Abstract
Objective
To compare the differences in adverse effects and efficacy profile between bacillus Calmette-Guerin (BCG) Danish 1331 and BCG Moscow-I strain in management of non-muscle invasive bladder cancer.
Methods
Clinical data of 188 cases of non-muscle invasive bladder cancer treated with BCG between January 2008 and December 2018 in our institute were collected prospectively and analysed retrospectively, and 114 patients who completed a minimum of 12 months of follow-up were analysed. Patient and tumor characteristics, strain of BCG, adverse effects, and tumor progression were included for analysis. Intravesical BCG was instilled in intermediate- and high-risk patients. Six weeks of induction BCG, followed by three weekly maintenance BCG at 3, 6, 12, 18, and 24 months was advised in high-risk patients.
Results
Overall 68 patients received BCG Danish 1331 strain and 46 patients received Moscow-I strain. Patient and tumor characteristics were well balanced between the two groups. The median follow-up period was 42.5 months and 34.5 months in Danish 1331 and Moscow-I groups, respectively. Adverse events like dropout rate, antitubercular treatment requirement, and need of cystectomy were higher in Moscow-I group (n=31, 67.4%) when compared to Danish 1331 strain (n=33, 48.5%) (p=0.046). On direct comparison between Danish 1331 and Moscow-I strain, there was similar 3-year recurrence-free survival (80.0% vs. 72.9%) and 3-year progression-free survival (96.5% vs. 97.8%).
Conclusion
Study results suggest no significant differences between Danish 1331 and Moscow-I strain in recurrence-free survival and progression-free survival, but a significantly higher incidence of moderate to severe adverse events in BCG Moscow-I strain.
Keywords: Adjuvant bacillus Calmette-Guerin, Bacillus Calmette-Guerin adverse effects, Danish 1331 strain, Intravesical therapy, Moscow-I strain, Non-muscle invasive bladder cancer
1. Introduction
Bladder cancer is the second most commonly diagnosed genitourinary malignancy with an estimated 549 400 newly diagnosed cases in 2018 worldwide and 18 926 in India, with 10 231 bladder cancer related deaths in 2018 in India [1,2]. Non-muscle invasive bladder cancer (NMIBC) accounts for a total of 75%–85% of patients with bladder cancer [3]. The global mortality rate is 4 per 100 000 in men [4].
The gold standard in the management of NMIBC is the complete removal of the bladder lesion by transurethral resection (TUR). There is a potential risk of progression to muscle-invasive disease (1%–45%) and high rates of recurrence after the transurethral resection of bladder tumor (TURBT) in the tune of 20% in low-risk disease (low-grade Ta ≤3 cm) and 24% in intermediate-risk disease (recurrent lesion or solitary Ta >3 cm or multiple low-grade Ta or T1 low-grade lesions) and 78% in high-risk disease (high grade recurrent or >3 cm or carcinoma in situ) [5].
Intravesical instillation of bacillus Calmette-Guerin (BCG) is considered the most effective adjuvant treatment to TUR for intermediate and high-risk NMIBC cases to prevent recurrence and progression of the disease [4]. BCG was first discovered by Albert Calmette and Camille Guerin, at the Pasteur Institute, France for tuberculosis vaccination in 1921 but the Food and Drug Administration approval for intravesical usage was obtained in 1990 [6]. BCG daughter strains prepared by subculturing have extensive differences in the level of expression of surface proteins and immunodominant protein antigens that influence their virulence and reactogenicity [7]. These genetic mutations will ultimately define their efficacy and adverse effects (AEs) profile. The precise immunological mechanism of BCG in carcinoma of the bladder is still unclear. BCG is a nonspecific immunostimulant; fibronectin protein attached to tumor cells causes internalization of BCG in tumor cells and induces inflammation. This local phenomenon in the urinary bladder causes intense inflammatory response initiated by a significant increase in T cells particularly CD4 which in turn secrete various cytokines like INF-γ, IL-1, IL-12, and TNF-α which further activates lymphocytes. CD4 T cells also secrete cytokines, which in turn mature cytotoxic T cells and natural killer cells and locally destroy bladder tumor cells.
Intravesical BCG administration is associated with complications and AEs ranging up to 40%–50%, and a further 20.3% of patients do not complete their treatment [8]. These complications may appear immediately or up to several months after BCG instillation making the diagnosis difficult. Mostly these complications are attributed to the local inflammatory reaction of the urinary bladder. Since long urologists have debated whether the differences in strains of BCG have an impact on AEs and/or efficacy.
In this study, a retrospective analysis of prospectively collected data was done between two different strains of BCG (Danish 1331 and Moscow-I [Russian strain]) used for adjuvant treatment of NMIBC at our institute. We studied the differences in AEs between two strains, and also prospectively compared the clinical outcomes in terms of recurrences and progression of the disease.
2. Patients and methods
2.1. Study design and patients
Clinical data of 188 cases of NMIBC that were treated with adjuvant BCG therapy between January 2008 and December 2018 were collected prospectively and analyzed retrospectively. We adhered to the principles of Helsinki Declaration, 1964 (amended in 2013). Analysis was done from 114 patients who completed minimum follow-up of 12 months and their complete medical records were available. Patient's demographic details, tumor details, nature of the surgical treatment, type of BCG used, AEs, and tumor recurrences were analyzed. Danish 1331 strain (produced by BCG vaccine laboratory, Chennai, India) was used during instillation in first 68 patients (60%) and Moscow-I strain (produced by SII, Pune, India) in next 46 patients (40%) as the former was discontinued by manufacturer (Table 1).
