Abstract
Purpose
To test the validity of the Vienna consensus laboratory key performance indicators (KPIs) to monitor the outcome of treatments involving women of different age ranges.
Methods
The retrospective cohort study included 862 complete IVF/ICSI cycles carried out between January 2014 and May 2021. All embryos of each cycle cohort were subject to extended culture. The overall population was divided into two groups according to female age: the Vienna consensus (≤ 39 years) and older female age (≥ 40 years). We compared outcomes of a selection of the Vienna performance indicators (PIs) and KPIs, with a focus on measures relevant to embryo cleavage and blastocyst formation. A possible association between total good blastocyst development rate (TGBDR) and cumulative clinical pregnancy rate (CPR) was also assessed.
Results
No differences were observed in fertilization and embryo cleavage KPIs between the Vienna consensus and the older female age group (standard IVF fertilization, 67.2 vs. 67.3; ICSI fertilization, 72.3 vs. 75.3; day 2 development, 57.6% vs 58.7%; day 3 development, 52.4% vs. 50.7%, respectively). TGBDR was lower in the older female age group (45.5% vs. 33.4% p < 0.001). Multivariate logistic regression analysis indicated female age as a factor independently associated with TGBDR. Clinical outcomes significantly decreased with increasing female age.
Conclusion
The study suggests that, while most laboratory outcome measures are reliably applicable irrespective of female age, KPIs describing extended embryo culture should be fine-tuned in consideration of older female age.
Keywords: IVF, Indicators, Fertilization, Embryo, Blastocyst, Pregnancy
Introduction
The clinical outcome of assisted reproduction technology (ART) treatments crucially depends on quality and quantity of embryos generated in the IVF laboratory [1]. At different developmental steps, several intrinsic and extrinsic factors can cause attrition of such embryos [2]. This demands methodical and precise monitoring of laboratory performance, to assure the highest standard of patient care performance indicators (PIs) can meet this need, representing an essential tool of quality management systems in the setting of clinical laboratories. Closing a gap that previously existed for decades, the ESHRE Special Group in Embryology and Alpha Scientists in Reproductive Medicine achieved the first consensus on the formulation of a systematic set of relevant PI and key PIs (KPIs) [3]. Like those adopted to monitor processes in other fields, IVF laboratory PIs are expected to be robust and able to detect weaknesses in the achievement of individual steps of a process, highlighting situations that might affect the desired outcome [4]. Indeed, the ESHRE/Alpha document—the “Vienna consensus”—sets for each PI or KPI minimum and aspirational values for competency and benchmarks levels [3]. Adopting such measures, embryologists can assess the laboratory efficiency in assisting specific developmental steps that lead to the formation of a blastocyst suitable for transfer or cryopreservation. Such efficiency measures can reveal influences of extrinsic factors that may affect essential segments of the IVF process, such as the impact of an operator’s skills on the rates of normal fertilization in ICSI cases [5–7]. PI outcomes may also be influenced by intrinsic gamete characteristics, which are often associated with specific patient typologies. Therefore, PIs and KPIs should not be applied to undefined patient populations, but rather to relatively homogeneous groups. The “reference population” of the Vienna Consensus is defined by female age ≤ 39 years, exclusion of PGT cases and use of own fresh oocytes and ejaculated sperm. While the definition of such a population is a step forward standardization of outcome measurements and correct inter-laboratory comparisons, it excludes important and large groups of ART patients. Specifically, the relevance of the Vienna PIs and KPIs to treatments of women older than 39 years remains untested. This knowledge gap raises concern for at least two reasons: (a) in addition to the well-known positive association with embryo aneuploidy, maternal age could affect diverse developmental parameters, questioning the robustness of the Vienna consensus KPIs [6, 8–11]; (b) as reported in national and international ART registries, older women represent an increasingly large proportion of IVF patients [12–14]. This suggests the need of “ad hoc” indicators to assess the laboratory performance of treatments concerning such patients.
Therefore, in relation to treatment of patients of different female age ranges, in this study, we assessed the performance of our IVF laboratory implementing the Vienna consensus PIs and KPIs. Our results suggest that most—but not all—non-clinical laboratory outcome measures may be universally applicable, calling for only a limited adjustment of the Vienna consensus indicators in consideration of maternal age.
