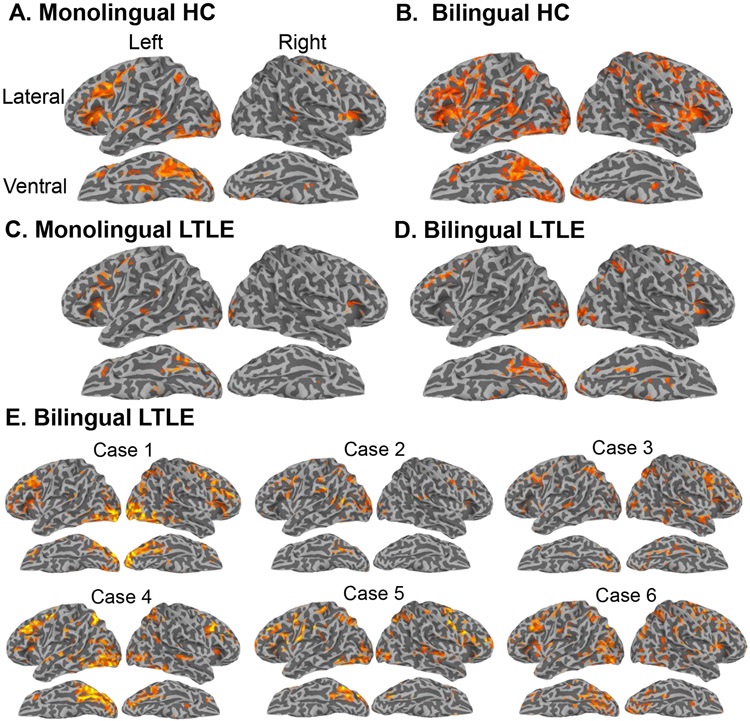
The semantic decision task activated a typical network of language regions in HC including the left inferior frontal, inferior parietal, lateral temporal, and ventral temporal (i.e., fusiform) regions, with weaker activations in the right inferior frontal and precentral gyrus (Figure 2A-B). A more bilateral pattern of activation was observed in B-HC. Figure 3C-D shows group-level surface activation maps for M- and B-LTLE. Activations in RTLE were similar to HC and are displayed in Supplementary Figure 1.
Figure 2. Individual and whole-brain statistical maps for the contrast of interest (novel words minus false fonts) that isolates lexical-semantic processing.
Whole-brain statistical t-maps are shown for: A) monolingual healthy controls (HC), B) bilingual HC, C) monolingual left TLE (LTLE), D) bilingual LTLE and E) single-subject maps for each B-LTLE participant that corresponds to a case number in Table 2. Monolingual group maps and B-LTLE individual subject maps were cluster-corrected at p < .05 with a voxel-wise correction of p < .01. We used a more liberal threshold for the two bilingual group maps for purposes of visualization and due to smaller sample size (<20 per group), using a cluster-correction of p < .05 and voxel-wise p < .10. Group maps for RTLE looked similar to controls and are presented in Supplementary Figure 1. Of note, all reported statistical analyses were carried out using ROIs in native space with a stringent correction (see Methods).
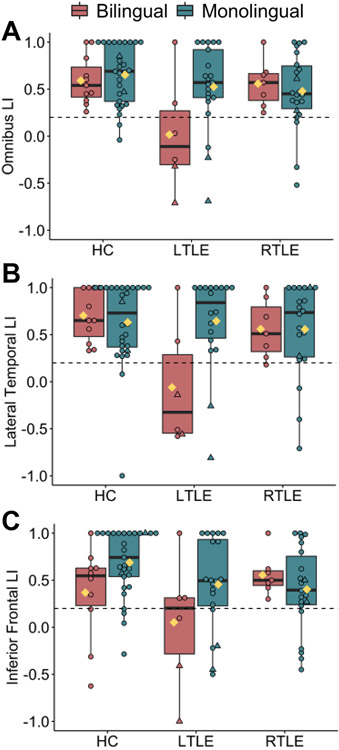
Figure 3. The effect of group on language lateralization is dependent on language status.
fMRI laterality index (LI) plotted separately by group (age-matched controls; HC, left TLE; LTLE, and right TLE; RTLE) and language status (bilingual, monolingual) for A) omnibus language LI, B) inferior frontal LI, and C) lateral temporal LI. Right- and left-handed/ambidextrous individuals are coded as circles and triangles, respectively. Yellow diamonds represent group means. Individuals below the horizontal dotted line have atypical language lateralization (LI ≤ 0.2). Rates of atypical (i.e., right or bilateral) lateralization based on omnibus LI were as follows: monolingual HC=7% (2/29), bilingual HC=0% (0/11), monolingual LTLE=14% (3/21), bilingual LTLE (67%; 4/6), monolingual RTLE=18% (4/22) and bilingual RTLE=0% (0/7). Boxplot denotes the median (bold bar), first and third quartiles (box limits), and ±1.5 times the interquartile range (whiskers).