Table 1.
Baseline demographic details and tumor characteristics of patients treated with adjuvant intravesical BCG Danish 1331 and BCG Moscow-I strain for non-muscle invasive bladder cancer.
| Variable | BCG Danish 1331 (n=68) |
BCG Moscow-I (n=46) |
p-Value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 57 (83.8) | 38 (82.6) | 0.864 |
| Female | 11 (16.2) | 8 (17.4) | |
| Age, median (range), year | 60.5 (34.0–78.0) | 61.5 (38.0–77.0) | 0.794 |
| Past history of TURBT, n (%) | |||
| Nil | 48 (70.6) | 33 (71.7) | 0.894 |
| 1 | 15 (22.1) | 11 (23.9) | |
| ≥2 | 5 (7.4) | 2 (4.3) | |
| Tumor status, n (%) | |||
| pTa | 25 (36.8) | 15 (32.6) | 0.735 |
| pT1 | 38 (55.9) | 25 (54.3) | |
| pTis | 3 (4.4) | 4 (8.7) | |
| pT1+pTis | 2 (2.9) | 2 (4.3) | |
| Tumor risk stratification, n (%) | |||
| Low | 8 (11.8) | 3 (6.5) | 0.723 |
| Intermediate | 33 (48.5) | 24 (52.2) | |
| High | 27 (39.7) | 19 (41.3) | |
BCG, bacillus Calmette-Guerin; TURBT, transurethral resection of bladder tumor.
2.2. Surgical management of NMIBC
Surgical management consisted of cystoscopy, and standard TURBT with or without random biopsy based on urine cytology. High grade and T1 tumors were subjected for re-resection after 1–3 weeks after initial TURBT. Patients who were referred from other institutes after TURBT were subjected to cystoscopy and/or TURBT for proper staging and to exclude residual disease. Immediate instillation of mitomycin was done in 25 patients in whom cystoscopic appearance was that of low-risk tumors.
2.3. Adjuvant BCG therapy
Intravesical BCG was typically started 2 weeks after TURBT. Six weekly instillations of 120 mg BCG in 50 mL normal saline with bladder retaining time of 1–2 h were performed. Maintenance BCG regimen with three weekly instillations at 3, 6, 12, 18, and 24 months was advised to high-risk patients; however, only 37% of the patients were compliant with the regimen. Follow-up protocol included three monthly cystoscopy and urine cytology for 2 years and then six monthly for 3 more years, yearly thereafter to identify recurrence and/or progression. Recurrence of the tumor was defined as return of tumor at any stage whereas grade progression was defined as recurrence with an increased stage and/or grade.
2.4. AEs
AEs were grouped as mild, moderate, and severe. If the patient had pre-existing lower urinary tract symptoms (LUTS) due to bladder outlet obstruction, they were treated and any increase in the symptoms after BCG instillations was considered as side effects. Mild AEs included mild irritative LUTS and occasional hematuria which subsided in 48 h without medication and were self-limited. Moderate AEs included prolonged severe LUTS requiring medications lasting more than 2–5 days, urinary tract infections requiring antibiotics, hematuria lasting more than 2–3 days which lead to delay or stoppage of BCG instillation without completing the cycles, reducing the dose to 80 mg, etc. Predominant urinary symptoms were frequency, urgency, burning urination, etc. Severe AEs included patients needing admission for BCG sepsis, severe BCG cystitis requiring antitubercular treatment, bladder contracture, and systemic manifestations. AEs were recorded when patient presented with one of the above mentioned features during routine outpatient follow-up or cystoscopy.
2.5. Statistical analysis
We used SPSS Statistics v20.0 (IBM Corp., Armonk, NY, USA) software for the analysis. Chi-square and Fischer-Exact tests were used to compare patient and tumor characteristics. Survival functions were calculated by using the Kaplan-Meier estimate and a log-rank test was used to compare survival functions in different groups and p-value and confidence intervals were used to measure significance. The p-value of <0.05 is taken as significant. Chi-square test analysis was used for the point estimate of survival at 3 and 4 years.
3. Results
3.1. Patients and tumour characteristics
Patients’ details of age, gender, tumor stage, grade, and risk grouping were well balanced between the two groups (Table 1). Median age of the patients was 60.5 (34.0–78.0) years in the BCG Danish 1331 group and 61.5 (38.0–77.0) years in the BCG Moscow-I group which were comparable. There were 95 (83.3%) males and 19 (16.7%) female patients in the study and distribution between the two groups was similar (p=0.864). Median follow-up for patients managed with BCG Danish 1331 was 42.5 months (95% confidence interval [CI] 34.0–46.0 months) and for BCG Moscow-I group was 34.5 months (95% CI 31.0–38.0 months).
