Abstract
Skeletal growth and bone health are very important in children. The effective role of vitamin D in bone mineral density has been observed in children and adolescents. This systematic review study evaluated the effects of vitamin D on bone density in healthy children with the help of valid databases and the website of clinical trials. The results of experimental, clinical, retrospective, prospective, double-blind, and randomized studies were used. Articles that appropriately covered the topic and had the proper content structure were selected for this review. Out of a total of 132 articles, finally, 13 articles were selected based on inclusion and exclusion criteria for further study, the results of which show the association between serum levels of vitamin D and bone mineral density and health. However, in some articles, the relationship between other influential variables such as age and nutrition on bone density in children was identified. In general, the current systematic review demonstrates the role of vitamin D on bone density in healthy children, so that in children studied, vitamin D is at different levels and complications related to bone density are observed in many children. It is recommended that more clinical and longitudinal studies be performed to further understand the role of vitamin D levels in bone health in children.
Keywords: Bone density, children, vitamin D, vitamin D deficiency
Introduction
Vitamin D along with calcium is greatly important for skeletal growth and bone health, also, has an important effect on body structure, soft tissue, fetal growth, and women’s health.[1] This vitamin is a secosteroid that could be obtained from the diet as ergocalciferol (vitamin D2), from plant sources as cholecalciferol (vitamin D3), and from animal sources. When 7-dehydrocholesterol is exposed to ultraviolet B (UVB) rays, vitamin D could be produced endogenously in the skin. Vitamin D is converted by 25-hydroxylase in the liver to 25-hydroxyvitamin D [25(OH) D]. It is the main circulating form of vitamin D and acts as a reservoir for the conversion of the active metabolite 25-hydroxyvitamin D [25(OH) D] by 1a-hydroxylase. This enzyme is located primarily in the cells of the proximal tubule of the kidney and to a lesser extent in the placenta, bone, and parathyroid glands.[1,2] The classic function of vitamin D is in calcium and phosphate homeostasis. The synthesis of 1,25-(OH) 2D in the kidney is highly regulated in response to serum ionized calcium (Ca2+) levels. This occurs with parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23). Low levels of Ca2+ stimulate PTH secretion, which simultaneously increases renal calcium uptake into the distal tubule and reduces proximal tubule phosphate reabsorption, which ultimately regulates the synthesis of 1,25-(OH) 2D. The main function of 1,25-(OH) 2D is to increase dietary calcium absorption through intestinal cells.[1,3]
25(OH) D is currently the best biochemical indicator of vitamin D status because hepatic hydroxylation of cholecalciferol to 25(OH) D depends only on the availability of conditions, whereas conversion of 25(OH) D to 1,25-(OH) 2D is fully regulated in response to serum Ca2+ and PTH. The half-life of 25(OH) D is significantly longer than 1,25-(OH) 2D: approximately 2–3 weeks compared with 4–6 h.[4]
There is little data to confirm the epidemiology of serum 25(OH) D in the general population, but several studies report a high prevalence of vitamin D deficiency (VDD).[5,6] Vitamin D is also absorbed in adults, but the fetus depends on the mother to absorb 25(OH) D, and 25(OH) D passes easily through the placenta during pregnancy, and maternal venous blood and umbilical cord have medium levels of 25(OH) D.[7,8]
Prevention of osteoporosis is a common and costly public health problem that is of great importance. The main cause of this disease is the low mineral density in bones, which is also the main cause of bone fractures. Low levels of vitamin D are very common in children and adolescents and are a major public health issue worldwide, including in the United Kingdom, many European countries, the United States, Lebanon, Australia, and New Zealand.[9,10] Low mineral density in bones is one of the risk factors for fractures in childhood. Evaluation of strategies to maximize bone mass in childhood has been identified as a research priority.[11]
Vitamin D deficiency is diagnosed by measuring serum 25(OH) D. Concentrations above 50 nmol/L are considered normal, which of course is debatable because although only lower amounts are commonly associated with clinical conditions such as rickets, the optimum level for bone health may be above 50 nmol/L.[12]
Randomized controlled trials of vitamin D supplementation have reported conflicting results for bone densitometry (bone density analysis)[13,14,15,16,17] and therefore evaluation of the effectiveness of vitamin D supplementation in improving bone density in children and the relationship between serum vitamin D levels and bone density is greatly important. This systematic review was conducted to determine the effect of vitamin D on bone density in healthy children, to identify the most effective components in children, and to determine the number of children with bone density problems in these studies.