Materials and methods
Study design
This was a retrospective, single-center cohort analysis of 862 ART cycles carried out between January 2014 and May 2021. The study was approved by the IRB (Ref. R04/PA 19—Rev. 0). Inclusion criteria were indication for IVF/ICSI, blastocyst culture of all embryos formed in each cohort, use of own ejaculated spermatozoa (fresh or frozen), and complete cycles, i.e., those whose all embryos were transferred, cryopreserved, or disposed of. Oocyte donation, canceled, and PGT cycles were not included. Blastocyst transfer policy was applied to all cases treated in the participating institution, not only those included in the study.
Clinical and laboratory protocols
As previously described [15], controlled ovarian stimulation was performed with either recombinant FSH or hMG, with starting doses ranging from 100 to 450 IU per day, according to hormonal and anthropometric parameters. Gonadotropin dose was adjusted according to individual follicular response, while GnRH antagonist was used to prevent the LH spontaneous surge.
Oocytes were retrieved transvaginally 35 to 36 h after hCG or decapeptyl administration; Fertilization was achieved by either standard IVF or ICSI. For fresh cycles, luteal support was initiated after oocyte retrieval, while for frozen embryo transfer estrogen and vaginal progesterone were administered in a sequential regimen aimed to mimic endometrium exposure to physiological hormone levels [16].
Approximately 4 h after oocyte pickup, standard IVF was carried out using a final motile sperm concentration of 100,000–200,000/mL. In ICSI cases [17], after 2 h from oocyte retrieval cumulus cells were removed from companion oocytes [15]. At fertilization check (16–18 h after insemination), oocytes displaying two pronuclei and two polar bodies were considered to be normally fertilized and further cultured. Embryos were cultured for 5–6 days and, if appropriate according to criteria of morphological quality, transferred and/or cryopreserved at the blastocyst stage. Blastocysts were evaluated according to the degree of expansion and quality of the inner cell mass and trophectoderm cell, as previously described [18]. During the study period, no changes were made to culture conditions (5% oxygen, 6% CO2), incubation equipment and type of sequential culture media (Cook IVF, Brisbane, Australia). Blastocyst was vitrified using a closed system device (Zacà et al., 2020) [19].
The good blastocyst development rate PI was defined as the fraction of good quality blastocysts obtained from the number of normally fertilized oocytes. Blastocyst quality was assessed according to the Istanbul consensus [20]. Blastocyst development rates were appraised according to the Vienna consensus reference values: competency ≥ 30%, benchmark ≥ 40% (day 5); competency ≥ 40%; benchmark ≥ 50% (day 5 and day 6) [3].
Embryology KPIs
We measured a selection of the Vienna performance indicators (PIs) and KPIs, with a focus on measures relevant to embryo cleavage and blastocyst formation. Assessment of fertilization, cleavage, and blastocyst rates was carried out at the time points recommended by the Istanbul consensus on embryo assessment [20]. To assess more comprehensively blastocyst quality and quantity, we estimated the total good blastocyst development rate (TGBDR); this outcome expresses the proportion of fertilized oocytes developing into blastocysts both on day 5 and day 6 and suitable for transfer or cryopreservation. Finally, blastocyst cryosurvival rate was assessed.
Clinical outcome
Implantation rate (IR) was calculated as the number of gestational sacs divided by the total number of embryos transferred [21]. This KPI provides an indication of the overall performance of the laboratory, although female age dependent. Cumulative clinical pregnancy rate (CPR) following transfer of day 5 or day 6 of fresh or cryopreserved blastocyst was also assessed. The cumulative analysis included complete cycles with at least one normally fertilized oocyte finally selected for the analysis. A complete cycle included oocyte retrieval, followed by fresh transfers or with cryopreserved embryos, until all of embryos were used or until pregnancy was achieved.
Data analysis/stratification
Data were assessed after splitting cycles into two female age groups (Vienna consensus reference population ≤ 39 and older female age ≥ 40 years). TGBDR was also compared between the two age groups in sub-populations that were homogeneous for number of retrieved oocytes. Finally, to assess the impact of blastocysts quantity and quality on clinical outcome, cumulative CPR was further sub-analyzed in the two age groups.
