Abstract
Objectives:
Urinary tract infections (UTI) are a common diagnosis within the pediatric emergency department (ED). Due to the necessary delay in obtaining urine culture results, clinicians must decide whether to prescribe antibiotics for a suspected UTI prior to urine culture results. The primary objective of this study was to identify the proportion of children given empiric antibiotics who subsequently did not meet consensus definition of an UTI. The secondary objective was to identify factors associated with return visits to the ED following an index visit for UTI. We also attempted to identify predictors of prescription of empiric antibiotics for children who did not have a UTI.
Methods:
This was a retrospective chart review of all patients between the ages of 2 months and 18 years diagnosed with a UTI between July 2016 and June 2017 in the ED of a single urban quaternary care center. Patients were excluded for the following reasons: use of bag for urine collection, subsequent admission to the hospital, receipt of antibiotics within the previous 3 days, use of antibiotics for an indication other than a UTI, and urine culture obtained at an outside facility.
Results:
Of 404 included patients, 389 (96.2%) were discharged on antibiotics and 243 (62.4%) did not have a UTI. On the multivariate analysis, age ≧ 36 months was associated with increased odds of receiving antibiotics and not having a UTI while both ≥1+ leukocyte esterase and ≥1+ nitrites on urinalysis were associated with decreased odds of receiving antibiotics and not meeting UTI criteria. 62 patients revisited the ED within 30 days of the initial visit, 24 (38.7%) of which met criteria for UTI during the index visit. Prescription of antibiotics at the time of the index visit was associated with decreased odds of reutilization, while an extended spectrum beta-lactamase producing organism cultured from urine at the index visit was associated with increased odds of reutilization.
Conclusions:
A high number of patients discharged on empiric antibiotics did not meet criteria for a UTI. We did not identify clinically useful factors that predicted prescription of empiric antibiotics for children who do not have a UTI. We believe unnecessary antibiotic prescriptions could be substantially decreased by decreasing empiric use of antibiotics coupled with reliable follow up for positive urine cultures.
Keywords: Urinary tract infections, Children, Empiric antibiotics
Introduction:
Urinary tract infections (UTIs) are diagnosed in 7% of febrile young children presenting to the Emergency Department.1 However, accurate diagnosis of UTI at the point of care can be difficult given the consensus definition of a pediatric UTI includes urine culture results, which traditionally are not available until a minimum of 24 hours after specimen collection. Therefore, clinicians rely on a combination of symptoms and biomarkers, including the results of a dipstick and urine microscopy (urinalysis (UA)), to diagnose a UTI. While the UA has good predictive accuracy for the diagnosis of UTI in young febrile children,2 its imperfect specificity means that children may be overtreated for UTI at the point of care. Indeed, recent work documented that approximately 50% of children treated for UTI in the Emergency Department (ED) did not meet the American Academy of Pediatrics’ (AAP) definition for a UTI.3,4
While many EDs have mechanisms in place to contact patients regarding urine culture results following discharge,3 there are still consequences of initial overtreatment. These include cost to the family, potential for adverse drug events, and the risk of development of antibiotic resistance. Further, emerging data suggests that even a single dose of antibiotics can significantly alter the microbiome,5 which has potentially adverse consequences.6 Conversely, the risks associated with delayed treatment of UTIs include potential spread of infection, increased healthcare reutilization due to worsened infection, and increased risk of renal scarring.7 Therefore, it is crucial to maintain good antibiotic stewardship within the ED to treat infections appropriately while avoiding unnecessary antibiotics. Identification of factors associated with use of antibiotics in children who do not ultimately have a UTI will improve the ability to appropriately prescribe empiric antibiotics at the point of care in the ED.
The primary objective of this study was to identify the proportion of children in the ED given empiric antibiotics who did not meet consensus definition of a pediatric UTI. We also attempted to identify predictors of prescription of empiric antibiotics for those children. The secondary objective was to identify factors associated with return visits to the ED following an index visit for UTI.
