Abstract
Purpose
The study aims to summarize current knowledge on the use of oil in embryo culture systems, with a focus on proper management of different types of oil and possible impact on culture systems.
Methods
PubMed was used to search the MEDLINE database for peer-reviewed English-language original articles and reviews concerning the use of oil in embryo culture systems. Searches were performed by adopting “embryo,” “culture media,” “oil,” and “contaminants” as main terms. The most relevant publications were assessed and discussed critically.
Results
Oils used in IVF are complex mixtures of straight-chain hydrocarbons, cyclic and aromatic hydrocarbons, and unsaturated hydrocarbons, whose precise composition influences their chemical and physical properties. Possible presence of contaminants suggests their storage at 4 °C in the dark to prevent peroxidation. Washing, generally performed by manufacturers prior to commercialization, may remove trace chemical contaminants. Oils reduce evaporation from culture media at rates depending on their chemical physical properties, culture system parameters, and incubator atmosphere. Contaminants — mainly metal ion and plastic components derived from refinement processes and storage — can pass to the aqueous phase of culture systems and affect embryo development.
Conclusions
Oils are essential components of culture systems. Their original quality and composition, storage, handling, and use can affect embryo development with significant efficiency and safety implications.
Keywords: Oil, Embryo, IVF, Culture media, Peroxidation
Introduction
The culture system is crucial in the setup of a clinical embryology laboratory, as it can influence embryonic development and the outcome of in vitro fertilization (IVF) treatments [1]. During the past decades, identification and increased control of several factors affecting embryo development and technological advancements have led to improved embryo culture conditions and higher clinical success rates. Although culture media are considered key components of culture systems, many other factors crucially contribute to the stability of the culture microenvironment. Factors that can influence embryo development and viability, and consequently ART success, include protein supplements, incubator atmosphere and humidity, plastic disposables, embryo culture density, temperature, pH, and osmolality [2]. With reference to osmolality and pH stability, the oil overlay represents a fundamental component of culture systems. As recently reported, the volume of mineral oil and humidity levels in the incubators play an important role in osmolality stabilization. The oil overlay has been used in embryo culture since the early sixties [3] to prevent culture medium evaporation and preserve proper pH and osmotic pressure. It also acts as a physical barrier protecting culture media from toxic compounds and microorganism contamination [4]. Studies carried out in recent years have demonstrated how oil is crucial for embryo culture, suggesting special consideration to the choice and management of the oil overlay in IVF. In fact, diverse compositions of commercially available mineral oils may have a different influence on oil chemical stability and ultimately the overall quality of the culture microenvironment. Many contaminants having a negative impact on embryo development may be detected in commercially available embryo culture oils [5]. Peroxidation represents one of the major sources of oil toxicity. It may be caused by suboptimal or inappropriate oil storage and management and UV light exposure and overheating [6, 7]. Although paraffin or mineral oil are extensively used in IVF, their interaction with other embryo culture media components and possible impact on safety and efficiency have long been neglected. The purpose of this review is to summarize recent knowledge on origins and use of oil in culture systems, proper management of different types of oil, and possible impact on embryo culture.
Methods
PubMed was used to search the MEDLINE database for peer-reviewed English-language original articles and reviews concerning the use of oil in embryo culture systems. Searches were performed by adopting “embryo,” “culture media,” “oil,” and “contaminants” as main terms. The most relevant publications were assessed and discussed critically.
Types of oil and chemical-physical properties
The structural diversity of hydrocarbons in mineral oil is vast [8], determining a huge complexity of mineral oil composition. This results from the process of mineral oil refinement, which is also complex as part of crude oil distillation. The product, also called mineral petrolatum, paraffin oil, and light or white mineral oil, contains complex mixtures of straight-chain hydrocarbons, cyclic and aromatic hydrocarbons, and unsaturated hydrocarbons. The different chain lengths of the hydrocarbons determine mineral oil viscosity [9]. A more rigorous definition mineral oil is based on the length of composing hydrocarbons, whose carbon numbers range from C15 to C50 (IARC 1984). Specific analytical techniques distinguish two fractions: mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH). MOSH are composed of straight and branched open-chain alkanes (paraffins) and largely alkylated cycloalkanes (naphthenes) [8, 10]. The proportion of straight-chain alkanes (n-alkanes, also called paraffin waxes) among MOSH is low because a dewaxing step is included in the manufacturing process. MOAH include also highly alkylated and often partially hydrogenated monoaromatic and/or polyaromatic hydrocarbons [8, 11]. Mineral oils approved for clinical purpose must be United States Pharmacopeia (USP) grade. USP-grade oil quality includes several requirements relevant to viscosity, sulfur content, and the amount of unsaturated, polycyclic (aromatic) hydrocarbons [9]. Refinement is not very efficient and oil nomenclature is not always resolutive in discriminating paraffin from mineral oils. Oil nomenclature is unclear in defining paraffin and mineral oils. Regardless, companies producing mineral oils for embryo culture often claim that paraffin oils are further refined and contain more saturated hydrocarbons than mineral oils, making them less reactive to oxygen or other chemicals. Mineral oil classification is therefore difficult because of the complexity of purification processes. High contents of saturated hydrocarbons make mineral oil less sensitive to reactive oxygen species and, consequently, more suitable for contact with gametes and embryos in vitro, in particular for prolonged embryo culture. A clear nomenclature recognizing paraffin and mineral oil as distinct chemical categories is still missing. Oil for use in culture systems should be therefore chosen based on purity, viscosity, density, and absolute water content.