3.3. Laterality analyses
3.3.1. Primary outcome: Omnibus LI
Our main analysis compared an omnibus language LI (i.e., a combination of frontal and temporal ROIs in Figure 1) across groups using a three (group) by two (language status) ANOVA. Figure 3A plots individual LIs. Supplementary Table 2 presents descriptive statistics and confidence intervals. Overall, LI significantly differed across groups (a main effect of group, F (2, 96) = 5.06; p=.008; ηp2=.10), such that HC had greater leftward asymmetry than LTLE (p=.006); no other group differences were significant (ps ≥.130). Overall, monolinguals tended to have more leftward asymmetry than bilinguals (a marginally significant effect of language status, F (1, 96) = 3.14; p=.080; ηp2=.03). Importantly, these main effects were qualified by a significant group by language status interaction of a medium effect size (F (2, 96) = 3.23; p=.044;ηp2=.07). A significant simple main effect of language status (F (1, 90) = 8.30; p=.005; ηp2=.08) suggested that B-LTLE had more right-lateralized asymmetry (M=0.02; [95% CI= −0.29, 0.33]) relative to M-LTLE (M=0.52; [95% CI=0.36, 0.69]), whereas the effect of bilingualism was not significant in RTLE (p=.632) or HC (p=.649). A direct comparison of the mean LI in B-LTLE versus M-LTLE revealed a large effect size difference (t(25) = −2.27; p=.032; Cohen’s d=0.96). The effect of bilingualism in LTLE approached significance when controlling for English vocabulary scores, although the effect size remained large (F (1, 26) = 3.95; p=.059; ηp2=.15).
In addition, the two-way interaction remained significant and of the same effect size when controlling for education (F (2, 96) = 3.45; p=.036; ηp2=.07). Given the relevance of age of seizure onset for language lateralization, we controlled for this variable in a separate model with the patient groups only. The two-way interaction between side of seizure focus and language status remained significant and became of a stronger effect size (F (1, 56) = 5.42; p=.024; ηp2=.10). Finally, we repeated the analysis using a one-way ANOVA and pairwise comparisons between the six groups, given our smaller sample of bilinguals and concern for low power. This analysis revealed a main effect of group of a large effect size (F (5, 96) = 2.93; p=.017; ηp2=.14). Pairwise comparisons demonstrated that B-LTLE showed greater rightward asymmetry compared to all other groups (all ps ≤ .012); no other group differences were significant.
3.3.2. Lateral temporal ROI
We repeated the two-way ANOVA for each of the language ROIs (Figure 3B-C). The interaction was significant in the lateral temporal region (F (1, 90) = 4.48; p=.014; ηp2=.10). A significant simple main effect of language status (F (1, 90) = 10.52; p=.002; ηp2=.11) revealed that B-LTLE had greater right-lateralized asymmetry (M=−0.06; [95% CI= −0.44, 0.31]) than M-LTLE (M=0.65; [95% CI= 0.43, 0.86]). This was confirmed by a direct comparison between the two groups (t(22) = −2.78; p=.011; Cohen’s d=1.22). The effect of bilingualism in LTLE remained significant and of a large effect size when controlling for English vocabulary scores (F (1, 24) = 6.11; p=.022; ηp2=.23). The effect of bilingualism was not significant in RTLE (p=.982) or HC (p=.655).
3.3.3. Inferior frontal ROI
The interaction approached significance in the inferior frontal region (F (1, 94) = 2.48; p=.089; ηp2=.05). Simple main effects revealed that B-LTLE showed a trend towards more right-lateralized activity (M=−0.05; [95% CI= −0.31, 0.41]) than M-LTLE (M=0.46; [95% CI= 0.26, 0.65]) that approached significance (F (1, 88) = 3.88; p=.052; ηp2=.04). A direct comparison between B-LTLE and M-LTLE did not reach significance in the inferior frontal LI (t(24) = −1.61; p=.121; Cohen’s d=.67). Similarly, B-HC showed a trend toward more right-lateralized asymmetry (M=0.37; [95% CI= 0.09, 0.65]) relative to M-HC (M=0.69; [95% CI= 0.53, 0.85]) that approached significance (F (1, 88) = 3.92; p=.051; ηp2=.04). However, a direct comparison between B-HC and M-HC was significant (t(37) = −2.23; p=.032; Cohen’s d=.74) with bilingual HC demonstrating more right-lateralized language than monolingual HC. The effect of bilingualism was not significant in RTLE (p=.423).
3.4. Individual subject analyses
Fisher’s exact tests produced consistent findings as the parametric tests, such that atypical language lateralization (defined as omnibus LI ≤ .20) was more frequently observed in B-LTLE (4/6; 67%) versus M-LTLE (3/21; 14%) (p=.024). The proportion of atypical lateralization did not differ among B-RTLE (0/7;0%) and M-RTLE (4/22; 18%) (p=.546) or among B-HC (0/11; 0%) and M-HC (2/29; 7%) (p=1.0). Figure 2E shows individual subject maps for B-LTLE. Using the following cut-offs for language lateralization based on omnibus LI (LI > 0.2= left; −0.2 < LI < 0.2= bilateral; LI < −0.2 = right), B-LTLE Cases 1, 3 and 5 showed right lateralization, Case 6 showed bilateral lateralization, and Cases 2 and 4 had left-lateralization (Table 2). In contrast, all seven bilingual RTLEs showed left lateralization (Table 2; see Supplementary Figure 1 for individual participant maps).
