Abstract
Purpose
Polycystic ovary syndrome (PCOS), the most common endocrinopathy in women, is typically accompanied by a defective oxidative defense system. Here, we investigated the effect of astaxanthin (AST) as a powerful antioxidant on the oxidative stress (OS) response and assisted reproductive technology (ART) outcomes in PCOS patients.
Methods
In this double-blind, randomized, placebo-controlled trial, PCOS patients were randomly assigned into two groups. The intervention group received 8 mg AST, and the control group received the placebo daily for 40 days. The primary outcomes were the serum and follicular fluid (FF) levels of the OS biomarkers and the expression levels of the specific genes and proteins in the oxidative stress response pathway. The secondary outcomes were considered ART outcomes.
Results
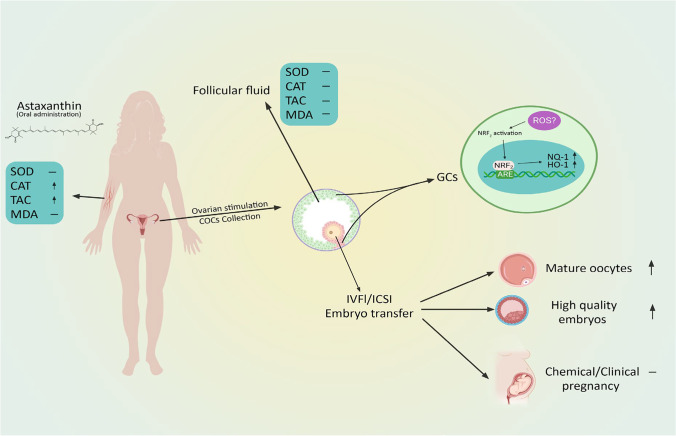
According to our findings, a 40-day course of AST supplementation led to significantly higher levels of serum CAT and TAC in the AST group compared to the placebo group. However, there were no significant intergroup differences in the serum MDA and SOD levels, as well as the FF levels of OS markers. The expression of Nrf2, HO-1, and NQ-1 was significantly increased in the granulosa cells (GCs) of the AST group. Moreover, the MII oocyte and high-quality embryo rate were significantly increased in the AST group compared to the placebo group. We found no significant intergroup difference in the chemical and clinical pregnancy rates.
Conclusion
AST treatment has been shown to increase both serum TAC levels and activation of the Nrf2 axis in PCOS patients’ GCs.
Trial Registration
ClincialTrials.gov Identifier: NCT03991286.
Keywords: Astaxanthin, PCOS, Antioxidant status, Nrf2/ARE, Granulosa cell
Introduction
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder in women of reproductive age. This disorder has a controversial etiology and is accompanied by hyperandrogenism manifestations and the polycystic morphology of the ovaries [1, 2].
PCOS phenotypes are usually associated with impaired glucose and lipid metabolism, leading to a higher prevalence of obesity, type 2 diabetes (T2D), depression, and cardiovascular diseases in the affected patients. Moreover, these patients are in a chronic hyper-inflammatory state, leading to elevated levels of C-reactive protein (CRP) and OS in most of the affected individuals [3, 4]. PCOS can be associated with a wide range of manifestations. Due to the heterogeneity in etiology and characteristics, the affected individuals may have endocrine, reproductive, metabolic, or even psychological involvements [3, 5], leading to a substantial health burden.
Although PCOS has long been studied, the related pathophysiology and etiology are not yet illustrated. Considering the PCOS pathophysiology, this problem is highly associated with excessive ROS production, the progressive elevation of serum OS biomarkers, and decreased antioxidant capacity. Thus, PCOS can be considered a challenging OS condition. On the other hand, many conditions associated with PCOS, including insulin resistance (IR), androgen excess, and abdominal and systemic obesity, may increase the local or systemic hyper-inflammation caused by the elevated OS, thereby mutually aggravating the metabolic abnormalities [6–9].
A recent study by Fatima et al. reported reduced levels of vitamin E, glutathione, and vitamin C in women with PCOS, indicating elevated oxidative stress. Moreover, they found a relationship between OS biomarkers and insulin-related parameters [7]. Another study reported that women with PCOS had elevated levels of oxidative stress, which were the highest in those with IR and infertility [10]. Also, a meta-analysis including 68 studies showed significantly higher levels of several OS by-products and promoters, such as homocysteine, asymmetric dimethylarginine (ADMA), and malondialdehyde (MDA) (which also enhanced ROS production), in the women with PCOS compared to the control group. Also, some circulating antioxidant markers, such as glutathione, were decreased in these individuals [11].
Finding an effective treatment for PCOS is still challenging. The current drug treatment has been restricted because of the contraindications in PCOS patients, the prevalence of the side effects, their related infertility, low efficiency, and patients’ preferences for alternative items [12–14]. However, other treatments, such as supplementation with bioactive molecules with remarkable antioxidant activity, are needed for patients with PCOS. Antioxidant supplementation could probably be a promising therapeutic intervention for PCOS patients with infertility and insulin resistance [14–18].
As a fat-soluble xanthophyll carotenoid, astaxanthin (AST), is an abundantly found carotenoid in Haematococcus microalgae. This bioactive molecule is a more potent scavenger of free radicals than other carotenoids, with several times the singlet oxygen quenching activity of Vitamin E (⍺-tocopherol) [19–21]. Numerous studies have established that AST exhibits significant antioxidant activity against various free radicals, reactive nitrogen species (RNS), and reactive oxygen species (ROS). Additionally, AST exhibited potent antioxidant activity against lipid peroxidation, which is explained by the fact that AST traps free radicals at its conjugated polyene chain as well as in its polar terminal ring moieties, providing antioxidant activity on both the membrane surface and inside the membrane through intra- and inter-molecular hydrogen bonding [22, 23]. More recently, a meta-analysis showed that AST might be effective in OS suppression by increasing the plasma total antioxidant capacity (TAC), reducing the total lipid peroxidation (isoprostane (ISP) and MDA), and improving the SOD [24]. It has been confirmed that the antioxidant effects of AST are exerted through the Nrf2 signaling pathway [25]. Nuclear factor erythroid 2-related Factor 2 (Nrf2) is the primary sensor of OS and a regulator of cellular redox homeostasis. Under stressful conditions, Nrf2 is released from its inhibitor Keap1, regulating the expression of different cytoprotective genes, including NADPH quinone oxidoreductase1 (NQ-1), heme oxygenase-1 (HO-1), and superoxide dismutase (SOD) [26, 27]. As previously reported by our laboratory, AST had a protective effect against OS in the ex vivo human granulosa cells (HGCs) by activating the Nrf2 signaling pathway. Moreover, it could suppress the OS in animal models with PCOS [28, 29].