Oil handling, storage, and drawbacks
Due to its sensitive nature, oil should be handled and stored appropriately to avoid alterations and contaminations. Many producers recommend storage at 4 °C protected from direct light to limit over-heating and photo-oxidation. Based on oil composition, if only completely saturated hydrocarbons are present, such measures may be unnecessary. However, these precautions may be appropriate in case of undetected low peroxidation. In fact, even if present in traces, contaminants present in crude oil — such as unsaturated/aromatic hydrocarbons, short volatile carbon chains, peroxides and zinc, and other undefined compounds — could affect the quality of oil used in IVF laboratories.
To avoid the risk of contamination, some procedures have been introduced to improve mineral oil handling. The practice of washing mineral oil, for instance, was described as a detoxifying method. For the first time, Fleming and colleagues demonstrated that cytotoxic mineral oil could be detoxified through washing [12]. Likewise, in a mouse model, Lee et al. [13] reported improved embryo development using washed oil. Morbeck et al. [5] focused on TX-100-contaminated oils, demonstrating that toxin contamination could be reduced after washing. Moreover, they also reported that peroxided oils could be detoxified through the same washing practice. The authors thoroughly investigated the specific capacity of the protein supplement used in the washing procedure to remove toxins. They compared water, Human Tubal Fluid (HTF) culture medium, and HTF containing human serum albumin (HSA) as washing agents at both room temperature and 37 °C for 24 h. They reported no differences between the different protocols, even after testing at different temperatures [5]. Although washing remains not universally accepted, many manufacturers wash their oils with water before commercialization. Depending on the type of oil, manufacturers also provide essential information about storage, quality control (QC) testing and washing practice, and more importantly, correct management of oil during culture dish preparation.
Oil storage and handling are critical to achieve maximum performance of culture systems. It is fundamental to follow the manufacturers’ instructions and adopt operational protocols to minimize the risk of evaporation and the formation of endogenous contaminants in embryo culture (Table 1).
Table 1.
Checklist of oil management to minimize the risk of evaporation and formation of endogenous contaminants in the embryo culture environment
| Oil type and use | Question | Consideration |
|---|---|---|
| Storage | Is oil correctly stored, as suggested by manufacturer? |
If oil is contained in transparent bottles, store it in the dark Reason: in case of undetected low peroxidation this precaution reduces further peroxidation during storage |
| Container size | Is oil contained in large- or small-volume bottles? |
Consider aliquoting into smaller volumes if the oil is provided into large-volume bottles (> 200 mL); for instance, oil may be aliquoted in 50-mL flasks or similar This precaution aims at reducing the risk that oil-atmosphere ratio in large bottles increases in favor of air during the usage period, with consequent increase in peroxidation risk |
| Viscosity | Is it high or low? |
If viscosity and density are high, consider to a) contacting the manufacturer to request details of pre-equilibration times (T 37 °C, CO2 5–6%, O2 5%) before dish preparation b) pre-equilibration in humidified incubator before culture dish preparation Reason: oil is not completely impermeable to water, but some molecules can be absorbed by hydrocarbons in a proportion depending on its composition. Oil water content is increased by pre-incubation; it facilitates permeability to CO2 and O2, and more importantly, prevents culture media evaporation |
| Culture dish and media volume | What volume of oil should be overlaid? |
Adjust the oil overlay volume to optimize the oil interface thickness between medium and atmosphere Reason: higher amounts of oil and consequent increase in the thickness of the overlay separating the medium from the surrounding atmosphere can help to reduce evaporation. The volume of culture medium drops is crucial to determine the appropriate amount of oil overlay. The thickness between medium drops and atmosphere should be at least 2 mm |
| Equilibration time | How long should the dishes equilibrated for? |
If higher volumes of oil overlay are used, equilibration times should be adequately prolonged If culture dishes are prepared the day before use, refrigerated or room temperature oil may be used. If cultured dishes are prepared on the same day of use, pre-incubated oil (at least the day before) should be used, bearing in mind that the amount and viscosity of oil overlay impacts on time of media equilibration We suggest to validate the right equilibration period in each own culture system, measuring pH and T° of culture medium in the prepared dish |