Materials and Methods
This study was performed according to the preferential reports to systematically evaluate the effects of vitamin D on bone density in healthy children during 2000–2020. This systematic review followed a predefined protocol that has been recorded in the international database of prospectively registered systematic reviews in health and social care (PROSPERO). No ethical approval was required for this study. The following databases were searched: PubMed, MEDLINE, CINAHL, EMBASE, Cochrane Library, and Clinical Trials website. The current clinical systematic review uses the results of experimental, clinical, retrospective, prospective, double-blind, and randomized studies.
Inclusion and exclusion criteria
Inclusion criteria were minimum treatment with vitamin D supplements and mention of vitamin D efficacy results. Studies in languages other than English and studies in which vitamin D was not used to treat osteoporosis were excluded.
Data collection
To collect information from the databases, keywords such as vitamin D, osteoporosis, rickets, bone density, etc., were used. Various methodological aspects of the articles including sampling techniques, reliability of equipment and study objectives, bone mineral density, and bone mineral content as a percentage change from baseline, as well as sex, age, stage of maturation, and basal serum vitamin D level were investigated. Finally, articles that properly covered the topic and had the right content structure were selected for this review. In total, 132 articles with related topics, content, and objectives to the subject of study, were selected for review.
Results
In the first stage, 132 titles were selected. In the initial screening stage, duplicate content, title, and, if necessary, abstract of articles were reviewed, and then 68 articles were selected. In the second stage, the full text of the articles was studied and 26 articles were removed due to differences in purpose. Out of the remaining 42 articles, 29 articles were excluded from the study based on inclusion/exclusion criteria, and finally, 13 full-text articles published in English on the relationship between vitamin D and bone mineral density in children were included in the final study. [Figure 1].
Figure 1.
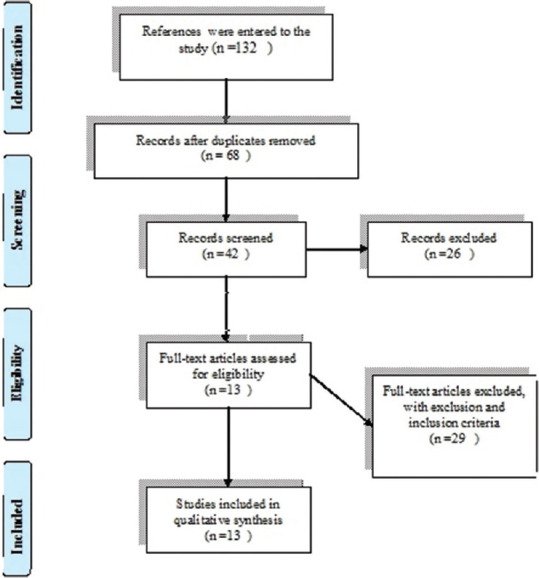
Diagram related to the selection of reviewed articles
The results of these 13 studies are summarized in Table 1. The results of these studies indicate a relationship between serum levels of vitamin D and bone density in children, and the findings confirm the control of bone density problems in children with the help of a diet containing vitamin D. Thus, the closer the level of vitamin D in the blood serum to normal, the higher the health of bone density. The lower the vitamin D, the higher the risk of bone problems in children.
Table 1.
