Abstract
NARCOLEPSY is characterized by excessive sleepiness and episodes of cataplexy brought on by emotional excitation. Cataplexy and sleep paralysis have been hypothesized to be produced by the triggering during waking of brain stem cholinergic mechanisms normally acting to induce atonia in REM sleep. We hypothesized that narcoleptics have an abnormal number of LDT and/or PPN cholinergic neurons. A comparison was made of cholinergic cell numbers in the brain stems of normal and narcoleptic canines. Cholinergic neurons were identified by NADPH-diaphorase histochemistry. We found increased numbers of cholinergic neurons at the R6-R7 level of the LDT and PPN in narcoleptic canines. This abnormality can explain alterations in cholinergic receptor number, acetylcholine release, and the occurrence of cataplexy and sleep paralysis that characterize narcolepsy.
Keywords: Narcolepsy, Canine, NADPH, Sleep, Acetylcholine, Pedunculopontine, Laterodorsal-tegmental
General Summary
Narcolepsy in the dog bears a strikingly similar resemblance to human narcolepsy. In both cases, the episodes of cataplexy that characterize this disease can be seen as a disorder of REM sleep in which brainstem mechanism normally acting to suppress muscle tone during REM sleep are triggered during waking. Such mechanisms have been shown to involve the activation of brainstem acetylcholine neurons. We hypothesized that narcoleptic dogs have an abnormal population of these neurons. Thus, NADPH-diaphorase histochemistry was used to compare cholinergic cell number in the brainstem of narcoleptic and non-narcoleptic dogs. The narcoleptic dogs in our study were found to have significantly more cholinergic neurons in the caudal portion of the pons than non-narcoleptics. This abnormality could explain the occurrence of cataplexy. In addition, the findings suggest that the development brainstem acetylcholine neurons in the narcoleptic dog is abnormal.
Introduction
Narcolepsy in both humans and canines is characterized by excessive sleepiness and episodes of cataplexy brought on by emotional excitation.1 Human narcoleptics experience hypnagogic hallucinations and sleep paralysis. Narcoleptic humans and canines also exhibit a significant reduction in latency to rapid eye movement (REM) sleep following sleep onset.1 Thus, narcolepsy has been hypothesized to be a disease of REM sleep control.2,3 According to this hypothesis, hypnagogic hallucinations represent a triggering of dream mentation in waking. Cataplexy and sleep paralysis are thought to be a result of the triggering during waking of the mechanism that normally serves to suppress muscle tone during REM sleep.
The neurophysiology underlying the production of muscle atonia during REM sleep has been well documented in the cat. Transection and lesion studies have localized an area in the pontine reticular formation (PRF) critical to the production of muscle atonia during REM sleep.4 This mechanism appears to be driven during REM sleep by the release of acetylcholine (ACh). Thus, several microinjection studies have demonstrated the induction of a REM sleep-like state with muscle atonia following micro-injection of Ach or the muscarinic receptor agonist carbachol into the PRF.5–8 Microdialysis studies indicate that Ach release in the PRF is increased during REM sleep as compared to that during waking or slow-wave sleep.9
Several studies suggest that cholinergic abormalities in the pons are involved in the induction of cataplexy in narcoleptic dogs. First, local perfusion of the muscarinic receptor agonist carbachol into the PRF increases the number of cataplectic episodes in narcoleptic dogs.10 In addition, Ach release in the PRF is increased during periods of cataplexy.11 Finally, narcoleptic dogs have an increased density of muscarinic M2 receptors in the brain stem.12
The abnormal density of brain stem muscarinic receptors in the narcoleptic dog could be a direct result of a genetic abnormality in the production of muscarinic receptors. Alternatively, the increased muscarinic receptor density could indirectly be a result of either a decreased basal release of Ach in the brainstem with consequent receptor up-regulation or a cholinergic hyperinnervation of the brainstem. We therefore hypothesized that narcoleptic dogs may have an abnormal population of brain stem cholinergic neurons. We have addressed this hypothesis by comparing numbers of cholinergic neurons in the pons of narcoleptic and normal canines using nicotinamide adenosine dinucleotide phosphate (NADPH)-diaphorase histochemistry. NADPH-diaphorase histochemistry has been shown to label cholinergic neurons in the brainstem laterodorsal tegmental (LDT) and pedunculopontine (PPN) nuclei.13,14
Materials and Methods
Three narcoleptic and four normal dogs were used in this study. All three narcoleptic animals were symptomatic for narcolepsy at the time of sacrifice. Animals were perfused under deep anaesthesia with 0.1 M phosphate buffered saline (PBS) at 4°C followed by 4% paraformaldehyde in 0.1M PBS at 4°C. The brain stem was dissected and then further fixed and cryoprotected at 4°C for 24 h in 4% paraformaldehyde and 20% sucrose in 0.1 M PBS.