Methods:
Patients:
This was a retrospective chart review of all patients between the ages of 2 months and 18 years diagnosed with a UTI between July 2016 and June 2017 in the ED of a single urban pediatric quaternary care center. Patients with the following ICD-10 discharge diagnoses were eligible for inclusion: N39.0, N30.0, N30.9, N34.1, N34.2, N34.3. Exclusion criteria included: use of bag for urine collection, admission to the hospital for further treatment, receipt of antibiotic therapy within the previous 3 days, use of antibiotics in the ED for an indication other than a UTI, and urine culture obtained at an outside facility. Clinical data, including urine culture source, UA results, culture results, empiric antibiotics and any subsequent changes, concurrent infectious diagnoses, and clinical outcomes including return visits to the ED, were collected through manual chart review. Symptoms were reported as documented in the provider’s note. This work was approved by our institution’s institutional review board.
Definitions:
A positive urine culture was defined as either ≥100,000 colony-forming units (CFU)/mL of a single organism in urine obtained by clean catch, or ≥50,000 CFU/ml of a single organism in urine obtained by catheterization or suprapubic aspiration. Samples with unidentified mixed bacterial growth were not considered to be positive cultures. UTI was defined as a positive urine culture in addition to either ≥1+ leukocyte esterase (LE), ≥1+ nitrites or ≥5 urine white blood cells per high power field in a symptomatic patient.8
Outcomes:
The primary outcome was proportion of patients who were discharged with empiric antibiotics for UTI who did not meet our criteria for UTI. The secondary outcome of interest was proportion of patients who had a revisit to the ED within 30 days of the index visit for a complaint related to the diagnosis or treatment of UTI.
Analysis:
As the primary outcome of interest in this study focused on patients discharged with empiric antibiotics, the analysis for the primary outcome only included this population. The analysis for the secondary outcome, which focused on revisits to the ED, included all patients. Continuous values were compared with Student’s T-Test or Mann-Whitney U, as appropriate. Categorical variables were compared with the Chi-square test, or Fisher’s exact, as appropriate. Variables that were significantly different between groups in the univariate analysis were included in the multi-variate analysis. Logistic regression was used to identify factors associated with the outcome of interest. For the secondary outcome, we completed multiple models with the number of predictors allowed by the number of patients with the outcome of interest. We chose the model with the lowest akaike information criterion for inclusion in this analysis. All analyses were completed with R Studio, version 1.1.447.9
Results:
583 patients were identified and reviewed for inclusion in this study. 179 patients were excluded, leaving 404 patients in the analysis (Figure 1). Of these patients, 389 (96.2%) were discharged on antibiotics, 243 (62.4%) of whom did not meet the study definition of UTI. 118 (48.6%) patients did not meet the study definition of UTI as they had urine cultures that grew mixed bacteria, 62 (25.5%) had negative urine cultures, 62 (25.5%) had positive urine cultures that did not meet the colony-count threshold, and 1 (0.4%) of the patients had a positive culture with a bacterial burden above the colony-count cutoff but did not have leukocyte esterase, nitrites, or pyuria on UA.
