Abstract
Background:
Sodium-glucose cotransporter-2 inhibitors (SGLT2 inhibitors) rarely cause euglycemic diabetic ketoacidosis (euDKA) in diabetic patients. The aim was to identify demographic, clinical, and predisposing factors for euDKA from published case reports.
Methods:
A systematic review of published case reports of euDKA in patients receiving SGLT2 inhibitors and meta-analysis of clinical trials to quantify the risk ratio (RR) of DKA in patients receiving SGLT2 inhibitors. PubMed and EMBASE databases were searched for the case reports of and clinical trials from January 2010 to August 2020. Studies published in English language were included and other languages were excluded. Data related to patients’ demography, clinical presentation, drug and dose of SGLT2 inhibitors, and concomitant medication were extracted. Incidence of diabetic ketoacidosis (DKA) extracted from clinical trials. Data related to demographic, clinical, and other parameters presented as ratios and proportions and incidence of DKA in RR using Review Manager 5.3.
Results:
Forty-seven of 160 reports with an aggregate of 77 patients were included in the analysis. The majority of the patients were females (67.53%), with T2DM and with gastrointestinal symptoms (58%). Surgery was the most common precipitating factor (n/N = 15/77). Canagliflozin (n/N = 34/77) was the commonest SGLT2 inhibitor reported along with metformin as the concomitant medication (63.6%). The pooled RR of DKA was 3.70 (95%CI 2.58, 5.29) and I2 = 0%.
Conclusion:
euDKA is commonly seen in middle-aged female, T2DM patients taking SGLT2 inhibitors along with metformin. The risk of DKA in patients receiving SGLT2 inhibitors increases by 3.7 times than the other medication.
Keywords: Diabetes mellitus, DKA, euglycemic diabetic ketoacidosis, SGLT2 inhibitors
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder associated with either deficiency of insulin called Type 1 diabetes mellitus (T1DM) or resistance to insulin action known as Type 2 diabetes mellitus (T2DM). T2DM is more common in adults and accounts for around 90% of all diabetes cases.[1] The sodium–glucose cotransporter 2 (SGLT2) inhibitors are one of the latest classes of antihyperglycemic medications and are currently highly recommended for the treatment of T2DM because of its multidimensional benefits on the cardiovascular system and renal system.[2,3] SGLT2 inhibitors (SGLT2i) act on the proximal tubular epithelium by inhibiting the reabsorption of glucose from the glomerular filtrate to the blood.[4] Glycosuria with SGLT2i usually results in a reduction of body weight and associated osmotic diuresis ends up in reducing the blood pressure; hence, they are quite beneficial in obese or overweight patients and hypertension with T2DM.[5] The cardiovascular benefits of empagliflozin are reported in “EMPA-REG Outcome trial,” which further makes these drugs a preferred choice for physicians. The cardiovascular benefits are due to the production of ketone bodies by SGLT2i, and its energy efficiency improves cardiovascular and renal functions.[6,7] With this evidence and insulin-independent mode of action, SGLT2i is a suitable class of drug for both T2DM and T1DM patients.[8,9] In view of such a broad clinical utility of SGLT2i in diabetes, it is crucial to keep a track of their associated adverse events (AEs). Usually, these agents have a low tendency to cause hypoglycemia, still, because of glycosuria, there are chances of genital and urinary tract infections (UTI) and occasional orthostatic hypotension due to mild dehydration.[10] In addition to the usual AEs, the US Food and Drug Administration (FDA) in May 2015, based on the 20 cases of diabetic ketoacidosis (DKA), published a warning with potential increased risk of DKA associated with the use of SGLT2i in both T2DM and T1DM patients.[11] Later by June 2015, the European Medicines Agency also spotted 147 cases of DKA in patients on treatment with SGLT2i.[12] Large trials like “DECLARE–TIMI 58 study” and “CREDENCE trial” have shown an increased probability of DKA with SGLT2i as compared to placebo.[13,14] The cases of SGLT2i-associated DKA were atypical in presentation as the glucose levels were mildly elevated (<200 mg/dL), hence can be called euglycemic diabetic ketoacidosis (euDKA).[10,15,16] The DKA is an emergency condition and is common in T1DM but is rare in T2DM. However, with the usage of SGLT2i in T2DM, the incidence of DKA is seen increasing.[10,16] Being a life-threatening condition, early diagnosis and treatment are crucial for a better prognosis of the patient, but euglycemia in euDKA poses a great challenge in diagnosing the underlying DKA; hence, high clinical intuition is needed for diagnosis.[17] Diabetes being a very prevalent noncommunicable disorder in the Indian population and with the evolution of SGLT-2 inhibitors in the therapy of DM, they are being currently used effectively and in the future might turn out to be a very commonly used drug by physicians. As the majority of the population in India lies in rural areas and inaccessibility of superspecialists physicians in the community, a major chunk of the diabetic patients is being treated by primary care and community physicians.[3] Hence, it is crucial for them to have an idea of euDKA associated with SGLT2i, which will help to manage the cases in a better way. In this article, we systematically reviewed published case reports and case series of euDKA, to understand the demographic characteristics, clinical presentation, precipitating factors, and predisposed drug and its dose relationship with euDKA with SGLT2i.
Methods
This review was synthesized by following a prespecified study protocol (unpublished) and reported by following the guidelines of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” statement.
Search strategy
To identify the case reports and case series on SGLT2i-associated euDKA, we systematically screened the PubMed/Medline and EMBASE database published up to Aug 2, 2020 for medical literature. The articles were screened by using the search string “(Euglycemic Diabetic Ketoacidosis) AND (Sglt2 Inhibitor).” Second, to identify the events of DKA we screened the PubMed and Embase for clinical trials using the string “(Diabetic ketoacidosis) AND (Sglt2 inhibitors) AND (placebo).”
Study selection
The prospective or retrospective descriptive case reports and case series of euDKA after treatment with SGLT2 inhibitors were included. The articles published in a language other than English and full text not available/accessible were excluded. Second, the clinical trials with SGLT2i were selected to assess the events of DKA in the drug group as compared to the placebo. All the potentially relevant articles were screened in two stages for the final eligibility. Initially, the abstracts of the selected articles were screened independently by two reviewers (TK, SD). Later, the full-text articles that met the inclusion criteria were extracted and independently reviewed.
Data extraction and synthesis
From the included case reports and case series, we extracted information like study characteristics, including author name, publication year, study type, number of patients, patient demographics (e.g., age and gender), details of SGLT2i (type and dose), and concomitant medicines. The events of DKA with the SGLT2i as compared to placebo were assessed from the included published clinical trials. The data was collated, disagreements were discussed, and differences were resolved between review authors. Grades of Recommendations, Assessment, Development, and Evaluations (GRADE) Pro GDT guidelines were used for grading the main outcome as per the GRADE recommendation. Online software was used for analysis.[18] Observed data were combined with Review Manager 5 (RevMan) Version 5.3. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).[19] Age is presented as mean and SD, sex, and ethnicity along with clinical presentation and precipitating factors presented in proportions. The incidence of DKA is summarized as a risk ratio (RR) with 95% CI. Heterogeneity was assessed based on the calculated I2 (the proportion of total variability explained by heterogeneity), estimated using the restricted maximum likelihood-based method. We set predecided criteria for significant heterogeneity as I2 >40%, and the analysis was done using a fixed-effect model even if heterogeneity is greater than 40% in the most pooled analysis, as heterogeneity was not taken care of by the random effect model. The reason for heterogeneity was assessed and explained and for high heterogeneity; inconsistency was downgraded for quality evidence in GRADE Pro analysis. To assess the publication bias, funnel plot assessment was applied.