Alternating 40 /um coronal sections from brain stem levels R9-R615 were processed for NADPH-diaphor-ase histochemistry. Slices were incubated in darkness at 37°C in a solution of 20 mM PBS with 2mg nitroblue tetrazolium, lOmg NADP, and 3% Triton-X for 30min. Slices were washed in 0.1 M PBS, mounted, and then counterstained with neutral red.
NADPH-diaphorase-positive neurons in the right hemisphere were counted in all NADPH-stained sections by a technician blind to the disease state of the animal. A correction for split cell error was used.16 Cell soma diameters were classified as small (<20 /um), medium (20–40/um) or large (>40/um). Average cell counts per slice were calculated for slices taken from millimeter length (anterior to posterior) areas of the brain stem according to the atlas of Lim.15
Results
NADPH-diaphorase-staining neurons were found mainly within the LDT and PPN nuclei. The average number of cells per slice taken between brain stem levels R7 and R6 was consistently higher in the PPN (p<0.03) and LDT (p<0.001) (ANOVA with Newman-Keuls post-hoc t-tests) of narcoleptic canines (Figs 1, 2). Cell numbers in this region of the LDT and PPN were increased by 245% in the PPN and 94% in the LDT. There was no overlap of total NADPH-positive cell number in area R7-R6 of narcoleptic and control animals. At this level, the range of total cell number for controls was 791–1675; the range for narcoleptic animals was 2413—4330. Across