Abstract
Objective
The study objective was to compare gut microbiome diversity and composition in SARS-CoV-2 PCR-positive patients whose symptoms ranged from asymptomatic to severe versus PCR-negative exposed controls.
Design
Using a cross-sectional design, we performed shotgun next-generation sequencing on stool samples to evaluate gut microbiome composition and diversity in both patients with SARS-CoV-2 PCR-confirmed infections, which had presented to Ventura Clinical Trials for care from March 2020 through October 2021 and SARS-CoV-2 PCR-negative exposed controls. Patients were classified as being asymptomatic or having mild, moderate or severe symptoms based on National Institute of Health criteria. Exposed controls were individuals with prolonged or repeated close contact with patients with SARS-CoV-2 infection or their samples, for example, household members of patients or frontline healthcare workers. Microbiome diversity and composition were compared between patients and exposed controls at all taxonomic levels.
Results
Compared with controls (n=20), severely symptomatic SARS-CoV-2-infected patients (n=28) had significantly less bacterial diversity (Shannon Index, p=0.0499; Simpson Index, p=0.0581), and positive patients overall had lower relative abundances of Bifidobacterium (p<0.0001), Faecalibacterium (p=0.0077) and Roseburium (p=0.0327), while having increased Bacteroides (p=0.0075). Interestingly, there was an inverse association between disease severity and abundance of the same bacteria.
Conclusion
We hypothesise that low bacterial diversity and depletion of Bifidobacterium genera either before or after infection led to reduced proimmune function, thereby allowing SARS-CoV-2 infection to become symptomatic. This particular dysbiosis pattern may be a susceptibility marker for symptomatic severity from SARS-CoV-2 infection and may be amenable to preinfection, intrainfection or postinfection intervention.
Trial registration number
NCT04031469 (PCR−) and 04359836 (PCR+).
Keywords: INTESTINAL MICROBIOLOGY, ENTERIC BACTERIAL MICROFLORA, BIFIDOBACTERIA, BACTERIAL INFECTION
Summary box.
What is already known about this subject?
The gut microbiome is intrinsically related to host immune response (eg, inflammation, Th1 vs Th2) and susceptibility to infection.
What are the new findings?
Patients with SARS-CoV-2 infections possess significantly less bacterial diversity, lower abundance of Bifidobacterium and Faecalibacterium and increased abundance of Bacteroides at the genus level compared with SARS-CoV-2-exposed controls. There are inverse associations between disease severity and the Shannon and Simpson diversity indices and also with Bifidobacterium and Faecalibacterium abundance. There is also a direct association between severity and Bacteroides abundance.
How might it impact on clinical practice in the foreseeable future?
Boosting of Bifidobacterium or Faecalibacterium through probiotic supplementation or faecal microbiota transplant is worthy of exploration in the management of patients with acute severe disease or protracted infection. If the changes that we document precede SARS-CoV-2 infection in those who are most severely affected, this therapeutic approach may be of particular interest. Conversely, if the reduction follows infection, then repopulation of the gut microbiome may reduce long-term effects related to gut microbiome composition changes with SARS-CoV-2 infection.
Introduction
The abundance of Bifidobacterium decreases with increasing age and body mass index (BMI)1 and Bifidobacterium is the active ingredient of many probiotics. In vitro studies have demonstrated the benefits of these Gram-positive bacteria to include enhanced ATP production, immune modulation and competence,2–8 mucosal barrier integrity, restriction of bacterial adherence to and invasion of the intestinal epithelium and modulation of central nervous system activity.9 10 Additionally, Bifidobacterium have anti-inflammatory properties: Bifidobacterium animalis, B. longum and B. bifidum decrease the function of the ‘master switch’2 proinflammatory tumour necrosis factor-α (TNF-α), increase the anti-inflammatory cytokine IL-10 and promote the Th1 while inhibiting the Th2 immune response.8 In a mouse model of inflammatory bowel disease (IBD), B. bifidum and B. animalis reduced proinflammatory cytokines and restored intestinal barrier integrity.8
With respect to SARS-CoV-2 infection, there is immunologic coordination between the gut and lungs.11–13 Numerous studies have suggested that a healthy gut microbiome may be associated with a decrease in SARS-CoV-2-related mortality14 and that probiotics should be considered for prophylaxis15 and/or treatment of SARS-CoV-2 or its associated secondary infections.15–17 However, as of February 2022, despite the publication of nearly 8000 studies on SARS-CoV-2 infection, few ongoing studies (clinicaltrials.gov: NCT04443075 and NCT04486482) and only five publications to date have examined gut microbiome changes in SARS-CoV-2-infected patients. Nevertheless, an association between the status of the gut microbiome and outcome from this infection has been suggested. Accordingly, increased abundances of the Streptococcus, Rothia, Veilonella and Actinomyces genera were associated with inflammation,18 whereas increased abundances of Collinsella aerofaciens, Collinsella tanakaei, Streptococcus infantis and Morganella morganii were associated with faecal samples with high SARS-CoV-2 infectivity,19 and increased Lachnospiraceae and Enterobacterioaceae abundances were associated with increased mortality and need for artificial ventilation.19 Species potentially protective against SARS-CoV-2 infection include Parabacteroides merdae, Bacteroides stercoris, Alistipes onderdonkii, Lachnospiracea bacterium19 and F. prausnitzii,19 20 while vulnerability to infection and increased severity were associated with decreased abundance of B.bifidum.20 21 A recent study correlated aspects of the gut microbiome with ‘Long-COVID’, including reduced levels of F. prausnitzii on admission.22 In short, there is still a compelling need to elucidate changes in the human gut microbiome due to SARS-CoV-2 and their relationship with clinical outcomes.
The scientific community and lay public are increasingly interested in the therapeutic potential of probiotics. Bifidobacteria have potential to improve clinical conditions ranging from IBD23 to Clostridioides difficile infections.23–26 Treatment with specific strains of Bifidobacterium in vitro has been shown to reduce toxins from C. difficile.25 In vivo, Bifidobacterium can restore colonic integrity,27 and B. longum administered intranasally in mice prior to exposure to influenza has been associated with reduction in mortality.4 Given that Bifidobacterium are common component of several probiotic products and appear to be associated with SAR-CoV-2 infections, one could ask if probiotics might have a role in SARS-CoV-2 therapy or prevention.
Herein, we evaluate the relationships between gut microbiome diversity and composition compared with clinical outcome in cross-sectional groups of SARS-CoV-2 PCR-confirmed positive patients (ranging from asymptomatic to severely symptomatic) versus SARS-CoV-2 PCR-confirmed negative controls. Our controls are SARS-CoV-2 exposed persons who remained PCR negative and asymptomatic. The controls likely had similar viral exposures, but appeared protected against infection, and our data suggest that some protection may reside in the microbiome.