Results related to the effects of vitamin D on bone density in children
| Authors/Year | Title | Population/Age/Type of research | Method | Results |
|---|---|---|---|---|
| Cheng et al.,[13] 2005 | Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10- to 12-year-old girls: a 2-year randomized trial. | This placebo-controlled intervention trial randomly assigned 195 healthy girls at Tanner stage I-II, aged 10-12 years | Dietary calcium intakes<900 mg/d to 1 of 4 groups: calcium (1,000 mg) + vitamin D3 (200 IU), calcium (1,000 mg), cheese (1,000 mg calcium), and placebo. Primary outcomes were bone indexes of the hip, spine, and whole body by X-ray and of the radius and tibia by peripheral quantitative computed tomography. | Calcium supplementation with cheese resulted in a higher percentage change in cortical thickness of the tibia than did placebo, calcium, or calcium + vitamin D treatment and in higher whole-body bone mineral density than did placebo treatment when compliance was >50%. With the use of a hierarchical linear model with random effects to control growth velocity, these differences disappeared. Increasing calcium intake by consuming cheese appears to be more beneficial for cortical bone mass accrual than the consumption of tablets containing a similar amount of calcium. |
| Kvammen et al.,[18] 2020 | Bone mineral density and vitamin D in pediatric intestinal failure patients receiving home parenteral nutrition | An observational cross-sectional study was performed at Oslo University Hospital and at the Department of Nutrition, University of Oslo/Nineteen IF patients and 50 healthy children were included. The m ean age of participants was 10 years. | Dual energy X-ray absorptiometry (DXA; Lunar Prodigy in IF patients and Lunar iDXA in healthy subjects) was performed to assess BMD and body composition. BMD z-score (BMD-z) was calculated for total body and lumbar spine L2-L4 based on the integrated reference population in the software. Weight and height were measured for growth assessment. Nutrient provision was assessed by a 4-day food record. Blood samples were analyzed for 25-hydroxy-vitamin D (25[OH] D). Physical activity was reported by a questionnaire. | Lower median BMD-z for total body and lumbar spine L2-L4 were found in the IF group compared with the healthy children. Vitamin D provision was significantly higher in IF patients (17 mg/d vs. 5.3 mg/d). Both groups were sufficient in 25(OH) D (IF patients 71 nmol/L vs. healthy 81 nmol/L). Nevertheless, IF patients had significantly lower 1,25-(OH) 2D than healthy children (71 pmol/L vs. 138 pmol/L). The IF group was significantly shorter (height for age z-score -1.5 vs. 0.1) and lighter compared with the healthy subjects. BMI-z did not differ; however, body fat percentage was significantly higher in IF patients compared with healthy children (34% vs. 25%). A lower frequency of physical activity was found in the IF group. |
| Mager et al.,[19] 2012 | Vitamin D and K status influences bone mineral density and bone accrual in children and adolescents with celiac disease | 54 children and adolescents (35 females and 19 males) aged 3-17 years with biopsy-proven CD at diagnosis and after 1 year on the GFD were studied. | bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry. Relevant variables included: diet, anthropometrics, vitamin D/K status, physical activity, and sunlight exposure. | Whole-body and lumbar-spine BMD-z scores were low at diagnosis (10%-20%) and after 1 year (30%-32%) in the children, independent of symptoms. Whole-body BMD-z scores and serum levels of 25(OH) vitamin D were significantly lower in older children (>10 years) when compared with younger children . Forty-three percent had suboptimal vitamin D status (25(OH)-vitamin D <75 nmol/l) at diagnosis; resolving in nearly half after 1 year on the GFD. Twenty-five percent had suboptimal vitamin K status at diagnosis; all resolved after 1 year. |
| White et al.,[14] 2019 | Bone Health, Cody composition, and Vitamin D Status of Black Preadolescent Children in South Africa |