| Embryo culture incubation time | How many days/hours are the dishes used for incubation including the equilibration time? |
Based on laboratory protocols of culture dish preparation and type and volume of oil, assessment and validation of incubation times needed to achieve appropriate pH and temperature should be performed Due to possible risk of oil peroxidation during prolonged embryo culture, consider a change-over step to refresh the oil overlay Also in case of use of a single-step culture medium for prolonged embryo culture, a change-over step should be implemented to avoid the risk of oil peroxidation |
Oil overlay as stabilizer of culture conditions
One of the main purposes for using the oil layer is to prevent evaporation from culture media. With the development of modern benchtop incubators, small chambers were introduced to better control chemical-physical parameters, such as temperature and gas exchanges. However, many of these modern incubators lack humidity. Early studies comparing embryo development in humidified or dry culture reported contrasting results. Evaporation from culture media can still occur under mineral oil overlay [14, 15] as that the use of 3 ml of oil results in higher evaporation compared with 5 or 7 ml in the same sized dish [16]. In a report presented at the ASRM 2018 Congress, the authors tested an incubator that can be operated in dry or humidified conditions. They evaluated the undisturbed culture of embryos from 83 patients in dry chambers reporting lower blastocyst development and slower development to the five-cell stage compared with embryos of 93 patients grown under the same conditions, but (same medium, dish, oil) in humidified chambers [17]. These findings confirm that evaporation still occurs even in the presence of oil overlay. For this reason, the quantity of oil used to prepare the culture dishes deserves special attention [15, 18–21]. Yumoto and colleagues investigated the stability of osmolality in microdrops under different conditions, evaluating the putative effect of four different oils and 3 different culture media, using increasing volumes of culture media (50 μL or 200 μL) in humidified or non-humidified incubators [21]. Authors found a significant increase in the osmolality of 50-μL and 200-μL microdrops covered with mineral oil during 5-day incubation in non-humidified benchtop incubators. The range of the increase was affected by microdrop volume and type of mineral oil used to cover the drops. In contrast, microdrop osmolality did not change during 5-day incubation in a humidified benchtop incubator. Interestingly, they also analyzed the absolute water content and water activity, dynamic viscosity, and density for each of the four oils tested. They demonstrated that the oil that showed the highest increase in osmolality during the 5 days of culture was characterized by the lowest water content and water activity compared to the other oils. Rather counterintuitively, oil is not completely impermeable to water, but some molecules can be absorbed by hydrocarbons in a proportion depending on its composition. In fact, the authors showed that the absolute water content of that oil with low water content increased significantly after overnight pre-equilibration in a humidified incubator from 24.8 ± 0.5 to 60.6 ± 0.5 ppm (P < 0.01); water activity value also increased significantly from 0.37 ± 0.01 to 0.80 ± 0.01 (P < 0.01) [21]. Pre-equilibration (humidification) of oil resulted in a small but significant reduction in the osmolality increase on day 5 of incubation (from 270 to 298.0 ± 1.4 mOsm/kg with non-humidified oil versus 292.3 ± 1.0 mOsm/kg with humidified oil).
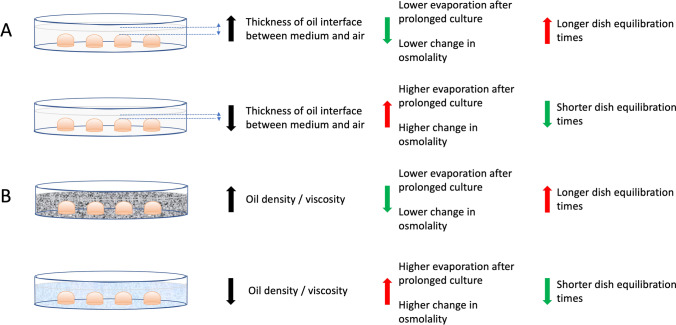
To minimize changes in osmolality, Mestres and colleagues demonstrated that using higher amounts of oil to increase the thickness of the overlay separating the medium from the surrounding atmosphere can help to reduce evaporation; the same effect can be obtained using higher volumes of medium [18]. Additionally, denser and heavier oils mitigate evaporation more effectively than lighter oils (Fig. 1), while high-viscosity oils are easier to manipulate and less likely to cause spillage or dish-lid sealing [15, 21]. Swain reported that a difference in density of 0.04 g/mL can significantly impact on evaporation rate [15]. This notion becomes particularly important when considering a prolonged and uninterrupted culture system without refreshments of culture dishes, in which the choice of a heavier and denser oil may reduce evaporation. However, heavier and denser oil may need pre-incubation in atmosphere and humidity conditions used for culture before dish preparation. Relevant manufacturers’ recommendations are essential.