Methods
Study design and patients
Individuals who were tested for SARS-CoV-2 infection either because they were symptomatic or had been exposed to a ‘case’ were eligible for enrolment the week following testing if either they or a household member was positive. Controls eligible for enrolment were PCR negative for SARS-CoV-2, remained antibody negative for 3–6 months and asymptomatic for 6–12 months. Additionally, controls had to either share a household with at least one symptomatic SARS-CoV-2-positive individual or be a healthcare worker who had been repeatedly exposed to symptomatic SARS-CoV-2-positive patients or numerous SARS-CoV-2-positive samples. All exposed controls were ones that, despite exposure to SARS-CoV-2, chose not to quarantine or take prophylaxis for SARS-CoV-2 infection and none had yet been vaccinated. Patients did not wear Personal Protective Equipment (PPE) inside their homes and staff did not wear full PPE (ie, did not wear masks) at the office because of its scarcity during this global pandemic. Patients undergoing treatment with total parenteral nutrition, or those with a history of significant gastrointestinal surgery (eg, bariatric surgery, total colectomy with ileorectal anastomosis, proctocolectomy, postoperative stoma, ostomy or ileoanal pouch) were excluded.
This study was performed between 1 March 2020 and 31 October 2021, with all but one subject recruited prior to 1 June 2021. During that time, alpha and epsilon variants predominated in the USA.28
Assessments
A self-administered questionnaire solicited information on symptom severity, previous medical history, current medication and probiotic use and exposure to recreational drugs or animals. Patients with SARS-CoV-2 infection were further classified as being either asymptomatic carriers or having mild, moderate or severe symptoms as per National Institute of Health, Clinical Spectrum of SARS-CoV-2 Infection criteria.29 30 Asymptomatic PCR-confirmed SARS-CoV-2-positive household members of SARS-CoV-2-infected patients were categorised as asymptomatic carriers. Patients and controls were classified as underweight, normal weight, overweight, obese or severely obese based on BMI criteria of the Center for Disease Control and Prevention.31
Stool sample collection and processing
Patients and controls within the same household collected stool samples within a week of the index case being positive. Patients had stool samples collected at baseline, prior to any treatment, and within 48 hours of symptom onset. No subjects had been commenced on antibiotics, SARS-CoV-2 infection treatments, over the counter (OTC) remedies (e.g., vitamins, antipyretics, analgesics) or supplemental oxygen between the time of symptom onset or demonstration of PCR positivity and stool collections. Subjects were instructed (and educated on the procedure and sterile methods) to collect 1 mL of fresh stool and place it directly in a DNA/RNA Shield Fecal Collection Tube (Zymo Research, Tustin, California) and then mix sample thoroughly. Here,1 mL of faeces is more than sufficient to capture the microflora of the gut accurately and consistently. This method is chosen to eliminate the need for whole stool mixing and aliquoting. The solution in the Fecal Collection Tube is designed to preserve samples at ambient temperature (4°C–25°C) for >2 years, or below −20°C indefinitely. Once samples reached our laboratory, they were immediately frozen at −20°C.
Following faecal collection, each individual sample DNA was extracted and purified with the Qiagen PowerFecal Pro DNA extraction kit. The isolated DNA was then quantitated using the Quantus Fluorometer with the QuantFluor ONE dsDNA kit. After DNA quantification, the DNA was normalised, that is, all samples begin library preparation (following DNA extraction and purification) with 100 ng of input DNA. Libraries were then prepared using shotgun methodology with Illumina’s Nextera Flex kit. Samples then underwent the shotgun metagenomic processing procedure of tagmentation, amplification, indexing and purification. Following completion of this shotgun metagenomic standard protocol, purified libraries were again normalised to standardise sequencing depth during the next-generation sequencing (NGS) run on the NextSeq 500/550. We achieved consistency of sequencing depth (ie, number of reads) by normalising the samples’ pooling concentrations (ie, molarity), loading the same number of samples per sequencing run, consistently using the same NextSeq High Output kits.
After completion of sequencing on the Illumina NextSeq with 500/550 High-Output Kits V.2.5 (300 cycles), the raw data were streamed in real time to Illumina’s BaseSpace cloud for FASTQ (Fast Quality, a standard text file type for storing biological sequence information) conversion. The FASTQ files were then sent through One Codex’s bioinformatics pipeline for metagenomic annotation and analyses to elucidate the microflora composition and relative abundances of the top genera and species for all patients and controls.
Data analysis
We assessed differences in relative abundance across taxa between the gut microbiome of SARS-CoV-2-infected patients and exposed controls and calculated Shannon and Simpson alpha diversity indices with One Codex’s bioinformatics analysis pipeline using Jupyter Notebook in Python. Specifically, the One Codex Database consists of ~114K complete microbial genomes (One Codex, San Francisco, California). During processing, reads were first screened against the human genome and then mapped to the microbial reference database using a k-mer-based classification. Individual sequences (NGS read or contig) were compared against the One Codex Database (One Codex) by exact alignment using k-mers, where k=31. Based on the relative frequency, unique k-mers were filtered to control for sequencing or reference genome artefacts.
The sequencing depth followed ProgenaBiome’s standard operating procedures and was 8 239 475 reads on average for this study. One should note that shallow metagenomic sequencing is typically only 0.5 million reads but is still considered sufficient for taxonomic phyla level analysis (and even genera for the most abundant bacteria).
The relative abundance of each microbial taxonomic classification was estimated based on the depth and coverage of sequencing across every available reference genome. Beta-diversity was calculated as weighted UniFrac distance visualised in a distance matrix using the phylum-level relative abundance obtained from One Codex. Thirteen genera were selected based on our experience and knowledge of critical players in the gut microbiome as well as similarity to other studies18 20 29
To compare patients across subgroups and patients to exposed controls, Analysis of Variation (ANOVA), Mann-Whitney U, Kruskal-Wallis tests and χ2 test statistics were conducted using GraphPad V.8 with p values <0.05 considered as significant. Dunn’s post-hoc was used for Kruskal-Wallis test, with correction for multiple comparisons in all situations.
All authors had access to study data and reviewed and approved the final manuscript.
Results
Patient characteristics
Demographic and clinical characteristics of patients (n=50) and exposed controls (n=20) are presented in online supplemental tables 1 and 2, and summarised in table 1. All patients were resident of USA, with states indicated in online supplemental table 1. Twenty-four of 50 (48%) patients and 7 of 20 (35%) of exposed controls were men. The mean±SEM age in years was 50.0±2.5 for patients and 44.4±3.6 for exposed controls. Fourty-four of 50 (88%) patients were non-Hispanic white; 5 of 50 (10%) were Hispanic and 1 of 50 (2%), Native American and 17 of 20 (85.0%) of exposed controls were non-Hispanic white; 2 of 20 (10.0%), Hispanic and 1 of 20 (5.0%), Black. Of patients, 28 of 50 (56%) had severe, 12 of 50 (24%) had moderate and 6 of 50 (12%) had mild disease and 4 of 50 (8%) were asymptomatic. Thirty-two of 50 (64%) patients and 12 of 20 (60.0%) exposed controls had underlying comorbidities considered risk factors for increased severity of SARS-CoV-2 infection by the Center for Disease Control (CDC).1 The mean±SEM BMI of the 46 patients with available data was 27.1±0.98 compared with 25.1±0.96 for the 20 exposed controls. There was no significant difference (p>0.2) in gender, age, racial demographics, loss of appetite, change in stool frequency, diet or presence of underlying comorbidities.
Table 1.