Statistics
Multivariable stratified analyses were performed to test for differences between groups. To this aim, we adopted the analysis of variance with the one-way ANOVA procedure for a quantitative dependent variable.
Study groups were compared in relation to maternal age, body max index, number of recovered oocytes, and sperm parameters. Data were presented as percentages or as the means ± SD. Quantitative variables were compared with Student’s t test for independent samples; chi-square analysis was performed for the comparison of categorical data. Differences were considered significant at p < 0.05 and highly significant at p < 0.01. Univariable and multivariable logistic regressions were performed to evaluate associations with TGBDR, including in the model patient and cycle characteristics. We tested all 2-way interactions between pairs of predictors included in our multivariable analyses and used a Bonferroni-correction (for multiple testing) P-value threshold of 0.05 to define statistical evidence of an interaction. The predictive value of the resulting model was assessed by calculating the area under the curve of the receiver operator characteristics (AUROC). The Hosmer–Lemeshow test was used to evaluate the level of agreement between the estimated and the observed probabilities (calibration). Statistical analysis was performed with IBM SPSS Statistics 27.
Results
Patient characteristics
Overall, the study included eight hundred sixty-two complete standard IVF/ICSI cycles. Female age, male age, and number of collected oocytes were different between the Vienna consensus and the older female age group (p < 0.001) (Table 1). Total sperm count and BMI were comparable. In our center, the SET policy is adopted with the exception of some patients (< 10% cycles) in which two blastocysts are transferred, in fact the mean number of blastocyst transferred was 1.1 ± 0.6.
Table 1.
Cycle characteristics and laboratory and clinical outcomes of study groups
| Total | Population groups | p-value | ||
|---|---|---|---|---|
| Vienna consensus | Older female age | |||
| ≤ 39 year | ≥ 40 years | |||
| n = 862 | n = 607 | n = 255 | ||
| Patients and cycles characteristics | ||||
| Female age (year) | 36.5 ± 4.6 | 34.3 ± 3.4 | 41.9 ± 1.6 | p < 0.001 |
| Male age (year) | 39.6 ± 5.9 | 37.7 ± 5.1 | 43.9 ± 5.1 | p < 0.001 |
| Number of total sperm | 36.4 ± 50.1 | 35.8 ± 50.6 | 38 ± 49.1 | NS |
| Number of recovered oocytes (m ± sd) | 9095 (10.6 ± 5.7) | 6861 (11.3 ± 5.8) | 2234 (8.8 ± 5.1) | p < 0.001 |
| BMI | 21 ± 3.2 | 21.9 ± 3.2 | 22.1 ± 3.4 | NS |
| Fertilization | ||||
| ICSI (%) | 72.9 | 72.3 | 75.3 | NS |
| Standard IVF (%) | 67.0 | 67.2 | 67.3 | NS |
| Embryo development | ||||
| Day 2 embryo development (%) | 57.9 | 57.6 | 58.7 | NS |
| Day 3 embryo development (%) | 52.0 | 52.4 | 50.7 | NS |
| Number of usable blastocysts (m ± sd) | 223 (2.6 ± 1.9) | 1778 (2.9 ± 2.0) | 458 (1.8 ± 1.4) | p < 0.001 |
| Total good blastocyst development rate (day 5–day 6) | 42.3 | 45.5 | 33.4 | p < 0.001 |
| Cryopreservation | ||||
| Blastocyst cryosurvival (%) | 95.0 | 95.2 | 94.2 | NS |
| Clinical results | ||||
| Implantation (%) | 28.3 | 34.3 | 16.9 | p < 0.001 |
| Cumulative clinical pregnancy (%) | 44.0 | 52.4 | 23.9 | p < 0.001 |
Fertilization KPIs
Fertilization rates were evaluated for both types of insemination techniques (Table 1). ICSI fertilization rates were comparable between the Vienna consensus and the older female age groups (72.3% and 75.3% respectively, p = NS). Likewise, comparison of standard IVF fertilization rates did not show significant differences (67.2% and 67.3% respectively, p = NS).