Fig. 1.
impact of mineral oil quantity and quality on the stability of microdrops of medium used for embryo culture. A Higher amounts of oil for culture dish preparation increase the thickness of oil overlay between medium and surrounding atmosphere reducing evaporation from medium. B More dense and viscous oil is also more effective in reducing evaporation from medium. Both conditions can help to stabilize medium osmolality preventing an increase due to evaporation, especially during prolonged culture
Recently in an experimental setting, Mullen analyzed the impact of nine different commercially available oils on osmolality increase in prolonged culture. Interestingly, he demonstrated that surface area-to-volume, low density, and low thickness of oil overlay directly affect the rate of osmolality increase. Moreover, a mathematical model of these three variables was developed predicting the rate of change in osmolality. This model has been proposed as a tool for embryologists to evaluate their culture systems and predict putative effects on osmolality [22]. Swain also demonstrated that the use of lids to cover culture dishes or washing of oil in dry incubators does not affect media evaporation rate [14]. Possible evaporation from media also raises the concern that oil itself could absorb water from culture media. However, media evaporation and the resulting osmolality increase can be avoided adding more oil overlay. Importantly, the time required for dish equilibration rises significantly in case of using more oil overlay. We suggest validating in each setting the proper time needed to reach the right pH and temperature when preparing culture dishes according to the volume and density of oil used. Together, these observations demonstrate that oil does not accumulate water from culture media, while passage through evaporation actually occurs [16].
Very recently, Mestres and colleagues described the differences between several oil brands, reporting their viscosity and density, proneness to peroxidation, capacity to minimize fluctuations of the culture conditions (temperature, pH, osmolality), and potential toxicity [23]. Authors analyzed 13 commercially available oils, finding differences in pH stability: following an exposure of 30 min to ambient conditions outside the incubator, this parameter did not increase in only two oils. Interestingly, higher viscosity was correlated with lower pH rise, while a non-linear correlation was observed between oil viscosity and capacity to mitigate pH fluctuations. Conversely, oil density did not seem to be directly related to the stabilization of pH. In the same study, the authors also evaluated possible differences in terms of thermal and osmolality stability. To assess the response to thermal fluctuations, pre-warmed dishes were placed on a heating stage. The authors found an initial 5-min acute temperature loss, in which cooling rate was high and comparable between all groups. On the other hand, osmolality and evaporation rates were different. One type of mineral oil, not specifically produced for IVF and with the highest viscosity among the tested samples, showed higher evaporation rate and osmolality rise, and was considered as an outlier in the further analysis. A non-linear correlation was established between viscosity and final osmolality in the culture medium for each type of oil. Authors also performed a Mouse embryo assay (MEA) test; remarkably, the oil with the highest evaporation rate did not pass the MEA test and was associated with the highest degeneration rate [23].
The use of higher volumes of oil should be encouraged in all the phases of IVF, since evaporation can also occur in the presence of an oil overlay at a rate depending on the thickness of the oil interface between medium and air. The choice of a heavier and denser oil or a light oil depends on the assessment of the culture system in its entirety, taking into account information on culture media osmolality reported by the manufacturer and duration of embryo culture (Table 2).
Table 2.
Schematic representation of studies investigating the impact of different types and modalities of use of mineral oils on embryo culture conditions and laboratory or clinical outcomes
| Oil overlay and osmolality (evaporation prevention) | |||
|---|---|---|---|
| Methods | Results | Materials | |
| Olds et al. (2015) |
Real-time pH data was evaluated following scheduled benchtop incubator openings in a microdrop culture system using a real-time pH monitoring device: the SAFE (Sterile Automated Fluoroscopic Evaluation) 35 µL or 50 µL of oil were used to cover 100 µL of culture media in SAFE Sens sensor cups and pH continuously evaluated in a benchtop incubator |
pH of culture media under 35 µL of oil overlay resulted higher compared to the control after repeated incubator opening. More stable pH was ensured by covering the media with 50 µL of oil | Continuous pH monitoring of SAFE Sens sensor cups |
| Swain et al. (2018) | 25-μL drops of media covered with 3.5 mL of 1 of 4 types of mineral oil to compare differences in osmolality after prolonged culture | Different mineral oils used for microdrop oil overlay result in different rates of media evaporation and resulting osmolality increase. Density of mineral oil seems to be an important factor to consider in terms of media evaporation | 35-mm culture dish |
| Swain et al. (2016) | Measure of osmolality in microdrops (25 µL) of culture medium, in dishes prepared daily for 7 days and cultured in humidified and non-humidified incubators | Uninterrupted culture for up to 7 days in a non-humidified incubator resulted in an increase in media osmolality over time, while osmolality of microdrops in a humidified incubator remained unchanged | 35-mm culture dish |
| Del Gallego et al. (2018) | Embryos cultured in time-lapse incubator in humidified and dry conditions (83 and 93 patients, respectively) | Significantly higher blastocyst rate in embryos cultured in humidified conditions (74.5% vs. 69.2%). No statistical differences in terms of pregnancy and miscarriage rates | Time-lapse multi-well dish |
| Yumoto et al. (2018) | 18 culture dishes containing six 50-µL microdrops of single-step medium. Dishes were divided into three groups: (A) light oil (10.8 mPa/s at 37 °C), (B) heavy oil (36.5 mPa/s at 37 °C), and (C) washed oil (10.4 mPa/s at 37 °C), and placed in a non-humidified benchtop incubator for 6 days | Results showed that the osmotic pressure of microdrops covered by light oil significantly increased on day 3 onward compared to that of microdrops covered by heavy oil | Dish size not reported |