Data were collected by means of a cross-sectional study on 84 conveniently sampled black preadolescent South African children (44 girls, 40 boys; mean±SD, age 8.5-1.4 years) from September to November (spring season), 2016 in Pretoria, South Africa, at a latitude of 25 S. Ethical approval was obtained from the Research Ethics Committee, Faculty of Health Sciences, University of Pretoria. | Body composition (fat-free mass [FFM], FM and body fat percentage [BF%]), and bone health parameters (BMD, BMC, and bone area) were measured by means of whole-body dual X-ray absorptiometry (DXA), using the Hologic Discovery-W densitometer. Weight estimated by the DXA was used for adjusting bone measurements. The total body less head (TBLH) and lumbar spine (LS) BMD were used in reporting the data due to the highly reproducible nature of these measures in pediatric measurements. | A quarter (25%) of children presented with low bone mass density for their chronological age and 7% with low BMC for age, whereas only 34% of the children had sufficient vitamin D status. Lean mass was the greatest body compositional determinant for variances observed in bone health measures. Body composition and bone health parameters were not significantly different across vitamin D status groups, except for lumbar spine bone mineral apparent density (LS-BMAD). No association was found between bone parameters at all sites and levels of 25(OH) D. Further research, using larger representative samples of South African children including all race groups is needed before any conclusions and subsequent recommendation among this population group can be made. |
| For TBLH bone mass, the Z-score is recommended to be adjusted by the height Z-score. | ||||
| Barnes et al.,[20] 2005 | Reduced Bone Density in Children on Long-Term Warfarin | 17 children in case control study were entered. | Bone density of the lumbar spine, incorporating L1- L4, on patients was assessed using Hologic QDR 4500 Elite densitometer (Hologic, Bedford, MA). The coefficient of variation using a spine phantom was 0.39%. Height was measured without shoes to the nearest 1.0 cm using a standard Harpenden wall-mounted stadiometer. Weight was measured with light indoor clothing, without shoes to the nearest 1.0 kg using standard electronic scales. Body mass index (BMI) was calculated using the standard formula. To facilitate interpretation of patient anthropometric data. |
marked reduction in bone mineral apparent density of lumbar spine between patients and controls. The lumbar spine areal bone mineral density Z-score of patients was reduced compared with controls [patients, –1.96 (95% CI, –2.52 to –1.40). This difference persisted after adjustment for age and body size. The etiology for the reduced bone density is likely to be multifactorial, however, screening of children on long-term warfarin for reduced bone density should be considered. The use of warfarin in children is rising and is directly related to the increasing incidence of thromboembolic disease in children. |
| Andersen et al.,[15] 2008 | Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomized double-blinded placebo-controlled intervention study. | This 1-year-long randomized double-blinded placebo-controlled intervention with vitamin D3 (10 and 20 µg/d) included girls (10.1-14.7 years), women (18.1-52.7 years) and men (17.9-63.5 years) of Pakistani origin living in Denmark | The main endpoints were serum 25-hydroxyvitamin D (S-25[OH] D), parathyroid hormone, bone turnover markers and bone mass. | Supplementation with 10 and 20 µg vitamin D3 per d increased S-25(OH) D concentrations similarly in vitamin D-deficient women (4-fold), and that 10 µg increased S-25(OH) D concentrations 2-fold and 20 µg 3-fold in men. S-25(OH) D concentrations increased at 6 months and were stable thereafter. Baseline S-25(OH) D concentrations tended to be lower in girls and women than in men. |
| Takuo Kubota et al.,[16] 2018 | Incidence rate and characteristics of symptomatic vitamin D deficiency in children: a nationwide survey in Japan | A questionnaire inquiring the number of new patients with vitamin D deficiency rickets and/or hypocalcemia for 3 years was sent to 855 randomly selected hospitals with a pediatrics department in Japan. In this survey, we found that 250 children were diagnosed with symptomatic vitamin D deficiency. | According to the Nationwide Epidemiologic Survey Manual issued by The Epidemiological Study Group of Specified Rare and Intractable Diseases, a questionnaire enquiring the number of new patients with vitamin D deficiency rickets and/or hypocalcemia visiting the target hospitals between April 1, 2013 and March 31, 2016 was sent to 855 hospitals (32%) with a pediatrics department, which were randomly selected from all hospitals (2,677) in Japan. | The estimated number of patients with symptomatic vitamin D deficiency per year was 183. The overall annual incidence rate among children under 15 years of age was 1.1 per 100,000 population. The second survey has provided detailed information on 89 patients with symptomatic vitamin D deficiency under 5 years of age in hospitals in the current research group. The nationwide estimated the overall annual incidence rate of symptomatic vitamin D deficiency in children under 5 years of age to be 3.5 per 100,000 population. The second survey revealed 83% had bowed legs, 88% had exclusive breastfeeding, 49% had a restricted and/or unbalanced diet, and 31% had insufficient sun exposure among the 89 patients. This is the first nationwide survey on definitive clinical vitamin D deficiency in children in Japan. Elucidating the frequency and characteristics of symptomatic vitamin D deficiency among children is useful to develop preventative public health strategies. |