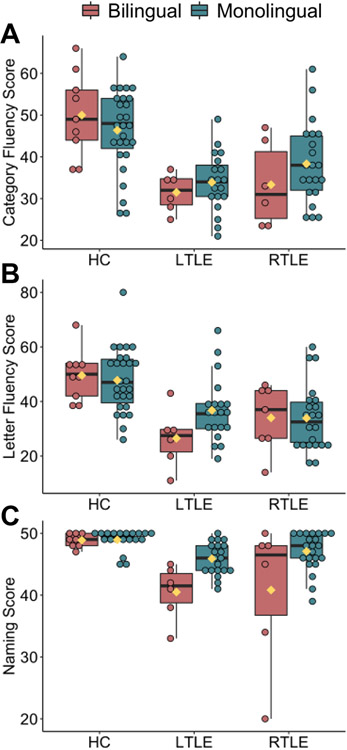
3.5. Relationship between laterality and language function
Figure 4A-C plots raw scores of three standardized language tests across groups. Supplementary Table 3 shows results of one-way ANCOVAs controlling for education. For category fluency, B-RTLE performed worse than B-HC (p=.010), and both B-RTLE (p=.004) and M-LTLE (p=.036) performed worse than M-HC. For letter fluency, B-LTLE (p=.016) and B-RTLE (p=.037) performed worse than M-HC. For naming, B-LTLE (p=.002) and B-RTLE (p=.003) performed worse than B-HC, both B-LTLE (p<.001) and B-RLTE (p<.001) performed worse than M-HC, and B-LTLE performed worse than M-RTLE (p=.007).
Figure 4.
Panels A-C plot raw scores on standardized language measures by group (left TLE; LTLE, right TLE; RTLE, healthy controls; HC) and language status (bilingual, monolingual) separately for A) category fluency, B) letter fluency, and C) auditory naming.
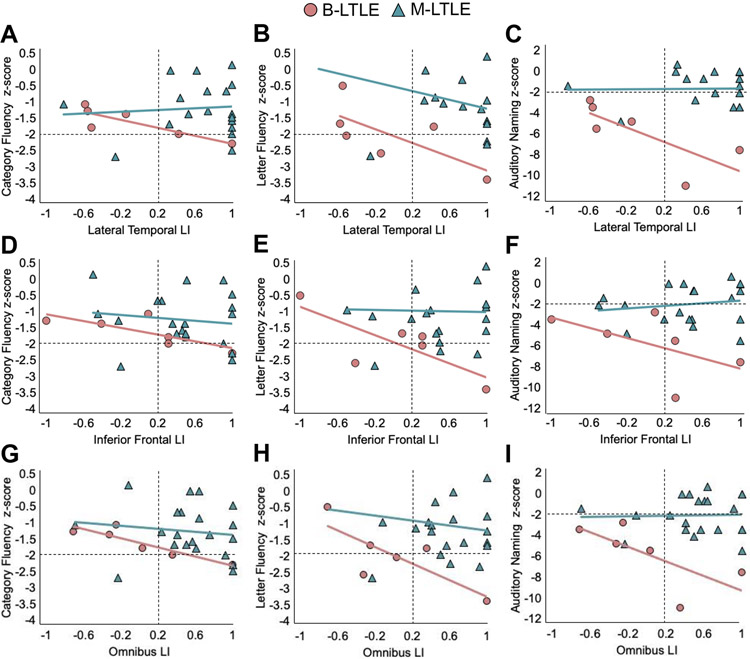
In a post-hoc analysis we examined whether language lateralization is associated with language function in TLE using partial Spearman-rank correlations between each of the LIs and each of the three language measures (z-scores relative to HC), controlling for age. First, we examined correlations in M-RTLE and B-RTLE combined, as these groups showed typical leftward asymmetry at the group level. These correlations were not significant (ps > .05) for the lateral temporal region (rs = −.14, .10, and .01 for category fluency, letter fluency, and auditory naming, respectively), nor the inferior frontal region (rs = .12, .18, and -.02 for category fluency, letter fluency, and auditory naming, respectively). Next, we examined correlations within B-LTLE and M-LTLE separately given that the difference between these two groups drove the interaction of interest (see Figure 5). Within B-LTLE, more rightward lateral temporal LI was associated with better scores on all three language measures (category fluency: rs = −.94; p=.016; letter fluency: rs = −.91; p=.031; auditory naming: rs = −.91; p=.031)—Figure 5A-C. More rightward inferior frontal LI was associated with better scores on letter fluency (rs = −.91; p=.033; Figure 5E) and the association with category fluency approached significance (rs = −.85; p=.070; Figure 5D). The association with auditory naming score was in the same negative direction but not significant (rs = −.77; p=.129; Figure 5F). Within M-LTLE, these correlations were not significant (ps > .05) for the lateral temporal region (rs = −.01, −.37, and -.11 for category fluency, letter fluency, and auditory naming, respectively)—Figure 5A-C, nor for the inferior frontal region (rs = −.28, −.05, and .03 for category fluency, letter fluency, and auditory naming, respectively)—Figure 5D-F. Associations with the omnibus LI showed a consistent pattern and are shown in Figure 5G-I.