Summary of demographics along with clinical and dietary characteristics of subjects
| SARS-CoV-2 negative; exposed control (n=20) |
SARS-CoV-2 positive | ||||||
| Asymptomatic (n=4) |
Mild (n=6) |
Moderate (n=12) |
Severe (n=28) |
Total (n=50) |
P value | ||
| Demographics | |||||||
| USA resident | 20 (100.00%) | 4 (100.00%) | 6 (100.00%) | 12 (100.00%) | 28 (100.00%) | 50 (100.00%) | ns |
| Male | 7 (35.00%) | 3 (75.00%) | 2 (33.33%) | 5 (41.66%) | 14 (50.00%) | 24 (48.00%) | 0.5706 |
| Mean age ±SEM | 44.40±3.62 | 47.50±9.40 | 37.67±4.65 | 50.58±5.92 | 52.82±3.33 | 50.00±2.50 | 0.5221 |
| Median age | 48 | 50.5 | 37 | 60 | 55.5 | 53 | |
| Race | |||||||
| White | 17 (85.00%) | 3 (75.00%) | 5 (83.33%) | 9 (75.00%) | 27 (96.43%) | 44 (88.00%) | 0.3826 |
| Hispanic | 2 (10.00%) | 1 (25.00%) | 0 (0.00%) | 3 (25.00%) | 1 (3.57%) | 5 (10.00%) | |
| Black | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Other | 0 (0.00%) | 0 (0.00%) | 1 (16.66%) | 0 (0.00%) | 0 (0.00%) | 1 (2.00%) | |
| Clinical/dietary characteristics | |||||||
| Severe COVID-19 comorbidities* | 12 (60.00%) | 3 (75.00%) | 3 (50.00%) | 10 (83.33%) | 16 (57.14%) | 32 (64.00%) | 0.5099 |
| Normal stool frequency | 20 (100.00%) | 4 (100.00%) | 6 (100.00%) | 10 (83.33%) | 24 (85.71%) | 44 (88.00%) | 0.2891 |
| Lack of appetite | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (8.33%) | 2 (7.14%) | 3 (6.00%) | 0.6663 |
| Omnivore diet | 19†(100.00%) | 4 (100.00%) | 6 (100.00%) | 12 (100.00%) | 28 (100.00%) | 50 (100.00%) | ns |
Numbers in cells indicate number of subjects with percentage in categories, except for age and BMI, which indicate value. P values calculated via one-way ANOVA (age and BMI) or χ2 (others). Normal stool frequency is defined as absence of diarrhoea. Total refers to sum of SARS-CoV-2-positive subjects, and it is not used in statistics.
*Comorbid conditions indicative of severe SARS-CoV-2 infection (not including hypertension), based on Center for Disease Control (CDC) definitions.44
†Nineteen total subjects had data available for diet within exposed control.
bmjgast-2022-000871supp001.pdf (99.3KB, pdf)
bmjgast-2022-000871supp002.pdf (95.5KB, pdf)
Of the exposed controls, 16 were household contacts of SARS-CoV-2-positive patients in the study, 2 were healthcare workers with extensive, non-protected, exposure to SARS-CoV-2-positive patients and 2 were laboratory personnel exposed to thousands of SARS-CoV-2 samples (healthcare workers and laboratory personnel did not wear full PPE, that is, did not wear a face mask, due its scarcity; see the Methods section). During the timeframe of the study, none of the patients or controls was on SARS-CoV-2 prophylaxis or treatment, and none had yet been vaccinated. No patients or exposed control were positive for SARS-CoV-2 prior to the study.
Gut microbiome diversity and composition
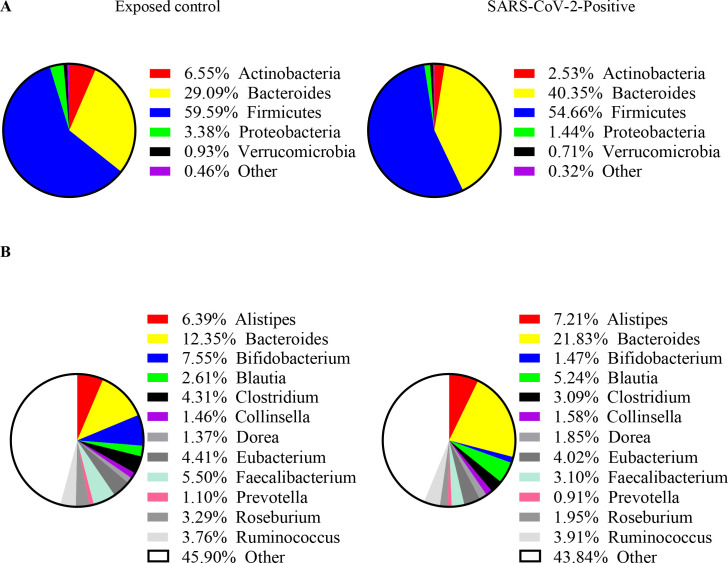
Figure 1 depicts pie charts of the composition of the gut microbiome for the exposed control at the phylum level (figure 1A) and genus level (figure 1B). At phylum level, Firmicutes and Bacteroides dominated, comprising 59.6% (exposed control) and 54.7% (SARS-CoV-2 positive) and 29.1% (exposed control) and 40.4% (SARS-CoV-2 positive) of all phyla, respectively. At the level of genus, Bacteroides contributed 12.4% (exposed control) and 21.8% (SARS-CoV-2 positive), Alistipes 6.4% (exposed control) and 7.2% (SARS-CoV-2 positive) and Bifidobacterium 7.6% (exposed control) and 1.5% (SARS-Cov-2 positive).
Figure 1.
Distribution of bacterial relative abundance in various (A) phlya and (B) genera for exposed control (n=20, left) and SARS-CoV-2 positive subjects (n=50, right).
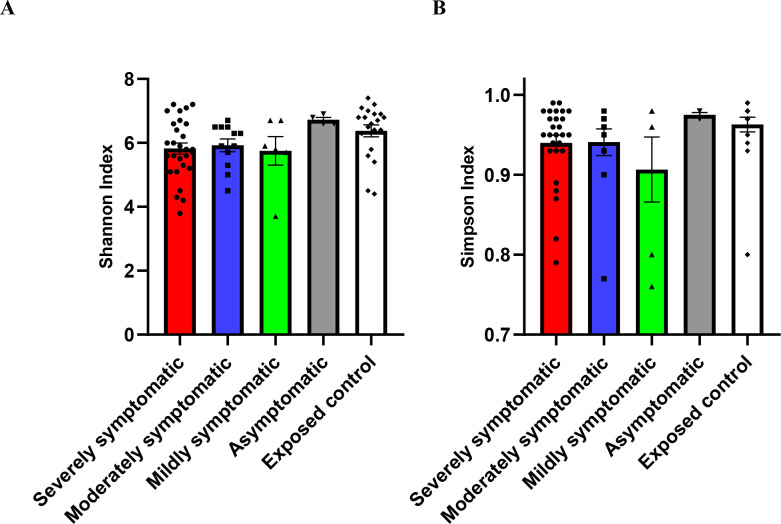
Figure 2 shows two diversity indices for all subgroups studied, namely, Shannon diversity (figure 2A) and Simpson diversity index (figure 2B). The overall p value for Shannon index (richness of bacterial composition) demonstrated a significant (p=0.0499) decrease in diversity with increased severity, and significance was seen for exposed control versus severely symptomatic (p=0.0201), analysed via Kruskal-Wallis test. The Simpson (evenness of bacterial composition) indexes showed a trend (p=0.0581) of a decrease in diversity with increased SARS-CoV-2 severity.
Figure 2.
