Abstract
Ischemic tolerance is a phenomenon whereby transient exposure to a non-injurious preconditioning stimulus triggers resistance to a subsequent lethal ischemic insult. Despite the fact that not only neurons but also astrocytes and endothelial cells have a unique response to preconditioning stimuli, current research has been focused mostly on the effect of preconditioning on neuronal death. Thus, it is unclear if the blood-brain barrier (BBB) can be preconditioned independently of an effect on neuronal survival. The release of tissue-type plasminogen activator (tPA) from perivascular astrocytes in response to an ischemic insult increases the permeability of the BBB. In line with these observations, treatment with recombinant tPA increases the permeability of the BBB and genetic deficiency of tPA attenuates the development of post-ischemic edema. Here we show that tPA induces ischemic tolerance in the BBB independently of an effect on neuronal survival. We found that tPA renders the BBB resistant to an ischemic injury by inducing TNF-α-mediated astrocytic activation and increasing the abundance of aquaporin-4-immunoreactive astrocytic end-feet processes in the neurovascular unit. This is a new role for tPA, that does not require plasmin generation, and with potential therapeutic implications for patients with cerebrovascular disease.
Keywords: Cerebral ischemia, preconditioning, tissue-type plasminogen activator, blood-brain barrier, plasmin
Introduction
Ischemic tolerance in the brain is an evolutionarily conserved form of cerebral plasticity 1 whereby resistance to an injurious ischemic insult is transiently augmented by previous exposure to a preconditioning stimuli. 2 There are two forms of ischemic tolerance: rapid and delayed. In rapid preconditioning, post-translational modifications such as protein phosphorylation and changes in ion channel permeability trigger a short-lasting protective effect within minutes of exposure to the preconditioning stimuli. 3 In contrast, in the delayed form gene activation and protein synthesis induce ischemic tolerance hours to days after the preconditioning event. 1
Inasmuch as a brief episode of non-lethal cerebral ischemia is the best known preconditioning event, ischemic tolerance can also be induced by exposure to other endogenous and exogenous stimuli such as hyperoxia, oxidative stress, caspases, inflammatory cytokines, hyperthermia and heat shock. 4 The identification and characterization of these tolerance-inducing stimuli is of pivotal translational importance because they may lead to the design of pharmacological tools to protect the brain of patients with cerebrovascular disease. In line with these observations, several clinical studies have evaluated the therapeutic success of different forms of ischemic preconditioning. 5
A growing body of experimental evidence indicates that not only neurons, but also astrocytes play a central role in the induction of ischemic tolerance. 6 Indeed, astrocytic activation triggers ischemic preconditioning via multiple mechanisms including HIF-1α upregulation, 7 the generation of an astrocytic-derived non-lethal proinflammatory environment, 8 transfer of energy substrates to neurons, 9 and erythropoietin production. 10 Remarkably, although astrocytes and endothelial cells have an unique response to preconditioning stimuli, 1 it is uncertain if it is possible to induce tolerance in the blood-brain barrier (BBB) independently of neuronal survival. Hence, although it has been shown that preconditioning with short periods of non-lethal ischemia attenuates the development of cerebral edema after transient middle cerebral artery occlusion (tMCAO), 11 it is unclear if the reported protective effect on the permeability of the BBB is an independent phenomenon or a consequence of the observed decrease in the volume of the ischemic lesion.
Tissue-type plasminogen activator (tPA) is a serine proteinase that catalyzes the conversion of the zymogen plasminogen into the protease plasmin. Despite the fact that for a long time it was believed that endothelial cells were the main reservoir of tPA and that its unique role was to promote plasmin-mediated degradation of fibrin in the intravascular space, 12 subsequent studies indicated that tPA is also found in neurons, astrocytes and microglia, and that its release is pivotal for the development of synaptic plasticity, 13 microglial activation, 14 and regulation of the permeability of the BBB.15,16 Furthermore, in vivo and in vitro experimental work have shown that tPA induces an NF-κB-mediated pro-inflammatory response in astrocytes, 17 and that preconditioning with tPA decreases the volume of the ischemic lesion when intravenously administered one, but not three or 24 hours before tMCAO. 18
The in vivo work presented here shows that intravenous administration of tPA three hours before tMCAO attenuates the development of ischemic edema without decreasing the volume of the ischemic lesion. In line with these observations, our in vitro studies indicate that preconditioning with tPA three, but not one or 24 hours before exposure to oxygen and glucose deprivation (OGD) conditions, attenuates the harmful effect of the hypoxic insult on the transendothelial electrical resistance (TEER) and permeability to albumin of the BBB, without having an effect on neuronal survival. 19 We found that this effect does not require plasmin generation, and is mediated by tPA’s ability to trigger TNF-α-mediated astrocytic activation, and increase the abundance of aquaporin-4-immunoreactive astrocytic end-feet processes in the neurovascular unit, which is pivotal to maintain the structural integrity and barrier function of the BBB. Our work reveals a new role for tPA as inductor of ischemic tolerance in the BBB with potential therapeutic implications for patients with cerebrovascular disease.
Materials and methods
Animals and reagents
Animals were 8–12 weeks-old male C57BL/6J mice. Animal procedures were conducted following the guidelines of the Guide for the Care and Use of Laboratory Animals, and with the approval of the Institutional Animal Care & Use Committee (IACUC) of Emory University, Atlanta GA. Experiments were reported following ARRIVE guidelines for how to report animal experiments. 20 Recombinant plasmin, and proteolytically active and inactive murine tPA [itPA: with an alanine for serine substitution at the active site Ser481 (S481A)] were acquired from Molecular Innovations (Novi, MI; Cat # MPLM, MTPA and MTPA – S481A, respectively). Other reagents were propidium iodide, DMEM without glucose, sample buffer 5X, bovine serum albumin 21 Alexa 488 conjugate, phalloidin Alexa 488, Hoechst, mouse anti-β actin antibodies, donkey anti-rabbit Alexa Fluor 594- and donkey anti-rat IgG (H +L) Alexa Fluor 488-conjugated antibodies (ThermoFisher; Grand Island, NY. Cat # P3566, 11966-025, 1859594, A13100, A122379, H3570, A1978, A21207 and A21208, respectively), rabbit anti-GFAP antibodies (Dako, Santa Clara; CA. Cat# Z0334), anti-TNF-α antibodies (R & D Systems; Minneapolis, MN. Cat # AF-410-NA), rabbit anti-aquaporin-4 and rabbit anti-Iba1 antibodies (Abcam, Cambridge, UK. Cat # ab46182 and ab178846, respectively), mouse anti-CD31 antibodies (BD biosciences; San Jose, CA. Cat # 550274), intercept (TBS) blocking buffer, IRDye 800CW donkey anti-rabbit and IRDye 680CW donkey anti-mouse antibodies (Li-Cor, Lincoln; NE. Cat # 927-60001, 926-32213 and 926-68072, respectively), 2,3,5-Triphenyltetrazolium chloride and Evans blue dye (TTC. MilliPore; Burlington, MA. Cat #: T8877 and E2129, respectively), RIPA buffer (TEKNOVA; Hollister, CA. Cat # R3792), paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA. Cat # 15714-S), primers for tnf [forward 5′-ACGGCATGGATCTCAAAGAC-3′ reverse 5′-CGGACTCCGCAAAGTCTAAG-3′ and actb [forward 5′-ACTGGGACGACATGGAGAAG-3′, reverse 5′-GGGGTGTTGAAGGTCTCAAA-3′ (Integrated DNA Technologies; Coralville, Iowa)], EmeraldAMp Max HS PCR master mix (Takara Bio USA; Mountain View, CA. Cat # RR330A), iScript cDNA Syntesis Kit (Bio-Rad; Hercules, CA. Cat # 1708891), Rneasy mini kit (Qiagen; Germantown, MD. Cat # 74106), and rat brain microvascular endothelial cells, rat microvascular endothelial cell growth medium and attachment factor solution (Cell Applications, Inc; San Diego, CA. Cat # R840-05a, R819 – 500 and 123–100, respectively).