Assessment of quality of evidence – GRADE pro analysis
The overall quality of evidence for each of the outcomes was assessed using GRADEpro GDT (guideline development tool) software based on the principles of GRADE.[19] Risk of bias, the directness of evidence, consistency and precision of results, risk of publication bias, magnitude of the effect, dose-response gradient, and influence of residual plausible confounding were assessed for grading the overall quality of evidence for individual outcomes. The final overall GRADE may be high, moderate, low, or very low. The online version of GRADE pro GDT software was accessed from the site: https://gradepro.org/.[20]
Ethical permission
The systematic review was synthesized from the data already published in open access and no direct contact with the patients was done; hence, ethical permission was not required.
Results
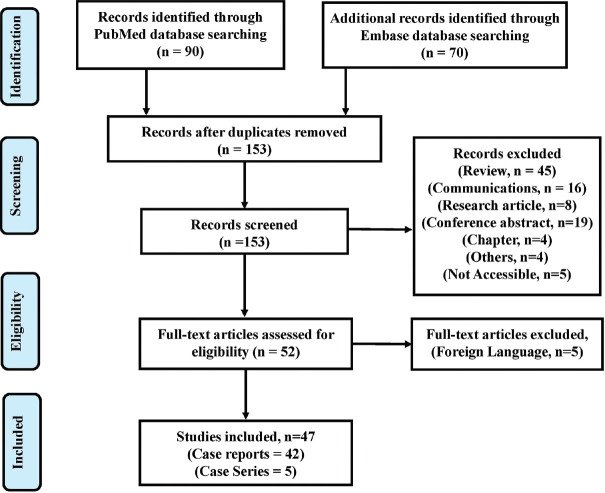
Our systematic search of the PubMed and EMBASE database resulted in an initial number of 160 potentially relevant articles. After removing seven duplicates, 153 articles were taken for the systematic review. Of 153 articles, 45 were review, 16 were communications, 19 were conference abstracts, 8 were research articles, 4 were book chapters, and five articles were not accessible hence excluded. Applying the inclusion/exclusion criteria on 52 full-text documents, five other language articles were excluded. Finally, 47 articles were included for the synthesis of this review.[21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] The details are illustrated in [Figure 1]. In 42 case reports and 5 case series, we got 77 patients with a mean age of 51.31 years (SD = 15.25). Basic characteristics of individual case reports and case series of euDKA with SGLT-2i are summarized in [Table 1]. The majority of the patients reported were females (67.5%, n = 52). The majority of the reports did not mention (n/N = 62/77) the ethnicity still among mentioned, Asians (46.6%, n = 7) was the most common. Among the disease distribution, T2DM was most prevalent, followed by T2DM with cardiovascular diseases like hypertension, coronary artery diseases, arrhythmia, etc., T1DM, T2DM with oncological disorders like benign or malignant cancers, T2DM with other endocrine disorders like hypothyroidism, etc., T2DM with renal dysfunction like acute or chronic kidney disease, etc., T2DM with multiple sclerosis, T1DM with other endocrine disorders like hypothyroidism, etc., and others which include multiple diseases and comorbidities [Table 2].
Figure 1.
PRISMA flow chart depicting the study selection process
Table 1.
Basic characteristics of individual case reports and case series of euglycemic DKA with SGLT-2 inhibitors
| Author/Year | Age/Gender | Drug and Dose | Disease distribution | Presenting complaints |
|---|---|---|---|---|
| Adachi J et al., 2017[59] | 27y, F | Canagliflozin 300 mg/day | T2DM | DKA |
| Alhassan S et al., 2018[60] | Case 1: 73y, F Case 2: 70y, M Case 3: 69y, F |
Case 1: Empagliflozin 25 mg daily Case 2: Canagliflozin 300 mg daily Case 3: Canagliflozin100 mg |
T2DM T2DM T2DM and hypertension |
Case 1: nausea, vomiting, DKA Case 2: Spinal stenosis was admitted for elective C3-C7 spinal fusion Case 3-: Admitted with ST elevation myocardial infarction (STEMI) |
| Allison R et al., 2019[61] | 47 y, M | Empagliflozin | Multiple Sclerosis with T2DM | Generalized weakness, known case of multiple sclerosis (MS) diagnosed 4 years ago |
| Andrews TJ et al., 2017[62] | 57 y, F | Canagliflozin 150 mg BD | T2DM, hypothyroidism, Hepatitis B, chronic obstructive pulmonary disease, coronary artery disease, myocardial infarction, pulmonary hypertension, depression, vitamin D deficiency, and restless leg syndrome | Progressive altered mental status for the past 2 days |
| Bader N et al., 2016[63] | 27 y, F | Canagliflozin | T1DM, depression, hypothyroidism | DKA |
| Badwal K et al., 2018[64] | 25 y, M | Dapagliflozin | T2DM with acute pancreatitis | One-day history of abdominal pain, nausea, and emesis |
| Benmoussa JA et al., 2016[65] | 39 y, F, Caucasian | Canagliflozin | T2DM with hypothyroidism | Nausea, Vomiting, anorexia, abdominal pain and myalgia |
| Brown F et al., 2018[22] | 53 y, M | Dapagliflozin | T2DM, Roux-En-Y Gastric bypass surgery 6 weeks prior, hypertension and hypercholesterolemia | One week history of nausea, vomiting, anorexia, and generalized abdominal pain |
| Bteich F et al., 2019[23] | 58 y, F | Empagliflozin 25 mg daily | T2DM with essential hypertension and obstructive sleep apnea | Altered mental status |
| Candelario N et al., 2016[21] | 61 y, F | Empagliflozin | T2DM with diet-controlled hypertension | Right upper quadrant abdominal pain for a day |
| Chao HY et al., 2020[24] | Case 1: 40 y, M Case 2: 60 y, M |
Case 1: Empagliflozin Case 2: Dapagliflozin |
Case 1: T2DM with alcoholic liver cirrhosis Case 2: T2DM with alcoholic liver cirrhosis |
Case 1: Nausea, fatigue, and dyspnea Case 2: Severe dyspnea and nausea |
| Chou YM et al., 2018[25] | 61 y, F | Dapagliflozin 10 mg OD | T2DM | Body weakness, dyspnea, nausea, vomiting, and mild abdominal pain for the past 2 days |
| Clement M et al., 2016[26] | 42 y, F | Canagliflozin 100 mg | T2DM, hypertension, obesity, psoriasis, hypothyroidism, and polycystic ovary syndrome | Shortness of breath |
| Dai Z et al., 2016[27] | 49 y, M | SGLT 2 inhibitors | T2DM and vasospastic angina | Suddenly lost consciousness while sightseeing, shortly after he complained of nausea |
| Diaz-Ramos A et al., 2019[28] | 44 y, F | Canagliflozin 100 mg daily | T2DM | Generalized weakness for 3 days |
| Dizon S et al., 2017[29] | Case 1: 55 y, F Case 2: 38 y, F Case 3: 45 y, M Case 4: 51 y, F Case 5: 29 y, F Case 6: 54 y, M Case 7: 54 y, F Case 8: 67 y, F Case 9: 74 y, M Case 10-74 y, F |
Case 1: Canagliflozin 300 mg daily Case 2: Canagliflozin 300 mg daily Case 3: Canagliflozin 300 mg daily Case 4: Canagliflozin Case 5: Dapagliflozin Case 6: Canagliflozin 300 mg daily Case 7: Dapagliflozin 10 mg daily Case 8: Dapagliflozin 5mg daily Case 9: Canagliflozin 100 mg daily Case 10: Canagliflozin 100 mg daily |
T2DM, Roux-en-Y gastric bypass surgery T2DM , Roux-en-Y gastric bypass surgery T2DM T2DM T1DM T2DM, laparoscopic cholecystectomy T2DM T2DM T2DM T2DM, operative repair of an intertrochanteric fracture |
Case 1: Roux-en-Y gastric bypass surgery, On POD #17, she developed nausea, vomiting, low-back pain, and dysphagia Case 2: Roux-en-Y gastric bypass surgery, 7 days after starting canagliflozin, she presented with nausea, vomiting, dizziness, and dehydration Case 3: Three-day history of shortness of breath and was found to have a mixed metabolic acidosis Case 4: Severe ketoacidosis Case 5: Nausea, vomiting and shortness of breath Case 6: Two months after starting canagliflozin, he had a laparoscopic cholecystectomy. On POD #1, he experienced shortness of breath and was found to have a pulmonary embolus Case 7: Presented with severe ketoacidosis Case 8: Cholecystitis, biliary sepsis, and ketoacidosis Case 9: Polydipsia, dizziness and loss of appetite Case 10: Admitted to hospital for operative repair of an intertrochanteric fracture. On POD #1, she restarted on all her oral diabetes medications On POD #5, she felt weak, confused, and generally unwell |