Diversity of gut microbiome composition of SARS-CoV-2 positive patients (severely symptomatic: n=28; moderately symptomatic: n=12; mildly symptomatic: n = 6; asymptomatic: n=4) versus exposed controls (n=20). (A) Shannon index (p=0.0499), (B) Simpson index (p=0.0581). Differences between severely symptomatic positive and exposed negative controls were analysed via Kruskal-Wallis test Dunn’s post-hoc, correcting for multiple comparisons, showing significant for Shannon index at p=0.0201.
Further metagenomic analysis comparing SARS-CoV-2 patients and controls revealed significant differences in relative abundance of specific bacteria. The relative abundance of SARS-CoV-2 positive (exposed control) versus negative subjects is presented in table 2, along with comparative p values via Mann-Whitney U test. Patients with SARS-CoV-2 infection showed a significantly decreased relative abundance of Bifidobacterium and Faecalibacterium, and significantly increased relative abundance of Bacteroides (table 2).
Table 2.
Relative abundances of Bacteroides increase and of Bifidobacterium, Faecalibacterium and Roseburium decrease in SARS-CoV-2 positive subjects versus SARS-CoV-2 negative exposed controls
| Genus (±species) | Relative abundance (mean±SEM) | P value | |
| Exposed controls | SARS-CoV-2 positive | ||
| Alistipes | 0.0639±0.0095 | 0.0721±0.0100 | 0.8709 |
| Bacteroides | 0.1235±0.0178 | 0.2183±0.0191 | 0.0025 |
| Bifidobacterium | 0.0755±0.0219 | 0.0147±0.0051 | <0.0001 |
| Blautia | 0.0261±0.0040 | 0.0524±0.0088 | 0.1349 |
| Clostridium | 0.0431±0.0075 | 0.0309±0.0039 | 0.9948 |
| Collinsella | 0.0146±0.0045 | 0.0158±0.0029 | 0.9948 |
| Dorea | 0.0137±0.0024 | 0.0185±0.0022 | 0.2777 |
| Eubacterium | 0.0441±0.0063 | 0.0402±0.0043 | 0.4786 |
| Faecalibacterium | 0.0550±0.0086 | 0.0310±0.0039 | 0.0137 |
| F. prausnitzii | 0.0542±0.0085 | 0.0313±0.0039 | 0.0153 |
| Prevotella | 0.0110±0.0086 | 0.0091±0.0066 | 0.6538 |
| Roseburium | 0.0329±0.0056 | 0.0195±0.0032 | 0.0097 |
| Ruminococcus | 0.0376±0.0079 | 0.0391±0.0056 | 0.9844 |
Mean±SEM relative abundances, as well as p value via Mann-Whitney U test are indicated, with bold marking p<0.05.
Table 3 lists the genera/species relative abundances (mean±SEM) for various levels of severity of SARS-CoV-2-positive patients versus exposed control. Analysed via Kruskal-Wallis test, the main effect (ie, overall p value) of these changes are shown in the left column. Table 3 proceeds to compare, correcting for multiple comparison, the three levels of severity in infected patients versus exposed control and asymptomatic groups. Specifically, increased disease severity was associated with decreased relative abundance of Bifidobacterium, Faecalibacterium, F. prausnitizii and Roseburium, along with an increased relative abundance of Bacteroides.
Table 3.
Relative abundance of Bacteroides increases and those of Bifidobacterium, Faecalibacterium and Roseburium decrease with increasing severity of disease
| Genus (±species) |
Relative abundance (mean±SEM) | |||||
| Main effect | Severe | Moderate | Mild | Asymptomatic | Exposed control | |
| A. Relative abundance of genera/species for various severities of SARS-CoV-2 positivity, as well as exposed controls (SARS-CoV-2 negative). Overall p value of Kruskal-Wallis test is indicated in ‘Main Effect’ column. | ||||||
| Alistipes | 0.8119 | 0.0793±0.0166 | 0.0520±0.0084 | 0.0664±0.0196 | 0.0901±0.0272 | 0.0639±0.0095 |
| Bacteroides | 0.0075 | 0.2187±0.0272 | 0.2849±0.0305 | 0.0912±0.0226 | 0.2058±0.0606 | 0.1235±0.0178 |
| Bifidobacterium | <0.0001 | 0.0018±0.0006 | 0.0037±0.0015 | 0.0507±0.0244 | 0.0840±0.0308 | 0.0755±0.0219 |
| Blautia | 0.2098 | 0.0495±0.0095 | 0.0426±0.0132 | 0.1037±0.0510 | 0.0260±0.0067 | 0.0261±0.0040 |
| Clostridium | 0.2721 | 0.0331±0.0062 | 0.0334±0.0055 | 0.0265±0.0083 | 0.0145±0.0025 | 0.0431±0.0075 |
| Collinsella | 0.7476 | 0.0145±0.0035 | 0.0107±0.0038 | 0.0203±0.0126 | 0.0334±0.0174 | 0.0146±0.0045 |
| Dorea | 0.4820 | 0.0218±0.0035 | 0.0126±0.0032 | 0.0174±0.0046 | 0.0152±0.0015 | 0.0137±0.0024 |
| Eubacterium | 0.9619 | 0.0436±0.0070 | 0.0367±0.0064 | 0.0366±0.0085 | 0.0332±0.0058 | 0.0441±0.0063 |
| Faecalibacterium | 0.0077 | 0.0209+0.0037 | 0.0428+0.0093 | 0.0359±0.0139 | 0.0597+0.0098 | 0.0550±0.0086 |
| F. prausnitzii | 0.0054 | 0.0220±0.0041 | 0.0417±0.0092 | 0.0356±0.0137 | 0.0589±0.0095 | 0.0542±0.0085 |
| Prevotella | 0.1687 | 0.0149±0.0118 | 0.0000±0.0000 | 0.0050±0.0046 | 0.0023±0.0020 | 0.0110±0.0086 |
| Roseburium | 0.0327 | 0.0146±0.0037 | 0.0245±0.0075 | 0.0268±0.0138 | 0.0274±0.0081 | 0.0329±0.0056 |
| Ruminococcus | 0.8033 | 0.0384±0.0076 | 0.0293±0.0073 | 0.0620±0.0250 | 0.0394±0.0139 | 0.0376±0.0079 |
| Genus (±species) |
P value vs exposed control | P value vs asymptomatic | |||||
| Mild | Moderate | Severe | Asymptomatic | Mild | Moderate | Severe | |
| B. P value for various levels of severity of symptomatic infection, compared to negative exposed controls and positive asymptomatic subjects, using Dunn’s post-hoc. | |||||||
| Alistipes | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Bacteroides | >0.9999 | 0.0078 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Bifidobacterium | >0.9999 | 0.0006 | 0.0026 | >0.9999 | >0.9999 | 0.0006 | <0.0001 |
| Blautia | 0.4116 | >0.9999 | 0.6373 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Clostridium | >0.9999 | >0.9999 | >0.9999 | 0.4944 | >0.9999 | 0.9841 | >0.9999 |
| Collinsella | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Dorea | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Eubacterium | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Faecalibacterium | >0.9999 | >0.9999 | 0.0082 | >0.9999 | >0.9999 | >0.9999 | 0.1422 |
| F. prausnitzii | >0.9999 | >0.9999 | 0.0109 | >0.9999 | >0.9999 | >0.9999 | 0.1568 |
| Prevotella | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | 0.8916 | >0.9999 |
| Roseburium | >0.9999 | >0.9999 | 0.0196 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
| Ruminococcus | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | >0.9999 |
For A and B, bold values indicate p<0.05. Note, no apparently significant (p<0.05) p values were observed in post-hocs, with main effects non-significant.