Animal model of cerebral ischemia
Transient occlusion of the middle cerebral artery (tMCAO) was induced in 8 – 12 weeks/old male C57BL/6J mice with a 6-0 silk suture advanced from the external carotid artery (ECA) into the internal carotid artery until the origin of the middle cerebral artery (MCA) as described elsewhere. 22 Briefly, a silicone-coated nylon monofilament (6-0, Ethicon; Issy Les Moulineaux, France) was introduced through the ECA and advanced up to the origin of the MCA. The suture was withdrawn after 60 minutes of cerebral ischemia, followed by 24 hours recovery times. Cerebral perfusion (CP) in the distribution of the MCA was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed Inc., North Royalton, OH), and only animals with a > 70% decrease in CP after occlusion and complete recovery after suture withdrawn were included in this study. The rectal and masseter muscle temperatures were controlled at 37 °C with a homoeothermic blanket.
Neuronal cultures and quantification of cell death
Cerebral cortical neurons were cultured, as described elsewhere, from E16-18 male C57BL/6J mice. 23 Briefly, the cerebral cortex was dissected from, transferred into Hanks' balanced salt solution containing 100 units/ml penicillin, 100 μg/ml streptomycin and 10 mM HEPES, and incubated in trypsin containing 0.02% DNase at 37 °C for 15 min. Tissue was triturated and the supernatant was re-suspended in GS21-supplemented neurobasal medium containing 2 mM l-glutamine, and plated onto 0.1 mg/ml poly-l-lysine-coated wells. To quantify cell survival neurons were preconditioned with 5 nM of tPA or a comparable volume of vehicle (control), followed 3 hours later by exposure to 60 minutes of oxygen and glucose deprivation conditions (OGD: < 0.1% oxygen and no glucose) in an anaerobic chamber (Don Whitley Scientific, Frederick, MD). The uptake of propidium iodide was quantified in each experimental group 24 hours later using the CellSens software and following manufacturer’s instructions, in micrographs taken with a 10x lens connected to a IX83 Olympus microscope. Values are presented as a percentage of neurons exhibiting propidium iodide uptake in relation with total number of Hoechst-positive cells.
Astrocytic cultures
Astrocytes were cultured, as described elsewhere, from the cerebral cortex of 1-day old male C57BL/6J mice. 23 Briefly, the cerebral cortex was dissected, transferred into Hanks' balanced salt solution containing 100 units/ml penicillin, 100 μg/ml streptomycin and 10 mM HEPES, and incubated in trypsin containing 0.02% DNase at 37 °C for 15 min. Tissue was triturated and the supernatant was re-suspended in GS21-supplemented neurobasal medium containing 2 mM l-glutamine, and plated onto 0.1 mg/ml poly-l-lysine-coated wells. The triturated tissue was then resuspended in 10% FBS DMEM and filtered through a 70 μm pore membrane. Cells were plated onto poly-l-lysine-coated T75 flasks. Ten-fourteen days later astrocytes were plated on a 1 µm size 24-well Millicell Hanging Cell Culture Insert as described below.
Quantification of volume of the ischemic lesion and Evans blue dye extravasation
Mice were intravenously treated with 0.9 mg/Kg of proteolytically active or inactive recombinant tPA, or with a comparable volume of saline solution (SS) three hours before undergoing tMCAO as described above. Each syringe was coded according to its load (i.e., tPA or SS) by a technician preparing the samples and not involved in the experiment. The surgeon was blind to the content of each syringe. Twenty-four hours later animals were divided in two groups. The first group was used to quantified Evans blue dye extravasation as described elsewhere. 15 Briefly, animals were intravenously injected with 1 ml of 2% Evans blue dye 60 minutes before transcardial perfusion with PBS. The brains were then removed, divided into ipsilateral and contralateral hemispheres, weighed, homogenized in 400 μl of N,N-dimethylformamide (Sigma-Aldrich), and centrifuged at 21,000 g for 30 minutes. Evans blue was quantified from the absorbance at 620 nm of each supernatant minus the background calculated from the baseline absorbance between 500 and 740 nm, and divided by the wet weight of each hemisphere. The second group of animals was transcardially perfused with paraformaldehyde 4%, brains were harvested and the volume of the infarcted brain was measure in TTC-stained sections and corrected for edema as previously described. 24 Measurements of volume of the ischemic lesion and Evans blue dye extravasation were performed by a technician blind to the experimental conditions (i.e., tPA or SS).
In vitro model of the blood-brain barrier
The in vitro model of the blood-brain barrier (BBB) was assembled as described elsewhere. 25 Briefly, cerebral cortical astrocytes cultured from male C57BL/6J mice as described above were plated either on the underside of a 1 µm size 24-well Millicell hanging cell culture insert, or on the bottom of a 24-well plate. Two hours later 250 μl of rat brain microvascular endothelial cells (Sigma; St. Louis, MO) previously trypsinized and suspended in 3 ml of rat brain endothelial cell growth medium (Sigma; St. Louis, Mo) were seeded on the upper side of the insert which was previously incubated with Attachment Solution Factor solution (Sigma, St. Louis, MO). Following 6 days of incubation in rat endothelial cell growth medium, fresh medium was added to a final volume of 400 μl and 800 μl in the upper and lower chambers, respectively. Cells were allowed to reach confluence at 37 C0/5% CO2. Inserts were then incubated with 5 nM of either proteolytically active or inactive tPA, or 100 nM of plasmin, or 40 ng/ml of anti-TNF-α blocking antibodies, alone or in combination with proteolytically active tPA. After 1, 3 or 24 hours of incubation, inserts were exposed to 60 minutes of oxygen and glucose deprivation (OGD) conditions in an anaerobic chamber (Don Withley Scientific; Bingley, UK) as described elsewhere. 25 To detect the presence of microglia, the underside of a subgroup of inserts (n = 5) was stained with anti-Iba1 antibodies. These experiments indicated that these inserts did not contain microglia (data not shown).
Quantification of transendothelial electrical resistance (TEER) and permeability coefficient to albumin
TEER was measured with a EVOM2 Epithelial Voltohmmeter (World Precision Instruments; Sarasota, Fl) in inserts maintained 60 minutes under either OGD conditions or normoxia in the presence of rat brain endothelial cell growth medium. The average of three TEER measurements for each insert exposed to OGD was compared to the average obtained in inserts kept under normoxia. Values are presented as percentage of TEER in controls. The permeability coefficient was then measured in each insert as described elsewhere. 25 Briefly, inserts were washed twice with PBS. Then, 400 μl of a 50 µg/ml solution of bovine serum albumin 21 conjugated to Alexa Fluor 488 and 800 μl of vehicle were added to the upper and lower chambers, respectively. The concentration of BSA conjugated to Alexa Fluor 488 in the upper and lower chambers was measured with the plate reader Synergy HT with filters set to excitation 485/20 nm and emission 520/20 nm. The permeability coefficient (P) was calculated using the following equation:
where C(B) and C(B) T are the concentrations (μg/mL) of BSA in the lower chamber at the start and at the end of the time interval, respectively, and V(B) is the volume of the lower chamber (in mL). C(A) and C(A) T are, respectively, the concentrations of BSA in the upper chamber at the start and at the end of the time interval and (C(A) + C(A) T) /2 is the average concentration over the time interval. T is the duration of the time interval (60 min), while A is the area of the filter (1.12 cm2).
Immunocytochemistry
Inserts preconditioned with either PBS or 5 nM of tPA and then exposed three hours later to 60 minutes of OGD conditions were fixed during 10 min with 4% paraformaldehyde, washed, permeabilized with 0.1% triton X-100 and blocked with 3% BSA in TBS. Inserts were then incubated overnight at 4 °C with rabbit polyclonal anti-GFAP antibodies (1:1000), and then washed again and incubated with a mixture of donkey anti-rabbit Alexa 488 antibodies, Alexa Fluor 594 Phalloidin (1:500) and Hoechst (1:5000). A sub-set of untreated inserts (n = 5) was incubated with anti-Iba1 antibodies (1:500). Each insert was then removed and mounted with ProLong gold antifade mounting media. Micrographs at 40X magnification were obtained with a DP80 camera attached to a fluorescent Olympus IX83 microscope. GFAP-immunoreactive area was quantified using CellSens dimension 1.17 Olympus software and normalized to the total number of Hoechst-positive nuclei in the underside of each insert. To study the effect of tPA on the distribution of aquaporin-4, astrocytes incubated during 3 hours with 5 nM of tPA or a comparable volume of vehicle (control) were fixed during 15 min with 4% PFA in TBS, washed with HBSS and incubated with 5 μg/ml of WGA-alexa 488 during 15 min. Cells were then washed, incubated with 0.1% triton and 3% BSA in TBS during 20 min at room temperature, incubated overnight with rabbit anti-aquaporin 4 antibody (1:100), and then washed and incubated with an anti-rabbit alexa 594 antibody (1:500). Coverslips were mounted using ProLong gold antifade mounting media and pictures were taken using a Fluoview FV10i automated confocal laser-scanning microscope (Olympus, Center Valley, PA) with the following settings: 60X lens NA 1.35 oil, pinhole 1 UA (50 µm), resolution 1024 x 1024 pixels and 16 bit. We then used the CellSens dimension 1.17 Olympus software to draw a 40 μm line along the WGA-positive plasma membrane, and quantified the proportion of this length that was immunoreactive to anti-aquaporin-4 antibodies. Data are presented as a percentage of aquaporin-positive plasma membrane in vehicle (control)-treated cells.