| Dull RB et al., 2017[30] | Case 1: 55 y, F Case 2: 62 y, M |
Case 1: Dapagliflozin 10 mg OD Case 2: Empagliflozin 25 mg OD |
T2DM, hyperlipidemia, hypothyroidism, and anemia T2DM, past medical history of heart failure with preserved ejection fraction, cerebrovascular accident, coronary artery disease, hypertension, hyperlipidemia, obstructive sleep apnea, morbid obesity, and neuropathy |
Case 1: Nausea and vomiting Case 2: Four-day history of dysuria, urinary frequency, fever, chills, and myalgia |
| Earle M et al., 2020[31] | 31y, F | Canagliflozin | T2DM | Dizziness and shortness of breath worsening over 1 week |
| Elshimy G et al., 2019[32] | 28 y, F | Dapagliflozin | T2DM | Sudden-onset abdominal pain and multiple episodes of nonbloody vomitus during the previous 24 h |
| Fukuda M et al., 2020[33] | 71 y, F | Canagliflozin 100 mg/day | T2DM | Malaise, nausea, and abdominal pain |
| Ghosh MSA, 2019[34] | 52 y, M | Empagliflozin | T2DM | Weakness, poor oral intake, malaise, and tightness of chest in the evening |
| Iqbal I et al., 2019[35] | 75 y, F | Dapagliflozin | T2DM, hypertension, chronic kidney disease stage III | Altered mental status and confusion |
| Jazi M et al., 2016[36] | 51 y, M | Canagliflozin | T2DM and Hypertension | Malaise, cough, and intermittent shortness of breath |
| Karakaya Z et al., 2018[37] | 72 y, F | Dapagliflozin | T2DM | Altered mental status two days after her hip prosthesis operation |
| Kelmenson DA et al., 2017[38] | 50 y, F | Canagliflozin 300 mg daily | T2DM | Four days of nausea, vomiting, abdominal pain, and decreased oral intake |
| Koch RA et al., 2018[39] | 61 y, F | Dapagliflozin | T2DM | Profound acidemia and ketonemia. |
| Kum-Nji JS et al., 2017[40] | Case 1: 48 y, M Case 2: 62 y, F Case 3: 37 y, F Case 4: 52 y, F Case 2: Canagliflozin 100 mg daily Case 3: Dapagliflozin 10 mg daily Case 4: Dapagliflozin 5 mg daily |
T2DM T2DM, metastatic pancreatic adenocarcinoma T2DM T2DM |
Case 1: Abdominal pain Case 2: Abdominal pain revealed metastatic pancreatic adenocarcinoma Case 3: Nausea, vomiting, and weakness Case 4: Generalized weakness, malaise, polydipsia, and polyphagia |
|
| Lane S et al., 2018[41] | 42 y, F | Canagliflozin 300 mg daily | T2DM, hypercholesterolemia, and gastroesophageal reflux | Presented for bariatric surgery with a body mass index of 40.1 kg/m2 |
| Lau A et al., 2017[42] | Case 1: 54 y, M Case 2: 58 y, M Case 3: 54 y, M |
Case 1: Empagliflozin 25 mg daily Case 2: Empagliflozin 25 mg daily Case 3: Empagliflozin 25 mg daily |
T2DM, DKA followed by cardiopulmonary bypass (CPB) T2DM, DKA followed by coronary artery bypass grafting (CABG) T2DM, DKA followed by CABG |
Case 1: Elective CABG Case 2: Elective CABG Case 3: Elective off-pump CABG (OPCAB) |
| Lee IH et al., 2020[43] | 76 y, F | Dapagliflozin 10 mg/day | T2DM | General weakness, fever, oliguria, nausea, and vomiting |
| Levine JA et al., 2017[44] | 60 y, M | Canagliflozin 300 mg daily | T2DM, coronary artery disease, arthritis | Total knee replacement |
| Lin YH, 2018[66] | 37 y, F | Empagliflozin 25 mg daily | T2DM, hypertension, hyperlipidemia | Acute gastroenteritis with nausea and abdominal pain |
| Lucero P et al., 2018[45] | Case 1: 22 y, F Case 2: 50 y, F Case 3: 74 y, M |
Case 1: none Case 2: Dapagliflozin 10 mg/day Case-3- none |
T2DM, history of DKA, hypothyroidism T2DM, hypertension, dyslipidemia, left-side breast cancer T2DM, CKD, Cryptogenic Liver Cirrhosis |
Case 1: Vomiting and abdominal pain Case 2: Abdominal pain, diarrhea, and fever Case 3: Passing liquid stools, together with emesis |
| Mackintosh C et al., 2019[46] | 68 y, F | Empagliflozin 10 mg once daily | T2DM, left temporal meningioma | Left temporal meningioma |
| Nappi F et al., 2019[47] | 67 y, F | Empagliflozin 25 mg/day | T2DM | Abdominal pain and impaired conscious level (Glasgow Coma Scale: 12), occurring after 1 week of fever, malaise, and dyspnea |
| Pace DJ et al., 2019[48] | Case 1: 66 y, F Case 2: 75 y, M |
Case 1: Canagliflozin Case 2: Dapagliflozin |
T2DM, pancreatic adenocarcinoma T2DM, pancreatic adenocarcinoma |
Case 1: Incidental finding of a body of pancreas mass on magnetic resonance imaging for follow-up of a stable ovarian cyst Case 2-Obstructive jaundice and elevated liver function tests |
| Papadokostaki E et al., 2019[49] | 64 y, M | Dapagliflozin | T2DM, HTN | Nausea, vomiting, and abdominal pain |
| Pereyra A M et al., 2017[50] | 17 y, F | Dapagliflozin 10 mg/day | T1DM | Metallic taste in mouth, thick saliva, and malaise |
| Peters AL et al., 2015[51] | Case 1: 40 y, F Case 2: 58 y, M Case 3: 27 y, F Case 4: 28 y, F Case 5: 31 y, F Case 6: 55 y, F Case 7: 26 y, F Case 8: 39 y, F Case 9: 64 y, F |
Case 1: Canagliflozin Case 2: Canagliflozin 300 mg/day Case 3: Canagliflozin 300 mg/day Case 4: Canagliflozin 300 mg/day Case 5: Canagliflozin 300 mg/day Case 6: Canagliflozin Case 7: Canagliflozin Case 8: Canagliflozin Case 9: Canagliflozin 300 mg/day |
T1DM T2DM T1DM T1DM T1DM T1DM T1DM T1DM T2DM |
Case 1: Tachypnea and tachycardia Case 2: Elective sigmoid colectomy Case 3: Upper respiratory tract infection (URI) Case 4: Vomiting and had a reduction in consciousness. She was admitted for eDKA Case 5: Presumed euglycemic ketosis, severe headache, and nausea not relieved with her migraine medications Case 6: Nausea and vomiting several hours after eating in a restaurant Case 7: Nausea and vomiting and presented to the hospital Case 8: eDKA Case 9: Elective bilateral cervical foraminotomy. |
| Pujara S et al., 2017[52] | 50 y, F | Dapagliflozin 10 mg daily | T2DM | Ten days of constipation and fatigue |
| Rafey MF et al., 2019[53] | Case 1: 44 y, M Case 2: 59 y, F |
Case 1: Canagliflozin 300 mg once daily Case 2: Empagliflozin 25 mg once daily |
T2DM T2DM, renal oncocytoma |
Case 1: Generalized weakness, lethargy, nausea, and anorexia, Six days post C5-C7 cervical decompression Case 2: Generalized weakness, dyspnea, and presyncope 3 days after elective laparoscopic right partial nephrectomy for removal of a renal oncocytoma |
| Sampani E i., 2020[54] | 51 y, F | Empagliflozin 25 mg once a day | T2DM, peptic ulcer, uterine fibroids | Weakness, tachypnea, anorexia, vomiting, and mild abdominal pain |
| Wang AY et al., 2017[55] | 61 y, F | Empagliflozin 25 mg per day | T2DM | Severe vomiting for 1 day |
| Wang KM et al., 2020[67] | 40 y, F | Empagliflozin | T2DM | Scheduled cerebral revascularization for moyamoya disease |
| Yamamoto M et al., 2019[56] | 51 y, M | Empagliflozin | T2DM | Nausea and vomiting |
| Yeo SM et al., 2019[57] | 23 y, F | Dapagliflozin 10 mg once a day | T2DM, acute pancreatitis due to hypertriglyceridemia | Severe abdominal pain |
| Zhang L et al., 2018[58] | 70 y, M | Empagliflozin | T2DM, paroxysmal atrial fibrillation, and dyslipidemia | Nausea, vomiting, and generalized weakness |
Table 2.