Although several studies have investigated the cellular mechanisms through which AST exerts its effects on the oxidative stress markers and the Nrf2-ARE pathway, no clinical trials have ever investigated its effects on the oxidative stress biomarkers in women with PCOS. Accordingly, the present study aimed to investigate the effect of AST on the expression regulation of some essential genes in the Nrf2-ARE pathway in the GCs of the women with PCOS, assess the FF and serum levels of some oxidative stress and antioxidant markers, and investigate the relationship of these markers with the ART outcome.
Materials and methods
The current RCT (randomized controlled trial) was conducted at the Fertility Clinic of Shariati Hospital, Tehran, Iran. The study was registered in the ClinicalTrials.gov Protocol Registration System (ClinicalTrials.gov identifier: NCT03991286). It was also approved by the Ethics Committee of Tehran University of Medical Sciences (the ethics committee reference number was IR.TUMS.MEDICINE.REC.1398.702.). All the participants submitted their informed consent. Prior to acquiring the written informed permissions from the research subjects, the investigators provided them with enough explanations.
Study population
Women aged 18–40 with PCOS according to the Rotterdam criteria, and with at least two of the following symptoms were recruited for the study during the period from December 2019 to September 2020: (a) clinical/biochemical signs of hyperandrogenism (HA); (b) oligo/anovulation; (c) polycystic ovarian morphology. The couples did not have a background of severe male factor infertility. The patients with FSH > 10 mg/ml, ovary tumors, severe endometriosis, endocrine diseases (e.g., hypo/hyperthyroidism, Cushing’s syndrome, and hyperprolactinemia), other female factors of infertility (e.g., excluding tubal and cervical factors), and autoimmune diseases such as systemic lupus erythematosus (SLE) were excluded from the study. All the endocrine evaluations were performed on the first day. The physical and demographic characteristics, drug history, menstrual history, and husbands’ sperm quality were documented in a questionnaire.
Randomization and blinding
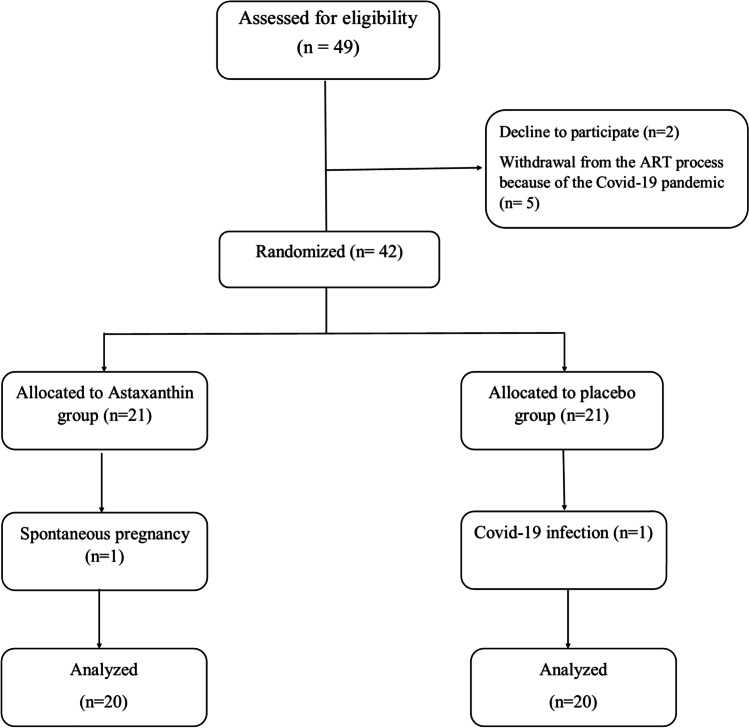
The patients who satisfied the inclusion criteria were randomly assigned to one of two groups, AST or placebo, by the use of the balanced block randomization method, with block sizes of 4. The patients and investigators were blinded to the treatment (Fig. 1).
Fig. 1.
Participant enrollment, randomization, and follow-up
Intervention and ovarian stimulation
The participants in the intervention (AST) group received AST 8 mg/day (2 × 4 mg capsules) orally (Algalife Pure astaxanthin, Reykjanesbær, Iceland) for 40 days (from day 1 of the previous cycle before the ovulation induction until the day of the egg retrieval). The 40-day period was selected based on previous studies and patient inclination [15, 30, 31]. The subjects in the placebo group were given oral placebo capsules, resembling AST in shape. The AST dose (8 mg/day) was selected based on a review of the previous studies on clinical trials of the natural AST, the approved dose levels, and the toxicological studies with natural and synthetic AST [30]. A flexible antagonist regime was applied for all the subjects. To summarize, all the patients took oral contraception (OCP-Ovocept LD®, Aburaihan, Iran) for a 21-day period before ovulation induction. Recombinant follicle‐stimulating hormone (150–300 IU/day, Gonal-F®, Merck Serono SA, Switzerland) was administered from day 3 of the menstrual cycle and continued until the HCG injection. The transvaginal sonography was performed for routine monitoring of the patients. Once two or more follicles reached ≥ 14 mm in size, the GnRH (gonadotropin-releasing hormone) antagonist (cetrorelix acetate Cetrotide 0.25 mg, Merck Serono SA, Switzerland) was administered. Cetrotide was discontinued after a minimum of two follicles achieved a diameter of ≥ 18 mm, and 10,000 IU HCG (Ovitrelle, Merck Serono SA, Switzerland) were injected for the final oocyte maturation. The oocyte retrieval took place 34–36 h following the transvaginal ultrasound-guided trigger injection. All the participants underwent the standard ICSI protocol.
Follicular fluid and blood collection and biochemical measurement
Two blood specimens were taken from all the patients: one prior to the intervention on day 1 of the last cycle and one following the end of the intervention, on the follicles puncture day. The specimens were centrifuged (1500 × g for 10 min) and the serum was separated.
In order to prevent blood contamination, FF was aspirated only from the first follicle during the oocyte retrieval (without any diluting fluid). The samples were centrifuged (300 × g for 10 min) and the clarified supernatants were collected. The serum and FF aliquots were stored at − 80 °C until the assay.
To reduce the systematic error and inter-assay variations, the biomarkers of the oxidative stress (the levels of TAC and MDA, as well as SOD and CAT activities) in FF and serum were measured in duplicate, a blinded fashion, in pairs (prior/post-intervention) simultaneously, and in the same analytical run randomly. All the tests were performed using the enzyme-linked immunosorbent assay kits (Zellbio, GmbH, Germany). The levels of the antioxidant enzymes were reported in unit/ml, and MDA and TAC as µM.
GC preparation
The primary granulosa cells (GCs) were purified by density centrifugation based on the previous studies with some modifications [32]. Briefly, at the time of oocyte retrieval, FF derived from all the follicles of each individual was pooled and immediately centrifuged at 700 × g for 10 min at room temperature (RT). The supernatant removed and the pellet was being resuspended in 2 mL phosphate-buffered saline (PBS) (Sigma, Germany). The suspension was gently layered on 3-mL Ficoll-Paque (Lymphodex, Inno-Train, Germany) density gradient media and centrifuged at 400 × g for 20 min. The GL cell layer at the interface of the Ficoll-Paque column was collected and washed three times with 3 mL of PBS. The purified GCs were immediately stored at − 80 °C until the RNA extraction.