Immunohistochemistry
Male C57BL/6J mice were preconditioned with either 0.9 mg/Kg of rtPA or with a comparable volume of saline solution 3 hours before 60 minutes of tMCAO. A different subgroup of animals was injected at bregma: 0 mm; lateral: 2 mm; ventral: 1 mm 26 with 2 μl of either saline solution (SS), or 2 μl of either saline solution, or a 5 nM solution of proteolytically inactive tPA (itPA), or a combination of itPA and 600 ng of anti-TNF-α blocking antibodies. Brains were harvested 24 hours later following transcardial perfusion with PBS, fixed with 4% PFA in 10% sucrose/PBS during 2 hours, transferred to 20% sucrose, and cut onto 30 µm sections with a Microm HM 450 microtome. Sections were then were permeabilized with 0.1% triton and blocked with 3% BSA in TBS during one hour at room temperature, followed by overnight incubation at 4 °C with rabbit anti-GFAP (1:1000), alone or in combination with either rat anti-mouse CD31 antibodies (1:100) or rabbit anti-aquaporin 4 (AQP4) antibodies (1:1000), or with rabbit anti-Iba1 antibodies alone (1:1000). Samples were then washed and incubated during one hour at room temperature with donkey anti-rabbit Alexa Fluor 594 (1:500) and donkey anti-rat IgG (H + L) Alexa Fluor 488 antibodies (1:500). Slices were washed and mounted with ProLong gold antifade mounting media. Micrographs at 60X magnification from the ischemic cortex were obtained with a Fluoview FV10i automated confocal laser-scanning microscope (Olympus, Center Valley, PA) with the following settings: NA 1.35 oil, pinhole 1 UA (50 µm), resolution of 1024 × 1024 pixels, 16 bit, voxel size 207 × 207 × 200 nm3 (XYZ), and total volume 212 × 212 × 20 µm3 (XYZ). Six blood vessels were randomly selected in each section with the CellSens dimension 1.17 Olympus software and the areas of GFAP-, Iba1, and CD31-immunoreactivity, were measured in a 10 µm-long region of interest drawn over the area of the blood vessel with maximum diameter. Results are expressed as a CD31-immunoreactive area covered by GFAP-immunoreactive astrocytes, and GFAP-immunoreactive area covered by AQP4-positive puncta, or area of each region of interest covered by either GFAP- or Iba1-immunoreactive cells.
Western blot analysis
To study the effect of tPA on GFAP expression, cerebral cortical astrocytes cultured from male C57BL/6J mice were treated 0, 1 or 3 hours with 5 nM of proteolytically active tPA, or 3 hours with 5 nM of proteolytically inactive tPA (itPA), or three hours with a combination of proteolytically active tPA and 40 ng/ml of anti-TNF-α blocking antibodies. At the end of each period of time, samples were homogenized with RIPA buffer and centrifuged 21000 g × 20 min at 4 °C, and equal amounts of protein, as measured with the BCA assay, were loaded into a mini-protean TGX stain-free 4-15% gel (Bio-Rad, Hercules, CA), transferred into a nitrocellulose membrane (Bio-Rad, Hercules, CA) and blocked with Odyssey blocking buffer (TBS). Membranes were then incubated overnight at 4 °C with rabbit polyclonal anti-GFAP (1:20000), rabbit anti-aquaporin-4 antibodies (1: 1000), and mouse anti-β-actin antibodies (1:50000), and then washed and incubated with IRDye 800CW donkey anti-rabbit (1:10000) and IRDye 680RD donkey anti-mouse secondary antibodies (1:10000). Infrared signal was detected and quantified with the LI-COR Odyssey Fc reader and IMAGE STUDIO 5.2. Values were expressed as a ratio of GFAP signal intensity normalized to β-actin normalized and then to GFAP/β-actin signal in controls.
Polymerase chain reaction
To study the effect of tPA on TNF-α mRNA expression, cerebral cortical astrocytes cultured from male C57BL/6J mice were treated 3 hours with 5 nM of proteolytically active tPA or a comparable volume of vehicle (control). RNA was extracted using the Rneasy mini kit and quantified with a Nanodrop system, cDNA was synthetized with 500 ng of RNA using iScript cDNA Synthesis kit, and PCR for Tnf (TNF-alpha) or Actb (actin) was done with the EmeraldAMp Max HS PCR master mix, using a MultiGene OptiMax Thermal Cycler [72 °C × 2 min; 30 cycles of 98 °C × 10 sec, 60 °C × 30 sec, 72 °C × 20 sec; and 72 °C × 10 min (Labnet International, Edison, NJ)] and the following primers: Tnf (forward 5′-ACGGCATGGATCTCAAAGAC-3′, reverse 5′-CGGACTCCGCAAAGTCTAAG-3′); and Actb (forward 5′-ACTGGGACGACATGGAGAAG-3′, reverse 5′-GGGGTGTTGAAGGTCTCAAA-3′). The amplified PCR product was detected with a 2% agarose gel and quantified with the LI-COR Odyssey Fc reader and IMAGE STUDIO 5.2.
Statistics
To confirm normal distribution of the data when the sample size was less than 10, we used visual inspection and the outliers test, as described elsewhere. Statistical analysis was performed with either two-tailed student’s t-test, or one- or two-way ANOVA with corrections, as deemed as appropriate and described in each figure legend. p-values ≤ 0.05 were considered as significant. The effect of the interaction between experimental groups was calculated using the Prism software (RRID: SCR_002798) with two-way ANOVA test.
Results
Effect of intravenous preconditioning with tPA
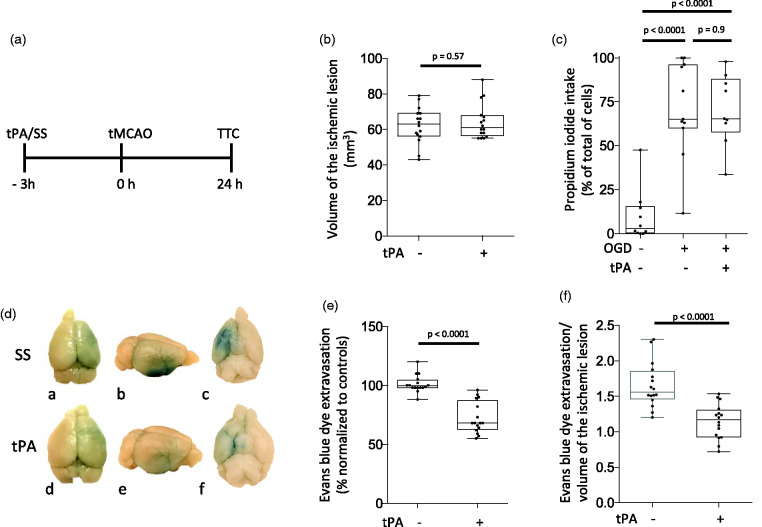
In our early studies we found a decrease in the volume of the ischemic lesion in mice intravenously treated with tPA one but not 24 hours before 60 minutes of tMCAO. 18 However, because clinical studies have shown that treatment with tPA within 3 - 4.5 hours of onset of symptoms is associated with complete or nearly complete recovery of neurological function in a substantial number of acute ischemic stroke patients,27,28 we decided to use the experimental paradigm depicted in Figure 1(a) to test the effect of preconditioning with tPA three hours before tMCAO. Furthermore, we quantified the uptake of propidium iodide (PPI) in cerebral cortical neurons treated with 5 nM of tPA 3 hours before exposure to 60 minutes of oxygen and glucose deprivation (OGD) conditions. We found that preconditioning with tPA three hours before tMCAO or OGD does not have an effect on the volume of the ischemic lesion (Figure 1(b)) or neuronal survival (Figure 1(c)). Because our early work shows that tPA has a direct effect on the permeability of the blood-brain barrier (BBB), 15 we quantified the extravasation of Evans blue dye in a separate cohort of animals subjected to the experimental conditions described above. We found that although preconditioning with tPA three hours before tMCAO does not have an effect on the volume of the ischemic lesion (Figure 1(b)), it effectively attenuates the development of ischemic edema (Figure 1(d) and (e), n = 16 mice per group; p < 0.0001). Furthermore, our finding of a decreased Evans blue dye extravasation/volume of the ischemic lesion ratio in animals preconditioned with tPA (Figure 1(f), n = 16 per group; p < 0.0001) indicates that tPA preconditions the BBB without having an effect on the volume of the ischemic lesion.
