Abstract
OBJECTIVE
Pituitary adenoma is one of the most common primary intracranial neoplasms. Most of these tumors are soft, but up to 17% may have a firmer consistency. Therefore, knowing the tumor consistency in the preoperative setting could be helpful. Multiple imaging methods have been proposed to predict tumor consistency, but the results are controversial. This study aimed to evaluate the efficacy of MR elastography (MRE) in predicting tumor consistency and its potential use in a series of patients with pituitary adenomas.
METHODS
Thirty-eight patients with pituitary adenomas (≥ 2.5 cm) were prospectively evaluated with MRI and MRE before surgery. Absolute MRE stiffness values and relative MRE stiffness ratios, as well as the relative ratio of T1 signal, T2 signal, and diffusion-weighted imaging apparent diffusion coefficient (ADC) values were determined prospectively by calculating the ratio of those values in the tumor to adjacent left temporal white matter. Tumors were classified into three groups according to surgical consistency (soft, intermediate, and firm). Statistical analysis was used to identify the predictive value of the different radiological parameters in determining pituitary adenoma consistency.
RESULTS
The authors included 32 (84.21%) nonfunctional and 6 (15.79%) functional adenomas. The mean maximum tumor diameter was 3.7 cm, and the mean preoperative tumor volume was 16.4 cm3. Cavernous sinus invasion was present in 20 patients (52.63%). A gross-total resection was possible in 9 (23.68%) patients. The entire cohort’s mean absolute tumor stiffness value was 1.8 kPa (range 1.1–3.7 kPa), whereas the mean tumor stiffness ratio was 0.66 (range 0.37–1.6). Intraoperative tumor consistency was significantly correlated with absolute and relative tumor stiffness (p = 0.0087 and 0.007, respectively). Tumor consistency alone was not a significant factor for predicting gross-total resection. Patients with intermediate and firm tumors had more complications compared to patients with soft tumors (50.00% vs 12.50%, p = 0.02) and also had longer operative times (p = 0.0002).
CONCLUSIONS
Whereas other MRI sequences have proven to be unreliable in determining tumor consistency, MRE has been shown to be a reliable tool for predicting adenoma consistency. Preoperative knowledge of tumor consistency could be potentially useful for surgical planning, counseling about potential surgical risks, and estimating the length of operative time.
Keywords: pituitary adenomas, MRE, MR elastography, consistency, pituitary surgery
PITUITARY adenomas represent 10%–25% of all intracranial neoplasms arising from the anterior lobe of the pituitary gland.1 They can be classified according to their size, into microadenomas (< 1 cm) and macroadenomas (≥ 1 cm), as well as by their clinical features.2 Resection plays an essential role in their management, with most tumors treated via a transsphenoidal approach.3 The majority of pituitary adenomas are soft and easily resected using suction and minimal curettage. However, 10%–17% tend to have a firmer consistency, making surgery more challenging, higher risk, and potentially substantially impacting operative time and planning.4,5
Since its introduction into the medical field in 1995, MR elastography (MRE) has been an important noninvasive tool to characterize tissue mechanical properties. MRE can “palpate” tissues and thus identify characteristics that would aid in preoperative surgical planning. MRE relies on imaging of shear waves, which are induced into tissues by an external driver, using a phase-contrast MR pulse sequence and generating an elastogram. MRE relies on two main tissue properties: shear stiffness, which is determined by the wavelength of the traveling waves; and shear viscosity, which is then determined by the energy loss as the waves travel through tissue.6,7
Preoperative determination of pituitary tumor consistency has been investigated in the past by different imaging methods. However, the results are controversial.8–14 Preoperative determination of the tumor consistency could optimize preoperative planning to achieve maximal safe resection. It can be helpful in preoperative counseling of patients and families about potential surgical risks.8,14
There have been limited reports delineating the utility of MRE to determine pituitary adenoma consistency preoperatively.15,16 In this report, we attempt to expand the value of MRE for predicting tumor consistency and its potential use in the preoperative setting. We also compared the value of the T2-weighted sequence and MR diffusion-weighted imaging (DWI).
Methods
With the approval of our institutional review board and following written consent obtained prospectively, we evaluated 40 consecutive patients with pituitary macroadenomas who underwent surgery between September 2013 and July 2019. Due to the resolution challenges of MRE, we only included patients with tumors ≥ 2.5 cm in maximal diameter in a coronal plane. In addition, the patient had to have availability, time, and willingness to undergo the examination. We excluded patients for whom the tumor consistency was not recorded in the postoperative note. All patients underwent an MRI and MRE session before surgery. MRE values were calculated preoperatively. Thus, the radiologist was unaware of surgical consistency (i.e., tumor consistency reported at surgery). The lead surgeon (J.J.V.G.) was blinded to the MRE results and reported tumor consistency in his operative notes. Surgical consistency was graded according to the following: 1) soft—primarily removed with suction; 2) intermediate—parts easily removed with suction but other portions difficult to remove with suction requiring mechanical techniques such a sharp dissection; 3) firm—unable to remove with suction requiring sharp dissection.
Tumor Volume Measurement and Extent of Resection
Volumetric analysis was performed in all cases using Aquarius iNtuition software using the free region of interest tool by a neurosurgeon (S.C.C.) and confirmed with a neuroradiologist (J.H.). The analysis was performed on coronal or axial gadolinium-enhanced T1-weighted images from the preoperative MRI. Nonenhanced images were used when contrast-enhanced studies were unavailable. Postoperative MRI (between 3 and 6 months of the operation) was used to calculate residual tumor volume. A comparison of pre- and postoperative tumor volumes was used to calculate the extent of resection (EOR). Gross-total resection (GTR) was defined as no evidence of residual tumor, near-total resection (NTR) if 90%–99% of the tumor was resected, and subtotal resection (STR) if less than 90% of the tumor was resected. A 3T MRI system (Signa Excite; GE Healthcare) was used for the acquisition of conventional MRI as well as MRE.