all brain stem levels analyzed (levels R9-R6), the total NADPH-positive cell count ranged between 3449 and 5735 for control animals and between 4352 and 9869 for narcoleptic animals. Total cell counts and average cell counts between levels R9 and R8 and between levels R8 and R7 were not significantly different. Although NADPH-diaphorase-positive cells differed in number between narcoleptic and control animals, the overall anatomical distribution of stained cells within the pons was similar. No significant differences in cell size were found. Figure 3 displays individual LDT nuclei from narcoleptic and normal animals at each of the three brainstem levels analyzed.
FIG. 1.
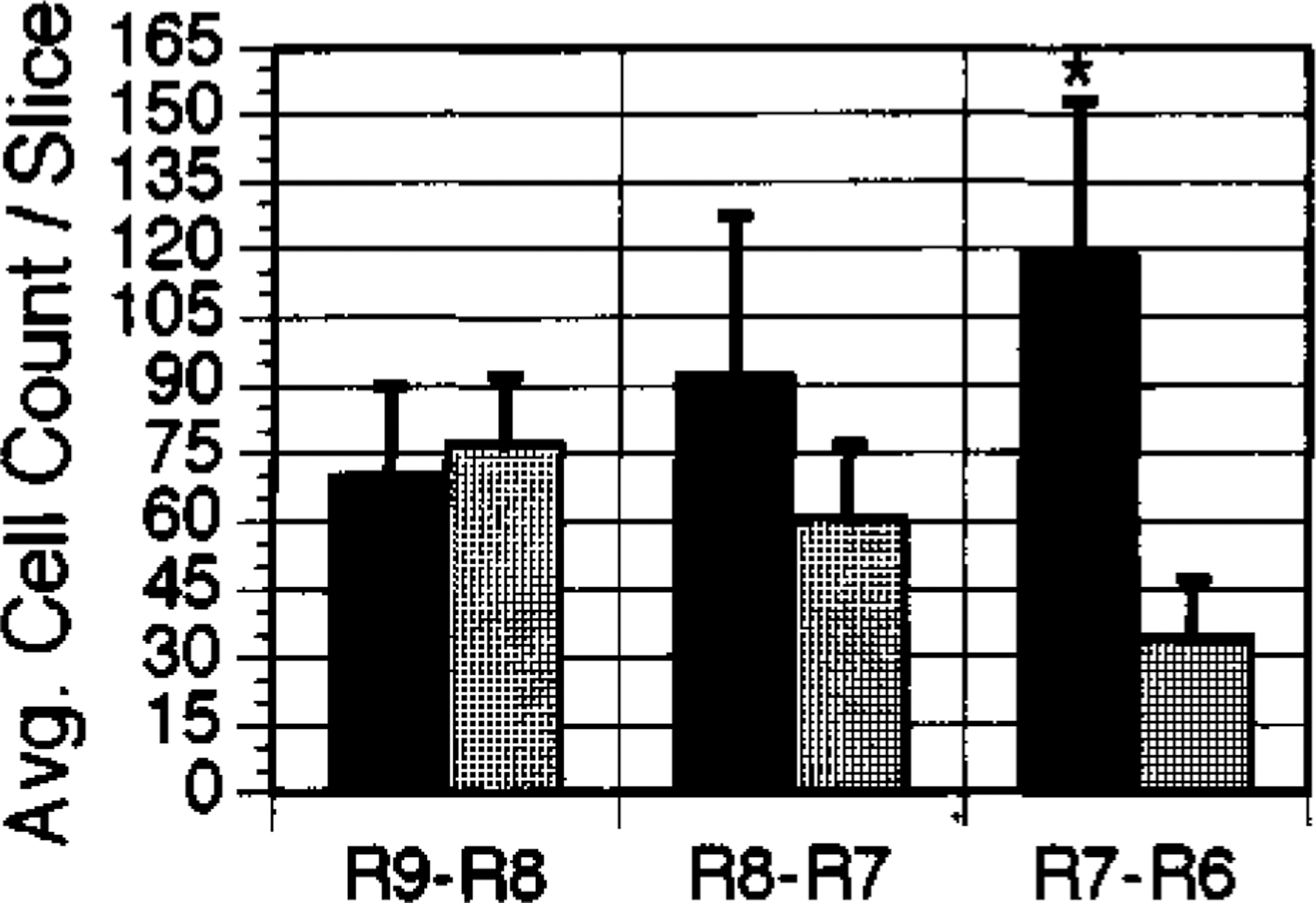
Average cell counts in the PPN for areas R9-R8, R8-R7, and R7-R6 of the canine brainstem. ■: narcoleptic,  : normal. Error bars indicate s.e.m. *p<0.03.
: normal. Error bars indicate s.e.m. *p<0.03.
FIG. 2.
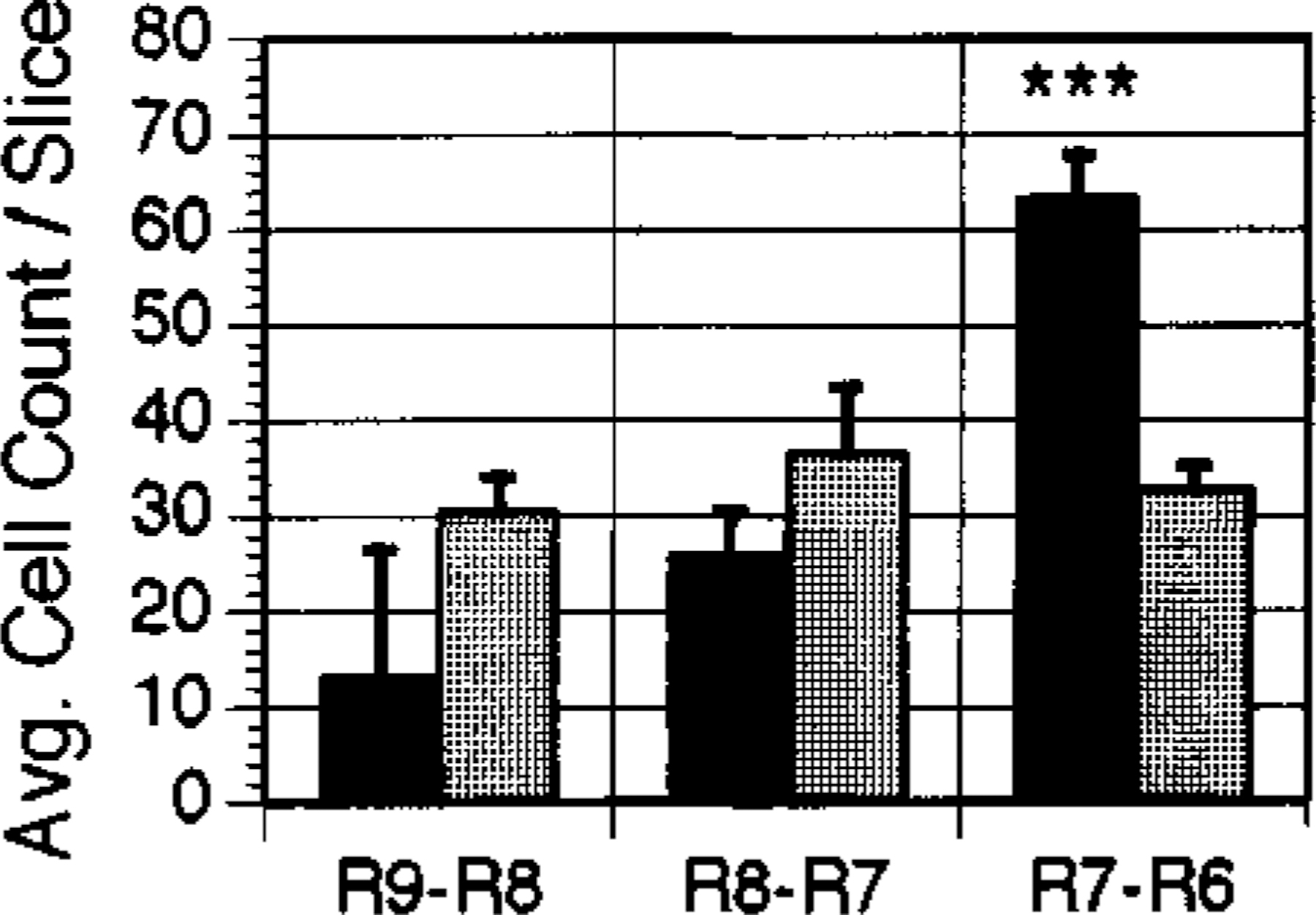
Average cell counts in the LDT for areas R9-R8, R8-R7, and R7-R6 of the canine brainstem. ■: narcoleptic.  : normal. Error bars indicate s.e.m. ***p<0.001.
: normal. Error bars indicate s.e.m. ***p<0.001.
FIG. 3.
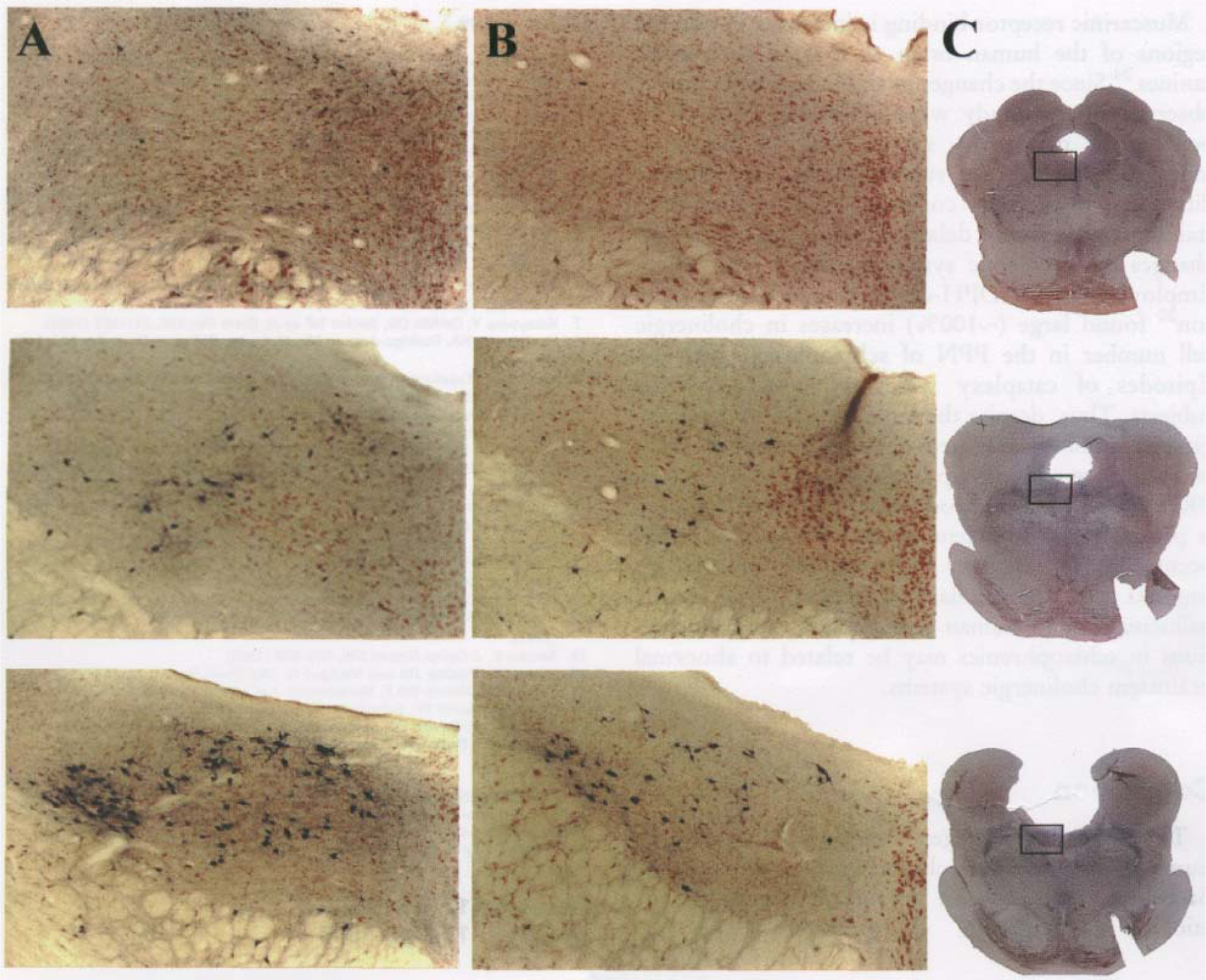
Comparison of NADPH-diaphorase staining in individual LDT nuclei of narcoleptic (A) and normal (B) dogs at brain stem levels (from top to bottom) R9-R8, R8-R7, and R7-R6. Magnification=40x. (C) Low power photographs of normal brain stem slices used in B. The outlined area corresponds to the high power photographs of A and B. Note the greater number of stained cells in the narcoleptic in area R7-R6.