| Yumoto et al. (2019) | Three single-step culture media were incubated for 5 or 6 days covered with four different mineral oils in non-humidified or humidified incubators to investigate the stability of osmolality in microdrops under different conditions. The absolute water content and water activity, dynamic viscosity, and density for each oil were also analyzed | Osmolality significantly increased in non-humidified benchtop incubators. The range of the increase was affected by microdrop volume and by the type of mineral oil used to cover the drops. In contrast, microdrop osmolality did not change during 5-day incubation in a humidified benchtop incubator. The oil that showed the highest osmolality increase was analyzed and reported the lowest water content and water activity | 35-mm plastic culture dishes |
| Mestres et al. (2021) | Several variables compared to assess the factors can affect evaporation and osmolality including media composition and supplementation, volume of mineral oil, incubator humidification, and the type of dish and incubator used |
Evaporation and consequently osmolality increased according to humidity levels inside the incubators, the volume of mineral oil, and the type of culture media Volume of oil is crucial to stabilize osmolality in dry incubators |
Six experiments were conducted in which different dishes and time-lapse dishes were compared with different volume of oil and culture conditions |
| Mullen (2021) | Analysis of 9 different commercially available oils and their effect on osmolality rise, toward the evaluation of surface area-to-volume, oil density and viscosity, oil overlay thickness, upon 7 days of culture in non-humidified benchtop incubator | The surface area-to-volume, low density, and low thickness of oil overlay directly affect the rate of osmolality increase. Moreover, a mathematical model of these three variables was developed predicting the rate of change of osmolality |
Three different dishes were tested under different culture conditions: 35-mm dishes 96-well dishes 4-well dishes |
| Mestres et al. (2021) | Comparison of 13 different commercially available oils, detailing their viscosity and density, proneness to peroxidation, capacity to minimize fluctuations of the culture conditions (temperature, pH, osmolality), and potential toxicity | pH fluctuations are better mitigated by high-viscosity oils and only two of them analyzed in the study are able to minimize pH arise after 30 min outside the incubator. Osmolality and evaporation rate vary in low viscous oils and one of them analyzed showed huge arise of osmolality after 7 days of embryo culture, demonstrated as embryo-toxic after MEA test was performed | Different oils were analyzed and tested |
Impact of oil overlay in different types of cell cultures
Human-assisted reproduction techniques are based on culture of gametes and early embryos up to the blastocyst stage. However, other culture system options are currently under development, such as in vitro maturation (IVM) or ovarian tissue culture. In fact, the process of animal embryo in vitro production (IVP) routinely involves IVM for embryo production. Proper culture conditions are necessary to increase the overall efficiency of IVP technology, whose efficiency also varies depending on the species of interest. The mineral oil overlay has been indicated as a component with significant impact on IVM systems for embryo IVP. The use of mineral oil overlay has been associated with delayed nuclear maturation and reduced oocyte developmental capacity in porcine IVP [24], as well as with deferred meiosis progression in mouse oocytes [25]. Such adverse effects may be caused by passage from the maturation medium and accumulation into the oil phase of steroid hormones and meiosis-activating sterols [24]. To investigate how the oil overlay can affect IVM rate in a porcine model, Martinez and colleague studied progesterone levels and timings of oocyte maturation in the presence of oil overlay or under high-humidity conditions. Although progesterone was progressively depleted from the medium with oil overlay, no delay in nuclear maturation was reported. They concluded that, despite its marked impact on progesterone concentration in the culture medium, mineral oil did not affect oocyte maturation kinetics or oocyte developmental competence [26].
The proper use of oil is of paramount importance in animal IVP industry because it can affect the efficiency of the culture system. Optimization and validation of its usage is therefore crucial.
Oil as a source of embryonic toxicants
Although evidence indicate that modality of usage and type of oil used for embryo culture systems can influence embryo in vitro development, only in recent years scientists have begun to investigate the presence of possible contaminants in products released in the marketplace. Identification of substances present in mineral oil is crucial to prevent exposure of embryo to harmful contaminants. As sometimes reported by manufacturer QC testing, zinc, Triton X-100, and peroxides [5, 7, 27, 28] may be found in oil used for embryo culture. Triton X-100, a detergent used in industrial manufacturing and research to permeabilize membranes or solubilize proteins, was found at different concentrations during manufacturing. Despite Triton X-100 is extremely toxic to mouse embryo, Morbeck and colleagues detected this agent in a batch of oil that passed the MEA, suggesting a bottle-to-bottle variation. More importantly, they found that the source of contamination was in the bottling and plastic used for storage [9]. In an early study, zinc was identified as a possible toxic contaminant of silicon oil in mouse embryo cultures [29]. Authors found that a batch of silicon oil performed differently using two types of culture media. When using CZB medium, mouse embryos developed to blastocysts with higher rates compared with KSOM medium, due to higher rates of 2-cell arrest in the latter group. Their results showed that the higher concentrations of ethylenediaminetetraacetic acid (EDTA) and bovine serum albumin BSA in CZB medium protected against the toxic component present in the oil and identified the contaminant as zinc ions. The EDTA chelating effect also was demonstrated to protect also against other impurities of the oil overlay [29].