MRE Acquisition and Processing
MRE was acquired according to protocols previously described in 3T MR scanners.17 Low-amplitude, 60-Hz frequency mechanical vibrations in the form of shear waves were delivered to the patient’s brain. The active component, composed of a waveform generator, an amplifier, and an acoustic speaker, was located outside the scan room and connected via a long flexible tube to a soft pillow-like passive driver positioned under the patient’s head in a standard receive-only 8-channel MRI head coil. The spin echo echo planar imaging MRE pulse sequence was used for imaging by synchronizing motion-encoding gradients to the applied mechanical vibrations. The images were acquired using the following parameters: TR/TE 3600/62 msec; 72 × 72 acquisition matrix reconstructed to 80 × 80; field of view 24 cm; section thickness 3 mm; 48 contiguous axial sections; bandwidth 250 kHz; parallel imaging acceleration factor 3; motion encoding in the positive and negative x, y, and z directions; and 8 phase offsets sampled during 1 period of motion at 60 Hz. The time for obtaining the images was less than 4 minutes.
The tumor stiffness was calculated as follows. First, the complex phase-difference wave images were calculated in the x, y, and z motion-encoding directions. The curl of the wave images was then calculated to remove the effects of longitudinal waves, and the curl images were smoothed with a filter of the form of (1 − x2)2(1 − y2)2(1 − z2)2, where x, y, and z are linearly spaced from −1 to 1 over 5 × 5 × 5 window.18 Finally, the complex shear modulus was calculated by direct inversion of the Helmholtz equation. Shear stiffness was reported as the magnitude of the complex shear modulus (|G*|, kPa).
A high-resolution 3D T1-weighted image was also acquired and used to generate tumor regions of interest (ROIs). The tumor ROI was manually drawn on each imaging slice from T1-weighted images following registration to the MRE space. To reduce the effects of surrounding brain tissue on the stiffness estimate of the tumor, the edges of the tumor mask were eroded by 1 pixel. Absolute tumor stiffness values were then reported as the median of all voxels in the eroded tumor ROIs. The relative tumor stiffness ratio was then calculated relative to the left temporal white matter.
Similarly, relative DWI apparent diffusion coefficient (ADC), precontrast T1, and T2 tumor signal intensity were reported in comparison to left temporal white matter signal intensity. ADC values were not available for 5 patients and thus they were excluded from this part of the analysis. Relative T1 signal intensity was considered hyperintense when comparative ratios were ≥ 1.1, isointense with ratios between < 1.1 and > 0.9, and hypointense with ratios ≤ 0.9. Similar cutoffs were used to determine relative T2 signal intensity. By reporting relative tumor signal intensities in comparison to a standard anatomical landmark, sampling errors were minimized and MRI signals are standardized to the individual’s MRI scan.
Statistical Analysis
JMP version 14 (SAS) was used for all statistical analyses. A one-way ANOVA with Tukey honestly significant difference for post hoc analysis was used to compare absolute tumor stiffness values (in kPa) and relative tumor stiffness ratios to surgical consistency grades, relative T1, and relative T2 signal intensity. One-way ANOVA (Tukey honestly significant difference) was also used to compare relative ADC values to surgical consistency grades. A chi-square test, or a Fisher exact test when necessary, was used for direct comparison of surgical consistency grades to relative T2 and T1 signal intensity and MRE stiffness grades. A p value < 0.05 was considered significant.
Results
Tumor Characteristics and Patient Demographics
This study included 38 patients with pituitary adenomas resected by the lead surgeon via an endoscopic endonasal approach (EEA). There were 22 (57.89%) men and 16 (42.11%) women with a median age at the time of surgery of 54 years (range 22–78 years). Thirty-two (84.21%) adenomas were nonfunctional, 5 (13.16%) were growth hormone–secreting adenomas, and 1 (2.63%) was an adrenocorticotropic hormone–secreting adenoma. Eight patients (21.05%) had a Knosp grade 2, 10 (26.32%) had a Knosp grade 3A, 2 (5.26%) had a Knosp 3B, and 18 (47.37%) had a Knosp grade 4. Four tumors were Hardy A (10.53%), 12 were Hardy B (31.58%), 3 were Hardy C (7.89%), 4 were Hardy D (10.53%), and 15 were Hardy E (39.47%). MRE suggested that 35 (92.11%) tumors were soft, 2 (5.26%) were intermediate, and 1 (2.63%) was firm. The mean preoperative tumor volume was 16.4 cm3 (SD 10.9 cm3, range 3.5–53 cm3). The mean maximum tumor diameter was 3.7 cm (SD 0.87 cm, range 2.5–6 cm). Ten patients (26.32%) had undergone a previous operation elsewhere. A GTR was achieved in 9 (23.68%) patients, NTR in 15 (39.47%), and STR in the remaining 14 (36.84%) patients (Tables 1 and 2).
TABLE 1.