Cleavage and blastocyst KPI
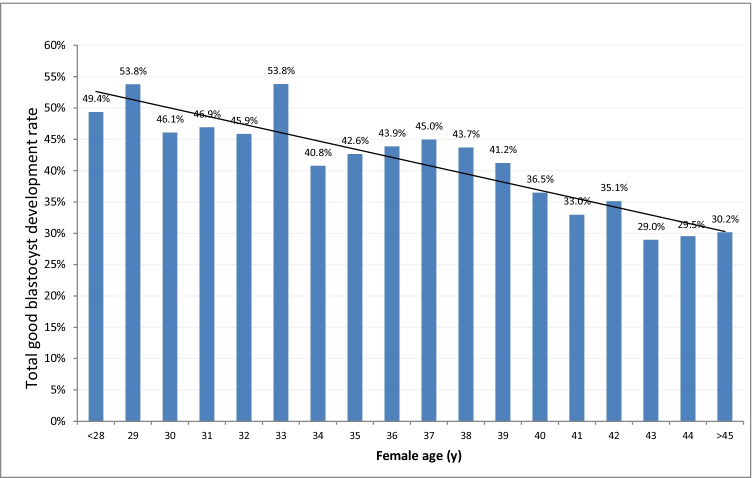
Embryo development rates on day 2 were 57.6% in the Vienna consensus and 58.7% in the older female age groups, respectively (p = NS). On day 3, such rates were 52.4% and 50.7%, respectively (p = NS). TGBDR was lower in the older female age group (45.5% and 33.4%, p < 0.001). This outcome decreased steadily with increasing female age (Fig. 1). Blastocyst cryosurvival rates were comparable between the two age groups (Table 1).
Fig. 1.
Total good blastocyst development rate in cycles of different female age
Implantation rate and cumulative CPR
Clinical outcome significantly decreased with increasing female age (Table 1). As anticipated, implantation rate decreased from 34.3% in the Vienna consensus group to 16.9% in the older female age group (p < 0.001). Cumulative CPR showed a similar trend decreasing from 52.4 to 23.9% (p < 0.001).
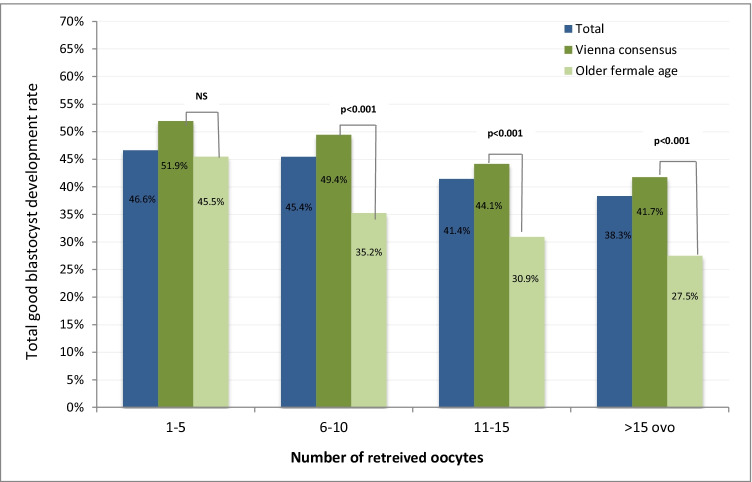
TGBDR and number of recovered oocytes
In the two populations, TGBDRwas further assessed after normalization of the number of retrieved oocytes (Fig. 2). Rates were comparable in cycles with only few (1–5 oocytes) collected oocytes (51.9% and 45.5%, p = ns). In cycles with 6–10, 11–15, and > 15 collected oocytes TGBDR was lower in older patient groups (p < 0.01) (Fig. 2).
Fig. 2.
Total good blastocyst development rate sub-analyzed according to number of retrieved oocytes and female age
Multivariate analysis
To further control for possible patient-specific confounding factors, female age, male age, number of retrieved oocytes, and day 3 embryo development rate (percentage of embryos with 8 cell on day 3) were evaluated in a univariate and multivariate logistic regression analysis (Table 2). All variables emerged as factors independently associated with TGBDR. Higher female, male age, and number of retrieved oocytes were associated with a reduced probability to achieve a TGBDR greater than 40%. Day 3 embryo development rate was positively associated with a higher TGBDR.
Table 2.