Figure 5. The relationship between language lateralization and language function.
Language z-scores as a function of laterality indices (LIs) derived from fMRI (x-axis), plotted separately for bilingual LTLE (B-LTLE; red circles) and monolingual LTLE (M-LTLE; green triangles). Panels A-C depict lateral temporal LI, panels D-F depict inferior frontal LI, and panels G-I depict the omnibus language LI by each language measure (category fluency, letter fluency, and auditory naming). X-axis values to the left of the dashed vertical black line represent atypical language lateralization, and y-axis values below the dashed horizontal black line represent impaired language scores.
Of interest, all four B-LTLEs with atypical language lateralization as measured by omnibus LI and lateral temporal LI demonstrated unimpaired category fluency scores (i.e., within two standard deviations of the mean of HC), whereas the two B-LTLEs with typical language lateralization had either borderline impaired or impaired category fluency. See Supplementary Table 4 for unadjusted bivariate Spearman rank correlations with 95% confidence intervals, which showed a similar pattern of results as the age-adjusted correlations.
4. Discussion
Despite extensive research on language lateralization in monolingual patients with TLE and healthy bilinguals, little is known about language laterality in bilinguals with TLE (for review see Bartha-Doering and Bonelli, 2019). Localization of naming sites in bilinguals with epilepsy has been previously examined using electrical stimulation mapping (Lucas et al., 2004) and electrocorticography (Cervenka et al., 2011). However, only two fMRI studies have examined language lateralization in a group of bilinguals with epilepsy (Centeno et al., 2014; Cheung et al., 2006). Our main finding was that bilinguals with a left-sided seizure focus showed greater rightward (i.e., atypical) language laterality than a matched group of monolingual patients with a left-sided seizure focus. This was driven by language laterality within the lateral temporal region, which showed a large effect size. On an individual level, 4 out of 6 of the B-LTLE patients showed atypical language lateralization, whereas only 3 out of 21 of the M-LTLE patients showed this pattern. Finally, rightward language lateralization was associated with better language performance in B-LTLE, in particular in lateral temporal regions for naming and fluency. Interestingly, all four B-LTLEs with atypical language lateralization in the lateral temporal LI had intact performances on a semantic fluency task, whereas the two B-LTLE patients with typical left-lateralized language had impaired scores. These preliminary findings provide some insight into the possibility that bilingualism may interact with LTLE to promote re-organization of language functioning to the right hemisphere, potentially resulting in better language function in this group.
4.1. Bilingualism contributes to neuroplasticity of language networks in LTLE
Our finding of a combined effect of bilingualism and left-hemisphere seizures on language lateralization may suggest that atypical language representation may be more likely to manifest in bilinguals who had a neurological insult to the left hemisphere, which is typically dominant for language. These two factors may collectively facilitate re-organization via recruitment of language homologues in the contra-lesional (right) hemisphere. Our finding of more bilateral activation in B-LTLE versus M-LTLE is consistent with a recent fMRI report of early (AoA <6 years) Spanish-English bilingual neurosurgical patients (primarily with brain tumors) demonstrating weaker left-hemisphere lateralization in both languages relative to matched monolingual patients (Połczyńska et al., 2017).
Our preliminary finding of more bilateral activation in bilinguals than monolinguals (primarily in LTLE, but with a weaker effect in HC) is in line with emerging literature suggesting that sustained and long-term use of more than one language can permanently change the amount of activation within the language network. Specifically, bilinguals overall tend to recruit additional brain regions during lexical-semantic tasks (Cargnelutti et al., 2019), and may recruit more right-hemisphere language regions (Palomar-García et al., 2015; Román et al., 2015; Wang et al., 2011) relative to monolinguals, especially for L2 (Evans et al., 2002; Park et al., 2012). Increased right hemisphere activation in the lateral temporal lobe specifically has been reported for bilinguals relative to monolinguals in a range of tasks including lexical decision (Park et al., 2012), picture naming (Palomar-García et al., 2015), semantic judgment (Román et al., 2015), and sentence processing (Jasinska and Petitto, 2013). These observations are consistent with our findings of the lateral temporal region showing the most robust effect of bilingualism on laterality.
Notably, we did not observe a robust effect of bilingualism in HC or RTLE across regions. However, B-HC showed more rightward asymmetry relative to M-HC in the inferior frontal region when directly comparing the two groups—and whole-brain maps suggested a more bilateral pattern of activation. In contrast, these effects were absent in RTLE. Although this may be related to limited power due to our sample size, it is also possible that the effect of bilingualism on re-organization is weaker or less consistent compared to the effect of left hemisphere seizures. One possibility is that in B-RTLE, the presence of right hemisphere seizures could have negated any potential for re-distribution of language to the right hemisphere associated with bilingualism, with B-RTLE resulting in left-lateralization similar to M-RTLE.