Clinical characteristics in 38 patients with pituitary adenoma
| Variable | No. of Cases (%) |
|---|---|
| Sex | |
| Female | 16 (42.11) |
| Male | 22 (57.89) |
| Prior surgery | 10 (26.32) |
| Type of adenoma | |
| Nonfunctional | 32 (84.21) |
| Functional | 6 (15.79) |
| Knosp grade | |
| 2 | 8 (21.05) |
| 3A | 10 (26.32) |
| 3B | 2 (5.26) |
| 4 | 18 (47.37) |
| Hardy grade | |
| A | 4 (10.53) |
| B | 12 (31.58) |
| C | 3 (7.89) |
| D | 4 (10.53) |
| E | 15 (39.47) |
| Tumor size | |
| ≥4 cm | 15 (39.47) |
| <4 cm | 23 (60.53) |
| Tumor vol | |
| ≥10 cm3 | 10 (26.32) |
| <10 cm3 | 28 (73.68) |
| Tumor consistency on MRE | |
| Soft | 35 (92.11) |
| Intermediate | 2 (5.26) |
| Firm | 1 (2.63) |
| Surgical consistency | |
| Soft | 32 (84.21) |
| Intermediate | 4 (10.53) |
| Firm | 2 (5.26) |
| EOR | |
| GTR | 9 (23.68) |
| NTR | 15 (39.47) |
| STR | 14 (36.84) |
TABLE 2.
Correlation between tumor and clinical characteristics and surgical consistency of 38 pituitary adenomas
| Characteristic | No. of Pts | Soft | Intermediate & Firm | p Value |
|---|---|---|---|---|
| No. of pts | 38 | 32 (84.21%) | 6 (15.79%) | |
| Sex | >0.99 | |||
| Male | 22 | 18 (56.25%) | 4 (66.67%) | |
| Female | 16 | 14 (43.75%) | 2 (33.33%) | |
| Mean age in yrs (± SD) | 53 ± 14.7 | 58 ± 16.6 | 0.50 | |
| Adenoma type | >0.99 | |||
| Functional | 6 | 5 (15.63%) | 1 (16.67%) | |
| Nonfunctional | 32 | 27 (84.38%) | 5 (83.33%) | |
| Knosp grade | 0.66 | |||
| 2–3A | 18 | 16 (50.00%) | 2 (33.33%) | |
| 3B-4 | 20 | 16 (50.00%) | 4 (66.67%) | |
| Hardy grade | 0.17 | |||
| A-C | 19 | 18 (56.25%) | 1 (16.67%) | |
| D-E | 19 | 14 (43.75%) | 5 (83.33%) | |
| Tumor size | >0.99 | |||
| ≥4 cm | 15 | 13 (40.63%) | 2 (33.33%) | |
| <4 cm | 23 | 19 (59.38%) | 4 (66.67%) | |
| Tumor vol | >0.99 | |||
| ≥10 cm3 | 28 | 23 (71.88%) | 5 (83.33%) | |
| <10 cm3 | 10 | 9 (28.13%) | 1 (16.67%) |
Pts = patients.
Shear Stiffness and Tumor Consistency
The shear stiffness of all adenomas had a mean value of 1.8 kPa (range 1.1–3.7 kPa), whereas the stiffness of the control ROIs in the left temporal white matter had a mean value of 2.7 kPa (range 2–3.2 kPa). Thus, our entire cohort’s mean relative tumor stiffness ratio was 0.66 (range 0.37–1.6). By surgical categorization, our cohort included 32 (84.21%) soft tumors, 4 (10.53%) intermediate tumors, and 2 (5.26%) firm tumors with mean stiffness values of 1.7, 2.1, and 2.8 kPa, respectively. Thus, each group’s relative tumor stiffness ratios were 0.61, 0.77, and 1.1, respectively.
Intraoperative tumor consistency was significantly correlated with the absolute MRE stiffness values (p = 0.0087) and relative MRE stiffness ratios (p = 0.007). Both absolute tumor stiffness and tumor stiffness ratio showed statistically significant differences between firm and soft tumors but not between soft and intermediate or intermediate and firm (Tables 3 and 4). MRE tumor stiffness values did not correlate with any other measured parameters, including relative T2 signal intensity, relative T1 signal intensity, the EOR, and tumor pathology.
TABLE 3.
Correlation between absolute MRE stiffness values and surgical findings
| Surgical Consistency | Mean kPa Ratio (SE) | p Value |
|---|---|---|
| Absolute MRE stiffness value | 0.0087 | |
| Soft | 1.62 (0.09) | |
| Intermediate | 2.0 (0.26) | |
| Firm | 2.77 (0.36) | |
| Post hoc Tukey pairwise comparison | ||
| Firm intermediate | 0.206 | |
| Soft firm | 0.010 | |
| Soft intermediate | 0.345 | |
SE = standard error.
Boldface type indicates statistical significance.
TABLE 4.
Correlation between relative MRE stiffness ratios and surgical findings
| Surgical Consistency | Mean kPa Ratio (SE) | p Value |
|---|---|---|
| Relative MRE stiffness value | 0.007 | |
| Soft | 0.61 (0.04) | |
| Intermediate | 0.77 (0.11) | |
| Firm | 1.13 (0.16) | |
| Post hoc Tukey pairwise comparison | ||
| Soft–firm | 0.008 | |
| Soft–intermediate | 0.382 | |
| Intermediate–firm | 0.168 | |
Boldface type indicates statistical significance.
There was no correlation between relative ADC values and intraoperative tumor consistency (p = 0.97) (Table 5). Furthermore, there was no correlation between intraoperative tumor consistency and either relative T2 signal intensity or relative T1 signal intensity.
TABLE 5.
Correlation between relative tumor ADC and surgical consistency
| Surgical Consistency | Mean Relative ADC Ratio (SE) | p Value |
|---|---|---|
| Soft | 1.03 (0.07) | |
| Intermediate | 1.01 (0.23) | |
| Firm | 0.97 (0.28) | 0.973 |
Five patients were excluded from this analysis due to lack of preoperative ADC maps.