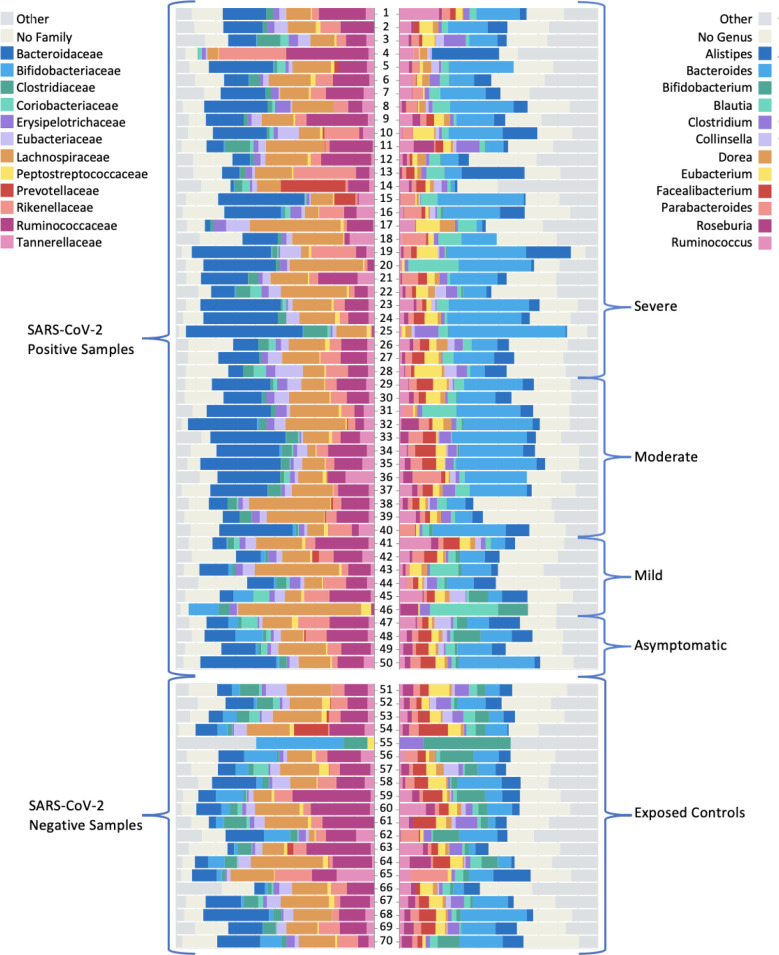
Depicted in figure 3 are the 12 most abundant families and the 12 most abundant genera for patients, stratified by disease severity and in comparison to exposed controls. Distinguished by colour, the bars represent the relative per cent bacterial families and genera abundance. Note the reduced diversity of the microbiome of SARS-CoV-2-positive patients shown in column B.
Figure 3.
Graphic of relative abundance of the 12 most common (A) families and (B) genera. The top group represents the SARS-CoV-2 positive samples (n=50), stratified by severity. The bottom group represents the exposed control samples (n=20). The coloured boxes represent the fraction of the entire rectangle composed of the given family/genera of bacteria.
Figure 4 summarises the microbiome changes according to SARS-CoV-2 positivity and severity, with green boxes depicting significant elevation and red boxes indicating significant depletion in genera/species abundance associated with SARS-CoV-2.
Figure 4.
Diagram of taxa comparing the gut microbiome of SARS-CoV-2 patients and exposed controls. Red or green background indicates a significant depletion or increase (due to positivity or severity), respectively, of the genus or species in SARS-CoV-2 positive subjects.
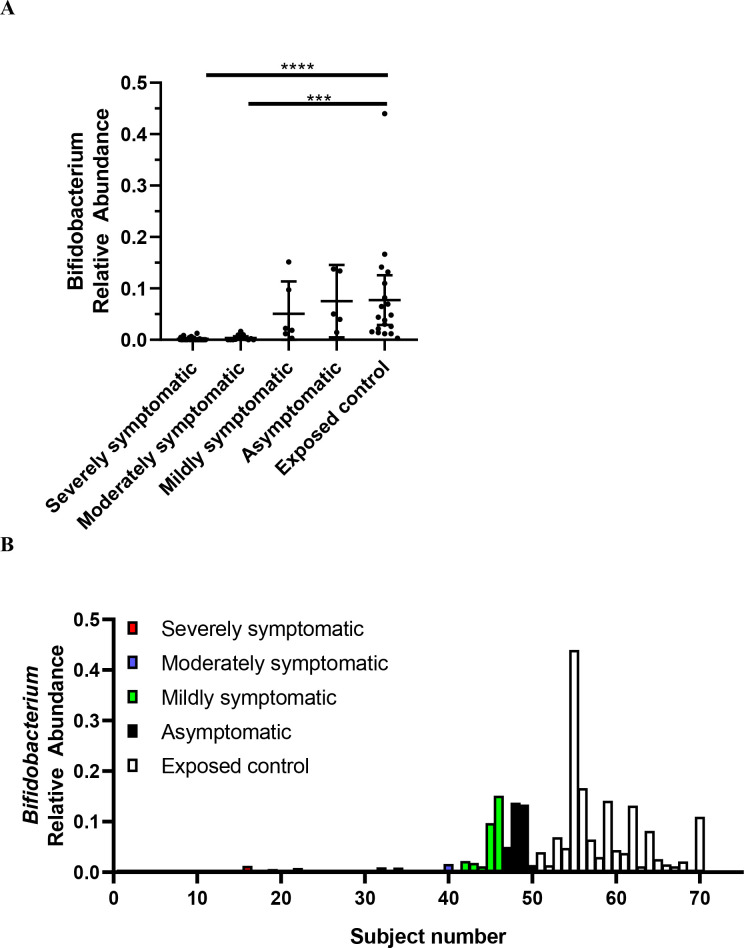
Figure 5A, B exhibit the relative abundance of Bifidobacterium for each subject, grouped by SARS-CoV-2 infection severity. This diagram, with subjects groups ordered by severity (from severe on left, to exposed controls on the right), depicts how Bifidobacterium abundance increases as severity decreases.
Figure 5.
Relative abundance of Bifidobacterium in SARS-CoV-2 positive patients (n=50) versus SARS-CoV-2 negative exposed controls (n=20). Data are plotted as (A) mean with error bars for 95% CI and (B) individual points of relative abundance for varying SARS-CoV-2 infection severity. Analysed via Kruskal-Wallis test, there were significant reductions in Bifidobacterium relative abundance for severely (p<0.0001) and moderately (p=0.0002) symptomatic patients. Subjects 1–28 = severely symptomatic; subjects 29–40 = moderately symptomatic; subjects 41–46 = mildly symptomatic subjects; subjects 47–50 = asymptomatic; subjects 51–70 = exposed control. Figure A, B depicts same data.