Gene expression
The total RNA from the GCs of each individual was extracted by TRIzol reagent (Invitrogen) as per the manufacturer’s instruction. Following the RNA extraction, the samples were eluted in the final volume of 30 µl of nuclease-free water. The quality and quantity of RNAs were evaluated by the use of electrophoresis and NanoDrop spectrophotometer, respectively. In the RT-PCR method, the RNA (1 µg) was reverse-transcribed into the complementary DNA (cDNA) using the cDNA synthesis kit (Yekta Tajhiz Azma, Iran). It is worth noting that, for the whole RNA cDNA synthesis, a combination of random hexamer and oligo(dt) was employed. The product of this procedure was utilized as a template for real-time PCR. Specific primers for Nrf2, NQ-1, HO1, and GAPDH (a housekeeping internal control gene) were designed for unique target amplification. (Table 1). The final qRT-PCR reaction volume was 20 µl comprising 12.5 µl RealQ and 2 × Master Mix Green (Amplicon, Denmark), 2 µl of primer mixture of both reverse and forward primers (10 µM), 1 µl of target cDNA, and 4.5 µl DEPC water. The running PCR procedure was proceeded by an amplification step of 40 cycles (95 °C for 15 s and 60 °C for 1 min) on a Real-Time ABI® StepOne thermocycler equipped with 48-well optical reaction plates. The related melting curve was calculated as described above. All the PCR reactions were carried out in duplicate. The 2ΔΔCt method was used to determine the relative expression levels of the genes.
Table 1.
Primers sequence and expected length of amplicons
| Target Gene | Forward sequence | Reverse sequence | Product length (bp) |
|---|---|---|---|
| NRF2 | 5′- TTCCTTCAGCAGCATCCTCTC-3′ | 5′-AATCTGTGTTGACTGTGGCATC-3′ | 86 |
| HO-1 | 5′- TGACACCAAGGACCAGAGC-3′ | 5′-TAAGGACCCATCGGAGAAGC-3′ | 149 |
| NQ-1 | 5′-TATCCTGCCGAGTCTGTTCTG-3′ | 5′-AACTGGAATATCACAAGGTCTGC-3′ | 104 |
| GAPDH | 5′-AGTCCACTGGCGTCTTCAC-3′ | 5′-ATCTTGAGGCTGTTGTCATACTTC-3′ | 148 |
Western blot analysis
The expressions of Nrf2 and Keap1 proteins were performed by the use of Western blot analyses according to the previous studies with some modifications [33, 34]. The RIPA lysis buffer was used to lyse the cells for Western blotting. The centrifugation (14,000 rpm, 30 min, 4 °C) was used to remove the lysates. The protein concentration was measured with the Bradford protein Quantification kit, as recommended by the manufacturer. The cell lysates were mixed with an equal volume of the 2 × Laemmli sample buffer. Then, 30 μg protein per lane was subjected to SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and after a 5-min boiling, transferred to an Immune-BlotTM polyvinylidene difluoride (PVDF) membrane (0.2 µm). The membranes were blocked with 5% BSA (Sigma-Aldrich, MO, USA) in 0.1% Tween 20 for 1 h, and incubated overnight with anti-Nrf2 (Cat No: ab92946, Abcam, UK), anti-Keap1 (Cat No: ab226997, Abcam, UK), and anti-beta actin-loading control antibodies (Cat No: ab8227, Abcam, UK) at RT. In the next step, the membranes were washed with Tris-buffered saline containing 0.1% Tween 20 (TBST) (3 times), followed by incubating with goat anti-rabbit IgG H&L (HRP) (Cat No: ab6721, Abcam, Cambridge, UK) secondary antibody for 1 h at RT. Eventually, the membranes were incubated for 5 min with enhanced chemiluminescence (ECL), and the signals were visualized using an ECL detection system. The expression of proteins was normalized against β-actin.
ART outcomes
On the puncture day, about 2 h after the surgery, the cumulus oocyte complexes (COCs) were denuded by the hyaluronidase enzyme. The quality and maturity of the oocytes were examined under a microscope [26] and categorized as GV (germinal vesicle), MI (metaphase I), and MII (metaphase II) concerning the maturity stage. The mature oocytes underwent intracytoplasmic sperm injection (ICSI) (day 0), and the fertilization was confirmed 17–20 h after insemination by the presence of two pronuclei (2PN) and two polar bodies (PBs). The fertilization rate was determined by dividing the number of fertilized oocytes by the total number of inseminated oocytes. The ASEBIR criteria [35] were used to determine the grades A and B with excellent quality of day 3 embryos according to the number of blastomeres, the cell symmetry, the percentage of fragmentation, the presence of pitting, the multi-nucleation, and the vacuoles.
The ART outcome was calculated as follows: MII oocyte rate: the numbers of the MII oocytes divided by the total number of the oocytes; the fertilization rate: the numbers of the fertilized oocytes divided by the total numbers of the inseminated oocytes; the high-quality embryo rate: the number of grade 1–2 embryos divided by the total number of the embryos. The rates were represented as a percentage. Individuals were checked again on days 12–14 following the embryo transfer, and chemical pregnancy rates (according to the—HCG test) and then the clinical pregnancy (based on the presence of the gestational sac on ultrasound) were determined.
Sample size and statistical analysis
Calculation of the sample size was based on variable TAC values over a 12-week treatment period [36]. A sample size of 17 in each group would yield 80% power (β = 0.2; two-tailed P = 0.05). Assuming a 15% loss of the follow-up and dropout (withdrawal) rates, a sample size of 20 in each group would yield 80% power at α = 0.05 for both the outcomes.
The data analysis was performed using the SPSS software version 22. Moreover, variables were described using the mean and standard deviation (SD). The data normality was evaluated using the Shapiro–Wilk and Kolmogorov–Smirnov tests. The continuous variables with normal distribution underwent intergroup and intragroup comparisons using the independent and paired t-tests, respectively. Moreover, the data without normal distribution were investigated using the non-parametric test of the Mann–Whitney U test. Also, intergroup comparisons of the chemical and clinical pregnancy rates were performed using Fisher’s exact and chi-squared tests. The correlations between the OS markers (both serum and FF levels) and the ART outcomes were investigated using Pearson’s correlation coefficient. In addition, all the statistical tests were two-sided. Eventually, the significance level was considered as 0.05, and the Graph Pad Prism software version 8 was used to plot the related graphs.
Results
Subjects
A total number of 49 individuals were chosen randomly (Fig. 1). Finally, 20 individuals in the treatment group and 20 individuals in the control group completed the research as planned, and these 40 participants were included in the final analysis. The recruiting period was from Dec. 2019 to Nov. 2020, and the follow-up ended in June 2021, when the study was terminated.