Peroxidation, which produces reactive oxygen species, is the major source of oil toxicity. It involves oxidation of double bonds of oil components. Among early studies reporting oil as a source of contaminants, those of Otsuki and colleagues demonstrated that oil degradation through peroxidation may affect embryo development [7, 27]. They observed that opening of a bottle of oil can result in increased peroxidation after several weeks of storage. Further studies demonstrated that peroxidation can affect fertilization, cleavage, and blastocyst formation in rodents [5, 9]. Peroxidation can occur in every moment of the shelf life of mineral oil. For this reason, the original quality of a MEA-tested oil can change over time. Progressive deterioration can increase peroxidation levels, depending on temperature and storage time, UV exposure, and other conditions. Martinez and colleagues produced evidence that oxidating agents can pass from oil to culture media. They reported severely reduced maturation, fertilization, cleavage, and blastocyst rate following the use of a batch of mineral oil suspected to be peroxided. The authors also observed that the oxidation status of culture media exposed to a batch of peroxided oil was higher than that of fresh control media incubated without oil overlay or with unaltered mineral oil [28]. In another study, the same authors focused on the comparison between a standard unaltered mineral oil used for porcine IVP and light paraffin oil claimed to be purer and saturated in its components. They reported that the use of mineral oil was associated with reduced cleavage and blastocyst rates. However, no differences in the oxidation state were observed between oils and culture media. This suggests that toxicity of unaltered oil can also reside in other contaminants. Indeed, the authors demonstrated that differences between type of oils in both the composition of volatile organic compounds (VOC) and the transfer rate of some of these VOCs (straight-chain alkanes and pentanal and 1,3-diethyl benzene) to the culture medium could affect embryo development [30]. As highlighted by Swain in a recent review, in particular with extended uninterrupted culture of embryos, proper precautions should be taken to avoid high VOC levels in the incubator atmosphere and prevent their accumulation into the oil overlay of culture dishes [16]. Commercial oils are tested for MEA; however, low levels of peroxidation can remain undetected. Moreover, oil quality can vary if handling and storage are not properly performed. To minimize storage and environmental effects on oil degradation, a more effective QC testing should be implemented, to solely allow commercialization of batches that are truly peroxide-free. Recently, some authors focused on this question, proposing an improvement of MEA test to increase its sensitivity to detect effects caused by peroxidation. They found that medium supplementation with HSA + alpha/beta-globulins or with HSA alone increased the test capacity to detect embryo toxicity compared with the use of bovine serum albumin (BSA). Moreover, the sensitivity of the MEA was greatly reduced when embryos were cultured in groups. Based on such findings, the authors proposed a new optimized MEA protocol to specifically detect peroxides in mineral oil samples prior to their release into the marketplace for human IVF use [31].
The original oil quality, also expressed as VOC levels, peroxidation, and ionic composition, may differ between oils [30] and can be affected by storage and handling. Therefore, reducing the possible detrimental impact of the oil overlay on embryo culture is crucial, especially in the case of prolonged uninterrupted culture. The oil overlay reduces evaporation from culture media, improving culture system performance. However, it also involves a toxicity risk. Several handling measures have been introduced to minimize this risk, while improved QC tests have been devised.
Conclusions
Oils used in IVF are USP-grade mixtures of straight-chain hydrocarbons, cyclic and aromatic hydrocarbons, and unsaturated hydrocarbons. Different chain lengths of hydrocarbons determine viscosity and other properties relevant to embryo culture. For example, oils including more saturated hydrocarbons are less exposed to reactive oxygen species and are therefore safer especially for prolonged culture. Oil overlay reduces, but not eliminates, evaporation. Changes in culture media osmolality caused by evaporation depend on several factors — including microdrop size and incubator atmosphere (humidified or dry) — and intrinsic oil characteristics, such as water content, viscosity, and density. As a general rule, denser and heavier oils reduce drop evaporation more effectively. Such factors can be controlled; for example, oil water content can be increased by overnight equilibration in a humidified atmosphere, while a thicker oil overlay minimizes evaporation. Computational approaches may also be adopted to predict and minimize osmolality changes. Chemical contamination causing peroxidation may derive from refinement processes, production for IVF. In this respect, detergents released by plastics of storage bottles can be a source of contamination. Storage is another delicate passage of oil use. Peroxidation levels may increase as a consequence of inappropriate storage time, temperature, and light exposure. Such contaminants can impact on embryo development with significant implication for the safety and efficiency of culture systems. This requires stringent and improved QC testing in the production pipeline, rigorous handling during storage, and rational use considering all culture system components.