These patients were categorized into low-risk, intermediate-risk, and high-risk NMIBC for recurrence and progression based on the number of tumors, grade, and depth of invasion and associated carcinoma in situ. Among 114 patients, 11 (10%) were low-risk; 57 (50%) were intermediate-risk; and 46 (40%) were high-risk patients. The distribution difference of these risk groups between two strain of BCG was not significant (p=0.723) (Table 1).
3.2. Tolerance and AE profile
AEs due to intravesical BCG usage were seen in 103 (90%) patients. The majority of patients who received adjuvant BCG therapy with either Danish 1331 and Moscow-I strain had no AEs or mild AEs. Mild to no AEs were seen in 35 patients (51.5%) and moderate to severe AEs were seen in 33 (48.5%) patients who received BCG Danish 1331 therapy. Whereas 15 (32.6%) patients had minimal or no AEs and 31 (67.4%) patients had moderate to severe AEs who received BCG Moscow-I strain. Thus, on comparison, the BCG Moscow-I strain group had an increased incidence of moderate to severe AEs (p=0.046) (Table 2). The number of patients who could not complete induction/maintenance cycles and/or required dose reduction was significantly higher in the Moscow-I strain group (20 [43.5%]) when compared to the Danish 1331 strain group (17 [25%]) with a p-value of 0.039. Antitubercular therapy was required in 5 (7.4%) patients for severe and persistent AEs in BCG Danish 1331 group and 9 (19.6%) patients in BCG Moscow-I group (p=0.051). The number of patients requiring cystectomy for BCG AEs were 2 (2.9%) in the BCG Danish 1331 group and 5 (10.9%) in the BCG Moscow-I group without statistical significance (p=0.093). A non-urological complication was seen only in one patient of the Danish group who had arthritis and was treated with antitubercular treatment.
Table 2.
Toxicity and adverse effects in BCG Danish 1331 and BCG Moscow-I strain groups.
| Variable | Danish 1331 strain (n=68) | Moscow-I strain (n=46) | p-Value |
|---|---|---|---|
| Side effect, n (%) | |||
| Nil to mild | 35 (51.5) | 15 (32.6) | 0.046 |
| Moderate to severe | 33 (48.5) | 31 (67.4) | |
| BCG discontinuation/dose reduction, n (%) | |||
| No | 51 (75) | 26 (56.5) | 0.039 |
| Yes | 17 (25) | 20 (43.5) | |
| Requiring antitubercular therapy, n (%) | |||
| No | 63 (92.6) | 37 (80.4) | 0.051 |
| Yes | 5 (7.4) | 9 (19.6) | |
| Requiring cystectomy for BCG side effect, n (%) | |||
| No | 66 (97.1) | 41 (89.1) | 0.093 |
| Yes | 2 (2.9) | 5 (10.9) | |
BCG, bacillus Calmette-Guerin.
3.3. Oncological outcomes
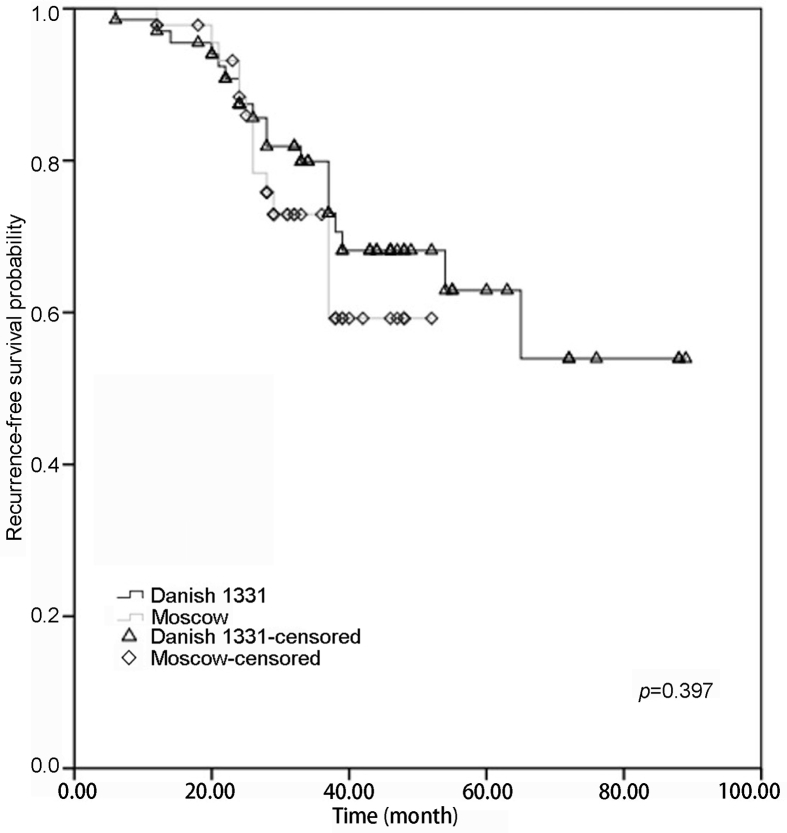
Tumor recurrences were noted in 19 patients in the BCG Danish 1331 group (n=68) and 14 patients in the BCG Moscow-I group (n=46) till the last follow-up with a p-value of 0.397 (Table 3). There was no difference in 3 years or 4 years recurrence-free survival (RFS) of patients treated with BCG Danish 1331 strain (80.0%, 95% CI 75.6%–84.4%; 68.2%, 95% CI 63.8%–72.6%) when compared to BCG Moscow-I strain (72.9%, 95% CI 70.9%–74.9%; 59.2%, 95% CI 57.2%–61.2%) (Fig 1).