Surgical Outcomes
GTR was achieved in 9 (23.68%) patients, NTR in 15 (39.47%) patients, and STR in the remaining 14 (36.84%). There were no significant differences between the EOR and the tumor consistency. The average operative time for soft tumors was 169 minutes, compared to 257 minutes for the intermediate and firm cases (p = 0.0002) (Table 6).
TABLE 6.
Surgical outcomes based on tumor consistency
| Outcome Measure | Soft Tumors, n = 32 | Firm & Intermediate Tumors, n = 6 | p Value |
|---|---|---|---|
| EOR | |||
| GTR | 8 (25.00%) | 1 (16.67%) | 0.65 |
| NTR | 13 (40.63%) | 2 (33.33%) | 0.73 |
| STR | 11 (34.38%) | 3 (50.00%) | 0.46 |
| Total pts w/ complications | 4 (12.50%) | 3 (50.00%) | 0.02 |
| Mean op time in mins (± SD) | 169 ± 38.6 | 257 ± 30.9 | 0.0002 |
Boldface type indicates statistical significance.
The most common complication was a postoperative CSF leak, which occurred in 4 patients (10.53%), and which required either a reoperation or a lumbar drain placement for management. The next most common complication was a new pituitary deficit postoperatively, which occurred in 3 patients (7.89%). One patient (2.63%) developed a transient cranial nerve (CN) III palsy (Table 7). From the 6 patients with intermediate and firm tumors, 3 (50.00%) had a complication, compared to 4 (12.50%) in the soft tumor group (p = 0.02).
TABLE 7.
Complications and tumor consistency
| Complications | Soft, n = 32 | Intermediate & Firm, n = 6 |
|---|---|---|
| CSF leak | 2 (6.25%) | 2 (33.33%) |
| New pituitary deficit | 2 (6.25%) | 1 (16.67%) |
| Transient CN palsy | 0 | 1 (16.67%) |
One patient had 2 complications.
Illustrative Cases
Case 1
A 75-year-old man presented with bitemporal hemianopia secondary to a nonfunctional pituitary adenoma. The tumor had bilateral cavernous sinus involvement and severe compression of the optic chiasm. Preoperative MRE showed a firm tumor with an elevated kPa of 5.4 (Fig. 1A). In the MRI T2-weighted (Fig. 1B) and T1-weighted (Fig. 1C) sequences the tumor was isointense. The patient underwent an EEA, which demonstrated a fibrous tumor. The pathology report showed a gonadotrophic adenoma with dense reticulin (Fig. 1D) deposition in large areas of the adenoma, consistent with the intraoperative impression of a firm tumor.
FIG. 1.
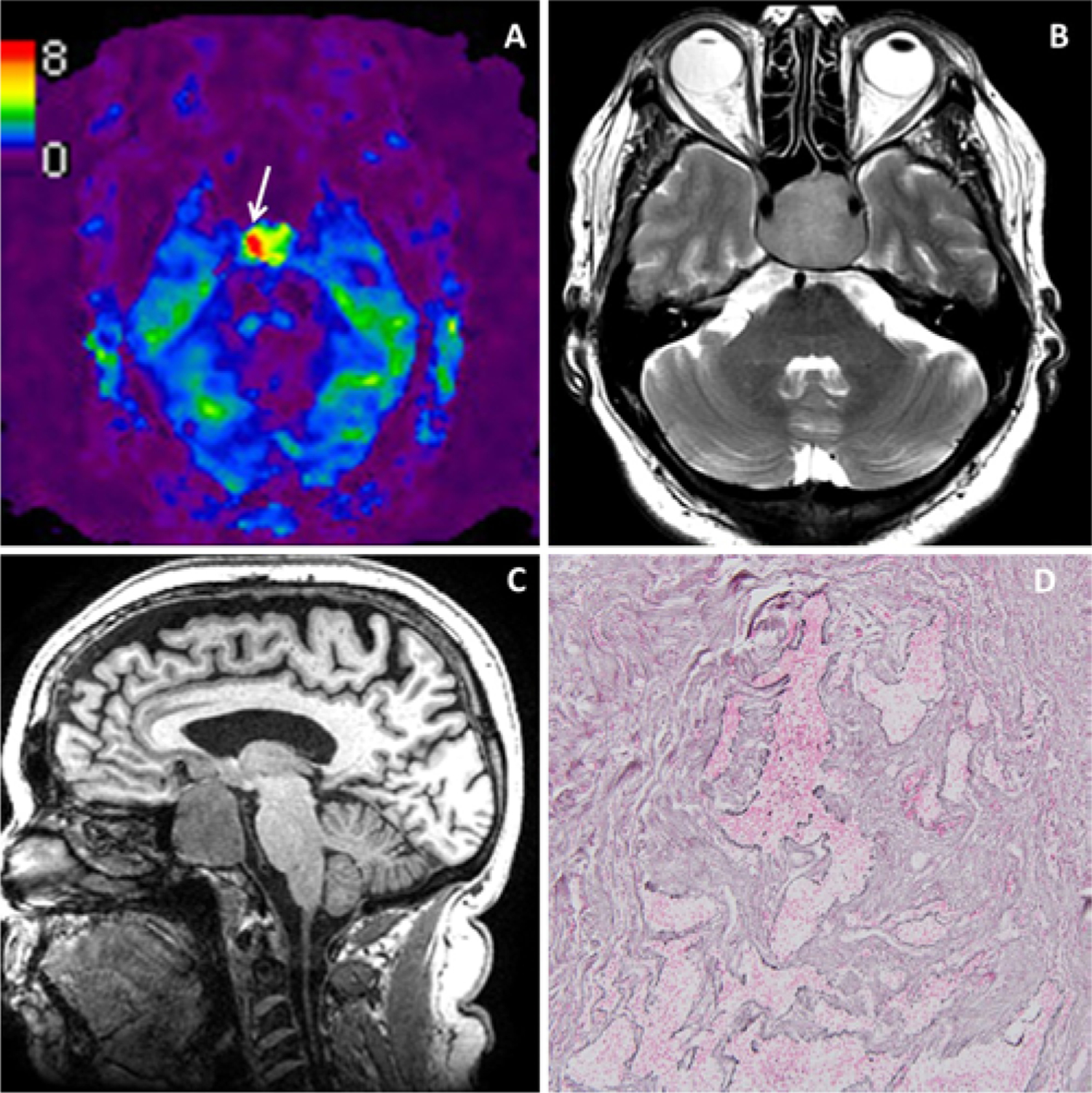
Illustrative case 1. Nonfunctional pituitary adenoma with a firm surgical consistency. MRE (A) with findings of a tumor firmer than the normal brain. The T2-weighted (B) and T1-weighted (C) sequences show a hyperintense and hypointense tumor, respectively. Reticulin staining (D) shows a dense reticulin deposition. Original magnification ×10. Figure is available in color online only.