Patients characteristics of included case reports for euglycemic diabetic ketoacidosis
| Characteristics | n/N (%) | ||
|---|---|---|---|
| Sociodemographic features | Gender | Male | 25/77 (32.47) |
| Female | 52/77 (67.53) | ||
| Ethnic Distribution | Asian | 7/77 (9) | |
| Caucasian | 4/77 (5.2) | ||
| White Irish | 2/77 (2.6) | ||
| African American | 1/77 (1.3) | ||
| Hispanic | 1/77 (1.3) | ||
| Not mentioned | 62/77 (80.5) | ||
| Disease distribution | T2DM | Without any comorbidities | 37/77 (48.20) |
| With multiple Sclerosis | 1/77 (1.30) | ||
| With cardiovascular diseases | 14/77 (18.00) | ||
| With renal disease | 2/77 (2.60) | ||
| With oncological disorder | 6/77 (7.80) | ||
| With endocrine disorders | 4/77 (5.20) | ||
| T1DM | Without any comorbidities | 9/77 (11.60) | |
| Endocrine disorders | 1/77 (1.30) | ||
| Others (Multiple diseases and comorbidities ) | 3/77 (3.90) | ||
| Drug distribution | Concomitant medications along with SGLT2 inhibitors | SGLT2i + Metformin | 49/77 (63.63) |
| SGLT2i + Insulin | 48/77 (62.33) | ||
| SGLT2i + Dipeptidyl peptidase-4 inhibitors | 16/77 (20.77) | ||
| SGLT2i + Sulfonylureas | 14/77 (18.18) | ||
| SGLT2i + Liraglutide or Dulaglutide | 5/77 (6.5%) | ||
| SGLT2i + Pioglitazone or Rosiglitazone | 2/77 (2.6%) | ||
Number of patients/Total Number of patients=n/N; SGLT2i: SGLT2 Inhibitor
The presenting complaints were mentioned in 45 of 77 patients of all the case reports. The commonest presenting complaints of euDKA (58%, n = 45) were nausea, vomiting, abdominal pain, malaise, and sometimes shortness of breath. The prevalent precipitating factor for DKA was surgery as about 33% of patients developed DKA in their postsurgical period. Other precipitating factors of DKA were associated with UTI, cardiovascular irregularities, acute gastroenteritis, etc.
Canagliflozin (44%, n = 34) was the commonest SGLT-2i reported in the selected studies followed by Dapagliflozin (27%, n = 21) and Empagliflozin (25%, n = 19). A dose-dependent increase in the number of cases of DKA was observed with all the three SGLT2i [Figure 2]. Among the concomitant medications used along with SGLT2i, metformin was the commonest, followed by Insulin, Dipeptidyl peptidase-4 (DPP-4) i, sulfonylureas along with vitamin B12, vitamin C, vitamin D, and multivitamins [Table 3].
Figure 2.
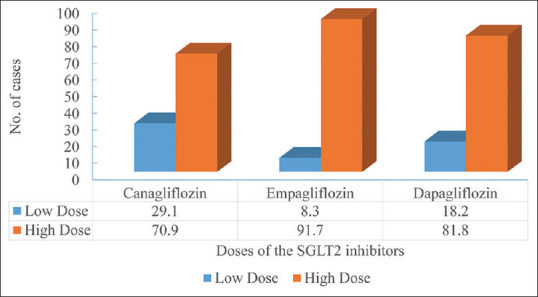
Dose-dependent number of euDKA cases reported in the literature
Table 3.
GRADE recommendation for diabetes ketoacidosis for use of SGLT-2 inhibitors in DM patients
| Certainty assessment | № of patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| № of studies Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Adverse events comparison in SGLT2 | Placebo group | Relative (95% CI) | Absolute (95% CI) | ||
| Events of DKA in SGLT2 inhibitors vs. Placebo | |||||||||||
| 16 RCT | not serious | not seriousa | not serious | not seriousb | strong associationc | 233/18956 (1.2%) | 31/12300 (0.3%) | RR 3.70 (2.58-5.29) | 7 more per 1,000 (from 4 more to 11 more) | ⨁⨁⨁⨁ HIGH | Critical |
| Events of DKA in SGLT2 inhibitors vs. Placebo - Certain DKA | |||||||||||
| 16 RCT | not serious | not seriousa | not serious | not seriousb | strong associationc | 192/17194 (1.1%) | 22/11303 (0.2%) | RR 4.08 (2.70-6.17) | 6 more per 1,000 (from 3 more to 10 more) | ⨁⨁⨁⨁ HIGH | Critical |
| Events of DKA in SGLT2 inhibitors vs. Placebo - Possible or Potential DKA | |||||||||||
| 2 RCT | not serious | not seriousa | not serious | not seriousb | strong associationc | 41/1762 (2.3%) | 9/997 (0.9%) | RR 2.60 (1.27-5.33) | 14 more per 1,000 (from 2 more to 39 more) | ⨁⨁⨁⨁ HIGH | Critical |
CI: Confidence interval; RR: Risk ratio; RCT: Randomized Clinical Trials Explanations. aAs I2=0%, hence low heterogeneity. bAs CI does not include one and overall information size is adequate; therefore, the outcome is precise. cAs RR is greater than 2, it is regarded as large effect
Incidence of DKA from the previously published clinical trials
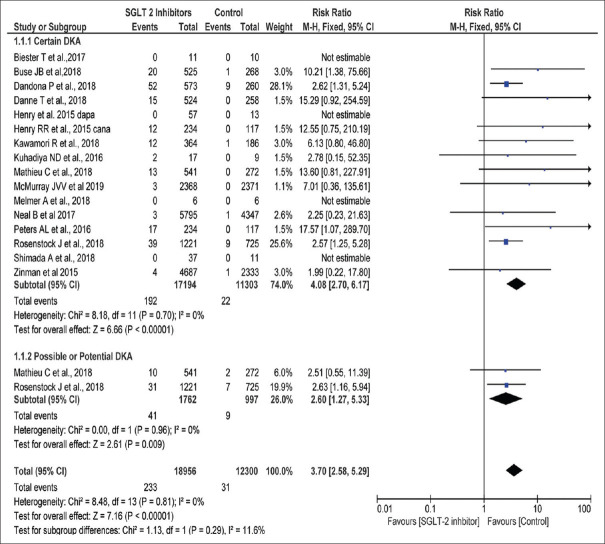
Sixteen studies comprising a total of 31,256 patients (18,956 in the SGLT2i group and 12,300 in the placebo group) were included for pooling the RR for DKA.[6,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] The pooled RR was 3.70 (95% CI 2.58, 5.29). I2 was 0% and the test overall effect was significant (P < 0.00001) [Figure 3]. High-quality evidence as per GRADE Pro analysis is shown in Table 3]. Publication bias was low as the funnel plot of 16 studies is symmetrical around the effect estimate [Figure 4].