Table 3.
Survival probability at 3 years and 4 years in BCG Danish 1331 and BCG Moscow-I strain groups.
| Variable | Median survival probability at 3 years, %; (95% CI) | Median survival at 4 years, %; (95% CI) | p-Value |
|---|---|---|---|
| RFS | |||
| BCG Danish 1331 | 80.0; (75.6–84.4) | 68.2; (63.8–72.6) | 0.397 |
| BCG Moscow-I | 72.9; (70.9–74.9) | 59.2; (57.2–61.2) | |
| PFS | |||
| BCG Danish 1331 | 96.5; (93.0–100.0) | 86.1; (82.5–89.7) | 0.703 |
| BCG Moscow-I | 97.8; (96.6–99.0) | 79.5; (78.3–80.7) | |
| OS | |||
| BCG Danish 1331 | 94.6; (91.6–97.6) | 88.2; (85.2–91.2) | 0.373 |
| BCG Moscow-I | 88.9; (87.6–90.2) | 83.5; (82.2–94.8) | |
| DSS | |||
| BCG Danish 1331 | 98.2; (96.5–99.9) | 94.9; (93.2–96.6) | 0.554 |
| BCG Moscow-I | 97.6; (96.8–98.4) | 94.0; (96.8–98.4) | |
BCG, bacillus Calmette-Guerin; PFS, progression-free survival; RFS, recurrence-free; OS, overall survival; DSS, disease-specific survival; CI, confidence interval.
Figure 1.
Probability with Kaplan-Meier analysis of recurrence-free survival in patients adequately treated with BCG Danish 1331 strain (black) and BCG Moscow-I strain (grey) for non-muscle invasive bladder cancer. BCG, bacillus Calmette-Guerin.
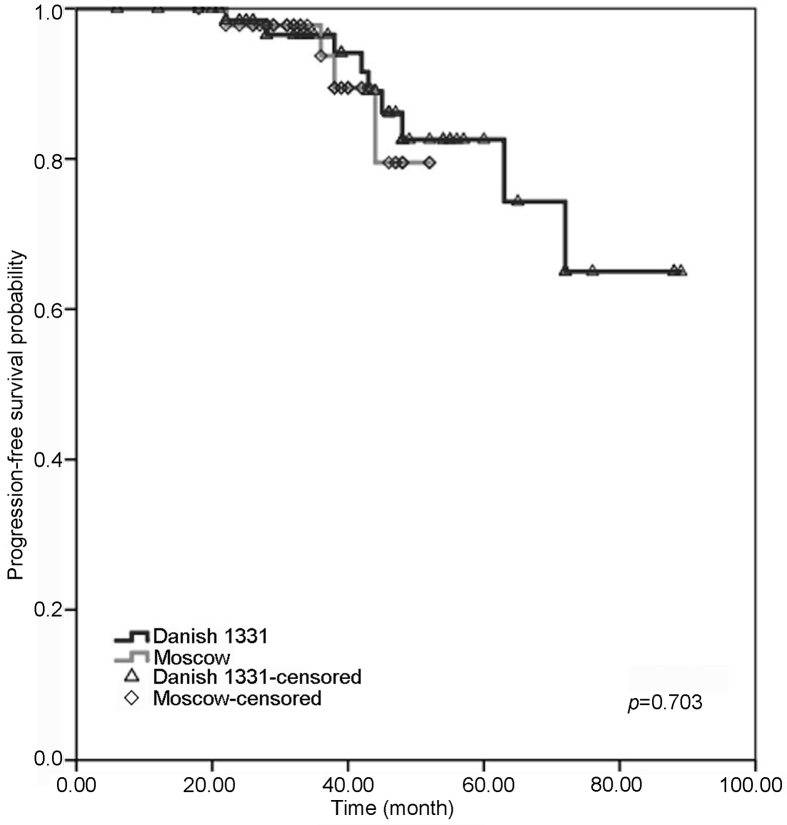
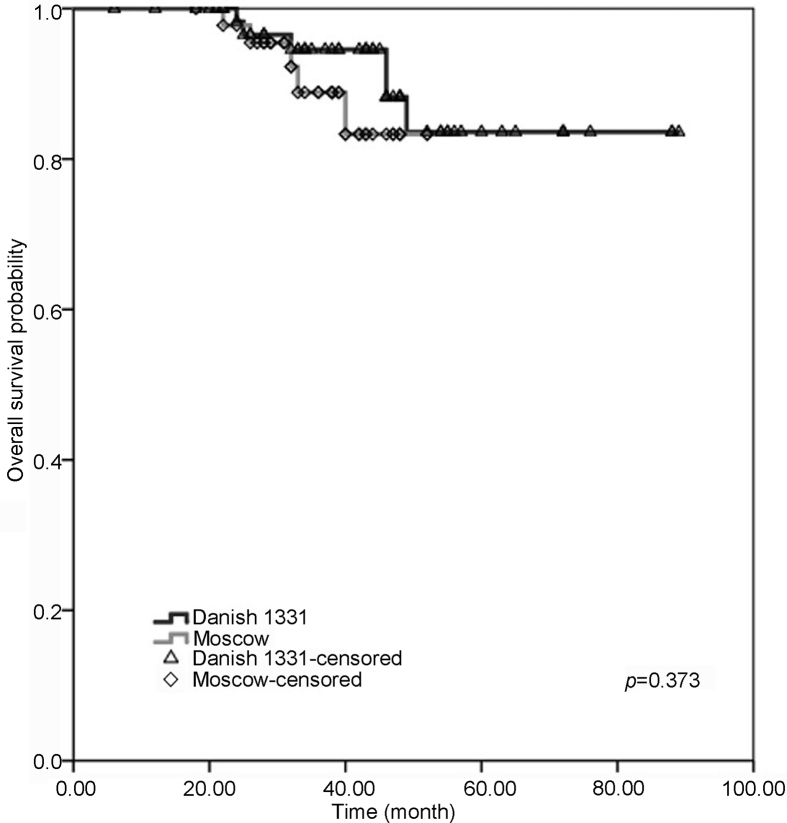
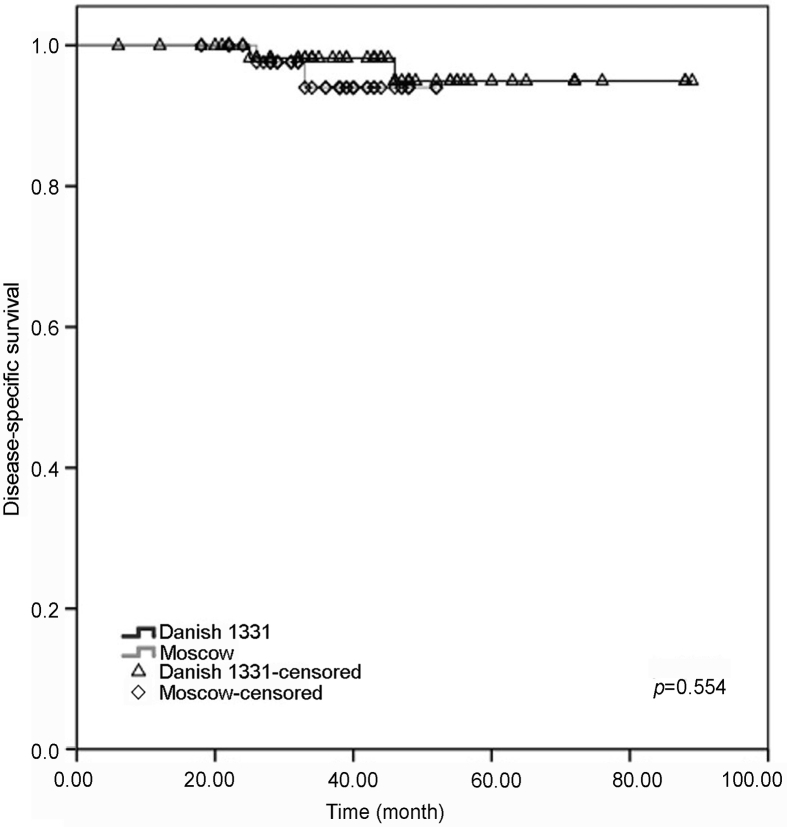