Multivariate logistic regression analysis of factors potentially impacting on total good blastocyst development rate (TGBDR)
| Characteristic | Categories | Univariable odds ratio of TGBDR (95% CI) | Multivariablea odds ratio of TGBDR (95% CI) | p-Valueb |
|---|---|---|---|---|
| Female age (years) | < 40 | 1 | 1 | < 0.001 |
| ≥ 40 | 0.489 (0.362–0.659) | 0.463 (0.318–0.674) | ||
| Male age (years) | < 43 | 1 | 1 | 0.009 |
| ≥ 43 | 0.56 (0.412–0.762) | 0.611 (0.417–0.894) | ||
| Number of retrieved oocytes | 1–5 | 1 | 1 | 0.001 |
| 6–10 | 1.116 (0.753–1.654) | 0.85 (0.54–1.338) | ||
| 11–15 | 0.969 (0.635–1.479) | 0.691 (0.423–1.13) | ||
| > 15 | 0.618 (0.384–0.995) | 0.395 (0.227–0.687) | ||
| Day 3 embryo development rate | < 45% | 1 | 1 | < 0.001 |
| 45%–70% | 3.397 (2.417–4.773) | 3.56 (2.499–5.069) | ||
| > 70% | 7.245 (5.037–10.421) | 8.52 (5.64–12.073) |
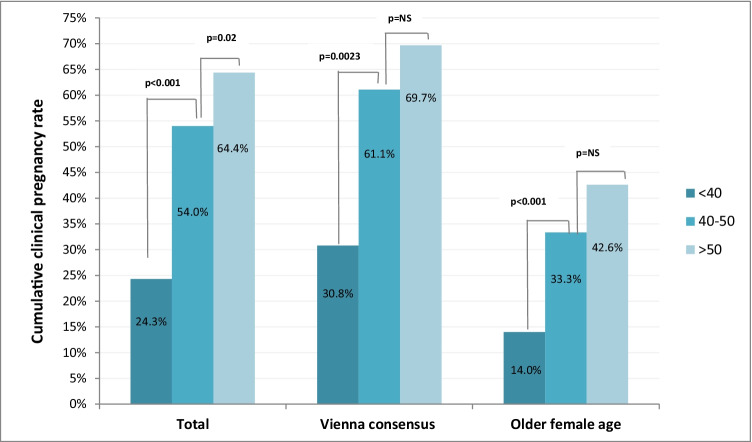
Impact of TGBDR on clinical outcome
To assess the impact of blastocysts quantity and quality on clinical outcome, cumulative CPR was further sub-analyzed in the two age groups. In the Vienna consensus group, cumulative CPR was 30.8 in cycles with TGBDR lower than the competence threshold (40%), while it reached 69.7% in cycles with TGBDR larger than the benchmark value (50%). In the older patients group, cumulative CPR was 14.0% and 42.6% in cycles with TGBDR below the competence value or above the benchmark limit, respectively (Fig. 3).
Fig. 3.
Cumulative clinical pregnancy rate sub-analyzed according to female age and rates of total good blastocyst development rate (TGBDR)
Discussion
In this study, we tested the validity of the Vienna consensus laboratory KPIs [3] to monitor the outcomes of treatments involving women of different age ranges. We observed that most outcome parameters are reliably applicable irrespective of female age. We also concluded that KPIs describing extended embryo culture require fine-tuning according to female age. However, limitations of the study associated with its retrospective nature and the associated risks of biases should be acknowledged.
The IVF laboratory is central to ART treatments, bringing in vitro the gap between the end of gametogenesis and embryo implantation. This is largely made possible by the astonishing developmental plasticity of the human embryos [22]. However, a multitude of factors can impact on gametic and embryonic functions underpinning preimplantation development. Some of such factors are procedural in nature and depend on both technical setups and human skills. Gamete preparation and insemination techniques, culture condition, and cryopreservation skills are examples of extrinsic elements that, if inadequate, may erode the developmental potential of an embryo. Therefore, the mission of the IVF laboratory can only be accomplished if performance is optimized, monitored, and maintained as constant as possible over time.