Case 2
A 48-year-old man presented with long-standing visual field deficit and a nonfunctional pituitary adenoma. Preoperative MRE (Fig. 2A) showed a soft tumor with a kPa of 1.6. In the MRI T2-weighted (Fig. 2B) and T1-weighted (Fig. 2C) sequences the tumor was hyperintense and hypointense, respectively. The patient underwent an EEA, which demonstrated a soft tumor that was easily removed with suction. The reticulin stain (Fig. 2D) demonstrates loss of the normal nested architecture of the anterior pituitary, supporting the diagnosis of a soft pituitary adenoma.
FIG. 2.
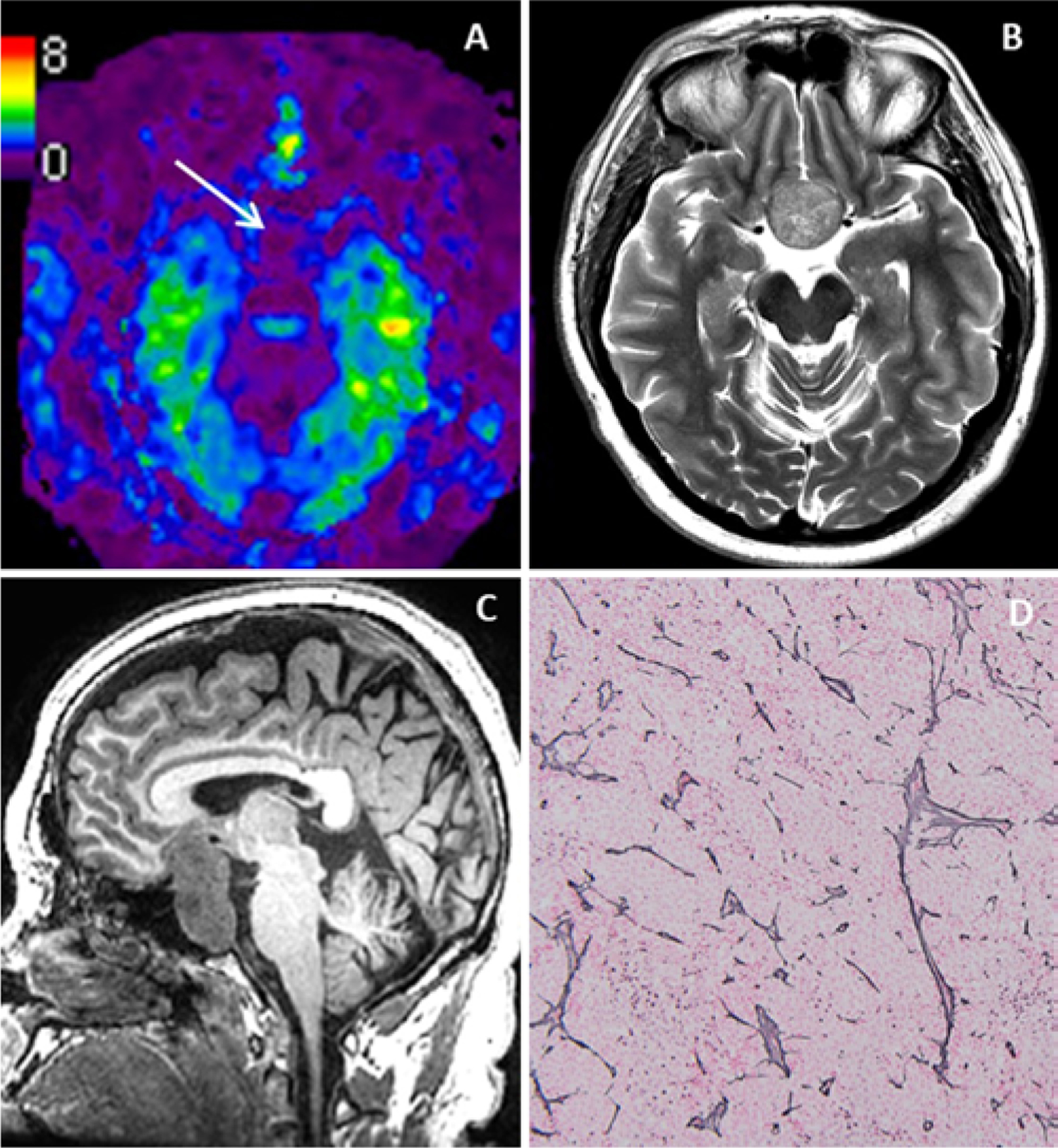
Illustrative case 2. Nonfunctional pituitary adenoma with a soft surgical consistency. MRE (A) with findings of a tumor softer than the normal brain. The T2-weighted (B) and T1-weighted (C) sequences show a hyperintense and hypointense tumor, respectively. Reticulin staining (D) shows a loose reticulin deposition. Original magnification ×10. Figure is available in color online only.