Figure 1:
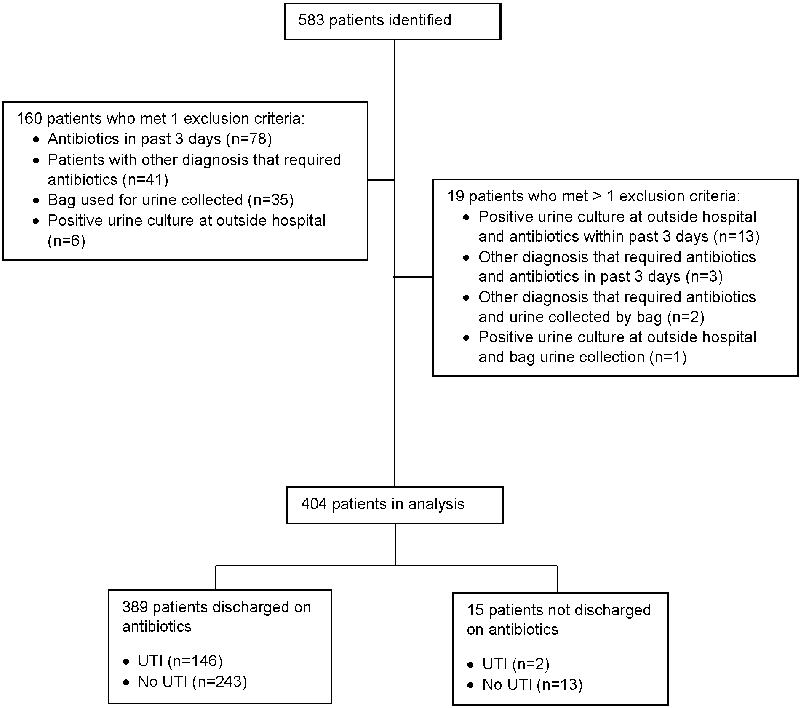
Patient flow diagram.
Among patients discharged with empiric antibiotics, those who did not meet criteria for UTI were older than those who did meet UTI criteria (median, interquartile range (IQR): 67 (33,109) months for no UTI; 30 IQR (9, 94) months for UTI, p<0.01) and there were a lower proportion of patients of Hispanic ethnicity (42.8% for no UTI, 62.3% for UTI, p<0.01). Patients treated with empiric antibiotics who did not have a UTI also had a higher proportion of urine samples collected by clean catch (71.6% no UTI, 35.6% UTI, p<0.01) and lower proportion of febrile patients compared to those who met UTI criteria and were treated with antibiotics (49.8% no UTI, 65.1% UTI, p<0.01) (Table 1).
Table 1:
Variables Associated with UTI Among Children Empirically Treated with Antibiotics
| UTI (n=146) |
No UTI (n=243) |
p-value | |
|---|---|---|---|
| Mean (SD) age (months) | 59.6 (63.8) | 76.6 (57.2) | 0.01 |
| Median (IQR) age (months) | 30 (9, 94) | 67 (33, 109) | <0.01 |
| Female (n (%)) | 113 (77.4%) | 202 (83.1%) | 0.21 |
| Black | 40 (27.4%) | 112 (46.1%) | <0.01 |
| Hispanic | 91 (62.3%) | 104 (42.8%) | <0.01 |
| Public insurance | 92 (63.0%) | 143 (58.8%) | 0.53 |
| Urine collection method | |||
| Catheter (n(%)) | 94 (64.4%) | 69 (28.4%) | <0.01 |
| Clean catch (n (%)) | 52 (35.6%) | 174 (71.6%) | <0.01 |
| Dip UA | 37 (25.3%) | 71 (29.2%) | 0.48 |
| Micro UA | 108 (74.0%) | 171 (70.4%) | 0.48 |
| ≥ 1+ LE | 137 (93.8%) | 205 (84.4%) | <0.01 |
| ≥ 1+ nitrites | 76 (52.1%) | 27 (11.1%) | <0.01 |
| More than 5 WBCs | 104 (71.1%) | 162 (66.7%) | 0.41 |
| Presenting symptom | |||
| Fever | 95 (65.1%) | 121 (49.8%) | <0.01 |
| Dysuria | 46 (31.45%) | 91 (37.4%) | 0.28 |
| Urinary frequency | 13 (8.9%) | 30 (12.3%) | 0.38 |
| Hematuria | 16 (11.0%) | 28(11.5%) | 0.99 |
| Vomiting | 40 (27.4%) | 59 (24.3%) | 0.35 |
| Abdominal pain | 36 (24.6%) | 88 (36.2%) | 0.02 |
| Nasal congestion | 31 (21.2%) | 48 (19.8%) | 0.83 |
| Coughing | 24 (16.4%) | 45 (18.5%) | 0.70 |
| Increased fussiness | 12 (8.2%) | 17 (7.0%) | 0.81 |
SD: standard deviation, IQR: interquartile rage. LE: leukocyte esterase; WBC: white blood cells
On the multivariate analysis, age ≧ 36 months was associated with increased odds of not meeting UTI criteria (Odds ratio (OR): 6.67, 95% confidence interval (CI) (3.53-12.92)). Presence of ≧1+ nitrites on urinalysis (OR 0.10, 95%CI (0.05-0.18)) and ≧ 1+ LE (OR 0.03, 95% CI (0.00-0.09)) were both associated with decreased odds of not meeting UTI criteria (Table 2A). The trend in these results did not change upon sensitivity analysis that included all patients with colony counts less than 50,000 cfu/ml who met all other UTI criteria (Table 2B).