Table 2 compares the demographic characteristics and the baseline laboratory results of the two groups. The age and BMI parameters were comparable among the groups. The median age in the AST and the placebo groups were 30.6 ± 1.115 and 30.45 ± 0.89 years, respectively. Among the 40 participants, 18 individuals (45 percent) were under the age of 30. In the AST and placebo groups, the mean BMIs were 27.72 ± 4.52 (18.9–39.7) and 28.9 ± 5.47 (21.1–38.3) kg/m2, respectively. The two groups’ hormonal profiles in serum, including LH, FSH, AMH, thyroid hormones, and the level of progesterone hormone, were similar. Moreover, all of the patients had primary infertility. The individuals had no background of chronic diseases, smoking and/or drinking histories, or taking particular medications. There was no background of significant male infertility in any of the couples. A flexible antagonist ovarian stimulation regimen was employed for all the patients.
Table 2.
Demographic and hormonal features of two groups of PCOS patients
| Characteristic | Treatment group (n = 20) | Control group (n = 20) | P value |
|---|---|---|---|
| Age (y) | 30.60 ± 4.98 | 30.45 ± 3.98 | 0.917 |
| BMI (kg/m2) | 28.91 ± 4.52 | 27.72 ± 5.47 | 0.458 |
| E2 (pg/mL) | 85.79 ± 46.76 | 80.12 ± 38.45 | 0.678 |
| P (ng/mL) | 1.26 ± 0.86 | 1.27 ± 0.87 | 0.978 |
| PRL (ng/mL) | 11.72 ± 6.31 | 12.14 ± 5.78 | 0.828 |
| LH (mU/mL) | 16.01 ± 7.47 | 14.06 ± 7.91 | 0.430 |
| FSH (mU/mL) | 4.79 ± 1.97 | 4.69 ± 1.44 | 0.857 |
| AMH | 12.28 ± 6.30 | 12.78 ± 6.29 | 0.800 |
| TSH | 2.18 ± 1.04 | 2.04 ± 0.98 | 0.658 |
Values are presented as mean ± SD. Treatment group received AST; control group received placebo. No statistically significant differences were found between the treatment group and the control group. BMI body mass index, E2 estradiol, P progesterone, PRL prolactin, LH luteinizing hormone, FSH follicle-stimulating hormone, AMH anti-Müllerian hormone, TSH thyroid-stimulating hormone
Serum and follicular fluid antioxidants and oxidative stress profile
Table 3 displays the data from all the assessments of the oxidative stress indicators. There was no significant difference in the CAT serum levels between the AST group and the control one after the intervention (P > 0.05). But, its levels in the Placebo group significantly decreased (P < 0.05), and the values were 6.83 ± 2.48 (U/mL) and 3.91 ± 2.61 (U/mL) in the baseline and on the day of oocyte retrieval, respectively.
Table 3.
Intragroup and Intergroup comparisons of oxidative stress markers
| Variable | Placebo mean ± SD | Astaxanthin mean ± SD | P value between groups | Follicular fluid mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before (baseline) | After | Paired t test P value | Before (baseline) | After | Paired t testP value | Placebo | Astaxanthin | t test P value | ||
| CAT (U/ml) | 6.83 ± 2.48 | 3.91 ± 2.61 | 0.0003*** | 7.52 ± 2.85 | 8.22 ± 2.38 | 0.114 | ˂0.0001**** | 3.83 ± 2.44 | 4.82 ± 2.07 | 0.179 |
| MDA (µM) | 92.39 ± 81.28 | 103.1 ± 79.44 | 0.498 | 92.83 ± 51.69 | 93.66 ± 43.47 | 0.934 | 0.644 | 4.94 ± 3.43 | 4.89 ± 2.49 | 0.96 |
| SOD (U/ml) | 23.17 ± 3.4 | 23.96 ± 3.7 | 0.245 | 22.35 ± 4.3 | 24.01 ± 4.7 | 0.114 | 0.969 | 44.25 ± 5.32 | 44.59 ± 4.76 | 0.836 |
| TAC (µM) | 0.495 ± 0.22 | 0.398 + 0.20 | 0.025* | 0.514 ± 0.198 | 0.599 ± 0.21 | 0.035* | 0.004** | 0.296 ± 0.189 | 0.381 ± 0.133 | 0.110 |
CAT catalase, MDA malondialdehyde, SOD superoxide dismutase, TAC total antioxidant capacity.
The levels of MDA and SOD in serum were not different between the two groups when compared to baseline values (P > 0.05).
Evaluating the second laboratory assessment (after treatment) in the two groups revealed that the blood level of TAC, reflecting the total antioxidant status, was significantly lower (P = 0.025) in the control group and significantly higher (P = 0.035) in the intervention group in comparison with the baseline, and also significantly differed between the two groups (P = 0.004). However, the FF levels of the antioxidant/OS parameters did not reveal any statistically significant difference between the two groups (P > 0.05).
Upregulation of Nrf2 and its downstream target genes by AST
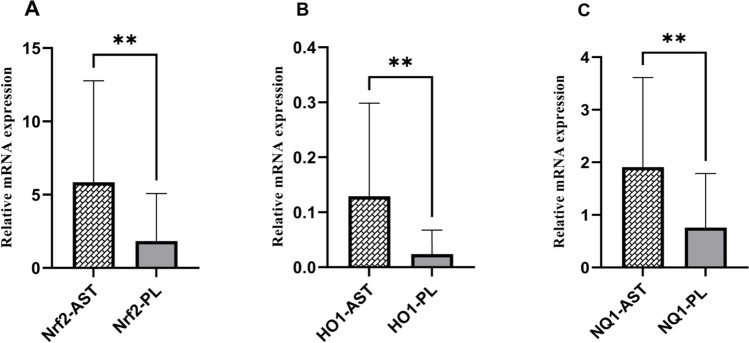
To investigate the differential gene expressions between the GCs from the women in the AST treatment group versus the placebo group, the relative mRNA levels were measured by RT-qPCR. The gene expression results showed that the expression level of Nrf2 significantly increased in the treatment group compared to the placebo group (P = 0.009). This increase was also observed in the expression levels of HO-1 and NQ1, the Nrf2 downstream targets (P = 0.001 and 0.007, respectively) (Fig. 2).
Fig. 2.