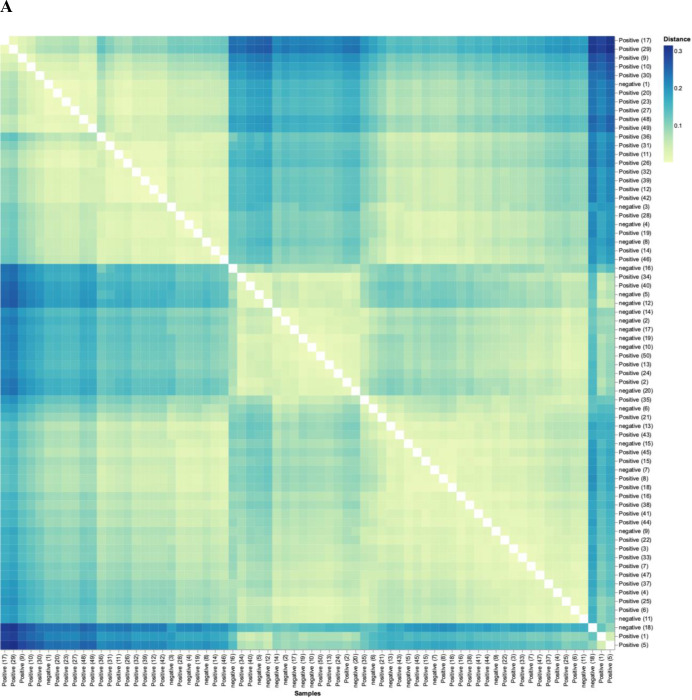
Analysis of the beta-diversity of subjects demonstrated that the diversity of control subjects cluster separately from that of patients. Figure 6A shows the beta-diversity-weighted (quantitative) UniFrac analysis featuring phyla bacterial profiles for all individuals in the study (n=70). Figure 6A reveals that, although there is a range of dissimilarity, the SARS-CoV-2-negative individuals are more similar to one another than they are to SARS-CoV-2-positive patients. The matrix also highlights clusters of similarity among SARS-CoV-2-positive patients, and darker quadrants of dissimilarity where positive and negative patients intersect. At a more granular level, figure 6B used principal component (PC) analysis of genera, where the axes depict the per cent of variance. In PC analysis, points closer together are more similar (less divergence with axis representing directions of divergence). Herein, the PC1 accounts for 43.16% of the variation, whereas PC2 accounts for 12.78%. This analysis reveals a clear divergence of a subset of SARS-CoV-2-positive patients clustering on the right side tracking along the x-axis (PC1), highlighting microbiota divergence as a function of disease. Thus, figure 6 shows that exposed controls cluster similar separately from SARS-CoV-2 patients; that is, patients are more similar in terms of their microbiome to each other than to controls.
Figure 6.
SARS-CoV-2 positive patients’ microbiome is more similar to each other than to that of exposed controls. (A) Weighted UniFrac distance matrix of phylum level SARS-CoV-2 positive (n=50) and exposed negative control samples (n=20). Distance of microbiome differences increases with increasing blue colour intensity (see legend top right). The centre of the diagram consists of negative subjects on both axis and is yellow indicative of less distance (ie, lessdifference in microbiome). The central area of the left as well as central-top side of diagram, consists of negative subjects on one axis and positive on the other, and are darker blue, indicative of more distance (more difference in microbiome). (B) Principal component analysis of microbiota from SARS-CoV-2 positive (n=50) and exposed negative controls (n=20). Dots closer in distance are more similar in microbiome composition. Axes depict the per cent of variance explained by principal component (PC) 1 and 2. Plots are based on bacterial genera relative abundance profiles.
Discussion
Immune function and health could be enhanced by bacterial abundance
Interactions between the host and gut microbiota are complex, numerous and bidirectional. Gut microbiota regulate the development and function of the innate and adaptive immune systems,32 potentially allowing them to protect against infections and infection severity. The primary findings of our study are that SARS-CoV-2 positivity and infection severity are associated with decreased levels of the protective Bifidobacterium and Faecalibacterium genera and with decreased bacterial diversity, as exemplified by the Shannon and Simpson indices. This accords with studies showing bacterial diversity inversely relates to the presence of various common disorders.33 Uniquely, our study compared SARS-CoV-2-exposed SARS-CoV-2-negative persons (ie, controls) with symptomatic and asymptomatic SARS-CoV-2-positive patients. Thus, we controlled for SARS-CoV-2 exposure.
The genus Bifidobacterium has important immune functions,8 is a major component of the microbiome and is frequently used in probiotics.34 Bifidobacterum increase Treg responses and reduce cell damage by inhibiting TNF-α and macrophages.35 Bifidobacterium also protects against intestinal epithelial cell damage independently from their effects on TNF-α production. The exopolysaccharide coat, which is a feature of some Bifidobacterium, plays a significant role in this protective effect.36 These immune functions of Bifidobacterium could be critical in relation to its SARS-CoV-2 infection-prevention effects.
Evidence has accumulated to support a beneficial effect from supplementation with Bifidobacterium in numerous disease states.37 The numbers of commensal Bifidobacterium have been shown to decrease with age and obesity, major SARS-CoV-2 infection risk factors. We demonstrate that patients with a more severe course of viral infection had decreased abundance of Bifidobacterium. However, it should be noted that there are no definitive studies concerning what constitutes a normal baseline abundance of Bifidobacterium in a ‘healthy’ individual.
The abundance of Faecalibacterium genus and F. prausnitzii species was also inversely related to SARS-CoV-2 positivity and SARS-CoV-2 infection severity in this analysis. Age and diabetes are risk factors for SARS-CoV-2 infection, and F. prausnitzii levels decline markedly in elder and diabetic populations.37 In fact, Faecalibacterium levels have been considered an indirect ‘indicator’ of overall human health.38 The abundance of F. prausnitzii is reduced by the ‘Western’ diet (consumption of more meat, animal fat, sugar, processed foods and low fibre), while it is enhanced by the high-fibre containing ‘Mediterranean’ diet of vegetables and fruits with low meat intake.39 Preliminary studies showed that reduced ingestion of a Mediterranean diet within the same country is associated with increased SARS-CoV-2-related death rates.40 In short, we show that F. prausnitzii levels negatively correlated to SARS-CoV-2 infection severity and prior studies show that reduced F. prausnitzii is associated with SARS-CoV-2 infection vulnerabilities such as age, diabetes, obesity and possibly diet.
SARS-CoV-2 positivity and severity were also associated with decreased abundance of Roseburium and increased abundance of Bacteroides. The implications of these changes remain unclear.