Figure 1.
Effect of preconditioning with tPA. A. Diagram of the experimental paradigm used to study the effect of preconditioning with tPA (SS: saline solution SS. TTC: 2,3,5-triphenyltetrazolium chloride). B. Male C57BL/6J mice were intravenously treated with either SS or tPA (n = 16 per group) three hours before 60 minutes of tMCAO. The volume of the ischemic lesion was measured in TTC-stained sections 24 hours later. Statistical analysis: two-tailed student’s t-test. C. Cerebral cortical neurons from male C57BL/6J mice were treated with 5 nM of tPA or PBS three hours before exposure to 60 minutes of oxygen and glucose deprivation (OGD) conditions. Cells were fixed 24 hours later to quantify the uptake of propidium iodide (PPI). Values are given as percentage of PPI-positive cells in relation to the total number of cells. n = 10 per experimental group with cells from three different cultures. Statistical analysis: one-way ANOVA with Holm-Sidaks multiple comparisons test. D. Representative photographs of the dorsal (a & d), lateral (b & e) and ventral (c & f) surface of the brain of male C57BL/6J mice injected with Evans blue dye 24 hours after 60 minutes of tMCAO performed 3 hours after the intravenous administration of either saline solution (SS; a–c), or tPA (tPA; d–f). Blue denotes areas with Evans blue dye extravasation in the ischemic hemisphere. E. Quantification of Evans blue dye extravasation in the ischemic hemisphere of mice preconditioned with tPA three hours before tMCAO, compared with Evans blue dye extravasation in the contralateral hemisphere, and normalized to Evans blue dye extravasation in mice pretreated with SS three hours before the ischemic injury. n = 16 mice per experimental group. Statistical analysis: two-tailed student’s t-test. F. Evans blue dye extravasation/stroke volume ratio in animals exposed to the experimental conditions described in D & E. n = 16 per experimental group. Statistical analysis: two-tailed student’s t-test.
Effect of preconditioning with tPA on the permeability of the blood-brain barrier
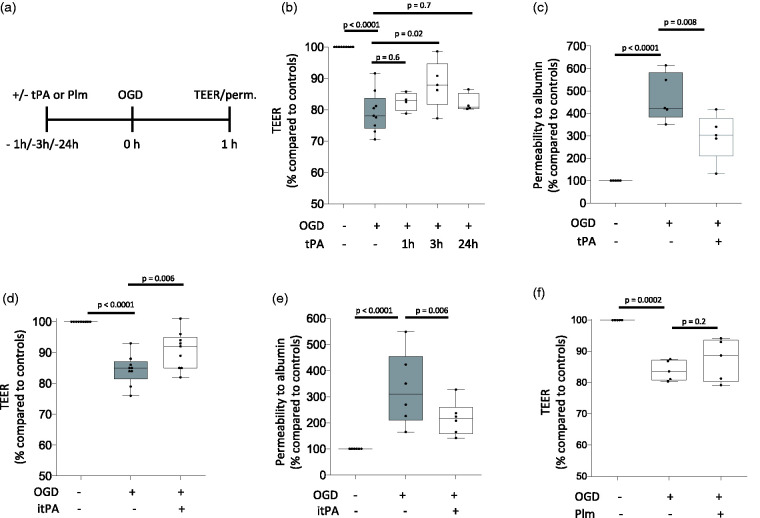
To further characterize our in vivo findings, we used the experimental design depicted in Figure 2(a) to measure the transendothelial electrical resistance (TEER) of an in vitro model of the BBB assembled with brain microvascular endothelial cells and astrocytes co-cultured on both sides of a 1 μm pore size insert and treated with vehicle (control) or 5 nM of tPA either one, or three, or 24 hours before exposure to 60 minutes of oxygen and glucose deprivation (OGD) conditions. Our results show that treatment with tPA three, but not one or 24 hours before the hypoxic insult effectively attenuates the harmful effects of OGD on the TEER of the BBB (Figure 2(b)). We then measured the permeability to FITC-conjugated albumin in BBB inserts treated with 5 nM of tPA three hours before exposure to 60 minutes of OGD. We found that compared to inserts maintained under normoxic conditions (11 inserts assembled with cells from 5 different cultures), OGD causes a 470.7 +/- 107.5% increase in the permeability to albumin (n = 12 inserts assembled with cells from 5 different cultures; p < 0.0001), and that preconditioning with tPA decreases this effect to 295.9 +/− 104.8% (n = 13 inserts assembled with cells from 5 different cultures. p = 0.008 compared to non-preconditioned inserts; Figure 2(c)). Importantly, this effect was independent of tPA’s ability to catalyze the conversion of plasminogen into plasmin as it was also observed in BBB inserts preconditioned with 5 nM of proteolytically inactive tPA (itPA; Figure 2(d) and (e)), but not with 100 nM of plasmin (Figure 2(f)).
Figure 2.
Effect of preconditioning with tPA on the permeability of the blood-brain barrier. a. Diagram depicting the experimental paradigm to study the effect of tPA and plasmin (Plm) on the permeability of an in vitro model of the blood brain barrier (BBB), assembled by brain microvascular endothelial cells and astrocytes co-cultured on both sides of a 1 μm pore size insert. b. The in vitro model of the BBB described in A was exposed to 60 minutes of oxygen and glucose deprivation (OGD) conditions either 1 (n = 15 inserts assembled with cells from 4 different cultures), or 3 (n = 12 inserts assembled with cells from 5 different cultures), or 24 hours (n = 10 inserts assembled with cells from 4 different cultures) after treatment with 5 nM of tPA. A subgroup of inserts was kept under physiological conditions (n = 16, assembled with cells from 9 different cultures). Values depict the transendothelial electrical resistance (TEER) in inserts exposed to OGD conditions compared to the TEER in inserts maintained under normoxic conditions. Statistical analysis: one-way ANOVA with Holm-Sidak’s multiple comparisons test. c. The in vitro model of the BBB described in A was treated with 5 nM of tPA (n = 13 inserts assembled with cells from 5 different cultures), or vehicle (control; n = 12 inserts assembled with cell from 5 different cultures) 3 hours before exposure to 60 minutes of OGD. The permeability to albumin in inserts exposed to OGD conditions was compared to the permeability of inserts maintained under normoxic conditions (n = 11, assembled with cells from 5 different cultures). Statistical analysis: one-way ANOVA with multiple comparisons test. d. TEER of the in vitro model of the BBB described in A and treated with either 5 nM of proteolytically inactive tPA (itPA; n = 19 inserts assembled with cells from 9 different cultures), or vehicle (control; n = 18 inserts assembled with cells from 9 different cultures) 3 hours before 60 minutes of OGD. Data were compared to the TEER in inserts maintained under normoxic conditions (n = 19, assembled with cells from 9 different cultures). Statistical analysis: one-way ANOVA with Holms-Sidak’s multiple comparisons test. e. Permeability to albumin in the in vitro model of the BBB described in A and treated with either 5 nM of proteolytically inactive tPA (itPA; n = 18 inserts assembled with cells from 5 different cultures), or vehicle (control; n = 17 inserts assembled with cells from 6 different cultures) 3 hours before exposure to 60 minutes of OGD. In each case data were compared to permeability to albumin in inserts maintained under normoxic conditions (n = 17, assembled with cells from 6 different cultures). Statistical analysis: one-way ANOVA with Holms-Sidak’s multiple comparisons test. f. TEER of the in vitro model of the BBB described in A and treated with either 100 nM of plasmin (Plm; n = 10 inserts assembled with cells from 5 different cultures), or vehicle (control; n = 10 inserts assembled with cells from 5 different cultures) three hours before exposure to 60 minutes of OGD. In each case data were compared to the TEER of inserts maintained under normoxic conditions (n = 9 inserts assembled with cells from 5 different cultures). Statistical analysis: one-way ANOVA with Holms-Sidak’s multiple comparisons test.