Figure 3.
Incidence of DKA reported in randomized clinical trials in patients taking SGLT2 inhibitors versus control in diabetes patients
Figure 4.

Funnel plot depicting publication bias for studies included in the review
RISK OF BIAS: RoB-2
The overall risk of bias was recorded as low in all the included studies. The weighted summary plot of ROB is shown in [Figure 5].
Figure 5.
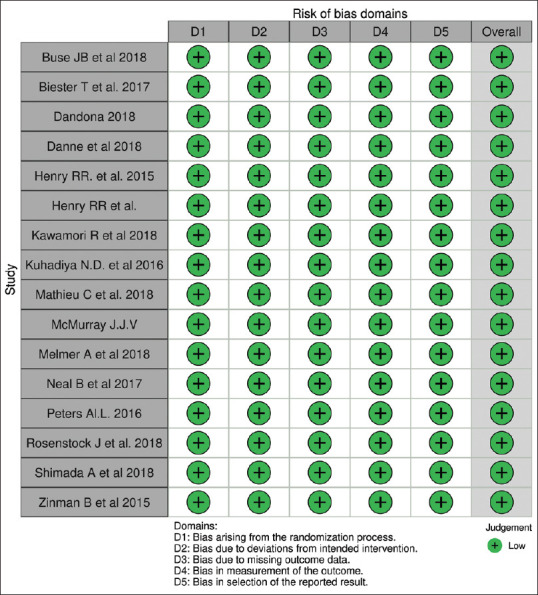
ROB-2: Weighted summary plot risk of bias in RCT evaluating SGLT2 inhibitors in diabetic patients
Discussion
SGLT2 inhibitors are emerging as a preferred drug because of its effectiveness in decreasing glycated hemoglobin levels along with weight loss, increasing peripheral insulin sensitivity, and preventing cardiovascular comorbidities in diabetic patients.[83] In addition, SGLT2i have a potentially beneficial role in decreasing morbidity and mortality in patients with congestive heart failure.[77] There are two different classes of SGLT transporters in the proximal convoluted tubule of the nephron, classified as SGLT-1 and SGLT-2. SGLT-2 itself reabsorbs nearly 90% of total filtered glucose into the nephron. SGLT2i decreases the renal threshold drastically, causes osmotic diuresis, and reduces plasma glucose concentration.[84] Blau et al.[85] observed that the risk of DKA is seven times more in diabetic patients taking the SGLT2i than the patient taking DPP-4 inhibitors. Though reported by Blau et al., the incidence of euDKA still not clear maybe because of its rare occurrence, underreporting as well as due to underdiagnosis.[51-85] The reason for euDKA and precipitating factors in DM patients still needs comprehensive research.
Recent FDA warning about euDKA associated with SGLT2i raised concern to understand the patient profile and presentation symptoms for risk evaluation in patients taking SGLT2i.[7] In this review, we studied patient demographic characteristics, clinical presentation, precipitating factors, and predisposed drug and its dose, from the published reports to enable treating physicians for early diagnosis and treatment of euDKA and prevent the potential adverse outcome.
Clinical presentation
In this review, we observed that half of the patients had T2DM without any comorbidities and about 1 in 5 patients had T2DM with cardiovascular disease, and 1 in 10 patients had T1DM. The majority of patients were middle age diabetic females (66%), maybe because of lower body mass index and poor glycogen stores.[51] The clinical presentation in these patients is similar to traditional symptoms of DKA, which include nausea, vomiting, abdominal pain, shortness of breath, etc. We also observed that diabetic patients during the postoperative period or patients with cardiovascular comorbidities prone to euDKA. This observation helps clinicians or treating physicians to create awareness among the patients and instruct them to report the clinician, in case they experience such symptoms. In the case of patients in postoperative care need to be screened for euDKA to avoid complications as a result of ketoacidosis. Infections were one of the precipitating factors for euDKA which was supported by the multicenteric cohort study done by Ata et al.[86] Treating community or primary care physicians also needs to be aware of these symptoms and high-risk individuals to avoid misdiagnosis or underdiagnosis. These symptoms and precipitating agents were similar to previously published articles.[83,87,88]
We observed that patients taking canagliflozin more prone to euDKA than dapagliflozin and empagliflozin. A multicenteric cohort study conducted by Ata et al.[86] also reported canagliflozin as the commonest SGLT-2 inhibitor causing euDKA. The reason for this difference may be because canagliflozin was the earliest drug approved under this class and it has been used by physicians for a longer duration than other drugs resulting in publishing more case reports.[7] We also observed the dose–response relationship with all three drugs as the incidence of euDKA proportionately increases as the dose of the drug was increased. This pattern may be because of the excessive release of glucagon in diabetic patients.[89] Wibawa et al. in their recent case reported euDKA with the use of empagliflozin.[90]
We also conducted a meta-analysis of randomized controlled trials, to see the overall events of DKA in patients taking SGLT2i compared to either active drug or placebo. Our findings show that patients taking SGLT2i are at the risk of experiencing DKA is about 2.58 to 5.29 times higher than the control group. This meta-analysis gives additional strength to our analysis in reinforcing the evidence showing that SGLT2i causes DKA including euDKA in both type 1 and type 2 diabetic patients. Blau et al.[85] showed that the risk of DKA is around 7-fold higher in T2DM patients and 5 to 12-fold in T1DM patients as reported in FDA adverse event reporting system (FAERS). In FAERS data, out of all DKA case reports, euDKA was reported in 71% of cases.
The current pandemic of COVID-19 has infected a large population around the world and people with comorbidities like DM are more prone to develop a severe form of the disease.[91,92] Recently, the cases of euDKA have also been seen in the COVID-19 patients and the probability might increase in COVID-19 because of the viral infection and its associated complications.[93,94] Vitale et al.[95] in their case series also reported cases of T2DM with no previous history of DKA and on SGLT-2 inhibitors presented with euDKA.
Mechanism
The pathophysiology of euDKA in patients receiving SGLT 2 inhibitors remains unclear, but there are multiple hypotheses explained to understand the mechanism of euDKA. The most popular hypothesis was that SGLT2 inhibitors increase glucagon secretion by binding to alpha cells of pancreatic islets and causing gluconeogenesis. Simultaneously, it decreases in blood glucose by hastening its renal execration from the plasma. A decrease in blood glucose cause decreased insulin secretion from islets which results in excess ketone body formation.[90] In addition to the above mechanism, it was observed that in type 1 diabetic patients, the decreased daily requirement of insulin causes increased lipolysis in adipose tissue and increased ketone body synthesis in the liver.[90] In addition, increased reabsorption of ketone bodies from the kidney due to positive electrochemical gradient generation by inhibition of SGLT 2 transporter.[96] Bonner C et al.[89] observed that treatment with dapagliflozin in mice resulted in inhibition of Solute Carrier Family 5 (Sodium/Glucose Cotransporter), Member 2 (SLC5A2) on pancreatic alpha cells thereby increasing glucagon. Also, increased glucagon gene expression results in increased glucagon synthesis leading to increased hepatic gluconeogenesis and excess fatty acid degradation leading to excess production of ketone bodies.
Strengths and Limitations
We did a comprehensive systematic review describing the data on demographic, clinical characteristics of euDKA in patients taking SGLT2 inhibitors and the dose–dependent relationship between euDKA and SGLT 2 inhibitors. There are chances of missing out on case reports published in local languages as we included only case reports published in the English language. We analyzed symptomatic reported cases. Case reports are good for generating hypotheses, but the potential risk of bias due to lack of external validity cannot be ruled out.