Relative mRNA expression of oxidative stress related genes (Nrf2 (A), HO-1 (B), NQ-1 (C)) in GCs of treatment and placebo groups. The expression level of the genes was expressed as fold change. Statistical significance (P < 0.05) was assessed by Mann–Whitney Test. The fold change levels of Nrf2, HO-1, and NQ-1 were significantly increased in the AST group (P < 0.01 for all)
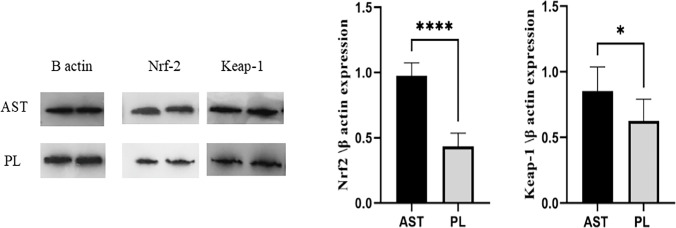
To confirm the effectiveness of astaxanthin in stimulating the Nrf2 antioxidant pathway, we also evaluated the expression of Nrf2 protein and its main inhibitor, Keap1, by Western blotting. The protein expression demonstrated a similar increase in Nrf2 in the AST treatment group (P < 0.001). But in contrast to the expectations, the expression of Keap1 protein, as Nrf2 inhibitor, increased in the AST group as well (P = 0.014) (Fig. 3).
Fig. 3.
Proteins levels of Keap1and Nrf2 in GCs of treatment and placebo groups. The relative intensities of Keap1and NRF2 normalized against β-actin. (Nrf2: 80 kDa, Keap1: 70 kDa, β-actin: 42 kDa). *P < 0.05 and ****P < 0.0001
Comparison of ART outcomes
Comparing the ovulation induction and the criteria of ART outcomes among the two groups, we observed that the MII oocyte rate and the high-quality embryo rate were substantially higher in the AST group (P = 0.029 and ˂ 0.001, respectively). However, there was no significant difference between the two groups in terms of the number of recovered oocytes, the total embryos, and the fertilization rate (Table 4).
Table 4.
Comparison of ART outcomes between astaxanthin and placebo groups
| Variable | Intervention (AST) | Placebo | P value (intervention vs placebo) |
|---|---|---|---|
| Number of retrieved follicles | 29.05 ± 13.00 | 32.37 ± 11.01 | 0.393 |
| Mature oocytes (M-II) rate (%) | 71.73 ± 11.61 | 63.00 ± 12.79 | 0.029* |
| Number of embryos | 15.50 ± 7.54 | 14.75 ± 4.88 | 0.71 |
| Fertilization rate (%) | 78.347 ± 18.56 | 77.04 ± 20.01 | 0.82 |
| High-quality embryo rate (%) | 52.93 ± 9.7 | 39.94 ± 13.04 | ˂0.001*** |
#Values are presented as the mean ± SD.
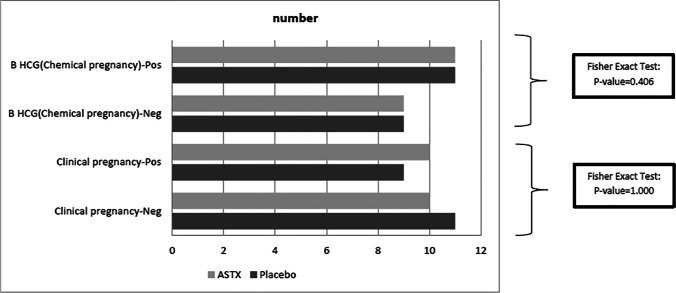
Also, we did not find any significant intergroup difference in the clinical and chemical pregnancy rates (Fig. 4). The chemical pregnancy rates were 55% (11/20) in both groups (Fisher’s exact test; two-tailed P = 0.406), while the clinical pregnancy rates were 50% (10/20) and 45% (9/20) in the AST and placebo groups, respectively (Fisher’s exact test; two-tailed P = 1.000).
Fig. 4.
Comparison of pregnancy (chemical/clinical) rate between AST and placebo groups
Correlation analysis of variables
Eventually, the antioxidant/OS markers in serum and FF on the oocyte retrieval day, in total patients, were assessed in regard to the ART outcomes. The serum MDA levels had a significant positive correlation with the total number of embryos (rs = 0.317, P = 0.046), while the follicular fluid MDA levels were negatively correlated with the MII oocyte rate (rs = − 0.359, P = 0.023). Moreover, the serum levels of CAT were positively correlated with the MII oocyte rate (rs = 0.376, P = 0.017). Also, the serum and follicular fluid TAC levels had a significant positive correlation with the high-quality embryo rate (rs = 0.492, P = 0.001 and rs = 0.373, P = 0.018, respectively). The mentioned results are presented in Table 5.
Table 5.
Correlations between oxidative stress markers with ART outcome
| Oxidative Stress markers | Total oocyte | MII rate | fertility rate | Embryo number | High-quality embryo rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | rs | p | rs | p | |
| Serum | ||||||||||
| CAT (U/ml) | 0.080 | 0.624 | 0.376* | 0.017 | − 0.089 | 0.584 | 0.168 | 0.300 | 0.220 | 0.172 |
| SOD (U/ml) | − 0.292 | 0.067 | 0.183 | 0.258 | 0.125 | 0.441 | − 0.091 | 0.577 | − 0.050 | 0.759 |
| TAC (µM) | − 0.236 | 0.142 | − 0.038 | 0.816 | − 0.010 | 0.951 | − 0.204 | 0.206 | 0.492** | 0.001 |
| MDA (µM) | 0.275 | 0.086 | 0.125 | 0.443 | 0.029 | 0.861 | 0.317* | 0.046 | − 0.099 | 0.542 |
| Follicular fluid | ||||||||||
| CAT (U/ml) | − 0.172 | 0.288 | 0.268 | 0.095 | 0.106 | 0.517 | − 0.035 | 0.831 | 0.071 | 0.665 |
| SOD (U/ml) | 0.007 | 0.964 | − 0.127 | 0.453 | 0.145 | 0.373 | 0.014 | 0.932 | − 0.272 | 0.090 |
| TAC (µM) | − 0.037 | 0.819 | − 0.041 | 0.804 | 0.122 | 0.454 | 0.116 | 0.477 | 0.373* | 0.018 |
| MDA (µM) | 0.007 | 0.964 | − 0.359* | 0.023 | − 0.050 | 0.760 | − 0.157 | 0.334 | − 0.175 | 0.279 |
rs: Pearson correlation coefficient, MDA malondialdehyde, SOD superoxide dismutase, TAC total antioxidant capacity, CAT catalase.
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
Discussion
To the best of our knowledge, the present study was the first clinical trial investigating the impact of astaxanthin on oxidative stress markers in PCOS patients. According to the present prospective, randomized, controlled clinical trial results, a 40-day course of AST administration could significantly increased the serum TAC and CAT levels in the intervention group. Moreover, our results indicating activation of the Nrf2/ARE axis and increased HO-1, NQ mRNA, Nrf2 mRNA, and Nrf2 protein expressions in the GCs in AST treatment group.
Despite the increasing prevalence of PCOS globally, the related etiology is largely unknown. It is extensively believed that OS is the main pathological process in PCOS. Due to the limited studies published, the relationship between OS and PCOS is not confirmed yet. According to several studies, women with PCOS have decreased serum SOD levels while elevated MDA levels [11]. A prospective study by Chen et al. reported that women with PCOS who underwent IVF had decreased serum SOD and increased serum MDA levels on the trigger day [37].