Table 2A:
Predictors in multivariate analysis of prescription of empiric antibiotics for patients who did not meet UTI criteria
| Predictor | Adjusted Odds Ratio (95% CI) |
|---|---|
| Male | 0.58 (0.28-1.21) |
| Age ≧ 36 months | 6.67 (3.53-12.92) |
| Hispanic ethnicity | 0.61 (0.27-1.33) |
| Black race | 1.36 (0.59-3.11) |
| ≧1+ nitrites | 0.10 (0.05-0.18) |
| ≧ 5 urine white blood cells | 0.88 (0.49-1.57) |
| ≧ 1+ Leukocyte esterase | 0.03 (0.01-0.09) |
| Fever | 1.15 (0.64-2.12) |
Table 2B:
Sensitivity analysis including patients with colony counts <50,000 cfu/mL: predictors in multivariate analysis of prescription of empiric antibiotics for patients who did not meet UTI criteria
| Predictor | Adjusted Odds Ratio (95% CI) |
|---|---|
| Male | 0.63 (0.31-1.27) |
| Age ≧ 36 months | 4.17 (2.25-7.94) |
| Hispanic ethnicity | 0.37 (0.17-0.78) |
| Black race | 1.02 (0.48-2.16) |
| ≧1+ nitrites | 0.07 (0.03-0.14) |
| ≧ 5 urine white blood cells | 0.71 (0.41-1.20) |
| ≧ 1+ Leukocyte esterase | 0.04 (0.01-0.10) |
| Fever | 1.18 (0.69-2.06) |
Of the 404 patients included in the initial analysis, 62 revisited the ED within 30 days of the initial visit. Eight of these revisits were for adverse drug events (ADE) (diarrhea, n=2; rash, n=2; bloody stools, n=2; diarrhea and rash, n=1; thrush, n=1). Forty-one patients had return visits with the same symptoms as on initial presentation, 11 of whom were admitted. Two of the 11 patients who revisited the ED with the same symptoms and were admitted were found to have alternate diagnoses, while four of the 11 patients were not discharged on empiric antibiotics at the index visit. Prescription of antibiotics at the time of the index visit was associated with decreased odds of revisit (OR 0.27, 95% CI(0.09-0.91)), while an ESBL-producing organism cultured from urine at the index visit was associated with increased odds of revisit (OR 6.30, 95%CI (1.22-34.72)) (Table 3).
Table 3:
Predictors in multivariate analysis for a return visit within 30 days (excluding visits for ADE)
| Predictor | Odds Ratio (95% CI) |
|---|---|
| Age ≧36 months | 0.74 (0.41-1.37) |
| Discharged on antibiotics | 0.27 (0.09-0.91) |
| ESBL-producing organism on urine culture | 6.30 (1.22-34.72) |
ADE: Adverse drug events, ESBL: extended-spectrum beta-lactamase
There was no difference in the class of empiric antibiotics between those with and without a 30-day revisit to the ED, both including and excluding revisits for ADE. While revisits were highest in patients discharged on beta-lactams, the only difference between the proportion of patients with and without revisits were within the subgroup of patients who did not receive empiric antibiotics. (Table 4).