Discussion
This study provides strong evidence toward the predictive value of MRE in predicting pituitary adenoma consistency. Relative tumor stiffness ratios showed a stronger pairwise correlation with intraoperative tumor consistency than did absolute tumor stiffness values. This may reflect superior reliability of standardized ratios where normalizing tumor stiffness to surrounding brain tissue stiffness could reduce any variations not specifically due to tumor consistency. The ratio of tumor stiffness showed a significant difference between soft and firm tumors but not between soft and intermediate or intermediate and firm tumors. This may be partially due to the small sample size and partially due to the qualitative nature of our reference standard.
The majority of adenomas are soft in consistency, and excellent resections can be achieved via a transsphenoidal approach.3 However, up to 17% of adenomas are firm in consistency and are more challenging lesions in which to achieve a GTR.4,5,19 In our series, only 5% of the tumors had a firm consistency. Accordingly, several studies have attempted to use different imaging modalities to preoperatively determine tumor consistency and thus alter the surgical approach and preoperative plans.12,20–23 This study cohort is, to our knowledge, the largest in which the efficacy of MRE in preoperatively determining pituitary adenoma consistency has been evaluated.15,16
MRE is a noninvasive tool that facilitates the prediction of tissue mechanical properties in vivo.6 It has been studied as a tool to assess both diffuse brain pathology, such as dementia and aging, and focal pathology including gliomas and meningiomas.16,24–26 MRE has shown promising results not only in determining tumor consistency, but also in predicting underlying pathology such as IDH mutations in gliomas.17
Previous data have shown an inconsistent value of conventional MR sequences in predicting tumor consistency.5,12, 20, 22, 27–29 In attempts to standardize measurements and increase accuracy, a number of studies have compared tumoral signal intensity to fixed anatomical landmarks such as temporal lobe white matter, cerebellar peduncles, or the pons; however, these results did not show a consistent predictive value for T2-weighted imaging.12,22,30,31
Studies addressing the ability of DWI to determine adenoma consistency have had incongruent findings. Pierallini et al., in a study conducted using 1.5T MRI, were able to correlate soft tumor consistency with lower ADC values.4 On the other hand, Boxerman et al. and Suzuki et al. found that softer tumors correlated with higher ADC values, or in other words, with less diffusion restriction.32,33 These conflicting results may be attributed to differences in the image acquisition protocol or large image distortion in the sella due to its proximity to the paranasal sinuses.23
In order to overcome voxel averaging and a relatively low signal-to-noise ratio in 1.5T and 3T T2-weighted and DWI MRI sequences, a number of different imaging tools have been studied. A study by Yamamoto et al. revealed a significant predictive value of contrast-enhanced 3D FIESTA MRI techniques for defining adenoma consistency.21 Vital anatomical structures in the vicinity of pituitary adenomas are also better visualized using contrast-enhanced 3D FIESTA MRI, increasing the importance of these sequences in preoperative planning.34 With the increasing availability of 7T MRI, a number of authors have proposed an increased predictive power of conventional sequences in which the higher resolution of 7T MRI is used. In a study by Yao et al. that compared the T2-weighted signal of soft and firm pituitary tumors, the results did not reveal a significant difference in average T2-weighted signal intensity. However, when voxelwise analysis—only feasible due to the high in-plane resolution of 7T MRI—was performed, softer tumors had significantly more voxels with higher signal intensity than local gray matter.10 Rutland et al. were able to detect a significant correlation between tumor consistency and ADC maps by using 7T MRI, with softer tumors having a greater ADC value.23
Prediction of adenoma firmness in the perioperative setting can lead to a better understanding of surgical risks. In this study, patients with softer tumors had fewer complications compared to patients with intermediate and firmer tumors. Firmer tumors require more traction and manipulation for resection than do softer tumors, placing the pituitary gland and surrounding neurovascular structures at risk. One patient in our series with a firm adenoma identified correctly with MRE developed a transient secondary CN III palsy. His immediate postoperative hematocrit revealed subarachnoid hemorrhage within the suprasellar and perimesencephalic cisterns, possibly from tumor manipulation.
By predicting a tumor’s firmness perioperatively, the surgeon can decide if a case is more suitable for extended exposure, a different resection strategy (i.e., extracapsular dissection for firmer tumors), or the need for different instrumentation like sharper instruments or ultrasonic aspirators.
Several factors are well recognized to impact the EOR. Cavernous sinus invasion, tumor size, and tumor volume are considered the most significant.35,36 The highest rate of cavernous sinus invasion and, consequently, the lowest EOR is seen in patients with Knosp 3B and 4 tumors.37 Tumors with a maximum diameter in any plane ≥ 4 cm and with a volume of ≥ 10 cm3 are associated with a higher likelihood of STR and increased morbidity.1,38 Tumor consistency is another factor that has been shown to impact the EOR.13,39 In a study recently published by Rutkowski et al.,19 patients with lower consistency grade tumors were significantly more likely to undergo GTR. However, this association was not significant on a multivariate model when controlling for size and invasion. In our study, consistency alone was not a significant factor that predicted GTR, although a trend is present.