As one of the most potent antioxidants known [38], AST has been reported to exert a protective effect against OS and can effectively improve the OS in humans and animals [38–42]. The present study did not find any significant CAT level changes in the AST group. However, the placebo group had significantly decreased levels of CAT. Therefore, AST possibly prevented the CAT level decrease that might have occurred during the ovarian stimulation cycle. Nonetheless, no significant change was observed in both groups’ MDA and SOD levels. Also, AST could increase the serum TAC levels in women with PCOS. According to these results, it is highly probable that AST significantly increased the serum antioxidant capacity. The results of other similar studies investigating the effects of antioxidants on PCOS are compatible with ours in this regard [43–45].
On the other hand, we did not find similar effects in the follicular fluid because there was no significant intergroup difference in the levels of OS markers in the follicular fluid, although we detected higher TAC and CAT levels in the follicular fluid of the AST group compared to the control group, the observed increase was not significant. These results are compatible with the study by Gong et al. which found no significant improvement in the SOD, MDA, and TAC levels in the follicular fluid of the individuals treated with growth hormone and the control group. However, growth hormone treatment could reduce the total oxidant status (TOS) and the OS Index (OSI) of the follicular fluid in women with PCOS [46]. Thus, future studies should investigate these markers, as well as the presence of AST and its oxidation fragments in FF. Also, these findings can be explained by the fact that follicular fluid is made of extravasated plasma and the secretions by the GCs and oocytes. Thus, the levels of OS markers in this fluid reflect the OS level of the ovaries and the systemic OS levels [47]. Moreover, systemic OS is closely associated with hyperandrogenism, obesity, and IR. According to recent studies, women with different phenotypes of PCOS respond differently to various treatments and have different ART outcomes [48, 49]. Therefore, the classification of the involved individuals based on their PCOS phenotypic characteristics may increase the accuracy of the results.
Despite the unknown underlying mechanism of antioxidant defenses in reducing the OS in the HGCs, some studies have noted the role of the Nrf2-Keap1/ARE pathway in this regard. According to Akino et al., Nrf2 can be a potent survival factor for follicle preservation [50]. Also, Wang et al. reported that humanin (HN), a mitochondrial-derived peptide, plays an essential role in the oxidative stress regulation in the GCs and ovaries of PCOS patients by regulating the Keap1/Nrf2 signaling pathway [51]. Therefore, targeting the Nrf2-signaling pathway may improve oxidative stress in women with PCOS.
Various studies have reported the ability of AST in inducing the Nrf2/ARE (antioxidant response elements) pathway [20, 52, 53]. In addition, our previous study indicated the improving effect of AST on the OS in HGCs by upregulating the HO-1 and NQ-1 expressions by activating the Nrf2 [28]. The results of our previous study are also compatible with the present study results, which demonstrated that AST could activate the Nrf2 defense system and significantly promote the Nrf2 expression, allowing it to manage the expression of downstream preserver genes, such as NQ-1 and HO-1. Keap1 is the most crucial negative regulator of Nrf2 activity. This molecule prevents the nuclear entrance of Nrf2 by binding to it [27]. Therefore, we expected decreased Keap1 expression by increasing the Nrf2 protein expression levels. However, Western blot analysis showed an increased Keap1 expression, which was incompatible with the results of an experimental study on the effect of AST on HGCs [28]. This observed effect can be due to the negative feedback loop between Keap1 and Nrf2. According to Lu et al., there is positive feedback between Nrf2 and Keap1, so increased Nrf2 expression will increase the Keap1 expression. In other words, there is an autoregulatory loop between Keap1 and Nrf2 that controls their cellular abundance [54].In addition to the Nrf2 cytosolic binding due to the Keap1 inhibitory effect, some other factors can also regulate the Nrf2 gene transcription. Some of these alternative mechanisms, including microRNAs[55], phosphorylation of Nrf2 by protein kinases [56–58], and acetylation of Nrf2 [59], were highlighted in recent studies. Also, phosphorylated p62 can activate the Nrf2 via direct competition with Nrf2 in binding to Keap1 [60, 61]. Therefore, the observed increase in Keap1 expression may be a cellular response to the increased NRF2 expression due to other regulatory pathways. Further studies can reveal the exact etiology of this observation.
According to our results, there was a significant intergroup difference in the rates of M-II oocytes and high-quality embryos. Therefore, the AST may improve the oocyte function and its potential for generating higher quality embryos, so the higher quality of embryos on day 3 may be due to the higher oocyte quality caused by AST. These results are compatible with some previous studies reporting the improving effect of antioxidants supplementation on the ART outcome in women with or without PCOS [31, 46, 62]. In addition, we found no intergroup difference in the chemical and clinical pregnancy rates (Fig. 5). Previous studies have established that numerous factors can affect the pregnancy outcome, such as sperm quality and other exogenous factors, including embryo handling, freezing and storage, and endometrial condition [63–67]. Therefore, oxidative stress and antioxidants status levels may not be the only factor affecting this process.
Fig. 5.
Astaxanthin-antioxidant impacts on PCOS patients. Astaxanthin increased serum TAC and CAT levels but did not affect follicular fluid antioxidant status. AST could increase the expression of the Nrf2 and its downstream genes in GCs and improve the oocytes maturation and good-quality embryos rates. It did not affect the pregnancy outcomes
According to our results, serum and FF levels of the TAC had a significant positive correlation with the high-quality embryo rate. Moreover, the serum levels of CAT were positively correlated with the MII oocyte rate. Also, the serum MDA levels had a significant positive correlation with the total number of embryos, while the MDA levels in the FF negatively correlated with the MII oocyte rate. A study by Liu et al. found that the TOC levels of serum and FF had a significant negative correlation with the blastocyst formation and high-quality embryo rate. However, they reported no significant relationship between the serum TAC and MDA levels with the IVF outcomes. Also, they reported that FF TAC levels were positively correlated with blastocyst formation, while the MDA levels had a significant negative relationship with the blastocyst formation and high-quality embryo rate [68]. Incompatible with the study by Liu et al., Naigaonkar et al. found no relationship between TAC levels and IVF-related parameters [69]. A recent prospective cohort study [37] found that the serum MDA and GPx levels were positively correlated with the IVF- or ICSI-related outcomes. However, they reported that mild systematic OS on the trigger day in non-obese young women with PCOS did not affect the live birth rate. The controversies in the mentioned studies can be due to different study populations and sample sizes. Therefore, it is recommended to perform further studies on different populations with larger sample sizes to illustrate the potential relationships between the OS markers and the ART outcomes.