GRADE Conclusion
The overall quality of the systematic review is high as the incidence of DKA has a high quality of evidence. This evidence suggests that further research is very unlikely to have an important impact on our confidence in the estimate and change the estimate.
Key points and Summary
DM is a prevalent noncommunicable disease in India.
SGLT-2 inhibitor is a crucial class of novel antidiabetic drugs in view of their additive cardiovascular benefits.
euDKA in case of diabetic ketoacidosis without hyperglycemia and is rarely seen with use with SGLT-2 inhibitors.
Type-2 DM patients with a long history and inadequate control of blood glucose seem to be more prone to develop euDKA
The cause of euDKA with SGLT-2 inhibitors might be an increase in glucagon secretion, excess ketone body formation, and increased reabsorption of ketone bodies from the kidney.
The symptoms of euDKA are lesser as compared to classical DKA; therefore, early detection of this potential complication might be a challenging task.
Physicians treating diabetic patients with SGLT-2 inhibitors should have a high level of suspicion to diagnose the condition early and treat it as normal glucose levels in these patients mask the diagnosis of DKA.
Conclusion
Systematic review of euDKA and the incidence of DKA with SGLT-2 inhibitors were performed, to assist the endocrinologists and primary care physicians in getting aware of these complications. This helps in the prevention, early diagnosis, and treatment of complications. With the increasing use of SGLT 2 inhibitors, there is a likelihood of an increase in the number of euDKA cases with this group of drugs. Hence, it is important to identify patient characteristics and precipitating factors to prevent similar cases in the future. We need multicenteric dedicated studies for the evaluation of risk factors of SGLT2 inhibitor-induced euDKA and associated biomarkers including pharmacogenomics of siRNA of SCL5A2 gene.
Author’s contribution
Study design and planning of systematic review – All of the authors.
Literature search – TK, SD, and SBV
Figures –SBV, SS, and SD
Tables – SBV, SA, and TK
Data collection and analysis – SD, TK, and SBV
ROB – SBV, SD, and SA
GRADE analysis – SS, SBV, and query resolved by all authors
Data interpretation – SD, SBV, SS, and SA
Writing – All authors
Corrections and final approval of manuscript – All of the authors
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful for GRADEpro GDT site for allowing us to generate the grading of evidence and summary of findings table using their online software. Available from: gradepro.org.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Lancet. Vol. 387. London, England: 2016. Worldwide trends in diabetes since 1980:A pooled analysis of 751 population-based studies with 4.4 million participants; pp. 1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pharmacologic management of type 2 diabetes:2016 interim update. Can J Diabetes. 2016;40:484–6. doi: 10.1016/j.jcjd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Dutta S, Sharma P, Mishra A. SGLT-2 inhibitors:An evidence-based perspective. In: Atta-ur-Rahman, editor. Frontiers in Clinical Drug Research-Diabetes and Obesity. Vol. 5. Sharjah, U.A.E.: Bentham Science Publishers; 2020. pp. 138–55. doi:10.2174/9781681087535120050007. [Google Scholar]
- 4.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport:Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–7. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors:A systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37:187–94. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes:Simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38:356–63. doi: 10.1016/j.jcjd.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes:A systematic review and meta-analysis. Sci Rep. 2017;7:44128. doi: 10.1038/srep44128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SS, Ahmed I. Sodium-glucose cotransporter 2 inhibitors:An evidence-based practice approach to their use in the natural history of type 2 diabetes. Curr Med Res Opin. 2016;32:907–19. doi: 10.1185/03007995.2016.1151774. [DOI] [PubMed] [Google Scholar]
- 11.FDA Drug Safety Communication:FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections [Internet] [Last accessed on 2020 Oct 14]. Available from:https://www.fda.gov/media/92185/download.
- 12.European Medicines Agency. Review of diabetes medicines called SGLT2 inhibitors started:Risk of diabetic ketoacidosis to be examined 2015. [Last accessed on 2020 Oct 15]. Available from:https://www.ema.europa.eu/en/documents/referral /sglt2-inhibitors-article-20-procedure-review-started_en.pdf.
- 13.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 14.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 15.Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019;63:9–14. doi: 10.1016/j.ejim.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis:A predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 17.Rawla P, Vellipuram AR, Bandaru SS, Pradeep Raj J. Euglycemic diabetic ketoacidosis:A diagnostic and therapeutic dilemma. Endocrinol Diabetes Metab Case Rep. 2017;2017 doi: 10.1530/EDM-17-0081. doi:10.1530/EDM-17-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, Updated October 2013. [Last accessed on 2020 Oct 20]. Available from:https://gdt.gradepro.org/app/handbook/handbook.html .
- 19.Copenhagen:The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. [Last accessed on 2020 Oct 20]. Available from:https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman .
- 20.GRADEpro GDT[Online software] [Last accessed on 2020 Oct 22]. Available from:https://gradepro.org/
- 21.Candelario N, Wykretowicz J. The DKA that wasn't:A case of euglycemic diabetic ketoacidosis due to empagliflozin. Oxf Med Case Rep. 2016;2016:144–6. doi: 10.1093/omcr/omw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown F, McColl T. Euglycemic diabetic ketoacidosis secondary to dapagliflozin use:A case report. J Emerg Med. 2018;54:109–11. doi: 10.1016/j.jemermed.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Bteich F, Daher G, Kapoor A, Charbek E, Kamel G. Post-surgical euglycemic diabetic ketoacidosis in a patient on empagliflozin in the intensive care unit. Cureus. 2019;11:e4496. doi: 10.7759/cureus.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao HY, Kornelius E. Two alcoholic liver cirrhosis patients developed diabetic ketoacidosis after SGLT2 inhibitors-prescription. J Formos Med Assoc. 2020;119:1886–7. doi: 10.1016/j.jfma.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Chou YM, Seak CJ, Goh ZN, Seak JC, Seak CK, Lin CC. Euglycemic diabetic ketoacidosis caused by dapagliflozin:A case report. Medicine (Baltimore) 2018;97:e11056. doi: 10.1097/MD.0000000000011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clement M, Senior P. Euglycemic diabetic ketoacidosis with canagliflozin:Not-so-sweet but avoidable complication of sodium-glucose cotransporter-2 inhibitor use. Can Fam Physician. 2016;62:725–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Z, Nishihata Y, Kawamatsu N, Komatsu I, Mizuno A, Shimizu M, et al. Cardiac arrest from acute myocardial infarction complicated with sodium-glucose cotransporter 2 inhibitor-associated ketoacidosis. J Cardiol Cases. 2017;15:56–60. doi: 10.1016/j.jccase.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Ramos A, Eilbert W, Marquez D. Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitor use:A case report and review of the literature. Int J Emerg Med. 2019;12:27. doi: 10.1186/s12245-019-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dizon S, Keely EJ, Malcolm J, Arnaout A. Insights into the recognition and management of SGLT2-inhibitor-associated ketoacidosis:It's not just euglycemic diabetic ketoacidosis. Can J Diabetes. 2017;41:499–503. doi: 10.1016/j.jcjd.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Dull RB, Spangler ML, Knezevich EL, Lau BM. Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter type 2 inhibitors in patients with type 2 diabetes mellitus receiving oral therapy. J Pharm Pract. 2019;32:240–3. doi: 10.1177/0897190017748049. [DOI] [PubMed] [Google Scholar]