Discussion and Conclusions
The increase in NADPH-diaphorase-staining neurons indicates that narcolepsy in canines is characterized by an abnormal development of brain stem cholinergic neurons. A likely consequence of this increase is an abnormality in the cholinergic innerva-tion of the PRF which is known to arise from neurons of the LDT and PPN.17,18 Such an abnormality might be related to the occurrence of cataplexy and the abnormal propensity for REM sleep exhibited by narcoleptic canines.
NADPH-diaphorase histochemistry labels neurons producing the enzyme nitric oxide (NO) sunthase.19 In the rat PRF, NADPH-diaphorase staining is localized to cholinergic neurons of the LDT and PPN nuclei identified by choline acetyltransferase (ChAT) immunohistochemistry.13 Thus, the present results indicate either an increase in cholinergic cell number or the presence of an additional cell type in narcoleptic canines. Although this possibility cannot be ruled out, the close correspondence between NADPH-diaphorase staining and ChAT immunohis-tochemical staining of brain stem cholinergic neurons in the rat and human PRF13,14 and the localization of NADPH-staining neurons in the present study to the PPN and LDT nuclei suggest that the current findings reflect differences in cholinergic cell number.
The results of this study are consistent with data indicating increased M2 receptor density in narcoleptic canines.12 Since the M2 muscarinic receptor is in part an autoinhibitory receptor found on cholinergic neurons, ° the simplest interpretation with regard to the present data would be that the previously reported M2 receptor increase is not a result of up-regulation, but a result of increased numbers of autoreceptors correlated with increased numbers of cholinergic neurons. Nevertheless, the fact that M2 receptors are also found on non-cholinergic neurons of the PRF19 leaves this question open.
The findings indicate that development of LDT/ PPN neurons in the narcoleptic canine is abnormal since increases in cell number would not be expected to result from postnatal degenerative changes. Reductions in normal apoptotic cell death could be responsible for higher numbers of cholinergic cells in adulthood.21 Alternatively, increased brain stem cholinergic neuronal number could be a function of abnormal differentiation or proliferation of cells during embryonic development. Insulin-like growth factors I and II, epidermal growth factor, and basic fibroblast growth factor all stimulate proliferation of embryonic mesopontine cholinergic cells.22,23 Overproduction of one or more of these factors or their receptors during development could be responsible for an abnormally high number of pontine cholinergic neurons.