Systems to monitor clinical and laboratory performance have gained much importance in medical practice [4, 23]. The Vienna Consensus has met the need for continued improvement and control of the IVF laboratory. It suggests the application of three types of indicators: reference indicators (RIs), offering information on ovarian response and, indirectly, oocyte quality: PIs, requiring monitoring and data storage, but not regular assessment for deviations from desired values; KPIs, expressing the “core business of the IVF laboratory” [3]. The Shewhart and the Levey-Jennings charts are widely used to monitor clinical and laboratory data. Recently, artificial intelligence-based tools have also been developed for early detection of adverse outcomes and identification of clinically relevant shifts in pregnancy rates [24].
The Vienna KPIs were conceived to assess the performance of embryo culture systems, by monitoring laboratory outcomes at crucial developmental landmarks. Among such indicators, those measuring the rate of blastocyst formation have progressively become more relevant, in parallel with the increased use of extended culture [25]. Blastocyst culture offers several advantages over the cleavage-stage embryo transfer (ET) [26], such as more stringent selection of embryos suitable for transfer or cryopreservation, systematic adoption of single ET, and higher implantation rates per ET [27–30].
Certain patient or gamete characteristics can clearly influence some KPIs, especially those more clinically relevant. Therefore, the Vienna consensus defined a reference population corresponding to the following criteria: (I) female patients ≤ 39 years old; (II) own fresh oocytes; (III) ejaculated spermatozoa (fresh or frozen); (IV) no PGD/PGS (PGT); and (V) all insemination methods (i.e., routine IVF and ICSI). While the adoption of this reference population reduces the source of possible biases and allows intra- and inter-laboratory performance comparisons, it implies the limitation to exclude from monitoring the treatments of women older than 39 years. This represents a major knowledge gap. Older age women already represent a large fraction of ART patients, with current trends suggesting further relative increase in the coming years [12–14].
Providing scope for this study, we addressed the question of the range of application of the Vienna consensus KPIs. To this end, we focused on female age, a factor which affects embryo chromosome constitution, but whose impact on preimplantation development is less clear. We identified our overall study group by adopting all criteria of the consensus reference population. Only exception was female age, with the inclusion of women older than 39. In such a group, all measured laboratory KPIs fell within the competency-benchmark interval values. Instead, as anticipated due to the inclusion of older women, blastocyst IR did not reach the competency threshold.
By splitting the original study group in two age subsets, we then gained specific information of the validity of the Vienna consensus outcome values to monitor treatment performance in older age women. In the younger group, defined by age < 40 years as indicated by the consensus, all measured KPIs (including IR) were above the competency threshold. In the older group, IR was lower and predictably below the competency threshold. Consistently, although not included in the consensus KPIs, cumulative pregnancy rate was also lower compared with the younger group. In the older group, we also ascertained adherence of fertilization (standard IVF and ICSI), cleavage (day 2 and day 3) and cryosurvival rates to the ranges indicated by the Vienna consensus. However, blastocyst parameters were influenced by age. Not only was the average number of good blastocysts lower in older women, as a probable consequence of a smaller number of collected oocytes. Good blastocyst rate (TGBDR measured on day 6) was also below the competency threshold.
Notably, a female age-dependent effect on embryo development emerges from our data only at the blastocyst stage. This may derive by sensitivity of a number of molecular, biochemical and cellular, and oocyte functions to female aging [31]. However, embryos of younger and older women developed with similar cleavage rates until day 3. On a purely speculative ground, this suggests that the differences observed at the blastocyst stage may result from alterations in the zygotic genome expression, which massively increases from day 3 [32].
Multivariate logistic regression analysis of factors potentially impacting on TGBDR shows also that male age negative impact on good blastocyst development; this outcome provides scope for future studies focused on the potential impact of male reproductive aging on embryo development and clinical outcomes.
Based on available evidence, we would tend to rule out the possibility that a lower good blastocyst rate is an effect of a lower oocyte yield in older women. Jones et al. [2] reported that quantity and quality of blastocysts formed on day 5 and day 6 are independent of the number of collected oocytes, but are negatively associated with female age. This is consistent with our sub-analysis showing that, with the exception of cycles with very few oocyte oocytes [1–5]—for which extended culture may be inappropriate—TGBDR decreases with female age in groups consistent for number of retrieved oocytes.