4.2. Is atypical language lateralization protective in B-LTLE?
Our preliminary finding that atypical language lateralization was associated with better language ability in B-LTLE supports the possibility that this type of re-organization may be protective for this group. This is consistent with reports that functional language re-organization to homologous right hemisphere regions is sometimes associated with better pre-operative (Chang et al., 2017; Rosazza et al., 2013) and post-operative (Binder et al., 2008; Rosazza et al., 2013; Sabsevitz et al., 2003) language performance in LTLE. It is possible that variability in these results is partly due to variable inclusion of bilingual subjects across different studies, calling for future work to incorporate language status into analyses.
Although B-LTLE showed more rightward lateralization relative to M-LTLE—and more bilateral language activation was associated with better language performance in B-LTLE—we did not find that B-LTLE necessarily performed better on language measures relative to the M-LTLE group. However, it is well accepted that monolinguals outperform bilinguals on speeded word retrieval and picture naming (Bialystok et al., 2008; Sandoval et al., 2010). Relatedly, naming scores did not lateralize bilingual patients with epilepsy as having a left versus right seizure focus as well as for monolinguals with epilepsy (Gooding et al., 2018). Reduced retrieval in bilinguals compared to monolinguals is considered to be a normal consequence of the need to juggle two languages, which may increase the processing load on the language production system. Interestingly, despite the expected bilingual ‘disadvantage’ in semantic fluency in particular (Gollan et al., 2002; Sandoval et al., 2010), B-LTLE did not differ from M-LTLE on category fluency (p = .45; Cohen’s d = 0.36). Although this coupled with our pattern of correlations within B-LTLE provide some support for the idea that an atypical language profile could be protective in this group, a more definitive test of whether increased neuroplasticity of language networks due to bilingualism helps preserve language in LTLE is needed in a larger patient sample.
4.3. Clinical implications
With further replication, our preliminary finding that bilingualism was associated with more bilateral and/or right-sided activation in LTLE could have implications for pre-surgical evaluation of language lateralization in TLE, which is commonly accomplished using fMRI (Szaflarski et al., 2017). Specifically, understanding how language lateralization profiles differ between bilingual and monolingual patients with TLE and determining whether bilingualism promotes an inter-hemispheric shift in language that is adaptive, could be important for evaluating risk for postoperative language decline. We believe that it is important to incorporate bilingual status as an additional factor influencing atypical laterality pre-surgically, along with known predictors such as a left-sided seizure focus, left handedness, earlier age of seizure onset, and the presence of MTS or a lesion (Dijkstra and Ferrier, 2013). This invites future, large-scale studies to examine the interactions among bilingualism, handedness, and left TLE status as predictors of atypical language lateralization that may be additive or synergistic in nature. Our findings also underscore the complexity of language lateralization in bilingual patients and converge with an existing literature suggesting that evaluation of language in bilingual epilepsy patients should not be interpreted by the same standards applied for monolingual speakers (Gooding et al., 2018).
Bilingualism might serve as cognitive reserve in epilepsy. A growing body of studies demonstrates that sustained engagement with an additional language modifies the structure and function of the brain, altering both gray and white matter networks (Hayakawa and Marian, 2019; Pliatsikas et al., 2020). For example, bilingualism may delay the diagnosis of Alzheimer’s disease by an average of 4-6 years (Anderson et al., 2020). In TLE, our lab has demonstrated that bilingualism served as a form of cognitive reserve, with bilingual patients showing equivalent executive functioning in the context of poorer white matter integrity compared to monolinguals (Reyes et al., 2018). However, further research and longitudinal designs are required to determine whether and how bilingualism may contribute to cognitive/brain reserve in TLE.
4.4. Limitations and future directions
Our study has several limitations, with the most notable being a small sample of bilinguals, especially those with LTLE. This partially reflects the fact that we only included patients that were carefully characterized proficient bilinguals, using both self-reported information about language background as well as an objective measure of expressive English vocabulary. Importantly, B-LTLE did not significantly differ from B-RTLE on AoA of L2 and did not significantly differ from any TLE group on English vocabulary, mitigating concerns that our main findings could be attributed to differences in English AoA or proficiency. Even with a series of six bilingual patients with LTLE, we found medium-to-large effects at the group level, with consistent results at an individual level. Although preliminary, these results may inspire new research that stratifies analyses by language status, and prospectively collects important language background variables (e.g., AoA, objective proficiency of both languages, language use patterns, etc).
Second, our fMRI and language measures were all conducted in English. Though our bilinguals reported English dominance and expressed preference for testing in English, emerging research suggests that it is important to test behaviorally and map all languages spoken given the possibility of divergent neural representation of L1 and L2 (Połczyńska et al., 2017; Bartha-Doering and Bonelli, 2019; Połczyńska and Bookheimer, 2021; Lucas et al., 2004; Cervenka et al., 2011). However, prior research on bilingual controls and neurosurgical patients suggested similar laterality values for L1 and L2 (Centeno et al., 2014; Hull and Vaid, 2007; Połczyńska et al., 2017), and more bilateral activation for both L1 and L2 when compared to monolinguals (Park et al., 2012; Połczyńska et al., 2017). Nonetheless, given a longstanding interest in understanding the neural differences between L1 and L2 language processing coupled with limited knowledge of this topic in epilepsy (Bartha-Doering and Bonelli, 2019), we acknowledge the need for future studies to examine all languages spoken by multilingual patients.