Table 4:
Classes of empiric antibiotics at discharge in patients with and without revisits
| All revisits | Revisit in 30 days (n=62) |
No Revisit in 30 days (n=341) |
p-value |
|---|---|---|---|
| No Antibiotics | 6 (9.7%) | 9 (2.6%) | 0.02 |
| Beta-lactam | 53 (85.5%) | 305 (89.4%) | 0.49 |
| Trimethoprim-sulfamethoxazole | 1 (1.6%) | 11 (3.2%) | 0.78 |
| Fluoroquinolones | 1 (1.6%) | 7 (2.1%) | 1.0 |
| Nitrofurantoin | 1 (1.6%) | 9 (2.6%) | 1.0 |
| Non-ADE-related revisits | Revisit in 30 days (n=54) |
No Revisit in 30 days (n=341) |
|
| No Antibiotics | 6 (11.1%) | 9 (2.6%) | >0.01 |
| Beta-lactam | 45 (83.3%) | 305 (89.4%) | 0.28 |
| Trimethoprim-sulfamethoxazole | 1 (1.9%) | 11 (3.2%) | 0.91 |
| Fluoroquinolones | 1 (1.9%) | 7 (2.1%) | 1.0 |
| Nitrofurantoin | 1 (1.9%) | 9 (2.6%) | 1.0 |
| Revisits related to index visit, non-ADE |
Revisit in 30 days (n=41) |
No Revisit in 30 days (n=341) |
|
| No Antibiotics | 4 (9.8%) | 9 (2.6%) | 0.06 |
| Beta-lactam | 34 (82.9%) | 305 (89.4%) | 0.32 |
| Trimethoprim-sulfamethoxazole | 1 (2.4%) | 11 (3.2%) | 1.0 |
| Fluoroquinolones | 1 (2.4%) | 7 (2.1%) | 1.0 |
| Nitrofurantoin | 1(2.4%) | 9 (2.6%) | 0.75 |
one patient did not have complete follow-up information, and was excluded from this analysis; ADE=Adverse drug events
Discussion:
In this study, we show that the majority of patients discharged from the ED with a diagnosis of UTI and prescribed empiric antibiotics do not meet criteria for UTI. Among children treated with empiric antibiotics, age ≥ 36 months increased odds of not meeting UTI criteria, while ≥1+ nitrites and ≥1+ leukocyte esterase on UA were associated with increased odds of meeting UTI criteria. Given that both leukocyte esterase and nitrites are part of the definition of UTI, this finding is expected within this analysis. We did not identify clinically useful factors that predicted prescription of empiric antibiotics for children who do not have a UTI. Our data suggests that prescription of an antibiotic at discharge is associated with decreased odds of revisit to the ED within 30 days of the initial visit, while growth of an ESBL-producing organism is associated with increased odds of revisit. Finally, we did not find an association between antibiotic class at discharge and revisit to the ED.
Our results show that 62% of children given empiric antibiotics for UTI in the ED did not meet our criteria for UTI. Other published studies have also documented that children who present with suspected UTI frequently are prescribed empiric antibiotics, yet many have negative urine cultures.3 Indeed, one study found that only 51% of children prescribed empiric antibiotics for UTI in the ED had confirmed UTI.10 Our results show an even higher rate of empiric antibiotic prescription for children who do not have UTI. One potential reason for the difference relates to the varying definitions of UTI used. Watson et al. used a cut-off of 50,000 cfu/mL for all culture results to define a positive urine culture, regardless of method of urine collection. In our work, we used a cut-off of 50,000 cfu/mL for urine collected by catheterization or suprapubic aspiration in accordance with the AAP’s UTI guideline for young children,8 and a cut-off of 100,000 CFU/mL for cultures obtained by clean catch. The AAP’s UTI guideline is specifically for children between 2 and 24 months of age. Given that we chose to include children up to 18 years of age, we chose to use growth of at least 100,000 CFU/mL as the cut-off to define a positive urine culture for clean catch samples in accordance with the classic, although admittedly arbitrary, cut-off used within the adult literature.11 This discrepancy may explain the different results, as 62 patients in our cohort did not meet UTI criteria on the basis of colony-counts.