Nine patients in the BCG Danish group and four in the BCG Moscow-I group had progression to a higher grade and/or stage with no statistical difference and p-value of 0.703. In both groups, three-year and four-year progression-free survival (PFS) outcomes were similar with no significant difference (BCG Danish 1331 [96.5%, 95% CI 93%–100%; 86.1%, 95% CI 82.5%–89.7%]; BCG Moscow-I strain [97.8%, 95% CI 96.6%–99%; 79.5%, 95% CI, 78.3%–80.7%]) (Fig 2). Three and four years overall survival and disease-specific survival were comparable between patients treated with BCG Danish 1331 and BCG Moscow-I strains (Table 3) (Fig 3, Fig 4).
Figure 2.
Probability with Kaplan-Meier analysis of progression-free survival in patients adequately treated with BCG Danish 1331 strain (black) and BCG Moscow-I strain (grey) for non-muscle invasive bladder cancer. BCG, bacillus Calmette-Guerin.
Figure 3.
Probability with Kaplan-Meier analysis of overall survival in patients adequately treated with BCG Danish 1331 strain (black) and BCG Moscow-I strain (grey) for non-muscle invasive bladder cancer. BCG, bacillus Calmette-Guerin.
Figure 4.
Estimated probability with Kaplan-Meier analysis of disease-specific survival in patients adequately treated with BCG Danish 1331 strain (black) and BCG Moscow-I strain (grey) for non-muscle invasive bladder cancer. BCG, bacillus Calmette-Guerin.