Innate immunity could be enhanced by increased level of beneficial bacteria
The pathological impact of SARS-CoV-2 infection includes both direct effects from viral invasion and complex immunological responses including, in its most severe form, the ‘cytokine storm’. The cytokine storm is the result of a sudden increase in circulating levels of proinflammatory cytokines produced by activated macrophages, mast cells, endothelial cells and epithelial cells during innate immune responses, which appear to be modulated by the abundance of Bifidobacterium and Faecalibacterium and bacterial diversity (5, 23, 25). Steroid treatment has situational success in SARS-CoV-2 infection, based on suppressing this over activation of the innate immune system, reviewed by Tang et al41
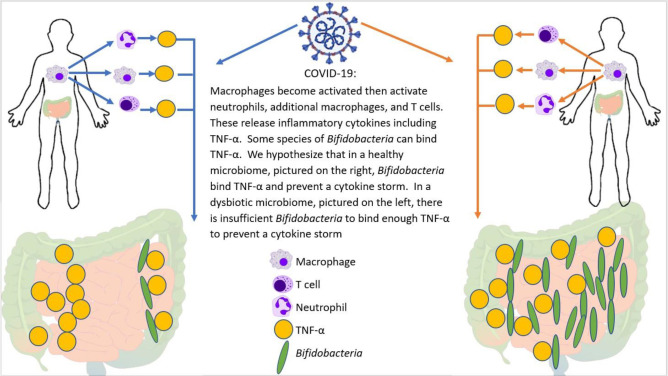
Zhao reported that elevated serum levels of proinflammatory cytokines such as IL-16 and IL-17 predict poor prognoses in patients with SARS-CoV-2 infection.42 Also, Tao et al showed that changes in gut microbiota composition might contribute to SARS-CoV-2-induced production of inflammatory cytokines in the intestine, which may lead to cytokine storm onset.29 Both authors report significantly reduced gut microbiota diversity and increased opportunistic pathogens in patients with SARS-CoV-2. Interestingly, the bloom of opportunistic pathogens positively correlated with the number of Th17 cells. Bozkurt and Quigley reported that IL-6 and IL-17 promote viral persistence by immune interactions through cellular autophagy via the inositol-requiring enzyme 1 pathway.16 Additionally, some species of Bifidobacterium are likely to prevent the replication of coronaviruses by reducing endoplasmic reticulum stress, also through the inositol-requiring enzyme 1 pathway. Reduced Bifidobacterium abundance has been observed in the gut microbiome of patients with IBD, which has mechanisms involving IL-17.31 Furthermore, the direct endoscopic delivery of Bifidobacterium has been shown to be effective in promoting symptom resolution and mucosal healing in IBD—an effect likely to be associated with the anti-Th17 effect of Bifidobacterium.(8) Figure 7 demonstrates how Bifidobacterium might hypothetically quell a heightened immune response by dampening the effect of the master switch TNF-α.
Figure 7.
Potential mechanism for cytokine storm and immune hyper-response in SARS-CoV-2 positive patients. In individuals infected with SARS-CoV-2, the macrophages become activated; these in turn activate T-cells, additional macrophages, and neutrophils―all of which release cytokines, including TNF-α. Bifidobacterium, when present in sufficient numbers, can bind to TNF-α and prevent the subsequent cytokine storm. Therefore, patients with a bifidobacterial dysbiosis characterised by low levels of Bifidobacterium lack this line of defense, which may lead to a cytokine storm.
Conclusions
Given our cross-sectional study design, it is not possible to determine whether the differences in Bifidobacterium levels observed between patients and exposed controls preceded or followed infection. If preceding infection, they could be a marker of susceptibility, and boosting Bifidobacterium levels might decrease the risk or severity of SARS-CoV-2 infection. If these changes followed infection, alteration of the gut microbiome (such as through faecal microbiota transplantation or possibly probiotic supplementation) to increase Bifidobacterium could be an area worth exploring for improved outcomes. If future studies can demonstrate improved outcomes, such therapy can be considered for complex cases of SARS-CoV-2 infection, such as ‘long-haulers’, and those with severe disease. Developing outbreaks within tightly closed communities such as nursing homes might be a good setting in which to assess susceptibility: faecal samples could be collected during the outbreak and run post hoc on ‘cases’ and ‘controls’. Future studies of individuals with baseline prepandemic microbiome data would be highly valuable, although acquiring such baseline preinfection microbiome data is still costly.
With the lack of data on the gut microbiome prior to onset of SARS-CoV-2 infection, we cannot completely rule out the confounding effect of illness on the microbiome. Nonetheless, we eliminated effects of treatment on the gut microbiome by sampling prior to administration of SARS-CoV-2 infection therapeutics of any kind and within 48 hours of symptom onset. Specifically, no subjects were given antibiotics, antivirals, anti-inflammatory medicines, oxygen or any other therapeutic agent between symptom onset or PCR positivity and stool sampling. We also note that the prevalence of appetite changes, alterations of stool frequency and gastrointestinal (GI) symptoms, in general, were not significantly different between any of the severity groups or controls (table 1), although the small sample sizes for some groups should be considered in evaluating these statistics.
SARS-CoV-2 infection presentation variability correlates with colon microbiome bacterial composition and overall diversity. The same changes we observe due to SARS-CoV-2 infection, namely reduced Bifidobacterium and/or Faecalibacterium abundance, are associated with SARS-CoV-2 infection risk factors, including old age, obesity and diabetes.9 37 39 43 Thus, colon microbiome diversity and relative abundance of Bifidobacterium and Faecalibacterium should be explored as potential markers for predicting SARS-CoV-2 infection severity.
In summary, we demonstrate in a study of PCR-positive and PCR-negative SARS-CoV-2-exposed subjects, reduced bacterial diversity and reduced levels of various genus/species are highly associated with both SARS-CoV-2 positivity and SARS-CoV-2 infection severity. These findings suggest that probiotic supplementation or faecal microbiota transplantation should be explored as a potential therapeutic avenue for patients with SARS-CoV-2. Additionally, individual colon microbiome evaluation may predict vulnerability to the development of severe SARS-CoV-2 infection. Finally, our data suggest a new area for exploration: if SARS-CoV-2 severity is found to be dependent on the microbiome, then accounting for microbiome differences could reduce variability in outcomes for SARS-CoV-2.
Acknowledgments
Medical writing assistance was provided by Sonya Dave, PhD (an author on the publication) and was funded by ProgenaBiome. The authors thank all clinicians for their involvement and contribution to the study. The authors thank Kate Hendricks, MD, MPH and TM for many helpful editorial suggestions. Finally, the authors owe a depth of gratitude to the late Sydney M Finegold, MD for mentorship that sparked the interest in the microbiome to many scientists, including authors of this paper.
Footnotes
Contributors: All authors (SH, NS, HSB, SD, AJP, JD, BDB, EMMQ and TJB) participated in the drafting, critical revision, and approval of the final version of the manuscript. SH led study design. SH and AJP conducted the bioinformatic analysis. SD conducted the statistical analysis and was a major contributor to writing the paper. SH was primarily responsible for interpretation of the study results, with contributions from all authors. EMMQ and TJB are senior authors who provided overall direction and advice. SH acts as the guarantor and accepts full responsibility for the work, has access to all data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SH declares that she has pecuniary interest in Topelia Pty Ltd in Australia, and Topelia Pty Ltd in USA where development of COVID-19 preventative/treatment options is being pursued. She has also filed patents relevant to Coronavirus treatments. She is the founder and owner of Microbiome research foundation, Progenabiome and Ventura Clinical Trials. TJB declares that he has pecuniary interest in Topelia Pty Ltd in Australia, and Topelia Therapeutics Inc. in USA developing COVID-19 preventative/treatment medications. He has also filed patents relevant to COVID-19 treatments. SD declares she has corporate affiliation to McKesson Specialty Health/Ontada and North End Advisory, LLC. SD is unaware of SARS-CoV-2 and microbiome projects and not directly involved in COVID-19 relevant projects at McKesson, but they may exist. AJP and BDB have corporate affiliations to Progenabiome. EMMQ serves as a consultant to Precisionbiotics, Novazymes, Salix, Biocodex and Axon Pharma and has received research support from 4D Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data available upon reasonable request from corresponding author, Dr. Sabine Hazan.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice and the Ethical and Independent Review Board. This study involves human participants and was approved by the 'Ethical and Independent Review Board' (IRB, IRB00007807). Participants gave informed consent to participate in the study before taking part.