- 31.Earle M, Ault B, Bonney C. Euglycemic diabetic ketoacidosis in concurrent very low-carbohydrate diet and sodium-glucose transporter-2 inhibitor use:A case report. Clin Pract Cases Emerg Med. 2020;4:185–8. doi: 10.5811/cpcem.2020.2.45904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elshimy G, Correa R. Sudden-onset hypoglycemia following fluid replacement in a patient with dapagliflozin-induced diabetic ketoacidosis without prior insulin use:Case report. Cureus. 2019;11:e5448. doi: 10.7759/cureus.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda M, Nabeta M, Muta T, Fukami K, Takasu O. Euglycemic diabetic ketoacidosis caused by canagliflozin:A case report. Int J Emerg Med. 2020;13:2. doi: 10.1186/s12245-020-0261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh MS. A case of euglycemic diabetic keto acidosis. Indian J Endocrinol Metab. 2019;23:500–1. doi: 10.4103/ijem.IJEM_302_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iqbal I, Hamid M, Khan MA, Kainat A, Tariq S. Dapagliflozin-induced late-onset euglycemic diabetic ketoacidosis. Cureus. 2019;11:e6089. doi: 10.7759/cureus.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jazi M, Porfiris G. Euglycemic diabetic ketoacidosis in type 2 diabetes treated with a sodium-glucose cotransporter-2 inhibitor. Can Fam Physician. 2016;62:722–4. [PMC free article] [PubMed] [Google Scholar]
- 37.Karakaya Z, Topal FE, Topal F, Payza U, Akyol PY. Euglisemic diabetic ketoacidotic coma caused by dapagliflozin. Am J Emerg Med. 2018;36:e1–2. doi: 10.1016/j.ajem.2018.08.054. 2136. [DOI] [PubMed] [Google Scholar]
- 38.Kelmenson DA, Burr K, Azhar Y, Reynolds P, Baker CA, Rasouli N. Euglycemic diabetic ketoacidosis with prolonged glucosuria associated with the sodium-glucose cotransporter-2 canagliflozin. J Investig Med High Impact Case Rep. 2017;5:2324709617712736. doi: 10.1177/2324709617712736. doi:10.1177/2324709617712736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch RA, Clark RF. Euglycemic ketoacidosis with sodium-glucose cotransporter-2 inhibitor. Am J Ther. 2018;25:e590–e1. doi: 10.1097/MJT.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 40.Kum-Nji JS, Gosmanov AR, Steinberg H, Dagogo-Jack S. Hyperglycemic, high anion-gap metabolic acidosis in patients receiving SGLT-2 inhibitors for diabetes management. J Diabetes Complications. 2017;31:611–4. doi: 10.1016/j.jdiacomp.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Lane S, Paskar D, Hamed S, Goffi A. When guidelines fail:Euglycemic diabetic ketoacidosis after bariatric surgery in a patient taking a sodium-glucose cotransporter-2 inhibitor:A case report. A A Pract. 2018;11:46–8. doi: 10.1213/XAA.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 42.Lau A, Bruce S, Wang E, Ree R, Rondi K, Chau A. Perioperative implications of sodium-glucose cotransporter-2 inhibitors:A case series of euglycemic diabetic ketoacidosis in three patients after cardiac surgery. Can J Anaesth. 2018;65:188–93. doi: 10.1007/s12630-017-1018-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee IH, Ahn DJ. Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes mellitus:A case report. Medicine (Baltimore) 2020;99:e20228. doi: 10.1097/MD.0000000000020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine JA, Karam SL, Aleppo G. SGLT2-I in the hospital setting:Diabetic ketoacidosis and other benefits and concerns. Curr Diab Rep. 2017;17:54. doi: 10.1007/s11892-017-0874-3. [DOI] [PubMed] [Google Scholar]
- 45.Lucero P, Chapela S. Euglycemic diabetic ketoacidosis in the ICU:3 Case reports and review of literature. Case Rep Crit Care. 2018;2018:1747850. doi: 10.1155/2018/1747850. doi:10.1155/2018/1747850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackintosh C, Tewari A, Siegel J, Wang RD, Freeman W. Postoperative euglycemic diabetic ketoacidosis and encephalopathy related to SGLT-2 inhibitors:A case report and discussion of diabetes treatment and “sweet pee encephalopathy”in perioperative hospital management. Neurohospitalist. 2020;10:51–4. doi: 10.1177/1941874419835035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nappi F, La Verde A, Carfora G, Garofalo C, Provenzano M, Sasso FC, et al. Nephrology consultation for severe SGLT2 inhibitor-induced ketoacidosis in type 2 diabetes:Case report. Medicina (Kaunas) 2019;55:462. doi: 10.3390/medicina55080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pace DJ, Dukleska K, Phillips S, Gleason V, Yeo CJ. Euglycemic diabetic ketoacidosis due to sodium-glucose cotransporter 2 inhibitor use in two patients undergoing pancreatectomy. J Pancreat Cancer. 2018;4:95–9. doi: 10.1089/pancan.2018.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadokostaki E, Liberopoulos E. Euglycemic diabetic ketoacidosis secondary to dapagliflozin in a patient with colon malignancy. Case Rep Endocrinol. 2019;2019:3901741. doi: 10.1155/2019/3901741. doi:10.1155/2019/3901741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereyra AM, Ramirez C, Roman R. Euglycemic ketosis in an adolescent with type 1 diabetes on insulin and dapaglifozin:Case report. Rev Chil Pediatr. 2017;88:404–10. doi: 10.4067/S0370-41062017000300015. [DOI] [PubMed] [Google Scholar]
- 51.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis:A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–93. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pujara S, Ioachimescu A. Prolonged ketosis in a patient with euglycemic diabetic ketoacidosis secondary to dapagliflozin. J Investig Med High Impact Case Rep. 2017;5:2324709617710040. doi: 10.1177/2324709617710040. doi:10.1177/2324709617710040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rafey MF, Butt A, Coffey B, Reddington L, Devitt A, Lappin D, et al. Prolonged acidosis is a feature of SGLT2i-induced euglycaemic diabetic ketoacidosis. Endocrinol Diabetes Metab Case Rep. 2019;2019:19–0087. doi: 10.1530/EDM-19-0087. doi:10.1530/EDM-19-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sampani E, Sarafidis P, Dimitriadis C, Kasimatis E, Daikidou D, Bantis K, et al. Severe euglycemic diabetic ketoacidosis of multifactorial etiology in a type 2 diabetic patient treated with empagliflozin:Case report and literature review. BMC Nephrol. 2020;21:276. doi: 10.1186/s12882-020-01930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang AY, Hou SK, Li SJ, Kao WF. Euglycemic diabetic ketoacidosis in type 2 diabetes with sodium glucose cotransporter 2 inhibitors. Am J Emerg Med. 2017;35:379 e5–6. doi: 10.1016/j.ajem.2016.08.055. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Ide N, Kitajima S, Obayashi M, Asada K, Matsushima S, et al. [Risk of euglycemic diabetic ketoacidosis due to low-carbohydrate diet while taking empagliflozin:A case report] Yakugaku Zasshi. 2019;139:1479–83. doi: 10.1248/yakushi.19-00120. [DOI] [PubMed] [Google Scholar]
- 57.Yeo SM, Park H, Paek JH, Park WY, Han S, Park SB, et al. Ketoacidosis with euglycemia in a patient with type 2 diabetes mellitus taking dapagliflozin:A case report. Medicine (Baltimore) 2019;98:e14150. doi: 10.1097/MD.0000000000014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Tamilia M. Euglycemic diabetic ketoacidosis associated with the use of a sodium-glucose cotransporter-2 inhibitor. CMAJ. 2018;190:E766–8. doi: 10.1503/cmaj.171319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adachi J, Inaba Y, Maki C. Euglycemic diabetic ketoacidosis with persistent diuresis treated with canagliflozin. Intern Med. 2017;56:187–90. doi: 10.2169/internalmedicine.56.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhassan S, Rudoni M, Alfonso-Jaume MA, Jaume JC. Protracted glycosuria after discontinuation of sodium-glucose cotransporter 2 inhibitors:Implications for weekly dosing and extended risk of euglycemic diabetes ketoacidosis. J. Diabetes. 2019;11:410–1. doi: 10.1111/1753-0407.12885. [DOI] [PubMed] [Google Scholar]