4. Discussion
4.1. Adjuvant BCG therapy in NMIBC
Intravesical BCG is one of the most successful immunotherapies as an adjuvant to TURBT in NMIBC. BCG is live attenuated mycobacterium which induces a massive local inflammatory response in the bladder and shows anti-tumoral activity [9]. Thus it has a preventive effect in tumor recurrence and progression of NMIBC which is proven by several studies [[10], [11], [12]]. BCG has shown to delay or prevent the progression of bladder carcinoma, thereby improving the RFS and PFS [10]. Meta-analysis has found BCG to be more efficacious than mitomycin in high-risk patients [13], and also revealed a 32% reduction in risk of recurrence after maintenance intra-vesical BCG when compared to mitomycin [14]. Even most of the guidelines (European Association of Urology and American Urological Association guidelines) recommend intravesical BCG instillation in intermediate- and high-risk groups.
We did a retrospective analysis of prospectively collected data in 114 patients who received intravesical BCG in cases of NMIBC in our institute to evaluate tolerance, AEs and oncological outcomes with the use of two BCG strains—Danish 1331 and Moscow-I.
4.2. Dose and duration of adjuvant BCG in NMIBC
The dose and duration of the BCG remain one of the most discussed topics. Morales et al. [15] published the first series on adjuvant immunotherapy for NMIBC; six instillations of 120 mg of BCG (Pasteur strain) were given over 6 weeks, 1 week apart, and initiated 3 weeks after TURBT. It was found to be effective in reducing recurrence and disease progression. A prospective study by Vijjan et al. [16] comparing three doses of BCG (120 mg, 80 mg, and 40 mg) showed a similar efficacy on recurrence rates and progression, but BCG toxicity was significantly lowered by using 80 mg when compared with the standard dose of 120 mg. In our patients, we used standard 120 mg of BCG instillation weekly for 6 weeks 2–3 weeks after TUR.
A trial conducted by Southwest Oncology Group demonstrated a significant impact of maintenance therapy when compared to induction only. The patients in the study arm received six-week induction BCG followed by three weekly instillations at 3 and 6 months and every 6 months up to 3 years. They showed no toxicity above Grade 3 and results showed RFS of 76.8 months as compared to 35.7 months in the control arm (p=0.0001). Five-year overall survival was 83% in the study arm compared to 78% in the control arm [13]. In contrast, the CUETO study by Martinez-Piñeiro et al. [17] and randomised control trial by Nakai et al. [18] reported no improvement in recurrence and PFS with maintenance therapy as compared to only induction therapy alone. There is limited evidence quoting that BCG maintenance is more effective than induction alone at reducing the risk of recurrence in high-risk NMIBC and responders of induction therapy. However, strength of evidence is low [19].
We advised patients with intermediate- and high-risk NMIBC for maintenance therapy with three weekly instillations at 3 and 6 months and then six monthly till 3 years. As patient acceptance was poor, only 11 (9.6%) patients (six in the Danish 1331 and five in the Moscow-I group) received maintenance BCG. Out of these, only 3 (2.6%) patients completed whole therapy.
4.3. AEs of the adjuvant BCG therapy
Complications induced by BCG can vary from self-limited irritative voiding symptoms to severe systemic sepsis [20]. In one of the most extensive studies by the European Organization for Research and Treatment of Cancer Genito-Urinary, intravesical BCG was instilled in 1316 patients, in which 30.6% developed systemic side effects, 62.8% reported local side effects, and 69.5% reported either local or systemic side effects [21]. Chemical cystitis (35%) and general malaise (15.5%) were more frequent, and 7.8% of patients discontinued due to complications. The most serious complication of BCG immunotherapy is disseminated infection referred to as “BCG-osis”. Complications can occur within a few hours and can present even after a few years after cessation of therapy. A systematic review of BCG vaccine complications highlighted significant substrain differences on AE profile [3]. There is not much literature available about the AEs of Moscow-I strain of BCG. One recent study comparing 80 mg and 120 mg of BCG Moscow-I did not show significant AEs [22].
Local complications include cystitis with or without haematuria. Lamm et al. [23] in his case series observed cystitis in 91% of his patients seen just 2–4 h after instillation. Cystitis manifests as dysuria, frequency, suprapubic pain, and sometimes haematuria. It usually resolves spontaneously with minimal supportive care within 48 h. Bladder contracture is an uncommon but severe form of a localised complication seen in 0.2% of patients [20]. Granulomatous prostatitis is one of the more common complications following BCG therapy, and 10% of them develop clinical prostatitis associated with discomfort, and more than 40% present with elevated prostate-specific antigen [24]. The mean interval between initiation and diagnosis is 11.5 months. MRI is a better investigative tool for adequate diagnosis; symptomatic patients may be treated with isoniazid and rifampicin for 3–6 months [25].
BCG sepsis is a life-threatening septic reaction that occurs in an estimated 1 in 15 000, characterized by chills, fever, and hypotension with potential progression to multisystem organ failure [26]. It is recommended to start antitubercular treatment with 40 mg prednisolone (tapered gradually) [21]. Predisposing factors for systemic complications are immunosuppression, geriatric patients, and chronic co-morbidities.