TPA induces astrocytic activation
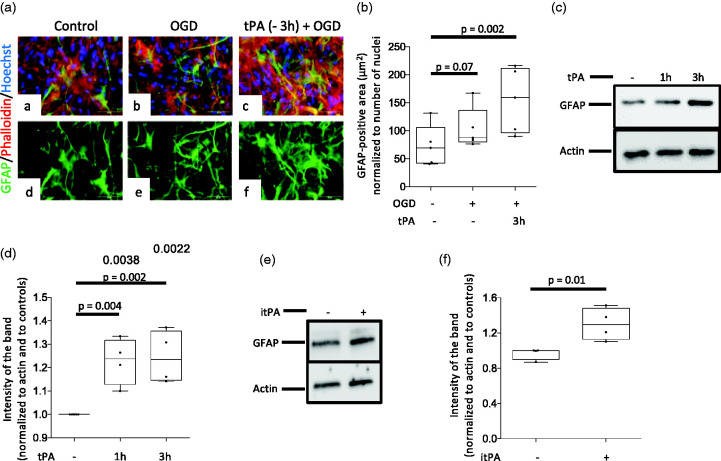
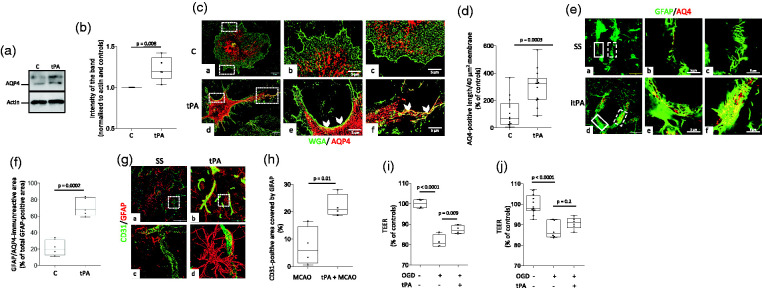
To investigate the mechanism whereby tPA preconditions the BBB, we used anti-GFAP antibodies to immunostain the underside of BBB inserts maintained under normoxic conditions (controls) or preconditioned with 5 nM of tPA or vehicle (control) 3 hours before exposure to 60 minutes of OGD conditions. These experiments revealed an increase in GFAP-immunoreactive astrocytes in inserts preconditioned with tPA (Figure 3(a) and (b)). In line with these observations, our immunoblottings indicate that 3 hours of treatment with either proteolytically active (Figure 3(c) and (d)) or inactive tPA (Figure 3(e) and (f)) effectively induce astrocytic activation, as denoted by an increase in the abundance of GFAP in both experimental groups.
Figure 3.
Preconditioning with tPA induces astrocytic activation. a. The in vitro model of the blood-brain barrier (BBB) was maintained under normoxic conditions (panels a & d), or treated with either PBS (panels b & e) or 5 nM of tPA (panels c & f) three hours before exposure to 60 minutes of OGD conditions. Inserts were then harvested and their underside was stained with phalloidin (red), Hoechst (blue) and anti- GFAP antibodies (green). Confocal micrographs were taken at 60 X magnification. B. GFAP-immunoreactive area normalized to the number of Hoechst-positive nuclei on the underside of the BBB inserts exposed to the experimental conditions described in b. n = 5 inserts per experimental group, assembled with cells from 3 different cultures. Statistical analysis: one way ANOVA with Holm-Sidak’s multiple comparisons. c & d. Representative Western blot analysis (C) and quantification of the intensity of the band normalized to actin (D) of GFAP abundance in astrocytes treated 1 or 3 hours with 5 nM of tPA. n = 4 per experimental group. Statistical analysis: one way ANOVA with Dunnett’s multiple comparisons test. e & f. Representative Western blot analysis (E) and quantification of the intensity of the band normalized to actin (F) of GFAP abundance in astrocytes treated during three hours with vehicle (control) or 5 nM of proteolytically inactive tPA (itPA). n = 4 per experimental group. Statistical analysis: two-tailed student’s t-test.
TNF-α mediates tPA-induced astrocytic activation and the preconditioning effect of tPA on the blood-brain barrier
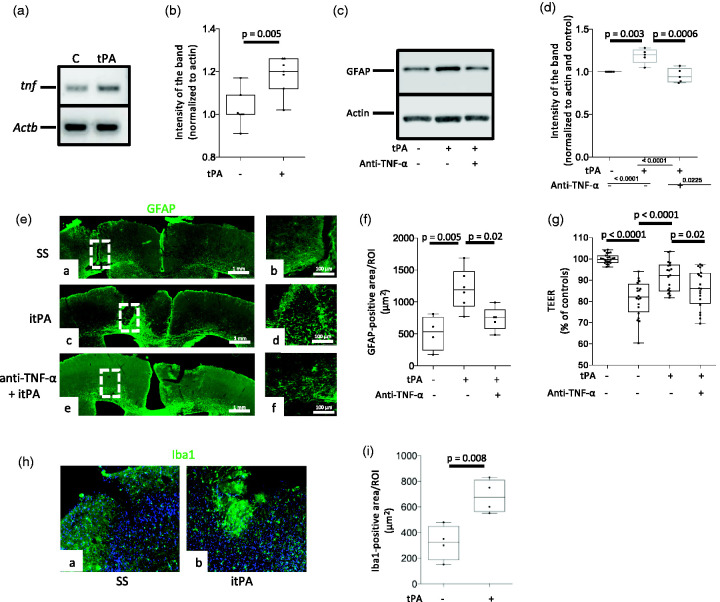
Because TNF-α mediates the development of ischemic preconditioning, 29 we then decided to study the effect of tPA on the expression of TNF-α mRNA in cerebral cortical astrocytes. We found that 3 hours of treatment with 5 nM of tPA increases the abundance of TNF-α mRNA (Figure 4(a) and (b)). Furthermore, our immunoblottings with extracts prepared from astrocytes treated during 3 hours with 5 nM of tPA, alone or in combination with 40 ng/ml of anti-TNF-α blocking antibodies, indicate that TNF-α mediates tPA-induced astrocytic activation (Figure 4(c) and (d)). To study the in vivo relevance of these observations and of our finding that tPA-induced astrocytic activation does not require plasmin generation (Figure 3(e) and (f)), we quantified the area of the frontal cortex of wild-type mice populated by activated astrocytes 24 hours after the intracerebral injection of either 5 nM of proteolytically inactive tPA (itPA), alone or in combination with 600 ng of anti-TNF-α blocking antibodies, or a comparable volume of saline solution. These in vivo experiments confirmed the finding that tPA induces astrocytic activation, and that this effect is mediated by TNF-α and does not require plasmin generation (Figure 4(e) and (f)). To determine if TNF-α mediates the preconditioning effect of tPA on the BBB, we quantified the TEER of the in vitro model of the BBB described in Figure 2, following preconditioning with 5 nM of tPA, alone or in combination with 40 ng/ml of anti-TNF-α blocking antibodies three hours before exposure to 60 minutes of OGD conditions. These results revealed that TNF-α mediates the preconditioning effect of tPA on the BBB (Figure 4(g)). Because microglia are an important source of TNF-α in the brain, we stained the underside of the inserts used to assemble the in vitro model of the BBB with anti-Iba1 antibodies. We failed to detect activated microglia before or after tPA treatment (data not shown). To further characterize the translational relevance of these observations, we quantified the area of the cerebral cortex covered by Iba1-positive microglia in the same cuts used for the experiments described in Figure 4(e) and (f). In contrast with our in vitro observations, these studies revealed a significant increase in the area covered by activated microglia in tPA-injected brains (Figure 4(h) and (i)).
Figure 4.