We did not find that choice of antibiotic prescribed was associated with return ED visits in our cohort. Data in adults has suggested that beta-lactams have an inferior cure rate for UTI when compared to other classes of antibiotics.12,13 This data is partially behind the Infectious Disease Society of America’s recommendation that beta-lactams not be used as first line treatments for uncomplicated cystitis in adults.14 However, beta-lactams are frequently used as treatment of UTIs in children,10 and were the most commonly prescribed antibiotic in our study. In our analysis, there was no difference in class of antibiotic prescribed at the index visit and reutilization. However, we may have been underpowered to detect a difference given the lower numbers of patients prescribed non-beta-lactam antibiotics.
The decision to initiate antibiotics at the point of care requires balancing the risk of renal scarring associated with delayed treatment7 with the risk of antibiotic resistance, and the as-of-yet not fully appreciated implications of changes in microbiomes.15,16 Emerging data suggest that exposure to antibiotics early in life is associated with later obesity,17 asthma,18 and allergic diseases,19 suggesting that careful consideration be given prior to antibiotic initiation in children. However, results of urine culture, the currently used confirmatory test for UTI, are not typically available for more than 24 hours after specimen collection. Because of this delay, clinicians need to decide whether or not to empirically begin antibiotics while the results of the urine culture are pending. Prior work in the literature demonstrates that there is an 0.8% increase in the risk of renal scarring for every 1 hour of fever before antibiotic treatment. Therefore, a delay in initiating antibiotic in a febrile child for 24 hours is associated with an 19% increased rate of renal scarring.7 Comparatively, there is no evidence to suggest that a delay in antibiotic initiation in an afebrile children is associated with an increased risk of renal scarring. The benefits of empiric treatment in afebrile children with suspected UTI may not outweigh the risks unnecessary antibiotic exposure. A potential method to deal with this clinical uncertainty is to empirically treat the high-risk group – those in whom the clinician has a high degree of suspicion for UTI and those at risk of complications, such as scarring. There are several novel candidate urinary biomarkers that may have greater predictive accuracy for UTI than the traditional UA and urine microscopy.20-22 Such biomarkers may play a future role in aiding clinical decisions around empiric antibiotic use for children being evaluated for UTIs.
There were several limitations to this study, including the single center design and the low number of patients who had return visits. Further, as this was a retrospective study, we relied on documentation in the medical record to determine symptoms on presentation, and therefore could not standardize this process. We also chose to include patients with complex chronic conditions as well as those with complex genitourinary conditions. We made this decision to accurately describe the antibiotic prescribing practices at our institution, but in doing so, have included a heterogenous population.
Conclusion:
62% of the patients who were prescribed empiric antibiotics for a UTI did not meet a consensus definition for UTI. Age≧ 36 months was associated with prescription of antibiotics for a patient who did not subsequently meet UTI criteria, while presence of leukocyte esterase and nitrites were associated with increased odds of meeting UTI criteria among patients prescribed empiric antibiotics for a UTI. We did not identify clinically useful factors that predicted prescription of empiric antibiotics for children who do not have a UTI. We believe the most effective way to substantially decrease unnecessary antibiotic prescriptions for suspected UTI in the ED will be to decrease the empiric use of antibiotics coupled with reliable follow up for positive urine cultures.
Funding:
This work was partially supported by National Institutes of Health (K12-HD-001339).
Footnotes
Conflict of Interest Disclosure: None of the authors have any conflicts of interest to disclose.