Funding
This work was funded by the authors’ institutions.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catello Scarica, Email: lello.scarica@gmail.com.
Antonio Monaco, Email: antoniomonaco10@hotmail.com.
Andrea Borini, Email: borini@nove.baby.
Elena Pontemezzo, Email: epontemezzo@newfertilitygroup.com.
Valentina Bonanni, Email: valentinabonanni.85.vb@gmail.com.
Lucia De Santis, Email: desantis.lucia@hsr.it.
Carlotta Zacà, Email: carlotta.zaca@9puntobaby.it.
Giovanni Coticchio, Email: giovanni.coticcho@nove.baby.
References
- 1.Gardner DK, Weissman A, Howles CM, Shoham Z (Eds). Textbook of Assisted Reproductive Techniques: Volume 2: Clinical Perspectives (5th ed.). CRC Press; 2018. 10.1201/9781351228244
- 2.Gardner DK, Kelley RL. Impact of the IVF laboratory environment on human preimplantation embryo phenotype. J Dev Origins Health Dis. 2017;8(4):418–435. doi: 10.1017/S2040174417000368. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL. A Method for in vitro cultivation of mouse ova from two-cell to blastocyst. Exp Cell Res. 1963;32(1):205–208. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainsworth AJ, Fredrickson JR, Morbeck DE. Improved detection of mineral oil toxicity using an extended mouse embryo assay. J Assist Reprod Genet. 2017;34(3):391–397. doi: 10.1007/s10815-016-0856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morbeck DE, Khan Z, Barnidge DR, Walker DL. Washing mineral oil reduces contaminants and embryotoxicity. Fertil Steril. 2010;94(7):2747–2752. doi: 10.1016/j.fertnstert.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Provo MB, Herr C. Washed paraffin oil becomes toxic to mouse embryos upon exposure to sunlight. Theriogenology. 1998;49(1):214. doi: 10.1016/S0093-691X(98)90567-2. [DOI] [Google Scholar]
- 7.Otsuki J, Nagai Y, Chiba K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil Steril. 2007;88(3):741–743. doi: 10.1016/j.fertnstert.2006.11.144. [DOI] [PubMed] [Google Scholar]
- 8.EFSA Panel on contaminants in the food chian. Scientific Opinion on Mineral Oil Hydrocarbons in Food. Efsa J. 2012;10.
- 9.Morbeck DE, Leonard PH. Culture systems: mineral oil overlay. Methods Mol Biol. 2012;912:325–31. 10.1007/978-1-61779-971-6_18 [DOI] [PubMed]
- 10.Biedermann M, Fiselier K, Grob K. Aromatic hydrocarbons of mineral oil origin in foods: method for determining the total concentration and first results. J Agric Food Chem. 2009;57(19):8711–21. 10.1021/jf901375e [DOI] [PubMed]
- 11.Pirow R, Blume A, Hellwig N, Herzler M, Huhse B, Hutzler C, Pfaff K, Thierse HJ, Tralau T, Vieth B, Luch A. Mineral oil in food, cosmetic products, and in products regulated by other legislations. Crit Rev Toxicol. 2019;49(9):742–89. 10.1080/10408444.2019.1694862 [DOI] [PubMed]
- 12.Fleming TP, Pratt HP, Braude PR. The use of mouse preimplantation embryos for quality control of culture reagents in human in vitro fertilization programs: a cautionary note. Fertil Steril. 1987;47(5):858–60. 10.1016/s0015-0282(16)59179-1 [DOI] [PubMed]
- 13.Lee S, Cho M, Kim E, Kim T, Lee C, Han J, et al. Renovation of a drop embryo cultures system by using refined mineral oil and the effect of glucose and/or hemoglobin added to a serum-free medium. J Vet Med Sci. 2004;66(1):63–66. doi: 10.1292/jvms.66.63. [DOI] [PubMed] [Google Scholar]
- 14.Swain JE, Schoolcraft WB, Bossert N, Batcheller AE. Media osmolality changes over 7 days following culture in a non-humidified benchtop incubator. Fertil Steril. 2016;106(3):e362. doi: 10.1016/j.fertnstert.2016.07.1028. [DOI] [Google Scholar]
- 15.Swain JE, Graham C, Kile R, Schoolcraft WB, Krisher RL. Media evaporation in a dry culture incubator; effect of dish, drop size and oil on media osmolality. Fertil Steril. 2018;110(4):e363–e364. doi: 10.1016/j.fertnstert.2018.07.1015. [DOI] [Google Scholar]
- 16.Swain JE. Controversies in ART: considerations and risks for uninterrupted embryo culture. Reprod Biomed Online. 2019;39(1):19–26. doi: 10.1016/j.rbmo.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Del Gallego R, Albert C, Marcos J, Larreategui Z, Alegre L, Meseguer M. Humid vs. dry embryo culture conditions on embryo development: a continuous embryo monitoring assessment. Fertil Steril. 2018;110(4):e362–e363. doi: 10.1016/j.fertnstert.2018.07.1012. [DOI] [Google Scholar]