Muscarinic receptor binding is increased in pontine regions of the human brain as it is in narcoleptic canines.24 Since the changes in cholinergic cell number observed in this study were quite large, it may be possible to perform a similar study using postmortem human brain stem, despite the inherent difficulties in properly controlling the conditions of staining as a result of delays in harvesting tissue and changes in narcoleptic symptomatology with aging. Employing the NADPH-diaphorase technique, Kar-son ° found large (~100%) increases in cholinergic cell number in the PPN of schizophrenic patients. Episodes of cataplexy were not noted in these subjects. Thus, despite the large increase in cholinergic cell number identified in the narcoleptic canine and the close relationship between Ach release in the PRF and REM sleep production, the present finding is probably not sufficient on its own to explain the occurrence of cataplexy. Nevertheless, this finding suggests that the similarities between hypnogogic hallucinations in human narcoleptics and hallucinations in schizophrenics may be related to abnormal brainstem cholinergic systems.
Conclusion
The present data suggest that an abnormally high number of brainstem cholinergic neurons in the narcoleptic caine is an important factor in the etiology of narcolepsy.
ACKNOWLEDGEMENTS:
This research was supported by the National Multisite Training Program for Basic Sleep Research, the Medical Research Service of the Veterans Administration, and USPHS grant NS14610. We thank David Rector and Gina Poe for help in the production of the anatomical figure.
References
- 1.Baker TL and Dement WC. Canine narcolepsy-cataplexy syndrome: evidence for an inherited monoaminergic-cholinergic imbalance. In: McGinty DJ, Drucker-Colin R, Morison A era/., eds. Brain Mechanisms of Sleep New York: Raven, 1985: 199–234. [Google Scholar]
- 2.Siege lJM, Nienhuis R, Fahringer HM era/. Science 262, 1315–1318(1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rechtschaffen A, Wolpert EA, Dement WC ef al. Electrophysiol Clin Neurophysiol 15, 599–609 (1963). [DOI] [PubMed] [Google Scholar]
- 4.Siege JM. Brainstem mechanisms generating REM sleep. In: Kryger M, Roth T and Dement W, eds. Principles and Practice of Sleep Medicine Philadelphia: WB Saunders, 1994: 125–144. [Google Scholar]
- 5.George R, Haslett WL and Jenden DJ. Int J Neuropharmacol 3, 541–552 (1964). [DOI] [PubMed] [Google Scholar]
- 6.Van Dongent RAM, Broekkamp CLE and Cools AR. Pharmacol Biochem Behav 8, 527–532 (1978). [DOI] [PubMed] [Google Scholar]
- 7.Katayama Y, DeWitt DS, Becker DP et al. Brain Res 296, 241–262 (1984). [DOI] [PubMed] [Google Scholar]
- 8.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RM et al. Brain Res 414, 245–261 (1987). [DOI] [PubMed] [Google Scholar]
- 9.Kodama T, Takahashi Y and Honda Y. Neurosci Lett 114, 277–282 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Reid MS, Tafti M, Gear JN et al. Neurosciences 59, 511–522 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid MS, Siegel JM, Dement WC et al. Neurosciences 59, 523–530 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilduff TS, Bowersox SS, Kaitin Kl et al. Sleep 9. 102–106 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Vincent SR, Satoh K, Armstrong DM et al. Neurosci Lett 43, 31–36 (1983). [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM, Geula C, Bothwell MA et al. J Comp Neurol 281, 611–633 (1989). [DOI] [PubMed] [Google Scholar]
- 15.Lim RKS, Liu C and Moffitt RL. A stereotaxic atlas of the dog’s brain Springfield: Charles C. Thomas, 1960. [Google Scholar]
- 16.Konigsmark BW. Methods for the counting of neurons. In: Nauta WJH and Ebbesson SOE, eds. Contemporary Research Methods in Neuroanatomy New York: Springer-Verlag, 1970: 315–340. [Google Scholar]
- 17.Lai YY, Clements JR and Siegel JM. J Comp Neurol 336, 321–330 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope BT, Michael GJ, Knigge KM et al. Proc NatlAcadSciUSA 88,2811–2814 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semba K J Comp Neurol 330, 543–556 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Vilaro MT, Palcios JM and Mengod G. Mol Brain Res 21, 30–46 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Karson CN, Garcia-Rill E, Biedermann J et al. Psy Res 40. 31–48 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Knusel B, Michel PP, Schwaber JS ef al. J Neurosci 10, 558–570 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Rill E, Davies DL, Skinner RD et al. Dev Brain Res 60, 267–270 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Aldrich MS, Desmond T and Frey KA. Steep Res 23, 215 (1994). [Google Scholar]