- 61.Allison R, Goldstein D, Musso MW. Challenges in the diagnosis of euglycemic diabetic ketoacidosis in a patient with multiple sclerosis taking a sodium-glucose cotransporter 2 inhibitor. J Emerg Med. 2019;57:e1–3. doi: 10.1016/j.jemermed.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Andrews TJ, Cox RD, Parker C, Kolb J. Euglycemic diabetic ketoacidosis with elevated acetone in a patient taking a sodium-glucose cotransporter-2 (SGLT2) inhibitor. J Emerg Med. 2017;52:223–6. doi: 10.1016/j.jemermed.2016.07.082. [DOI] [PubMed] [Google Scholar]
- 63.Bader N, Mirza L. Euglycemic diabetic ketoacidosis in a 27 year-old female patient with type-1-diabetes treated with sodium-glucose cotransporter-2 (SGLT2) inhibitor canagliflozin. Pak J Med Sci Q. 2016;32:786–8. doi: 10.12669/pjms.323.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badwal K, Tariq T, Peirce D. Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient presenting with acute pancreatitis. Case Rep Endocrinol. 2018;2018:6450563. doi: 10.1155/2018/6450563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benmoussa JA, Clarke M, Penmetsa A, Leykina L, Diaz K, Otterbeck P. Euglycemic diabetic ketoacidosis:The clinical concern of SGLT2 inhibitors. J Clin Transl Endocrinol Case Rep. 2016;2:17–9. [Google Scholar]
- 66.Lin Y. Sodium-glucose cotransporter-2 inhibitors induced eu-glycemic diabetic ketoacidosis:The first report in a type 2 diabetic (T2D) Taiwanese and literature review of possible pathophysiology and contributing factors. J Formos Med Assoc. 2018;117:849–54. doi: 10.1016/j.jfma.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Wang K, Isom R. SGLT2 inhibitor–induced euglycemic diabetic ketoacidosis:A case report. Kidney Med. 2020;2:218–21. doi: 10.1016/j.xkme.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes:The North American inTandem1 study. Diabetes Care. 2018;41:1970–80. doi: 10.2337/dc18-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biester T, Aschemeier B, Fath M, Frey M, Scheerer MF, Kordonouri O, et al. Effects of dapagliflozin on insulin-requirement, glucose excretion and ss-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017;19:1635–9. doi: 10.1111/dom.12975. [DOI] [PubMed] [Google Scholar]
- 70.Dandona P, Mathieu C, Phillip M, Hansen L, Tschope D, Thoren F, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes:The DEPICT-1 52-week study. Diabetes Care. 2018;41:2552–9. doi: 10.2337/dc18-1087. [DOI] [PubMed] [Google Scholar]
- 71.Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes:The European inTandem2 study. Diabetes Care. 2018;41:1981–90. doi: 10.2337/dc18-0342. [DOI] [PubMed] [Google Scholar]
- 72.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes:A randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412–9. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 73.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38:2258–65. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 74.Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, Miyamoto Y, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes:Glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;20:2200–9. doi: 10.1111/dom.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuhadiya ND, Ghanim H, Mehta A, Garg M, Khan S, Hejna J, et al. Dapagliflozin as additional treatment to liraglutide and insulin in patients with type 1 diabetes. J Clin Endocrinol Metab. 2016;101:3506–15. doi: 10.1210/jc.2016-1451. [DOI] [PubMed] [Google Scholar]
- 76.Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study):24-week results from a randomized controlled trial. Diabetes Care. 2018;41:1938–46. doi: 10.2337/dc18-0623. [DOI] [PubMed] [Google Scholar]
- 77.McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 78.Melmer A, Kempf P, Lunger L, Pieber TR, Mader JK, Stettler C, et al. Short-term effects of dapagliflozin on insulin sensitivity, postprandial glucose excursion and ketogenesis in type 1 diabetes mellitus:A randomized, placebo-controlled, double blind, cross-over pilot study. Diabetes Obes Metab. 2018;20:2685–9. doi: 10.1111/dom.13439. [DOI] [PubMed] [Google Scholar]
- 79.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 80.Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39:532–8. doi: 10.2337/dc15-1995. [DOI] [PubMed] [Google Scholar]
- 81.Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes:The EASE trials. Diabetes Care. 2018;41:2560–9. doi: 10.2337/dc18-1749. [DOI] [PubMed] [Google Scholar]
- 82.Shimada A, Hanafusa T, Yasui A, Lee G, Taneda Y, Sarashina A, et al. Empagliflozin as adjunct to insulin in Japanese participants with type 1 diabetes:Results of a 4-week, double-blind, randomized, placebo-controlled phase 2 trial. Diabetes Obes Metab. 2018;20:2190–9. doi: 10.1111/dom.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Introduction:Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S1–2. doi: 10.2337/dc19-Sint01. [DOI] [PubMed] [Google Scholar]
- 84.Imprialos KP, Stavropoulos K, Doumas M, Karagiannis A, Athyros VG. The effect of SGLT2 inhibitors on cardiovascular events and renal function. Expert Rev Clin Pharmacol. 2017;10:1251–61. doi: 10.1080/17512433.2017.1370371. [DOI] [PubMed] [Google Scholar]
- 85.Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment:Analysis of FAERS data. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2924. doi:10.1002/dmrr.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ata F, Yousaf Z, Khan AA, Razok A, Akram J, Ali EA, et al. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. 2021;11:10293. doi: 10.1038/s41598-021-89752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vellanki P, Umpierrez GE. Diabetic ketoacidosis:A common debut of diabetes among African Americans with type 2 diabetes. Endocr Pract. 2017;23:971–8. doi: 10.4158/EP161679.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Legaspi R, Narciso P. Euglycemic diabetic ketoacidosis due to gastroparesis, A local experience. J Ark Med Soc. 2015;112:62–3. [PubMed] [Google Scholar]
- 89.Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–7. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 90.Wibawa K, Kuhuwael FV, Putra CRJ, Widiastuti SU, Suciadi LP. Euglycemic diabetic ketoacidosis associated with empagliflozin in patients hospitalized with acute pulmonary embolism. Clin. Diabetol. 2021;10:204–8. [Google Scholar]
- 91.Dutta S, Kaur RJ, Bhardwaj P, Charan J, Bist SK, Detha MD, et al. Household transmission of COVID-19:A cross-sectional study. Infect Drug Resist. 2020;13:4637–42. doi: 10.2147/IDR.S285446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahai R, Dutta S, Kumar T. Anticoagulants in Covid-19 therapy:An evidence-based review. Int J Pharm Sci Rev Res. 2020;63:191–5. [Google Scholar]
- 93.Ozer O, Yorulmaz G. Euglycemic diabetic ketoacidosis associated with empagliflozin use in the course of the SARS-Cov-2 pandemic. J Coll Physicians Surg Pak. 2020;30:110–1. doi: 10.29271/jcpsp.2020.supp2.110. [DOI] [PubMed] [Google Scholar]
- 94.Dass B, Beck A, Holmes C, Morton G. Euglycemic DKA (euDKA) as a presentation of COVID-19. Clin Case Rep. 2020;9:395–8. doi: 10.1002/ccr3.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vitale RJ, Valtis YK, McDonnell ME, Palermo NE, Fisher ND. Euglycemic diabetic ketoacidosis with COVID-19 infection in patients with type 2 diabetes taking SGLT2 inhibitors. AACE Clin Case Rep. 2021;7:10–3. doi: 10.1016/j.aace.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–52. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Somagutta MR, Agadi K, Hange N, Jain MS, Batti E, Emuze BO, et al. Euglycemic diabetic ketoacidosis and sodium-glucose cotransporter-2 inhibitors:A focused review of pathophysiology, risk factors, and triggers. Cureus. 2021;13:e13665. doi: 10.7759/cureus.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]