References:
- 1.Shaikh N, Morone NE, Bost JE, et al. : Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr. Infect. Dis. J 2008; 27: 302–308. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18316994. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder AR, Chang PW, Shen MW, et al. : Diagnostic accuracy of the urinalysis for urinary tract infection in infants. Pediatrics 2015; 135: 965–71. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26009628, accessed April 18, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Saha D, Patel J, Buckingham D, et al. : Urine Culture Follow-up and Antimicrobial Stewardship in a Pediatric Urgent Care Network. Pediatrics 2017; 139: e20162103. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28302674, accessed April 12, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Alghounaim M, Ostrow O, Timberlake K, et al. : Antibiotic Prescription Practice for Pediatric Urinary Tract Infection in a Tertiary Center. Pediatr. Emerg. Care 2019: 1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/30829843, accessed March 11, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Doan T, Arzika AM, Ray KJ, et al. : Gut Microbial Diversity in Antibiotic-Naive Children After Systemic Antibiotic Exposure: A Randomized Controlled Trial. Clin. Infect. Dis 2017; 64: 1147–1153. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28402408, accessed April 12, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitre E, Susi A, Kropp LE, et al. : Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018: e180315. Available at: 10.1001/jamapediatrics.2018.0315, accessed April 12, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaikh N, Mattoo TK, Keren R, et al. : Early Antibiotic Treatment for Pediatric Febrile Urinary Tract Infection and Renal Scarring. JAMA Pediatr. 2016; 170: 848. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27455161, accessed September 12, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Roberts KB, Downs SM, Finell SME, et al. : Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011; 128: 595–610. [DOI] [PubMed] [Google Scholar]
- 9.Computing RF for S: R: A language and environment for statistical computing. 2013. [Google Scholar]
- 10.Watson JR, Sánchez PJ, Spencer JD, et al. : Urinary Tract Infection and Antimicrobial Stewardship in the Emergency Department. Pediatr. Emerg. Care 2018; 34: 93–95. [DOI] [PubMed] [Google Scholar]
- 11.KASS EH: Pyelonephritis and bacteriuria: A major problem in preventive medicine. Ann. Intern. Med 1962; 56: 46–53. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Baño J, Picón E, Navarro MD, et al. : Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli. Clin. Microbiol. Infect 2012; 18: 894–900. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21985560. [DOI] [PubMed] [Google Scholar]
- 13.Hooton TM, Scholes D, Gupta K, et al. : Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 2005; 293: 949–955. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15728165. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, Hooton TM, Naber KG, et al. : International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis 2011; 52: e103–20. Available at: 10.1093/cid/ciq257, accessed June 24, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Korpela K, Salonen A, Virta LJ, et al. : Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding. JAMA Pediatr. 2016; 170: 750. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27294842, accessed May 3, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Yassour M, Vatanen T, Siljander H, et al. : Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med 2016; 8: 343ra81–343ra81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27306663, accessed March 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korpela K, Zijlmans MAC, Kuitunen M, et al. : Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017; 5: 26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28253911, accessed March 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadizar F, Vijverberg SJH, Arets HGM, et al. : Early life antibiotic use and the risk of asthma and asthma exacerbations in children. Pediatr. Allergy Immunol 2017; 28: 430–437. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28423467, accessed March 11, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch AG, Pollak J, Glass TA, et al. : Early-life antibiotic use and subsequent diagnosis of food allergy and allergic diseases. Clin. Exp. Allergy 2017; 47: 236–244. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27562571, accessed March 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaikh N, Martin JM, Hoberman A, et al. : Host and Bacterial Markers that Differ in Children with Cystitis and Pyelonephritis. J. Pediatr 2019; 209: 146–153.e1. Available at: 10.1016/j.jpeds.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster CS, Loechtenfeldt AM, Shah SS, et al. : Urine neutrophil gelatinase-associated lipocalin in girls with recurrent urinary tract infections. Pediatr. Nephrol 2020; 35: 2121–2128. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh N, Martin JM, Hoberman A, et al. : Biomarkers that differentiate false positive urinalyses from true urinary tract infection. Pediatr. Nephrol 2020; 35: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]