Third, limited statistical power prevented us from examining important variables that have been shown to affect lateralization in epilepsy, including handedness and age of seizure onset. Indeed, two B-LTLEs and two M-LTLEs who were also left-handed showed atypical language representation (see Figure 3 and Table 2). Thus, there is a potential influence of both bilingualism and handedness on language lateralization that we were unable to test in the current study. Relatedly, given a limited sample of bilinguals, we were unable to explore the moderating effect of important bilingual variables such as AoA and proficiency. In addition, given the retrospective design of the study, we did not have information on the frequency of current and past use of each language. Many of these factors have been previously shown to affect the distribution and lateralization of language networks. In addition to these variables, it will be fruitful for future studies to examine the role of linguistic distance between L1 and L2, as well as to compare fMRI activation between logographic (e.g., Chinese) versus alphabetic (e.g., English) languages, and between spoken and signed languages, as these factors have all been shown to affect language activation in healthy bilinguals (Połczyńska and Bookheimer, 2021 for review).
Fourth, we did not have a thorough assessment of L2 proficiency in our B-HC. Whereas B-TLE learned English as their second language and we were confident in their bilingual status, the majority of B-HC learned English first and information on their age of L2 acquisition was unavailable. Finally, although all patients showed scalp and/or intracranial EEG evidence of a temporal lobe seizure focus, a subset of patients could not be localized to the mesial versus lateral temporal lobe based on available EEG patterns and clinical semiology. It is possible that localization of the seizure focus can affect language lateralization, although we were unable to examine this given the limited sample.
4.5. Conclusions
Our preliminary findings suggest that bilingualism may increase the likelihood of atypical language lateralization in TLE, with a likely mechanism being a greater tendency for re-organization and/or adaptation in language networks in bilinguals compared to monolinguals. This process may be protective pre-operatively and could help to mitigate language decline post-operatively in LTLE. Moving forward, it will be important to deconstruct the complex aspects of bilingualism (e.g., proficiency, age of acquisition, language use patterns, acculturation) to paint a more complete picture of how bilingualism may affect language networks in the presence of epilepsy. Bilingualism is just one of several potential factors of reserve, such as education, musical training, and physical activity. Detailed study of these factors will allow for exciting new investigations to increase our understanding of how life experiences may contribute to neuroplasticity and/or cognitive reserve in neurological disease.
Supplementary Material
Acknowledgements
The authors thank Sanam Lalani and Donatello Arienzo for helpful discussion, Anna Christina Macari, Daniel Asay, and Jun Rao for assistance with data collection and processing, and the patients of the UCSD Epilepsy Center for their participation and contribution to science. This research was supported by funding from the National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS065838 and R21 NS107739) to C.R.M., National Institute on Deafness and Other Communication Disorders (K01 DC016904) to M.P., and National Institute on Aging (U01 AG052564-01) to M.D. A.S. was supported by a F32 from NINDS (F32NS119285-01A1) and A.R. was supported by a F31 from NINDS (1F31NS111883-01). Some of the study data were collected and managed using REDCap, a secure web platform for building and managing online databases and surveys. The REDCap software system provided by the UCSD Clinical and Translational Research Center is supported by Award Number UL1TR001442 from the National Center For Research Resources.
Footnotes
Declarations of interest: none
Three B-TLEs were simultaneous bilinguals; labeled as “SB” in Table 2.