Our data confirm the sensitivity of the rates of blastocyst formation as indicators of the laboratory performance and/or oocyte quality, as proposed by the Vienna consensus. A recent study confirmed this notion, reporting that day 5 and TGBDR are mutually complementary to promptly identify a deterioration in the laboratory outcome, prior to adverse changes in clinical outcomes [33]. Another recent study including almost 8000 cycles also stressed the importance of laboratory KPIs—in such a case fertilization rate—as predictors of clinical outcome [6]. Our study also suggests a positive association between TGBDR and cumulative CPR. Therefore, this outcome should be closely monitored to strive for maximum clinical outcome for treatments of both younger and older women.
Conclusions
By assessing laboratory performance in treatment groups of different female age, in this study, we responded to the recommendation of the Vienna consensus to validate KPIs in consideration of relevant laboratory organization and patient characteristics. In final analysis, our data confirms the general validity of the Vienna consensus KPIs; nevertheless, with a focus on female age, they also indicate a need for a fine-tuning of indicators expressing blastocyst formation. Consistent with these findings, to assess the outcome of older female age (≥ 40 years) cycles, we suggest to adopt TGBDR of 25% and 40% for competency and benchmark values, respectively. We intend the proposed performance interval as a suggestion requiring further validation, in syntony with the methodology and approach of the Vienna consensus. Therefore, our study may be considered a starting point for further discussion and research to validate laboratory KPIs in relation to diverse settings and patient populations or following the introduction of novel procedures or technologies.
Funding
The study was funded by the authors’ institution.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–2196. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 2.Jones CA, Acharya KS, Acharya CR, Raburn D, Muasher SJ. Patient and in vitro fertilization (IVF) cycle characteristics associated with variable blastulation rates: a retrospective study from the Duke Fertility Center (2013–2017) Middle East Fertil Soc J. 2020;24(1):4. doi: 10.1186/s43043-019-0004-z. [DOI] [Google Scholar]
- 3.ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. Electronic address: coticchio.biogenesi@grupposandonato.it The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017;35(5):494–510. doi: 10.1016/j.rbmo.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Salinas M, López-Garrigós M, Gutiérrez M, Lugo J, Sirvent JV, Uris J. Achieving continuous improvement in laboratory organization through performance measurements: a seven-year experience. Clin Chem Lab Med. 2010;48(1):57–61. doi: 10.1515/CCLM.2010.003. [DOI] [PubMed] [Google Scholar]
- 5.ESHRE Guideline Group on Good Practice in IVF Labs. De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, et al. Revised guidelines for good practice in IVF laboratories (2015) Hum Reprod. 2016;31(4):685–6. doi: 10.1093/humrep/dew016. [DOI] [PubMed] [Google Scholar]
- 6.Scaravelli G, Zacà C, Levi Setti PE, Livi C, Ubaldi FM, Villani MT, et al. Fertilization rate as a novel indicator for cumulative live birth rate: a multicenter retrospective cohort study of 9,394 complete in vitro fertilization cycles. Fertil Steril. 2021;116(3):766–773. doi: 10.1016/j.fertnstert.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer ST, Mortimer D. Quality and risk management in the IVF laboratory. Cambridge: Cambridge University Press; 2015. [Google Scholar]
- 8.Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365(6460):1466–1469. doi: 10.1126/science.aav7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshaviak M, Kalma Y, Carmon A, Samara N, Dviri M, Azem F, et al. The effect of advanced maternal age on embryo morphokinetics. Front Endocrinol (Lausanne) 2019;10:686. doi: 10.3389/fendo.2019.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang A, Santistevan A, Hunter Cohn K, Copperman A, Nulsen J, Miller BT, et al. Freeze-only versus fresh embryo transfer in a multicenter matched cohort study: contribution of progesterone and maternal age to success rates. Fertil Steril. 2017;108(2):254–261.e4. doi: 10.1016/j.fertnstert.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Irani M, O’Neill C, Palermo GD, Xu K, Zhang C, Qin X, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95–102.e1. doi: 10.1016/j.fertnstert.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, Calhaz-Jorge C, et al. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open. 2017;2017(2):hox012. doi: 10.1093/hropen/hox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European IVF-monitoring Consortium (EIM)‡ for the European Society of Human Reproduction and Embryology (ESHRE) Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020(3):hoaa032. doi: 10.1093/hropen/hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012†. Hum Reprod. 2020;35(8):1900–1913. doi: 10.1093/humrep/deaa090. [DOI] [PubMed] [Google Scholar]