TNF-α mediates tPA-induced astrocytic activation. A & B. Representative PCR analysis (a) and mean expression of tnf and Actb mRNA (b) in astrocytes incubated during 3 hours with 5 nM of tPA or vehicle (C: control). n = 7 per experimental group. Statistical analysis: two-tailed student’s t-test. C & D. Representative Western blot analysis (c) and quantification of the intensity of the band (d) of GFAP abundance in astrocytes treated during three hours with vehicle (control) or 5 nM of tPA, alone or in the presence of 40 ng/ml of anti-TNF-α blocking antibodies. n = 5 per experimental group. Statistical analysis: one-way ANOVA with Tukey’s multiple comparisons test. (e). Representative micrographs at 10X magnification of GFAP expression (green) in the frontal cortex of wild-type mice 24 hours after the intracerebral injection of 2 μl of either saline solution (SS; panels a & d), or a 5 nM solution of proteolytically inactive tPA (itPA; panels b & e), or a combination of itPA and 600 ng of anti-TNF-α blocking antibodies (panels c & f). Panels d, e, and f are 5X magnifications of the areas depicted by the dashed squares in panels a, b and c. (f) GFAP-positive area in regions of interest (ROI) drawn over the frontal cortex of animals exposed to the experimental conditions described in E. n = 4 (SS), 6 (itPA) and 5 (itPA + anti-TNF-α blocking antibodies). Statistical analysis: one-way ANOVA with Holm-Sidak’s multiple comparison’s test. (g) TEER of the in vitro model of the blood-brain barrier (BBB) assembled by brain microvascular endothelial cells and astrocytes co-cultured on both sides of a 1 μm pore size insert and pretreated with either 5 nM of tPA (n = 19 inserts assembled with cells from 4 cultures), or vehicle (control; n = 20 inserts assembled with cells from 3 different cultures), or a combination of tPA and 40 ng/ml of anti-TNF-α blocking antibodies (n = 20 inserts assembled with cells from 4 different cultures), 3 hours before exposure to 60 minutes of OGD conditions. Data are presented in relation to the TEER of inserts maintained under normoxic conditions (n = 24 inserts assembled with cells from 3 different cultures). Statistical analysis: one-way ANOVA with Tukey’s multiple comparisons test. (h) Representative micrographs at 20X magnification of Iba1 expression (green) in the frontal cortex of wild-type mice 24 hours after the intracerebral injection of 2 μl of either saline solution (SS; panel a) or a 5 nM solution of proteolytically inactive tPA (itPA; panels b). (i) Iba1-immunoreactive area in regions of interest (ROI) drawn over the frontal cortex of animals exposed to the experimental conditions described in H. n = 4 animals per experimental group. Statistical analysis: student’s t-test test.
Preconditioning with tPA increases the abundance of aquaporin-4-immunoreactive astrocytic end-feet processes in the neurovascular unit
Because early upregulation of aquaporin-4 has a protective effect against the development of post-ischemic edema, 30 we decided to study the effect of tPA on its abundance and recruitment to astrocytic end-feet processes. Our immunoblottings and confocal microscopy studies indicate that tPA not only increases the abundance of aquaporin-4 in cerebral cortical astrocytes (Figure 5(a) and (b)) but also triggers its redistribution to peripheral astrocytic processes (Figure 5(c) and (d)). To study the in vivo relevance of these observations, and to determine if the effect of tPA on aquaporin-4 requires the conversion of plasminogen into plasmin, we quantified the expression of aquaporin-4 in astrocytic end-feet processes surrounding the neurovascular unit in the frontal cortex of Wt mice 24 hours after the intracerebral injection of 5 nM of proteolytically inactive tPA or a comparable volume of saline solution (SS). Our data reveal that treatment with tPA increases the area of astrocytes immunoreactive to aquaporin-4 from 21.13 +/− 16.02% in SS-treated animals to 70.97 +/− 43.05% (Figure 5(e) and (f), n = 5 mice per condition, 6 micrographs per animal. p = 0.0002, two-tailed student’s t-test). Because aquaporin-4 regulates the motility of astrocytic end-feet processes 31 and their interaction with endothelial cells, 32 and since both events are pivotal to maintain the TEER and barrier function of the BBB,19,33 we decided to quantify the area of CD31-positive blood vessels colocalizing with GFAP-immunoreactive astrocytes (activated astrocytes) in the peri-ischemic cortex of male C57BL/6J mice intravenously preconditioned with either saline solution or tPA three hours before 60 minutes of tMCAO. Our confocal microscopy studies revealed that preconditioning with tPA increases the abundance of activated astrocytes in the neurovascular unit (Figure 5(g) and (h)). To determine if the formation of these contacts mediates the preconditioning effect of tPA, we quantified the TEER of two different models of the BBB preconditioned with tPA or vehicle (control) three hours before exposure to 60 minutes of OGD conditions. In the first model, astrocytic end-feet processes were in direct contact with endothelial cells, as astrocytes and endothelial cells were plated on opposite sides of the same insert. In the second model, astrocytic end-feet processes were not in direct contact with endothelial cells, as astrocytes were plated on the bottom of the lower chamber and endothelial cells were grown on the upper side of the insert. We found that preconditioning with tPA preconditions the BBB when astrocytes are in direct contact with endothelial cells (Figure 5(i)) but not when they are plated on the bottom of the well (Figure 5(j)).
Figure 5.
Preconditioning with tPA increases the abundance of activated astrocytes and aquaporin-4-immunoreactive astrocytic end-feet processes in the neurovascular unit. A & B. Representative Western blot analysis (a) and quantification of the intensity of the band (b) of aquaporin-4 (AQ4) abundance in astrocytes incubated for three hours with vehicle (control) or 5 nM of tPA. n = 5 per experimental group. Statistical analysis: two-tailed student’s t-test. C. Representative confocal micrographs at 60X magnification of cerebral cortical astrocytes immunostained with the membrane marker Wheat Germ Agglutinin-Alexa Fluor 488 Conjugate (WGA; green) and anti-aquaporin-4 antibodies (AQP4; red) 3 hours after treatment with vehicle (control: C; panels a - c) or 5 nM of tPA (tPA; panels d - f). Panels b & c and e & f correspond to a 5X magnification of the area depicted by the white squares in a & b, respectively. Arrowheads in panels e & f show areas of the plasma membrane immunoreactive to anti-AQP4 antibodies in tPA-treated astrocytes. (d) Quantification of AQP4 immunoreactivity in end-feet processes of astrocytes exposed to the experimental conditions described in C. Values are given as percentage of control-treated cells. n = 14 cells from three different cultures per experimental group. Statistical analysis: two-tailed student’s t-test. (e & f) Representative confocal micrographs at 60 X magnification (a & d) of GFAP (green) and AQP4 (red) immunoreactivity in blood vessels of the frontal cortex of male C57BL/6J mice 24 hours after the intracerebral injection of 2 μl of either saline solution (SS; panel a), or a 5 nM solution of proteolytically inactive itPA (itPA; panel d). Panels b & c and d & f correspond to magnifications of the areas depicted by the continuous (b & e) and dashed (c & f) squares in a and d, respectively. F. Percentage of the total GFAP-immunoreactive area in the blood vessel wall that co-localizes with AQP4. n = 5 brains per experimental condition. Each dot represents the average of 6 micrographs per brain. Statistical analysis: two-tailed student’s t-test. (g) Representative three-dimensional reconstruction of confocal micrographs taken from the area surrounding the necrotic core of male C57BL/6J mice 24 hours after 60 minutes of tMCAO. Three hours before tMCAO animals were intravenously treated with either saline solution (a & c) or tPA (b & d). Green: CD31; red: GFAP. Magnification: 60X in a & b. Panels c & d correspond to a 400 X magnification of the area depicted by the dashed squares in a & b. (h) Quantification of the area immunoreactive to CD31 and GFAP in blood vessels in the zone surrounding the necrotic core of animals subjected to the experimental conditions described in G. n = 4 animals per experimental group, 6 micrographs per animal. Statistical analysis: two-tailed student’s t-test. (i & j) The in vitro model of the blood-brain barrier (BBB) assembled with brain microvascular endothelial cells cultured on the upper side of a 1 μm pore size insert and astrocytes plated either on the underside of the insert (i), or in the bottom of the lower chamber (j), were preconditioned with 5 nM of tPA (n = 4 in G and 6 in H) or vehicle (control; n = 4 in G and 6 in H), followed 3 hours later by exposure to 60 minutes of OGD conditions. The transendothelial electrical resistance (TEER) was quantified immediately after OGD and compared with the TEER of inserts maintained under normoxic conditions (n = 4 in G and 12 in H). Statistical analysis: one-way ANOVA with Tukey’s multiple comparisons test.