For calculation of z-scores, bilingual and monolingual HC were combined given no significant difference between groups on language measures, and given a small number of bilingual HC.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM, 2003. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage 18, 423–438. 10.1016/S1053-8119(02)00013-7 [DOI] [PubMed] [Google Scholar]
- Anderson JAE, Hawrylewicz K, Grundy JG, 2020. Does bilingualism protect against dementia? A meta-analysis. Psychon. Bull. Rev 27, 952–965. 10.3758/s13423-020-01736-5 [DOI] [PubMed] [Google Scholar]
- Bartha-Doering L, Bonelli S, 2019. Epilepsy and Bilingualism. A Systematic Review. Front. Neurol 10. 10.3389/fneur.2019.01235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik F, Luk G, 2008. Cognitive control and lexical access in younger and older bilinguals. J. Exp. Psychol. Learn. Mem. Cogn 34, 859–873. 10.1037/0278-7393.34.4.859 [DOI] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM, 2008. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 49, 1377–1394. 10.1111/j.1528-1167.2008.01625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnelutti E, Tomasino B, Fabbro F, 2019. Language Brain Representation in Bilinguals With Different Age of Appropriation and Proficiency of the Second Language: A Meta-Analysis of Functional Imaging Studies. Front. Hum. Neurosci 0. 10.3389/fnhum.2019.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno M, Koepp MJ, Vollmar C, Stretton J, Sidhu M, Michallef C, Symms MR, Thompson PJ, Duncan JS, 2014. Language dominance assessment in a bilingual population: Validity of fMRI in the second language. Epilepsia 55, 1504–1511. 10.1111/epi.12757 [DOI] [PubMed] [Google Scholar]
- Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE, 2011. Language Mapping in Multilingual Patients: Electrocorticography and Cortical Stimulation During Naming. Front. Hum. Neurosci 5. 10.3389/fnhum.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Javadi SS, Bahrami N, Uttarwar VS, Reyes A, McDonald CR, 2018. Mapping lexical-semantic networks and determining hemispheric language dominance: Do task design, sex, age, and language performance make a difference? Brain Lang. 179, 42–50. 10.1016/j.bandl.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Kemmotsu N, Leyden KM, Kucukboyaci NE, Iragui VJ, Tecoma ES, Kansal L, Norman MA, Compton R, Ehrlich TJ, Uttarwar VS, Reyes A, Paul BM, McDonald CR, 2017. Multimodal imaging of language reorganization in patients with left temporal lobe epilepsy. Brain Lang. 170, 82–92. 10.1016/j.bandl.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Marshall A, Bahrami N, Mathur K, Javadi SS, Reyes A, Hegde M, Shih JJ, Paul BM, Hagler DJ, McDonald CR, 2019. Differential sensitivity of structural, diffusion, and resting-state functional MRI for detecting brain alterations and verbal memory impairment in temporal lobe epilepsy. Epilepsia 60, 935–947. 10.1111/epi.14736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Chan AS, Chan Y, Lam JMK, 2006. Language lateralization of Chinese-English bilingual patients with temporal lobe epilepsy: A functional MRI study. Neuropsychology 20, 589–597. 10.1037/0894-4105.20.5.589 [DOI] [PubMed] [Google Scholar]
- Corballis MC, 2014. Left Brain, Right Brain: Facts and Fantasies. PLoS Biol. 12, e1001767. 10.1371/journal.pbio.1001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, 2001. Delis-Kaplan Executive Function System:Technical Manual. Harcourt Assessment Company, San Antonio, Texas. [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E, 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra KK, Ferrier CH, 2013. Patterns and predictors of atypical language representation in epilepsy. J. Neurol. Neurosurg. Psychiatry 84, 379–385. 10.1136/jnnp-2012-303141 [DOI] [PubMed] [Google Scholar]
- Evans J, Workman L, Mayer P, Crowley K, 2002. Differential bilingual laterality: mythical monster found in Wales. Brain Lang. 83, 291–299. 10.1016/s0093-934x(02)00020-2 [DOI] [PubMed] [Google Scholar]
- Garcia DL, Gollan TH, 2021. The MINT Sprint: Exploring a Fast Administration Procedure with an Expanded Multilingual Naming Test. J. Int. Neuropsychol. Soc. JINS 1–17. 10.1017/S1355617721001004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Montoya RI, Werner GA, 2002. Semantic and letter fluency in Spanish-English bilinguals. Neuropsychology 16, 562–576. [PubMed] [Google Scholar]
- Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM, 2012. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Biling. Lang. Cogn 15, 594–615. 10.1017/S1366728911000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding A, Cole JR, Hamberger MJ, 2018. Assessment of Naming in Non-native English Speakers with Epilepsy. J. Int. Neuropsychol. Soc 24, 1057–1063. 10.1017/S1355617718000632 [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, 2015. Object naming in epilepsy and epilepsy surgery. Epilepsy Behav. EB 46, 27–33. 10.1016/j.yebeh.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, 2003. Auditory and visual naming tests: normative and patient data for accuracy, response time, and tip-of-the-tongue. J. Int. Neuropsychol. Soc. JINS 9, 479–489. 10.1017/s135561770393013x [DOI] [PubMed] [Google Scholar]