- 18.Mestres E, García-Jiménez M, Casals A, Cohen J, Acacio M, Villamar A, et al. Factors of the human embryo culture system that may affect media evaporation and osmolality. Hum Reprod. 2021;36(3):605–613. doi: 10.1093/humrep/deaa370. [DOI] [PubMed] [Google Scholar]
- 19.Olds S, Stemm K, Wachter K, Wiemer K. Analysis of embryo culture media pH changes during incubator use and media evaporation under oil using a continuous pH monitoring system. Fertil Steril. 2015;104(3):e318–e319. doi: 10.1016/j.fertnstert.2015.07.997. [DOI] [Google Scholar]
- 20.Yumoto K, Iwata K, Sugishima M, Yamauchi J, Nakaoka M, Matsumoto I, et al. Mineral oil viscosity affects the osmotic pressure of human embryonic culture medium microdrops in non-humidified incubators. Fertility and Sterility. 2018;110(4):e52. doi: 10.1016/j.fertnstert.2018.07.161. [DOI] [Google Scholar]
- 21.Yumoto K, Iwata K, Sugishima M, Yamauchi J, Nakaoka M, Tsuneto M, et al. Unstable osmolality of microdrops cultured in non-humidified incubators. J Assist Reprod Genet. 2019;36(8):1571–1577. doi: 10.1007/s10815-019-01515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen SF. Toward a predictive theoretical model for osmolality rise with non-humidified incubation: a randomized, multivariate response-surface study. Hum Reprod. 2021;36(5):1230–1241. doi: 10.1093/humrep/deab015. [DOI] [PubMed] [Google Scholar]
- 23.Mestres E, Matia-Algué Q, Villamar A, Casals A, Acacio M, García-Jiménez M, et al. Characterization and comparison of commercial oils used for human embryo culture. Hum Reprod. 2021;37(2):212–225. doi: 10.1093/humrep/deab245. [DOI] [PubMed] [Google Scholar]
- 24.Shimada M, Kawano N, Terada T. Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction. 2002;124(4):557–64. 10.1530/rep.0.1240557 [DOI] [PubMed]
- 25.Segers I, Adriaenssens T, Coucke W, Cortvrindt R, Smitz J. Timing of nuclear maturation and postovulatory aging in oocytes of in vitro-grown mouse follicles with or without oil overlay. Biol Reprod. 2008;78(5):859–868. doi: 10.1095/biolreprod.107.062539. [DOI] [PubMed] [Google Scholar]
- 26.Martinez CA, Nohalez A, Cuello C, Vazquez JM, Roca J, Martinez EA, et al. The use of mineral oil during in vitro maturation, fertilization, and embryo culture does not impair the developmental competence of pig oocytes. Theriogenology. 2015;83(4):693–702. doi: 10.1016/j.theriogenology.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Otsuki J, Nagai Y, Chiba K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril. 2009;91(5):1745–1749. doi: 10.1016/j.fertnstert.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Martinez CA, Nohalez A, Ceron JJ, Rubio CP, Roca J, Cuello C, et al. Peroxidized mineral oil increases the oxidant status of culture media and inhibits in vitro porcine embryo development. Theriogenology. 2017;103:17–23. doi: 10.1016/j.theriogenology.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Erbach GT, Bhatnagar P, Baltz JM, Biggers JD. Zinc is a possible toxic contaminant of silicone oil in microdrop cultures of preimplantation mouse embryos. Hum Reprod. 1995;10(12):3248–3254. doi: 10.1093/oxfordjournals.humrep.a135897. [DOI] [PubMed] [Google Scholar]
- 30.Martinez CA, Nohalez A, Parrilla I, Motas M, Roca J, Romero I, García-González DL, Cuello C, Rodriguez-Martinez H, Martinez EA, Gil MA. The overlaying oil type influences in vitro embryo production: differences in composition and compound transfer into incubation medium between oils. Sci Rep. 2017;7(1):10505. 10.1038/s41598-017-10989-5 [DOI] [PMC free article] [PubMed]
- 31.Mestres E, Garcia-Jiménez M, Faes L, Vanrell I, Bogaert V, Jonckheere I, et al. Parameters of the Mouse Embryo Assay that affect detection of peroxides in mineral oil. Reprod Biomed Online. 2019;39(4):547–555. doi: 10.1016/j.rbmo.2019.05.008. [DOI] [PubMed] [Google Scholar]