Women with PCOS may have different phenotypic characteristics. Therefore, the main limitation of the present study was the sample size, which was too small to analyze the outcomes related to the PCOS subtypes. Moreover, factors such as reactive oxygen species and homocysteine, a significant marker of oxidative stress, as well as reductive stress markers, were not measured in this study. As a result, it is unknown whether the enrolled patients were under oxidative stress at baseline. Measuring these markers may aid in determining the efficacy and mechanism of action of AST. Nevertheless, the present study results provided a scientific basis for further RCTs.
Conclusion
Astaxanthin supplementation in women with PCOS was associated with increased serum antioxidant parameters. Also, AST could increase the expression of the Nrf2 and Nrf2-regulated downstream genes in GCs and improve the oocyte maturation and rates of high-quality embryos. However, it did not affect the pregnancy outcome. Eventually, additional research will be required to determine the efficacy and mechanism of action of AST, the optimal dosage, duration, and timing of treatment, as well as the therapeutic effect of AST administration in various PCOS subgroups.
Author contribution
Fardin Amidi, Ashraf Alyasin, and Roghaye Gharaei designed the research. Roghaye Gharaei and Fardin Amidi performed all the experiments and wrote the manuscript. Forough Mahdavinezhad and Zhaleh Ashrafnezhad involved in analyzing and interpreting the data. Esmaeil Samadian reviewed & edited the manuscript. All the authors read and approved the final manuscript.
Funding
This study was funded and supported by the Tehran University of Medical science (Grant Number: 98–01-30–41687).
Declarations
Ethics approval
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (the ethics committee reference number: IR.TUMS.MEDICINE.REC.1398.702.).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roghaye Gharaei, Email: gharaei_r@yahoo.com.
Ashraf Alyasin, Email: ivfshariati69@gmail.com.
Forough Mahdavinezhad, Email: mahdaviforough@gmail.com.
Esmaeil Samadian, Email: essamadian@gmail.com.
Zhaleh Ashrafnezhad, Email: Zhashrafnezhad@yahoo.com.
Fardin Amidi, Email: Famidi@tums.ac.ir.
References
- 1.Dumesic DA, Oberfield SE, Stener-Victorin E, et al. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran S, Sagili H. Metabolic syndrome in women with polycystic ovary syndrome. Obstet Gynaecol. 2018;20:245–252. doi: 10.1111/TOG.12519. [DOI] [Google Scholar]
- 4.Pinola P, Puukka K, Piltonen TT, et al. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril. 2017;107:788–795.e2. doi: 10.1016/j.fertnstert.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Vannuccini S, Clifton VL, Fraser IS, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22:104–115. doi: 10.1093/humupd/dmv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Ruan X, Li Y, et al. (2019) Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch Gynecol Obstet. 2019;3005(300):1413–1421. doi: 10.1007/S00404-019-05305-7. [DOI] [PubMed] [Google Scholar]
- 7.Fatima Q, Amin S, Kawa IA, et al. Evaluation of antioxidant defense markers in relation to hormonal and insulin parameters in women with polycystic ovary syndrome (PCOS): a case-control study. Diabetes Metab Syndr Clin Res Rev. 2019;13:1957–1961. doi: 10.1016/j.dsx.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102:1167–1174.e4. doi: 10.1016/j.fertnstert.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Zhang T, Gao H, et al. Tempol ameliorates polycystic ovary syndrome through attenuating intestinal oxidative stress and modulating of gut microbiota composition-serum metabolites interaction. Redox Biol. 2021;41:101886. doi: 10.1016/J.REDOX.2021.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turan V, Sezer ED, Zeybek B, Sendag F. Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2015;28:119–123. doi: 10.1016/j.jpag.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Murri M, Luque-ramírez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (pcos): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 12.Arentz S, Smith CA, Abbott J, et al. Combined lifestyle and herbal medicine in overweight women with polycystic ovary syndrome (PCOS): a randomized controlled trial. Phyther Res. 2017;31:1330–1340. doi: 10.1002/ptr.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szczuko M, Kikut J, Szczuko U, et al. Nutrition strategy and life style in polycystic ovary syndrome—narrative review. Nutrients. 2021;13:1–18. doi: 10.3390/nu13072452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arentz S, Abbott JA, Smith CA. Bensoussan A (2014) Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med. 2014;141(14):1–19. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenjian S, Moini A, Yamini N, et al. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol. 2020;83:1–10. doi: 10.1111/aji.13186. [DOI] [PubMed] [Google Scholar]
- 16.Gharaei R, Mahdavinezhad F, Samadian E, et al. Antioxidant supplementations ameliorate PCOS complications: a review of RCTs and insights into the underlying mechanisms. J Assist Reprod Genet. 2021 doi: 10.1007/s10815-021-02342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, et al. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101:4322–4328. doi: 10.1210/jc.2016-1858. [DOI] [PubMed] [Google Scholar]
- 18.Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme q10 and/or vitamin e in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;104:319–327. doi: 10.1210/jc.2018-01221. [DOI] [PubMed] [Google Scholar]
- 19.Donoso A, González-Durán J, Muñoz AA, et al. Therapeutic uses of natural astaxanthin: an evidence-based review focused on human clinical trials. Pharmacol Res. 2021;166:105479. doi: 10.1016/J.PHRS.2021.105479. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Guo C, Wu J. Astaxanthin in liver health and disease: a potential therapeutic agent. Drug Des Devel Ther. 2020;14:2275–2285. doi: 10.2147/DDDT.S230749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faraone I, Sinisgalli C, Ostuni A, et al. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: a systematic review. Pharmacol Res. 2020;155.10.1016/j.phrs.2020.104689. [DOI] [PubMed]
- 22.Jannel S, Caro Y, Bermudes M, Petit T. Novel insights into the biotechnological production of haematococcus pluvialis-derived astaxanthin: advances and key challenges to allow its industrial use as novel food ingredient. J Mar Sci Eng. 2020;8:1–48. doi: 10.3390/jmse8100789. [DOI] [Google Scholar]
- 23.Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta Biomembr. 2001;1512:251–258. doi: 10.1016/S0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Xu H, Chen J, Zhang L. Effects of astaxanthin supplementation on oxidative stress. Int J Vitam Nutr Res. 2019;90:179–194. doi: 10.1024/0300-9831/a000497. [DOI] [PubMed] [Google Scholar]
- 25.Kohandel Z, Farkhondeh T, Aschner M, Samarghandian S. Nrf2 a molecular therapeutic target for astaxanthin. Biomed Pharmacother. 2021;137:111374. doi: 10.1016/j.biopha.2021.111374. [DOI] [PubMed] [Google Scholar]
- 26.Loboda A, Damulewicz M, Pyza E, et al. (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;7317(73):3221–3247. doi: 10.1007/S00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40.10.1128/mcb.00099-20. [DOI] [PMC free article] [PubMed]
- 28.Eslami M, Esfandyari S, et al. Astaxanthin protects human granulosa cells against oxidative stress through activation of NRF2 / ARE pathway and its downstream phase II enzymes. Cell J. 2021;23. 10.22074/cellj.2021.7222. [DOI] [PMC free article] [PubMed]