- Hayakawa S, Marian V, 2019. Consequences of multilingualism for neural architecture. Behav. Brain Funct 15, 6. 10.1186/s12993-019-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM, 2010. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage 50, 175–183. 10.1016/j.neuroimage.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R, Vaid J, 2007. Bilingual language lateralization: A meta-analytic tale of two hemispheres. Neuropsychologia 45, 1987–2008. 10.1016/j.neuropsychologia.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Jasinska KK, Petitto LA, 2013. How age of bilingual exposure can change the neural systems for language in the developing brain: a functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev. Cogn. Neurosci 6, 87–101. 10.1016/j.dcn.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner E, Reyes A, Macari AC, Chang Y-H, Paul BM, Hermann BP, McDonald CR, 2019. Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy. Epilepsia 60, 1627–1638. 10.1111/epi.16283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas TH, McKhann GM, Ojemann GA, 2004. Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J. Neurosurg 101, 449–457. 10.3171/jns.2004.101.3.0449 [DOI] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, Sherfey JS, Devinsky O, Kuzniecky R, Dolye WK, Cash SS, Leonard MK, Hagler DJ, Dale AM, Halgren E, 2010. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. NeuroImage 53, 707–717. 10.1016/j.neuroimage.2010.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergen F, Kuruoglu G, 2021. Lateralization of lexical processing in monolinguals and bilinguals. Int. J. Biling 13670069211018842. 10.1177/13670069211018842 [DOI] [Google Scholar]
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T, 2009. Atypical language lateralization in epilepsy patients. Epilepsia 50, 1505–1516. 10.1111/j.1528-1167.2008.02000.x [DOI] [PubMed] [Google Scholar]
- Navarro V, Delmaire C, Chauviré V, Habert M-O, Footnick R, Lehéricy S, Baulac M, Pallier C, Cohen L, 2009. “What is it?” A functional MRI and SPECT study of ictal speech in a second language. Epilepsy Behav. EB 14, 396–399. 10.1016/j.yebeh.2008.10.020 [DOI] [PubMed] [Google Scholar]
- O’Grady C, Omisade A, Sadler RM, 2016. Language lateralization of a bilingual person with epilepsy using a combination of fMRI and neuropsychological assessment findings. Neurocase 22, 436–442. 10.1080/13554794.2016.1233987 [DOI] [PubMed] [Google Scholar]
- Palomar-García M-Á, Bueichekú E, Ávila C, Sanjuán A, Strijkers K, Ventura-Campos N, Costa A, 2015. Do bilinguals show neural differences with monolinguals when processing their native language? Brain Lang. 142, 36–44. 10.1016/j.bandl.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Park HRP, Badzakova-Trajkov G, Waldie KE, 2012. Language lateralisation in late proficient bilinguals: a lexical decision fMRI study. Neuropsychologia 50, 688–695. 10.1016/j.neuropsychologia.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Pliatsikas C, DeLuca V, Voits T, 2020. The Many Shades of Bilingualism: Language Experiences Modulate Adaptations in Brain Structure. Lang. Learn 70, 133–149. 10.1111/lang.12386 [DOI] [Google Scholar]
- Połczyńska MM, Bookheimer SY, 2021. General principles governing the amount of neuroanatomical overlap between languages in bilinguals. Neurosci. Biobehav. Rev 130, 1–14. 10.1016/j.neubiorev.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Połczyńska MM, Japardi K, Bookheimer SY, 2017. Lateralizing language function with pre-operative functional magnetic resonance imaging in early proficient bilingual patients. Brain Lang. 170, 1–11. 10.1016/j.bandl.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Reyes A, Paul BM, Marshall A, Chang Y-HA, Bahrami N, Kansal L, Iragui VJ, Tecoma ES, Gollan TH, McDonald CR, 2018. Does bilingualism increase brain or cognitive reserve in patients with temporal lobe epilepsy? Epilepsia 59, 1037–1047. 10.1111/epi.14072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román P, González J, Ventura-Campos N, Rodríguez-Pujadas A, Sanjuán A, Ávila C, 2015. Neural differences between monolinguals and early bilinguals in their native language during comprehension. Brain Lang. 150, 80–89. 10.1016/j.bandl.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Rosazza C, Ghielmetti F, Minati L, Vitali P, Giovagnoli AR, Deleo F, Didato G, Parente A, Marras C, Bruzzone MG, D’Incerti L, Spreafico R, Villani F, 2013. Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri-ictal, pre- and post-operative language performance: An fMRI study. NeuroImage Clin. 3, 73–83. 10.1016/j.nicl.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, 2012. SUMA. NeuroImage 62, 768–773. 10.1016/j.neuroimage.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, Mueller WM, Binder JR, 2003. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology 60, 1788–1792. 10.1212/01.wnl.0000068022.05644.01 [DOI] [PubMed] [Google Scholar]
- Sandoval TC, Gollan TH, Ferreira VS, Salmon DP, 2010. What causes the bilingual disadvantage in verbal fluency? The dual-task analogy*. Biling. Lang. Cogn 13, 231–252. 10.1017/S1366728909990514 [DOI] [Google Scholar]
- Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Hader WJ, Jetté N, 2011. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia 52, 857–869. 10.1111/j.1528-1167.2011.03022.x [DOI] [PubMed] [Google Scholar]
- Stewart CC, Swanson SJ, Sabsevitz DS, Rozman ME, Janecek JK, Binder JR, 2014. Predictors of language lateralization in temporal lobe epilepsy. Neuropsychologia 60, 93–102. 10.1016/j.neuropsychologia.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Gloss D, Binder JR, Gaillard WD, Golby AJ, Holland SK, Ojemann J, Spencer DC, Swanson SJ, French JA, Theodore WH, 2017. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 88, 395–402. 10.1212/WNL.0000000000003532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen T, McDonald CR, Carlson C, Doyle W, Cash S, Sherfey J, Felsovalyi O, Girard H, Barr W, Devinsky O, Kuzniecky R, Halgren E, 2012. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat. Commun 3, 1284. 10.1038/ncomms2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiang J, Vannest J, Holroyd T, Narmoneva D, Horn P, Liu Y, Rose D, deGrauw T, Holland S, 2011. Neuromagnetic measures of word processing in bilinguals and monolinguals. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 122, 1706–1717. 10.1016/j.clinph.2011.02.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.