Discussion
TPA is a serine proteinase assembled by a fibronectin- and EGF-like domains, and two kringles and a serine proteinase region. 34 Because it is abundantly found in endothelial cells 35 and has high affinity for fibrin via its second kringle and EGF-like domains, 36 it was initially believed that its only role was to trigger intravascular fibrinolysis by its ability to catalyze the conversion of plasminogen into plasmin. 37 This led to the successful use of intravenous tPA to treat acute ischemic stroke patients within 3–4.5 hours of onset of symptoms.27,28
However, subsequent studies indicated that tPA is also abundantly found in the central nervous system 38 and that its release in the ischemic tissue 39 by neurons, astrocytes and microglia has a plethora of effects that not always require plasmin generation, 40 such as the development of synaptic plasticity,13,41 cell survival, 23 microglial activation 14 and regulation of the permeability of the BBB.15,16 The studies presented here reveal that preconditioning with tPA attenuates the harmful effects of cerebral ischemia on the structural integrity and permeability of the BBB when administered three hours before the ischemic insult. We show that this effect is independent of neuronal survival, does not require plasmin generation, and is mediated by tPA’s ability to trigger TNF-α-induced astrocytic activation, and increase the abundance of activated astrocytes and the neurovascular unit.
The phenomenon of ischemic tolerance was initially described in the heart 42 and then in the brain, 2 and although in both cases the preconditioning event was a brief period of ischemia/hypoxia, 43 it was soon recognized that many other stimuli such as inflammation, seizures, hypothermia, hyperthermia and cortical spreading depression are also capable to render the brain resistant to a subsequent lethal ischemic injury.44–46 Remarkably, the clinical relevance of these findings is underscored by the observation that brief episodes of non-lethal cerebral ischemia, known as transient ischemic attacks, also precondition the human brain. 47
The interaction between endothelial cells, perivascular astrocytes and the basement membrane is pivotal to maintain the structural and functional integrity of the BBB. 19 Significantly, in vitro studies have shown that hypoxic preconditioning has a protective effect on endothelial cells, 48 and that astrocytes play a central role in the development of ischemic tolerance.6,7 Furthermore in vivo work has revealed that ischemic preconditioning attenuates the development of cerebral edema. 11 However, because the final outcome in these studies was cell death and the volume of the ischemic lesion, it is not clear if it is possible to precondition the BBB independently of a beneficial effect on neuronal survival. Our studies indicate that treatment with tPA three, but not one or 24 hours before 60 minutes of either tMCAO or exposure to OGD conditions, attenuates the harmful effect of a hypoxic/ischemic injury on the TEER and permeability of the BBB, without decreasing the volume of the ischemic lesion or attenuating neuronal death. Importantly, in contrast with its effect on the BBB, our early studies indicate that the preconditioning effect of tPA on neuronal survival is evident when it is administered either 5 minutes before OGD, or 60 minutes before tMCAO. 18 Together with our previous work, these results indicate that tPA is able to induce delayed ischemic tolerance in the BBB and rapid preconditioning in neurons. Our findings suggest that while neuronal preconditioning most likely requires rapid tPA-induced post-translational modifications, the preconditioning effect of tPA on the BBB is mediated by gene induction and activation of proinflammatory pathways. Although we do not know if there is a time-point for the administration of tPA to precondition both, neurons and the BBB, our data indicate that tPA may be used to protect the brain of patients scheduled to undergo interventional procedures with high risk of cerebral ischemia, or in those suffering transient episodes of cerebral ischemia, which are known to increase the risk of a subsequent ischemic stroke. Furthermore, it is important to keep in mind that the preconditioning effect of tPA does not require plasmin generation, and thus is devoid of plasmin-induced hemorrhagic complications.
The molecular pathway that mediates the preconditioning effect of tPA on the BBB is still unknown. However, although our previous studies indicate that the interaction between tPA and the low density lipoprotein receptor-related protein-1 (LRP-1) in astrocytic end-feet processes has a direct effect on the permeability of the BBB,15,16 further studies are required to determine if LRP-1 also mediates the preconditioning effect of tPA on the BBB. It has long been recognized that cytokines that induce a stress response are capable to trigger ischemic tolerance, 49 and in line with these observations, pretreatment with TNF-α induces ischemic tolerance in an in vivo model of cerebral ischemia. 50 Our earlier work indicates that tPA induces NF-κB activation in astrocytes, 51 and our current studies show that the preconditioning effect of tPA on the BBB is mediated by astrocytic activation and is partially abrogated by anti-TNF-α antibodies. The temporal profile of tPA-induced TNF-α-mediated BBB preconditioning agrees with genomic studies indicating that activation of toll- like receptors (TLR) is an effective inductor of ischemic tolerance when the stimulus is applied three hours before the ischemic insult.52,53 We acknowledge that TNF-α is not the only mediator of ischemic preconditioning. In agreement with this observation, our studies indicate that anti-TNF-α antibodies have a significant but still incomplete inhibitory effect on the preconditioning effect of tPA on the permeability of the BBB. Thus, further research is necessary to identify other molecular pathways whereby tPA renders the BBB tolerant to an ischemic injury. Furthermore, it is unknown if estrogens play a role on the preconditioning effect of tPA on the BBB, as our study only included male mice This is an issue of high translational relevance that is currently under investigation.
Experimental evidence reveals that ischemic preconditioning induces astrocytic activation, as shown by an increase in the abundance of GFAP. 54 Our in vitro and in vivo studies show that tPA induces astrocytic activation, as denoted by an increase in GFAP expression in cultured astrocytes incubated with tPA. Significantly, we found that tPA increases the abundance of astrocytic TNF-α mRNA, and that inhibition of TNF-α abrogates not only tPA-induced astrocytic activation, but also the preconditioning effect of tPA. These findings are in line with the reported preconditioning effect of TNF-α in in vivo and in vitro models of hypoxia and ischemia.29,55 Importantly, our immunocytochemical studies show that the inserts used to assemble our in vitro model of the BBB are devoid of microglia, and thus that these cells are not the source of TNF-α following tPA treatment. In contrast, our in vivo experiments indicate that tPA treatment induces microglial activation in the cerebral cortex. Thus, it is possible that these cells are also a source of TNF-α following preconditioning with tPA.
Aquaporin-4 regulates the movement of water across cell membranes, and although its expression increases within one hour of the onset of cerebral ischemia, 56 it is unclear if this effect contributes or attenuates the development of ischemic edema. The role of aquaporin-4 in astrocytic end-feet processes has been the focus of intense research. Accordingly, it has been shown that under non-ischemic conditions aquaporin-4 mediates the movement of fluid between the paravascular space and the brain interstitium. 57 However, the role of aquaporin-4 in astrocytic end-feet processes under ischemic conditions seems to be different. Indeed, aquaporin-4 has been reported to play a central role in the reabsorption of fluid from the extracellular space, and in the bidirectional movement of water from and to the brain. 58 In line with these observations, it has been shown that genetic deletion of aquaporin-4 impairs the cellular uptake of water and increases the severity of cerebral edema in animal models of cerebral ischemia and traumatic brain injury.58,59 These observations agree with our data indicating that upregulation of aquaporin-4 in astrocytic end-feet processes induced by preconditioning with tPA renders the BBB resistant to the deleterious effects of an ischemic injury. Importantly, it is unknown if sex plays a role on the preconditioning effect of tPA on the BBB, as our study only included male mice.
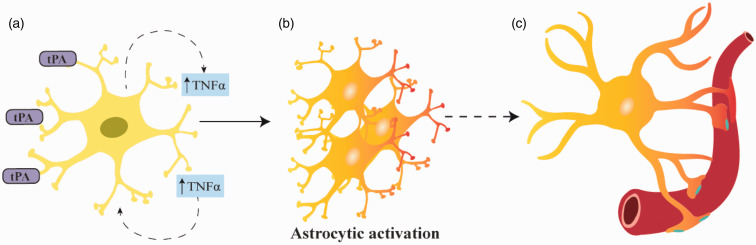
In summary, we propose a model (Figure 6) in which tPA-induced release of TNF-α from cerebral cortical astrocytes triggers their activation and increases their abundance in the neurovascular unit. Our data indicate that this sequence of events renders the BBB tolerant to an ischemic/hypoxic injury without having an effect on neuronal death. This is a new role for tPA in the central nervous system with potential therapeutic implications for patients with cerebrovascular disease.
Figure 6.
Proposed mechanism whereby tPA preconditions the blood-brain barrier. Treatment with tPA induces the release of TNF-α from cerebral cortical astrocytes (A), which then triggers their activation (B). TPA also increases the abundance of aquaporin-4 and prompts its redistribution to astrocytic end-feet processes (blue dots in C), which by entering in contact with the neurovascular unit prevent the harmful effects of the ischemic injury on the permeability of the BBB.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211060395 for Tissue-type plasminogen activator induces TNF-α-mediated preconditioning of the blood-brain barrier by Ariel Diaz, Yena Woo, Cynthia Martin-Jimenez, Paola Merino, Enrique Torre and Manuel Yepes in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Institutes of Health Grant NS-NS091201 (to M.Y) and VA MERIT Award IO1BX003441 (to M.Y).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: Ariel Diaz: designed and performed experiments, and analyzed data. Paola Merino: performed experiments. Yena Woo: performed experiments. Cynthia Martin-Jimenez: performed experiments. Enrique Torre: performed experiments. Manuel Yepes: designed experiments, analyzed data, and wrote paper.