References
- 1.De Vuyst L, Moens F, Selak M, et al. Summer meeting 2013: growth and physiology of bifidobacteria. J Appl Microbiol 2014;116:477–91. 10.1111/jam.12415 [DOI] [PubMed] [Google Scholar]
- 2.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010;20:87–103. 10.1615/CritRevEukarGeneExpr.v20.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins AKS, Martins FS, Gomes DA, et al. Evaluation of in vitro antagonism and of in vivo immune modulation and protection against pathogenic experimental challenge of two probiotic strains of Bifidobacterium animalis var. lactis. Arch Microbiol 2010;192:995–1003. 10.1007/s00203-010-0626-0 [DOI] [PubMed] [Google Scholar]
- 4.Groeger D, Schiavi E, Grant R, et al. Intranasal Bifidobacterium longum protects against viral-induced lung inflammation and injury in a murine model of lethal influenza infection. EBioMedicine 2020;60:102981. 10.1016/j.ebiom.2020.102981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konieczna P, Akdis CA, Quigley EMM, et al. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes 2012;3:261–6. 10.4161/gmic.20358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konieczna P, Ferstl R, Ziegler M, et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One 2013;8:e62617. 10.1371/journal.pone.0062617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiavi E, Plattner S, Rodriguez-Perez N, et al. Exopolysaccharide from Bifidobacterium longum subsp. longum 35624™ modulates murine allergic airway responses. Benef Microbes 2018;9:761–73. 10.3920/BM2017.0180 [DOI] [PubMed] [Google Scholar]
- 8.Ruiz L, Delgado S, Ruas-Madiedo P, et al. Bifidobacteria and their molecular communication with the immune system. Front Microbiol 2017;8:2345. 10.3389/fmicb.2017.02345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marras L, Caputo M, Bisicchia S, et al. The role of bifidobacteria in predictive and preventive medicine: a focus on eczema and hypercholesterolemia. Microorganisms 2021;9. 10.3390/microorganisms9040836. [Epub ahead of print: 14 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: a long debate. Front Immunol 2020;11:2192. 10.3389/fimmu.2020.02192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlawat S, Sharma KK. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res 2020;286:198103. 10.1016/j.virusres.2020.198103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Follmer C. Viral infection-induced gut dysbiosis, neuroinflammation, and α-synuclein aggregation: updates and perspectives on COVID-19 and neurodegenerative disorders. ACS Chem Neurosci 2020;11:4012–6. 10.1021/acschemneuro.0c00671 [DOI] [PubMed] [Google Scholar]
- 13.Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung axis in respiratory disease. Ann Am Thorac Soc 2015;12 Suppl 2:S150–6. 10.1513/AnnalsATS.201503-133AW [DOI] [PubMed] [Google Scholar]
- 14.Janda L, Mihalčin M, Šťastná M. Is a healthy microbiome responsible for lower mortality in COVID-19? Biologia 2021;76:819–29. 10.2478/s11756-020-00614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari SK, Dicks LMT, Popov IV, et al. Probiotics at war against viruses: what is missing from the picture? Front Microbiol 2020;11:11. 10.3389/fmicb.2020.01877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozkurt HS, Quigley EM. The probiotic Bifidobacterium in the management of Coronavirus: A theoretical basis. Int J Immunopathol Pharmacol 2020;34:2058738420961304. 10.1177/2058738420961304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X, Lian J-S, Hu J-H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020;69:1002–9. 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu S, Chen Y, Wu Z, et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis 2020;71:2669–78. 10.1093/cid/ciaa709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo T, Liu Q, Zhang F, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021;70:276–84. 10.1136/gutjnl-2020-322294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeoh YK, Zuo T, Lui GC-Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698–706. 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu K, Cai H, Shen Y. Management of COVID-19: the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020;49:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022;71:544–52. 10.1136/gutjnl-2021-325989 [DOI] [PubMed] [Google Scholar]
- 23.Din AU, Hassan A, Zhu Y, et al. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J Nutr Biochem 2020;79:108353. 10.1016/j.jnutbio.2020.108353 [DOI] [PubMed] [Google Scholar]
- 24.Nitzan O, Elias M, Peretz A, et al. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol 2016;22:1078–87. 10.3748/wjg.v22.i3.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdés-Varela L, Hernández-Barranco AM, Ruas-Madiedo P, et al. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front Microbiol 2016;7:738. 10.3389/fmicb.2016.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y, Yang F, Wu Q, et al. Protective Effects of Bifidobacterial Strains Against Toxigenic Clostridium difficile. Front Microbiol 2018;9:888. 10.3389/fmicb.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philippe D, Heupel E, Blum-Sperisen S, et al. Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int J Food Microbiol 2011;149:45–9. 10.1016/j.ijfoodmicro.2010.12.020 [DOI] [PubMed] [Google Scholar]
- 28.Overview of variants in countries. Available: https://covariants.org/per-country
- 29.Tao W, Zhang G, Wang X, et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol 2020;5:100023. 10.1016/j.medmic.2020.100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagisawa MFP, Hanaki H, Ōmura S. Global trends in clinical studies of ivermectin in COVID-19. Japanese Journal of Antibiotics 2021;74:44–94. [Google Scholar]
- 31.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489–99. 10.1053/j.gastro.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negi S, Das DK, Pahari S, et al. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol 2019;10:2441. 10.3389/fimmu.2019.02441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med 2016;8:51. 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghouri YA, Richards DM, Rahimi EF, et al. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 2014;7:473–87. 10.2147/CEG.S27530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes KR, Harnisch LC, Alcon-Giner C, et al. Bifidobacterium breve reduces apoptotic epithelial cell shedding in an exopolysaccharide and MyD88-dependent manner. Open Biol 2017;7:160155. 10.1098/rsob.160155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fanning S, Hall LJ, Cronin M, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci U S A 2012;109:2108–13. 10.1073/pnas.1115621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arboleya S, Watkins C, Stanton C, et al. Gut bifidobacteria populations in human health and aging. Front Microbiol 2016;7:1204. 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira-Halder CV, Faria AVdeS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol 2017;31:643–8. 10.1016/j.bpg.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 39.Ganesan K, Chung SK, Vanamala J, et al. Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci 2018;19. 10.3390/ijms19123720. [Epub ahead of print: 22 Nov 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene MW, Roberts AP, Frugé AD. Negative association between Mediterranean diet adherence and COVID-19 cases and related deaths in Spain and 23 OECD countries: an ecological study. Front Nutr 2021;8:591964. 10.3389/fnut.2021.591964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang L, Yin Z, Hu Y, et al. Controlling cytokine storm is vital in COVID-19. Front Immunol 2020;11:570993. 10.3389/fimmu.2020.570993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents 2020;55:105982. 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 2010;5:e10667. 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Center for Disease Control . People with certain medical conditions. Available: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2022-000871supp001.pdf (99.3KB, pdf)
bmjgast-2022-000871supp002.pdf (95.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data available upon reasonable request from corresponding author, Dr. Sabine Hazan.