- 15.Borini A, Lagalla C, Bonu MA, Bianchi V, Flamigni C, Coticchio G. Cumulative pregnancy rates resulting from the use of fresh and frozen oocytes: 7 years’ experience. Reprod Biomed Online. 2006;12(4):481–486. doi: 10.1016/S1472-6483(10)62002-0. [DOI] [PubMed] [Google Scholar]
- 16.Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril. 2002;77(5):956–960. doi: 10.1016/S0015-0282(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 17.Borini A, Bafaro MG, Bianchi L, Violini F, Bonu MA, Flamigni C. Oocyte donation programme: results obtained with intracytoplasmic sperm injection in cases of severe male factor infertility or previous failed fertilization. Hum Reprod. 1996;11(3):548–550. doi: 10.1093/HUMREP/11.3.548. [DOI] [PubMed] [Google Scholar]
- 18.Zacà C, Bazzocchi A, Pennetta F, Bonu MA, Coticchio G, Borini A. Cumulative live birth rate in freeze-all cycles is comparable to that of a conventional embryo transfer policy at the cleavage stage but superior at the blastocyst stage. Fertil Steril. 2018;110(4):703–709. doi: 10.1016/j.fertnstert.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Zacà C, Coticchio G, Tarozzi N, Nadalini M, Lagalla C, Garolla A, et al. Sperm count affects cumulative birth rate of assisted reproduction cycles in relation to ovarian response. J Assist Reprod Genet. 2020;37(7):1653–1659. doi: 10.1007/s10815-020-01807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 21.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, et al. Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update. 2021;27(5):848–865. doi: 10.1093/humupd/dmab016. [DOI] [PubMed] [Google Scholar]
- 23.Leandro G, Rolando N, Gallus G, Rolles K, Burroughs AK. Monitoring surgical and medical outcomes: the Bernoulli cumulative SUM chart. A novel application to assess clinical interventions. Postgrad Med J. 2005;81(960):647–52. doi: 10.1136/pgmj.2004.030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bormann CL, Curchoe CL, Thirumalaraju P, Kanakasabapathy MK, Gupta R, Pooniwala R, et al. Deep learning early warning system for embryo culture conditions and embryologist performance in the ART laboratory. J Assist Reprod Genet. 2021;38(7):1641–1646. doi: 10.1007/s10815-021-02198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saket Z, Källén K, Lundin K, Magnusson Å, Bergh C. Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Hum Reprod Open. 2021;2021(3):hoab021. doi: 10.1093/hropen/hoab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Electronic address: asrm@asrm.org Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril. 2018;110(7):1246–52. doi: 10.1016/j.fertnstert.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 28.Desai NN. The road to blastocyst transfer. Hum Reprod. 1998;13(12):3292–3294. doi: 10.1093/oxfordjournals.humrep.a019678. [DOI] [PubMed] [Google Scholar]
- 29.Tsirigotis M. Blastocyst stage transfer: pitfalls and benefits. Too soon to abandon current practice? Hum Reprod. 1998;13(12):3285–9. doi: 10.1093/humrep/13.12.3285. [DOI] [PubMed] [Google Scholar]
- 30.Gardner DK, Schoolcraft WB. No longer neglected: the human blastocyst. Hum Reprod. 1998;13(12):3289–3292. doi: 10.1093/oxfordjournals.humrep.a019677. [DOI] [PubMed] [Google Scholar]
- 31.Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162(2):R19–33. doi: 10.1530/REP-21-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassena R, Boué S, González-Roca E, Aran B, Auer H, Veiga A, et al. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development. 2011;138(17):3699–3709. doi: 10.1242/dev.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammond ER, Morbeck DE. Tracking quality: can embryology key performance indicators be used to identify clinically relevant shifts in pregnancy rate? Hum Reprod. 2019;34(1):37–43. doi: 10.1093/humrep/dey349. [DOI] [PubMed] [Google Scholar]