ORCID iD: Manuel Yepes https://orcid.org/0000-0002-5224-9663
Supplemental material: Supplemental material for this article is available online.
References
- 1.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 2006; 7: 437–448. [DOI] [PubMed] [Google Scholar]
- 2.Schurr A, Reid KH, Tseng MT, et al. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res 1986; 374: 244–248. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Pinzon MA, Xu GP, Dietrich WD, et al. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab 1997; 17: 175–182. [DOI] [PubMed] [Google Scholar]
- 4.Cadet JL, Krasnova IN. Cellular and molecular neurobiology of brain preconditioning. Mol Neurobiol 2009; 39: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 2009; 8: 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia 2005; 50: 307–320. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama Y, Ikeda-Matsuo Y, Notomi S, et al. Astrocyte-mediated ischemic tolerance. J Neurosci 2015; 35: 3794–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morizawa YM, Hirayama Y, Ohno N, et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat Commun 2017; 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romera C, Hurtado O, Botella SH, et al. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci 2004; 24: 1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci 2002; 22: 10291–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masada T, Hua Y, Xi G, et al. Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cereb Blood Flow Metab 2001; 21: 22–33. [DOI] [PubMed] [Google Scholar]
- 12.Collen D. The plasminogen (fibrinolytic) system. Thromb Haemost 1999; 82: 259–270. [PubMed] [Google Scholar]
- 13.Qian Z, Gilbert ME, Colicos MA, et al. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 1993; 361: 453–457. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, An J, Haile W, et al. Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain. J Cereb Blood Flow Metab 2009; 29: 1946–1954. [DOI] [PubMed] [Google Scholar]
- 15.Yepes M, Sandkvist M, Moore E, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 2003; 112: 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polavarapu R, Gongora M, Yi H, et al. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood 2007; 109: 3270–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polavarapu R, Gongora MC, Winkles JA, et al. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappaB pathway activation. J Neurosci 2005; 25: 10094–10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haile WB, Wu J, Echeverry R, et al. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J Cereb Blood Flow Metab 2012; 32: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. NatRev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 20.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobsar I, Berghoff M, Samsam M, et al. Preserved myelin integrity and reduced axonopathy in connexin32-deficient mice lacking the recombination activating gene-1. Brain 2003; 126: 804–813. [DOI] [PubMed] [Google Scholar]
- 22.Diaz A, Merino P, Manrique LG, et al. Urokinase-type plasminogen activator (uPA) protects the tripartite synapse in the ischemic brain via ezrin-mediated formation of peripheral astrocytic processes. J Cererb Blood Flow Metab 2019; 39: 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Echeverry R, Wu J, Haile W, et al. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest 2010; 120: 2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990; 10: 290–293. [DOI] [PubMed] [Google Scholar]
- 25.Haile WB, Echeverry R, Wu J, et al. The interaction between tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 promotes the recruitment of neutrophils into the ischemic brain. J Cereb Blood Flow Metab 2010; 30: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press Inc., 2001, pp.1–93. [Google Scholar]
- 27.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 28.The National Institute of Neurological Disorders Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 29.Ginis I, Jaiswal R, Klimanis D, et al. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab 2002; 22: 142–152. [DOI] [PubMed] [Google Scholar]
- 30.Hirt L, Ternon B, Price M, et al. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab 2009; 29: 423–433. [DOI] [PubMed] [Google Scholar]
- 31.Ciappelloni S, Bouchet D, Dubourdieu N, et al. Aquaporin-4 surface trafficking regulates astrocytic process motility and synaptic activity in health and autoimmune disease. Cell Rep 2019; 27: 3860–3872. [DOI] [PubMed] [Google Scholar]
- 32.Michinaga S, Koyama Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Ijms 2019; 20: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchaud C, Le Bert M, Dupouey P. Are close contacts between astrocytes and endothelial cells a prerequisite condition of a blood-brain barrier? The rat subfornical organ as an example. Biol Cell 1989; 67: 159–165. [PubMed] [Google Scholar]
- 34.Pennica D, Holmes WE, Kohr WJ, et al. Cloning and expression of human tissue-type plasminogen-activator CDNA in Escherichia coli. Nature 1983; 301: 214–221. [DOI] [PubMed] [Google Scholar]
- 35.Emeis JJ, Eijnden-Schrauwen Y, van den Hoogen CM, et al. An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol 1997; 139: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Zonneveld AJ, Veerman H, Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A 1986; 83: 4670–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lijnen HR, Collen D. Interaction of plasminogen activators and inhibitors with plasminogen and fibrin. Semin Thromb Hemost 1982; 8: 2–10. [DOI] [PubMed] [Google Scholar]
- 38.Sappino AP, Madani R, Huarte J, et al. Extracellular proteolysis in the adult murine brain. J Clin Invest 1993; 92: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YF, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med 1998; 4: 228–231. [DOI] [PubMed] [Google Scholar]
- 40.Yepes M, Roussel B, Ali C, et al. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci 2009; 32: 48–55. [DOI] [PubMed] [Google Scholar]
- 41.Jeanneret V, Wu F, Merino P, et al. Tissue-type plasminogen activator (tPA) modulates the postsynaptic response of cerebral cortical neurons to the presynaptic release of glutamate. Front Mol Neurosci 2016; 9: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124–1136. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa K, Matsumoto M, Kuwabara K, et al. Ischemic tolerance' phenomenon detected in various brain regions. Brain Res 1991; 561: 203–211. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann C, Ginis I, Furuya K, et al. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res 2001; 895: 59–65. [DOI] [PubMed] [Google Scholar]
- 45.Chopp M, Chen H, Ho KL, et al. Transient hyperthermia protects against subsequent forebrain ischemic cell damage in the rat. Neurology 1989; 39: 1396–1398. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi S, Harris VA, Welsh FA. Spreading depression induces tolerance of cortical neurons to ischemia in rat brain. J Cereb Blood Flow Metab 1995; 15: 721–727. [DOI] [PubMed] [Google Scholar]
- 47.Wegener S, Gottschalk B, Jovanovic V, et al.; MRI in Acute Stroke Study Group of the German Competence Network Stroke. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke 2004; 35: 616–621. [DOI] [PubMed] [Google Scholar]
- 48.Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab 2003; 23: 1348–1355. [DOI] [PubMed] [Google Scholar]
- 49.Tasaki K, Ruetzler CA, Ohtsuki T, et al. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res 1997; 748: 267–270. [DOI] [PubMed] [Google Scholar]
- 50.Nawashiro H, Tasaki K, Ruetzler CA, et al. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab 1997; 17: 483–490. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Polavarapu R, She H, et al. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol 2007; 171: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenzel-Poore MP, Stevens SL, Xiong Z, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 2003; 362: 1028–1037. [DOI] [PubMed] [Google Scholar]
- 53.Stenzel-Poore MP, Stevens SL, King JS, et al. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke 2007; 38: 680–685. [DOI] [PubMed] [Google Scholar]
- 54.Gesuete R, Orsini F, Zanier ER, et al. Glial cells drive preconditioning-induced blood-brain barrier protection. Stroke 2011; 42: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 55.Ginis I, Schweizer U, Brenner M, et al. TNF-alpha pretreatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol 1999; 276: C1171–C1183. [DOI] [PubMed] [Google Scholar]
- 56.Clement T, Rodriguez-Grande B, Badaut J. Aquaporins in brain edema. J Neurosci Res 2020; 98: 9–18. [DOI] [PubMed] [Google Scholar]
- 57.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra111–147ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zador Z, Stiver S, Wang V, et al. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol 2009; 190: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papadopoulos MC, Manley GT, Krishna S, et al. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. Faseb J 2004; 18: 1291–1293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211060395 for Tissue-type plasminogen activator induces TNF-α-mediated preconditioning of the blood-brain barrier by Ariel Diaz, Yena Woo, Cynthia Martin-Jimenez, Paola Merino, Enrique Torre and Manuel Yepes in Journal of Cerebral Blood Flow & Metabolism