- 29.Ebrahimi F, Rostami S, Nekoonam S, et al. The effect of astaxanthin and metformin on oxidative stress in granulosa cells of BALB C mouse model of polycystic ovary syndrome. Reprod Sci. 2021 doi: 10.1007/s43032-021-00577-4. [DOI] [PubMed] [Google Scholar]
- 30.Brendler T, Williamson EM. Astaxanthin: How much is too much? A safety review. Phyther Res. 2019;33:3090–3111. doi: 10.1002/ptr.6514. [DOI] [PubMed] [Google Scholar]
- 31.Bahramrezaie M, Amidi F, Aleyasin A, et al. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: a triple-blind randomized clinical trial. J Assist Reprod Genet. 2019;36:1701–1712. doi: 10.1007/s10815-019-01461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HM, Klausen C, Leung PCK. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100:585–592.e1. doi: 10.1016/j.fertnstert.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Song X, Feng Y, et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radic Biol Med. 2020;152:854–864. doi: 10.1016/j.freeradbiomed.2020.01.185. [DOI] [PubMed] [Google Scholar]
- 34.Huang CC, Chen MJ, Lan CW, et al. Hyperactive CREB signaling pathway involved in the pathogenesis of polycystic ovarian syndrome revealed by patient-specific induced pluripotent stem cell modeling. Fertil Steril. 2019;112:594–607.e12. doi: 10.1016/j.fertnstert.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Balaban B, Brison D, Calderón G, et al. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. In: Human Reproduction. 2011. pp 1270–1283 [DOI] [PubMed]
- 36.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. 2011;66:363–369. doi: 10.1007/s11130-011-0258-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Huang X, Liu Y, et al. Systematic oxidative stress is not associated with live birth rate in young non-obese patients with polycystic ovarian syndrome undergoing assisted reproduction cycles: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;253:154–161. doi: 10.1016/j.ejogrb.2020.07.049. [DOI] [PubMed] [Google Scholar]
- 38.Sztretye M, Dienes B, Gönczi M, et al. Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longev. 2019;2019. [DOI] [PMC free article] [PubMed]
- 39.Park JS, Chyun JH, Kim YK, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab. 2010;7:1–10. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira CPM, Souza ACR, Vasconcelos AR, et al. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review) Int J Mol Med. 2021;47:37–48. doi: 10.3892/ijmm.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baralic I, Djordjevic B, Dikic N, et al. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phyther Res. 2013;27:1536–1542. doi: 10.1002/ptr.4898. [DOI] [PubMed] [Google Scholar]
- 42.Choi HD, Kim JH, Chang MJ, et al. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phyther Res. 2011;25:1813–1818. doi: 10.1002/ptr.3494. [DOI] [PubMed] [Google Scholar]
- 43.Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, et al. The effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Elem Res. 2018;184:300–307. doi: 10.1007/s12011-017-1198-5. [DOI] [PubMed] [Google Scholar]
- 44.Akbari M, Ostadmohammadi V, Lankarani KB, et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50:271–279. doi: 10.1055/s-0044-101355. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Guo Q, Pei YH, et al. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC Womens Health. 2020;20:1–9. doi: 10.1186/s12905-020-00930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Y, Luo S, Fan P, et al. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-75107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi L, Gagliardi A, Landi C, et al. Protein pathways working in human follicular fluid: the future for tailored IVF? Expert Rev Mol Med. 2016;18:1–14. doi: 10.1017/erm.2016.4. [DOI] [PubMed] [Google Scholar]
- 48.De Vos M, Pareyn S, Drakopoulos P, et al. Cumulative live birth rates after IVF in patients with polycystic ovaries: phenotype matters. Reprod Biomed Online. 2018;37:163–171. doi: 10.1016/j.rbmo.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Mumusoglu S, Yildiz BO. Polycystic ovary syndrome phenotypes and prevalence: differential impact of diagnostic criteria and clinical versus unselected population. Curr Opin Endocr Metab Res. 2020;12:66–71. doi: 10.1016/j.coemr.2020.03.004. [DOI] [Google Scholar]
- 50.Akino N, Wada-Hiraike O, Terao H, et al. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol Cell Endocrinol. 2018;470:96–104. doi: 10.1016/j.mce.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Li N, Zeng Z, et al. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol Hum Reprod. 2021;27.10.1093/molehr/gaaa081. [DOI] [PubMed]
- 52.Tripathi DN, Jena GB. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res Toxicol Environ Mutagen. 2010;696:69–80. doi: 10.1016/J.MRGENTOX.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Xiao J, Liu H, et al. Astaxanthin attenuates oxidative stress and immune impairment in d-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-?B pathway. Food Funct. 2020;11:8099–8111. doi: 10.1039/d0fo01663b. [DOI] [PubMed] [Google Scholar]
- 54.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282:36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 55.Cheng X, Ku CH, Siow RCM. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 58.Rojo AI, Medina-Campos ON, Rada P, et al. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: role of glycogen synthase kinase-3. Free Radic Biol Med. 2012;52:473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP Augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/mcb.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 Activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 62.Yu K, Wang RX, Li MH, et al. Melatonin reduces androgen production and upregulates heme oxygenase-1 expression in granulosa cells from PCOS patients with hypoestrogenia and hyperandrogenia. Oxid Med Cell Longev. 2019;2019.10.1155/2019/8218650. [DOI] [PMC free article] [PubMed]
- 63.Oehninger S, Mayer J, Muasher S. Impact of different clinical variables on pregnancy outcome following embryo cryopreservation. Mol Cell Endocrinol. 2000;169:73–77. doi: 10.1016/S0303-7207(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 64.Pan Y, Hao G, Wang Q, et al. Major factors affecting the live birth rate after frozen embryo transfer among young women. Front Med. 2020;7:1–8. doi: 10.3389/fmed.2020.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapuis A, Gala A, Ferrières-Hoa A, et al. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. 2017;27:1–9. doi: 10.1186/s12610-016-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Sun YF, Xu YM, et al. Effect of endometrium thickness on clinical outcomes in luteal phase short-acting GnRH-a long protocol and GnRH-Ant protocol. Front Endocrinol (Lausanne) 2021;12:1–11. doi: 10.3389/fendo.2021.578783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bu Z, Yang X, Song L, et al. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol. 2019;17:1–6. doi: 10.1186/s12958-019-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Yu Z, Zhao S, et al. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J Assist Reprod Genet. 2021;38:471–477. doi: 10.1007/s10815-020-02014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naigaonkar A, Dadachanji R, Hinduja I, Mukherjee S. Altered redox status may contribute to aberrant folliculogenesis and poor reproductive outcomes in women with polycystic ovary syndrome. J Assist Reprod Genet. 2021 doi: 10.1007/s10815-021-02241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]