Limitations
This study is also faced with several limitations, including the large average size of the adenomas and single-surgeon intraoperative assessment of tumor consistency. Furthermore, our results are currently of limited application due to the specialized hardware required, which is not widely available in clinical practice. However, with increasing evidence and imaging advancement, MRE could be integrated into routine imaging protocols. Another limitation was that even though the post hoc Tukey test for tumor stiffness ratio showed a statistically significant difference between firm and soft tumor consistency, the pairwise comparisons were not significant between other consistency groups. We only included tumors ≥ 2.5 cm because measuring the stiffness within smaller tumors does not provide an accurate stiffness estimate without significantly improved image resolution or further advancements in postprocessing techniques to correct the edge artifacts. Future studies with a higher-resolution, distortion-free technique might resolve this issue.
Conclusions
Our study reveals that MRE is a valuable tool for preoperative prediction of tumor consistency in pituitary macroadenomas. Unfortunately, conventional T1- and T2-weighted signal intensity, even with the high resolution of 3T MRI, could not accurately predict tumor consistency, thus limiting its utility as a surgical planning tool. Nevertheless, knowledge of tumor consistency in the preoperative setting can have vast clinical applications like improving surgical planning, counseling the patient about potential surgical risks, and estimating the length of operation. Further research, including possible correlation of pathological findings with MRE and enhanced technology, will hopefully increase the validity and applicability of MRE in clinical practice.
Acknowledgments
This work was supported by grants from the NIH (R01EB001981, R01 NS113760, and U01EB02445).
Disclosures
Dr. Ehman has direct stock ownership in Resoundant, Inc., and he is a patent holder with and receives royalties from the Mayo Clinic. Dr. Huston and the Mayo Clinic have a financial relationship with Resoundant, Inc.; they have intellectual property rights and a potential financial interest in some of the technologies used in this study.
ABBREVIATIONS
- ADC
apparent diffusion coefficient
- CN
cranial nerve
- DWI
diffusion-weighted imaging
- EEA
endoscopic endonasal approach
- EOR
extent of resection
- GTR
gross-total resection
- MRE
MR elastography
- NTR
near-total resection
- ROI
region of interest
- STR
subtotal resection
Footnotes
Supplemental Information
Previous Presentations
Part of this work was presented at The North American Skull Base Society (NASBS) on Februry 9, 2020, in San Antonio, Texas.
References
- 1.Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B, et al. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J Neurosurg. 2014; 121(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 2.Nishioka H, Inoshita N. New WHO classification of pituitary adenomas (4th edition): assessment of pituitary transcription factors and the prognostic histological factors. Brain Tumor Pathol. 2018; 35(2): 57–61. [DOI] [PubMed] [Google Scholar]
- 3.Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008; 33: 151–199. [DOI] [PubMed] [Google Scholar]
- 4.Pierallini A, Caramia F, Falcone C, Tinelli E, Paonessa A, Ciddio AB, et al. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging—initial experience. Radiology. 2006; 239(1): 223–231. [DOI] [PubMed] [Google Scholar]
- 5.Naganuma H, Satoh E, Nukui H. Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas. Neurol Med Chir (Tokyo). 2002; 42(5): 202–213. [DOI] [PubMed] [Google Scholar]
- 6.Yin Z, Romano AJ, Manduca A, Ehman RL, Huston J III. Stiffness and beyond: what MR elastography can tell us about brain structure and function under physiologic and pathologic conditions. Top Magn Reson Imaging. 2018; 27(5): 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunevicius A, Schregel K, Sinkus R, Golby A, Patz S. RE-VIEW: MR elastography of brain tumors. NeuroImage Clin. 2020; 25: 102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepin KM, McGee KP. Quantifying tumor stiffness with magnetic resonance elastography: the role of mechanical properties for detection, characterization, and treatment stratification in oncology. Top Magn Reson Imaging. 2018; 27(5): 353–362. [DOI] [PubMed] [Google Scholar]
- 9.Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008; 29(6): 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao A, Rutland JW, Verma G, Banihashemi A, Padormo F, Tsankova NM, et al. Pituitary adenoma consistency: direct correlation of ultrahigh field 7T MRI with histopathological analysis. Eur J Radiol. 2020; 126: 108931. [DOI] [PubMed] [Google Scholar]
- 11.Thotakura AK, Patibandla MR, Panigrahi MK, Mahadevan A. Is it really possible to predict the consistency of a pituitary adenoma preoperatively? Neurochirurgie. 2017; 63(6): 453–457. [DOI] [PubMed] [Google Scholar]
- 12.Smith KA, Leever JD, Chamoun RB. Prediction of consistency of pituitary adenomas by magnetic resonance imaging. J Neurol Surg B Skull Base. 2015; 76(5): 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alimohamadi M, Sanjari R, Mortazavi A, Shirani M, Moradi Tabriz H, Hadizadeh Kharazi H, Amirjamshidi A. Predictive value of diffusion-weighted MRI for tumor consistency and resection rate of nonfunctional pituitary macroadenomas. Acta Neurochir (Wien). 2014; 156(12): 2245–2252. [DOI] [PubMed] [Google Scholar]