In our series, AEs due to the BCG instillation were predominantly mild in nature. This included dysuria, frequency of urination, mild haematuria, and pain. These were self-limiting and subsided in 2 days. Mild to no AEs were seen in 35 (51.5%) and 15 (32.6%) patients in the Danish 1331 and Moscow-I group, respectively. Significantly, more patients had moderate to severe AEs in the BCG Moscow-I group (n=31, 67.4%) when compared to patients who received the BCG Danish 1331 strain (n=33, 48.5%) which was statistically significant (p=0.046). Maintenance therapy was discontinued due to AEs in 20 (43.5%) patients in Moscow-I strain and 17 (25%) patients in Danish 1331 strain (p=0.039). Patients with AEs requiring antitubercular treatment were higher in Moscow-I strain (n=9, 19.6%) as compared to Danish 1331 strain (n=5, 7.4%) (p=0.051). Cystectomy was required in 5 (10.9%) patients in Moscow-I strain and 2 (2.9%) patients in Danish 1331 strain (p=0.93) due to severe AEs. The incidence of cystectomy was higher in our series as compared to contemporary series [27]. All the five patients had severe debilitating LUTS refractory even after treatment with anticholinergics, anti-tubercular drugs, and pentosan sulphate. In additional to these, two patients had documented bladder contractures on cystoscopy with bladder capacity of less than 100 mL. Both these patients opted to undergo cystectomy rather than bladder augmentation. Out of other three patients, one patient had high grade recurrence along with small capacity bladder. He refused to restart the induction BCG and chose to undergo radical cystectomy. Our data support that different strains vary in their side effect profile. Mostly this is attributed to the amount of inflammatory and immune reaction induced by different strains of BCG [28].
4.4. Oncological outcomes of adjuvant BCG therapy
The meta-analysis by Shelley et al. [29] reported that TUR with intravesical BCG provided significantly better prophylaxis for tumor recurrence in Ta and T1 bladder cancers than TUR alone. The tumor recurrence at 12 months was 28.7% in the BCG with the TUR group as compared to 56% in TUR alone group. Variation in efficacy post BCG with different strains was greatly studied and continues to engage the researchers. Rentsch et al. [28] in his prospective randomised control trial highlighted the Connaught strain was significantly more effective than the BCG Tice strain in terms of 5-year RFS (74% in Connaught vs. 48% in Tice strain with p=0.001). On the other hand, Sengiku et al. [30] reported that there are no significant differences in RFS and AEs between the BCG Connaught and Tokyo strains.
Production of the Danish 1331 strain which was widely used earlier in our country was suspended and we started getting the Moscow-I strain for intravesical BCG use. We compared the results of two strains in our tertiary health care facility. Recurrence and progression of disease were seen in 19 (27.9%) and 9 (13.2%) patients in BCG Danish 1331 group whereas 14 (30.4%) and 4 (8.7%) patients in BCG Moscow-I group without statistical difference (p=0.397; p=0.703, respectively). In BCG Danish 1331 group, three-year and four-year RFS outcomes were seen in 80.0% and 68.2% of patients, and three-year and four-year PFS outcomes were seen in 96.5% and 86.1% of patients; in BCG Moscow-I group, three-year and four-year RFS outcomes were seen in 72.9% and 59.2% of patients, and three-year and four-year PFS outcomes were seen in 97.8% and 79.5% of patients. Our series demonstrated that there was no statistically significant difference in the recurrence and progression rates between two strains. Similar results were seen for overall and disease-specific survival (Table 3).
The present study has few limiting factors. It is a retrospective review from single-institute experience, and associated with inherent biases. The study population was small and maintenance therapy was not used to the full extent. Longer follow-up is needed to evaluate the prognosis and strengthen the study.
5. Conclusions
In this retrospective study, evaluating the differences in AEs and efficacy profile of the BCG Danish 1331 and the Moscow-I strain of intravesical BCG, the Moscow-I strain had a significantly higher incidence of moderate to severe AEs. However, there was no statistical difference in RFS, PFS, and also in overall and disease-specific survival between these two strains of BCG. A large prospective randomized trial with more number of patients and longer follow-up is required to confirm the AEs induced by different strains of BCG in the management of NMIBC.
Author contributions
Study concept and design: Yuvaraja B. Thyavihally.
Data acquisition: Abhinav Pednekar, Preetham Dev
Data analysis: Yuvaraja B. Thyavihally.
Drafting of manuscript: Preetham Dev, Santosh Waigankar.