- 14.Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011; 2(1): 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes JD, Fattahi N, Van Gompel J, Arani A, Ehman R, Huston J III. Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary. 2016; 19(3): 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai N, Takehara Y, Yamashita S, Ohishi N, Kawaji H, Sameshima T, et al. Shear stiffness of 4 common intracranial tumors measured using MR elastography: comparison with intraoperative consistency grading. AJNR Am J Neuroradiol. 2016; 37(10): 1851–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepin KM, McGee KP, Arani A, Lake DS, Glaser KJ, Manduca A, et al. MR elastography analysis of glioma stiffness and IDH1-mutation status. AJNR Am J Neuroradiol. 2018; 39(1): 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano AJ, Bucaro JA, Ehnan RL, Shirron JJ. Evaluation of a material parameter extraction algorithm using MRI-based displacement measurements. IEEE Trans Ultrason Ferroelectr Freq Control. 2000; 47(6): 1575–1581. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski MJ, Chang KE, Cardinal T, Du R, Tafreshi AR, Donoho DA, et al. Development and clinical validation of a grading system for pituitary adenoma consistency. J Neurosurg. 2021; 134(6): 1800–1807. [DOI] [PubMed] [Google Scholar]
- 20.Yiping L, Ji X, Daoying G, Bo Y. Prediction of the consistency of pituitary adenoma: a comparative study on diffusion-weighted imaging and pathological results. J Neuroradiol. 2016; 43(3): 186–194. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto J, Kakeda S, Shimajiri S, Takahashi M, Watanabe K, Kai Y, et al. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. AJNR Am J Neuroradiol. 2014; 35(2): 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei L, Lin SA, Fan K, Xiao D, Hong J, Wang S. Relationship between pituitary adenoma texture and collagen content revealed by comparative study of MRI and pathology analysis. Int J Clin Exp Med. 2015; 8(8): 12898–12905. [PMC free article] [PubMed] [Google Scholar]
- 23.Rutland JW, Loewenstern J, Ranti D, Tsankova NM, Bellaire CP, Bederson JB, et al. Analysis of 7-tesla diffusion-weighted imaging in the prediction of pituitary macroadenoma consistency. J Neurosurg. 2021; 134(3): 771–779. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JD, Fattahi N, Van Gompel J, Arani A, Meyer F, Lanzino G, et al. Higher-resolution magnetic resonance elastography in meningiomas to determine intratumoral consistency. Neurosurgery. 2015; 77(4): 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ElSheikh M, Arani A, Perry A, Boeve BF, Meyer FB, Savica R, et al. MR elastography demonstrates unique regional brain stiffness patterns in dementias. AJR Am J Roentgenol. 2017; 209(2): 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry A, Graffeo CS, Fattahi N, ElSheikh MM, Cray N, Arani A, et al. Clinical correlation of abnormal findings on magnetic resonance elastography in idiopathic normal pressure hydrocephalus. World Neurosurg. 2017; 99: 695–700.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabortty S, Oi S, Yamaguchi M, Tamaki N, Matsumoto S. Growth hormone-producing pituitary adenomas: MR characteristics and pre- and postoperative evaluation. Neurol Med Chir (Tokyo). 1993; 33(2): 81–85. [DOI] [PubMed] [Google Scholar]
- 28.Snow RB, Johnson CE, Morgello S, Lavyne MH, Patterson RH Jr. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990; 26(5): 801–803. [DOI] [PubMed] [Google Scholar]
- 29.Romano A, Coppola V, Lombardi M, Lavorato L, Di Stefano D, Caroli E, et al. Predictive role of dynamic contrast enhanced T1-weighted MR sequences in pre-surgical evaluation of macroadenomas consistency. Pituitary. 2017; 20(2): 201–209. [DOI] [PubMed] [Google Scholar]
- 30.Mastorakos P, Mehta GU, Chatrath A, Moosa S, Lopes MB, Payne SC, Jane JA Jr. Tumor to cerebellar peduncle T2-weighted imaging intensity ratio fails to predict pituitary adenoma consistency. J Neurol Surg B Skull Base. 2019; 80(3): 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen XY, Ding CY, You HH, et al. Relationship between pituitary adenoma consistency and extent of resection based on tumor/cerebellar peduncle T2-weighted imaging intensity (TCTI) ratio of the point on preoperative magnetic resonance imaging (MRI) corresponding to the residual point on point on postoperative MRI. Med Sci Monit. 2020; 26: e919565–1–e919565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR Am J Roentgenol. 2010; 195(3): 720–728. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki C, Maeda M, Hori K, Kozuka Y, Sakuma H, Taki W, Takeda K. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J Neuroradiol. 2007; 34(4): 228–235. [DOI] [PubMed] [Google Scholar]
- 34.Xie T, Zhang XB, Yun H, Hu F, Yu Y, Gu Y. 3D-FIESTA MR images are useful in the evaluation of the endoscopic expanded endonasal approach for midline skull-base lesions. Acta Neurochir (Wien). 2011; 153(1): 12–18. [DOI] [PubMed] [Google Scholar]
- 35.Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH. Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 2016; 96: 36–46. [DOI] [PubMed] [Google Scholar]
- 36.Cusimano MD, Kan P, Nassiri F, Anderson J, Goguen J, Vanek I, et al. Outcomes of surgically treated giant pituitary tumours. Can J Neurol Sci. 2012; 39(4): 446–457. [DOI] [PubMed] [Google Scholar]
- 37.Micko ASG, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015; 122(4): 803–811. [DOI] [PubMed] [Google Scholar]
- 38.Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012; 15(3): 450–463. [DOI] [PubMed] [Google Scholar]
- 39.Yun JJ, Johans SJ, Shepherd DJ, Martin B, Joyce C, Borys E, et al. The utility of using preoperative MRI as a predictor for intraoperative pituitary adenoma consistency and surgical resection technique. J Neurol Surg B Skull Base. 2020; 81(6): 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]