Critical revision of the manuscript: Yuvaraja B. Thyavihally, Santosh Waigankar, Preetham Dev, Nevitha Athikari, Archan Khandekar, Abhijit Raut, Naresh Badlani, Ashish Asari.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We thank Dr. Neha Sanwalka for the support with the statistical analysis and Dr. Meenal Hastak and Dr. Bijal Kulkarni for their continued support and in discussion of pathological aspects of disease.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Canc. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer today. http://gco.iarc.fr/today/home Available from:
- 3.Gan C., Mostafid H., Khan M.S., Lewis D.J.M. BCG immunotherapy for bladder cancer—the effects of substrain differences. Nat Rev Urol. 2013;10:580–588. doi: 10.1038/nrurol.2013.194. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M., Burger M., Compérat E.M., Gontero P., Mostafid A.H., Palou J., et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT 1 and carcinoma in situ)—2019 Update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Quan Y., Jeong C.W., Kwak C., Kim H.H., Kim H.S., Ku J.H. Dose, duration and strain of bacillus Calmette-Guerin in the treatment of nonmuscle invasive bladder cancer: Meta-analysis of randomized clinical trials. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamat A.M., Flaig T.W., Grossman H.B., Konety B., Lamm D., O'Donnell M.A., et al. Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 2015;12:225–235. doi: 10.1038/nrurol.2015.58. [DOI] [PubMed] [Google Scholar]
- 7.Brosch R., Gordon S.V., Garnier T., Eiglmeier K., Frigui W., Valenti P., et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007;104:5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meijden A.P.M., Sylvester R.J., Oosterlinck W., Hoeltl W., Bono A.V. Maintenance bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: Results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol. 2003;44:429–434. doi: 10.1016/s0302-2838(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor R., Vijjan V., Singh P. Bacillus Calmette-Guérin in the management of superficial bladder cancer. Indian J Urol. 2008;24:72–76. doi: 10.4103/0970-1591.38608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester R.J., van der Meijden A.P.M., Lamm D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 11.Han R.F., Pan J.G. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Duchek M., Johansson R., Jahnson S., Mestad O., Hellström P., Hellsten S., et al. Bacillus Calmette-Guérin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol. 2010;57:25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Shelley M.D., Court J.B., Kynaston H., Wilt T.J., Coles B., Mason M. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2003;3:CD003231. doi: 10.1002/14651858.CD003231. [DOI] [PubMed] [Google Scholar]
- 14.Malmström P.U., Sylvester R.J., Crawford D.E., Friedrich M., Krege S., Rintala E., et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Morales A., Eidinger D., Bruce A.W. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 16.Vijjan V., Mandhani A., Kapoor R., Dubey D., Srivastava A., Ansari M.S., et al. A randomized trial comparing low dose (40 or 80 mg) with standard dose (120 mg) of bacillus Calmette-Guerin for superficial bladder cancer. Indian J Urol. 2006;22:317–321. [Google Scholar]
- 17.Martínez-Piñeiro L., Portillo J.A., Fernández J.M., Zabala J.A., Cadierno I., Moyano J.L., et al. Maintenance therapy with 3-monthly bacillus Calmette-Guérin for 3 years is not superior to standard induction therapy in high-risk non-muscle-invasive urothelial bladder carcinoma: Final results of randomised CUETO study 98013. Eur Urol. 2015;68:256–262. doi: 10.1016/j.eururo.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Nakai Y., Anai S., Tanaka N., Chihara Y., Haramoto M., Otani T., et al. Insignificant role of bacillus Calmette-Guérin maintenance therapy after complete transurethral resection of bladder tumor for intermediate- and high-risk non-muscle-invasive bladder cancer: Results from a randomized trial. Int J Urol. 2016;23:854–860. doi: 10.1111/iju.13167. [DOI] [PubMed] [Google Scholar]
- 19.Chou R., Selph S., Buckley D.I., Fu R., Griffin J.C., Grusing S., et al. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: A systematic review and meta-analysis. J Urol. 2017;197:1189–1199. doi: 10.1016/j.juro.2016.12.090. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Lu J., Huang Y., Ma L. Clinical spectrum of complications induced by intravesical immunotherapy of bacillus Calmette-Guérin for bladder cancer. J Oncol. 2019:6230409. doi: 10.1155/2019/6230409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brausi M., Oddens J., Sylvester R., Bono A., van de Beek C., van Andel G., et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: Results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65:69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Sood R., Sharma H., Sharma B., Parekh S., Pujari P., Shewale S. A prospective comparative study to assess the efficacy and tolerability of 2 different doses of intravesical bacillus Calmette-Guerin (BCG) in patients with non-muscle-invasive bladder cancer. Urol Oncol. 2020;38:433–439. doi: 10.1016/j.urolonc.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Lamm D.L., van der Meijden P.M., Morales A., Brosman S.A., Catalona W.J., Herr H.W., et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 24.Humphrey P.A. BCG prostatitis. J Urol. 2012;188:961–962. doi: 10.1016/j.juro.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Vasudeva S., Baffoe-Bonnie A. Intravesical BCG therapy and side effects—case reports and a review of literature. Mycobact Dis. 2018;8:4. https://www.omicsonline.org/open-access/intravesical-bcg-therapy-and-side-effectscase-reports-and-a-review-ofliterature-2161-1068-1000270-105270.html [Google Scholar]
- 26.Liaw F., Tan Y.Y., Hendry D. Systemic BCG-osis following intravesical BCG instillation for bladder carcinoma. Clin Case Rep. 2017;5:1569–1572. doi: 10.1002/ccr3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orihuela E., Herr H.W., Pinsky C.M., Whitmore W.F., Jr. Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer. 1987;60:326–333. doi: 10.1002/1097-0142(19870801)60:3<326::aid-cncr2820600309>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Rentsch C.A., Birkhäuser F.D., Biot C., Gsponer J.R., Bisiaux A., Wetterauer C., et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol. 2014;66:677–688. doi: 10.1016/j.eururo.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 29.Shelley M.D., Kynaston H., Court J., Wilt T.J., Coles B., Burgon K., et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs. transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 30.Sengiku A., Ito M., Miyazaki Y., Sawazaki H., Takahashi T., Ogura K. A prospective comparative study of intravesical bacillus Calmette-Guérin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol. 2013;190:50–54. doi: 10.1016/j.juro.2013.01.084. [DOI] [PubMed] [Google Scholar]