| Du et al.,[21] 2004 | School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10-12 years in Beijing | A 2-year milk intervention trial was carried out with 757 girls, aged 10 years, from nine primary schools in Beijing (April 1999 to March 2001). | Schools were randomized into three groups: group 1, carton of 330 mL milk; group 2, quantity of milk additionally fortified with 5 or 8 µg cholecalciferol; group 3, control girls. Anthropometric and bone mineralization measurements, as well as dietary, health and physical-activity data, were collected at baseline and after 12 and 24 months of the trial. | Those subjects receiving additional cholecalciferol compared with those receiving the milk without added 25-hydoxycholecalciferol had significantly greater increases in the change in (size-adjusted) total-body bone mineral content (2.4% vs. 1.2%) and bone mineral density (5.5% vs. 3.2%). The milk fortified with cholecalciferol significantly improved vitamin D status at the end of the trial compared with the milk alone or control groups. It is concluded that an increase in milk consumption, e.g., by means of school milk programs, would improve bone growth during adolescence, particularly when Ca intake and vitamin D status are low. |
| El-Hajj et al.,[22] 2006 | Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab | One hundred seventy-nine girls, ages 10-17 year, were randomly assigned to receive weekly oral vitamin D doses of 1,400 IU (equivalent to 200 IU/d) or 14,000 IU (equivalent to 2,000 IU/d) in a double-blind, placebo-controlled, 1-year protocol. | Areal bone mineral density (BMD) and bone mineral content (BMC) at the lumbar spine, hip, forearm, total body, and body composition were measured at baseline and 1 year. Serum calcium, phosphorus, alkaline phosphatase, and vitamin D metabolites were measured during the study. | In the overall group of girls, lean mass increased significantly in both treatment groups (P<or=0.05); bone area and total hip BMC increased in the high-dose group (P<0.02). In premenarcheal girls, lean mass increased significantly in both treatment groups, and there were consistent trends for increments in BMD and/or BMC at several skeletal sites, reaching significance at lumbar spine BMD in the low-dose group and at the trochanter BMC in both treatment group |
| Ong YL et al.,[23] 2016 | The association of maternal vitamin D status with infant birth outcomes, postnatal growth and adiposity in the first 2 years of life in a multiethnic Asian population: The Growing Up in Singapore Toward healthy Outcomes cohort study | In a mother-offspring cohort in Singapore, maternal plasma vitamin D of 991 women were measured between 26 and 28 weeks of gestation, and anthropometric measurements were obtained from singleton offspring during the first 2 years of life with 3-month follow-up intervals to examine birth, growth and adiposity outcomes. | Associations were analyzed using multivariable linear regression. | did not find an association between maternal vitamin D deficiency and infant birth outcomes or postnatal growth and adiposity measures in our cohort. We think that this can be explained by the low prevalence of severe vitamin D deficiency within this population. Future studies using standardized and established cut-offs defining vitamin D deficiency will enable findings across studies to be more comparable. |
| Viljakainen et al.,[24] 2006 | A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention | Altogether, 228 girls (mean age, 11.4±0.4 years) participated. | BMC was measured by DXA from the femur and lumbar spine. Serum 25-hydroxyvitamin D [S-25(OH) D], intact PTH (S-iPTH), osteocalcin (S-OC), and urinary pyridinoline (U-Pyr) and deoxypyridinoline (U-Dpyr) were measured. | A dose-response effect was observed in the vertebrae (ANCOVA, P=0.039), although only with the highest dose. The mean concentration of S-25(OH) D increased (P<0.001) in the 5-µg group by 5.7±15.7 nM and in the 10-µg group by 12.4±13.7 nM, whereas it decreased by 6.7±11.3 nM in the placebo group. Supplementation had no effect on S-iPTH or S-OC, but it decreased U-DPyr (P=0.042). |
| Yali Ren et al.,[25] 2019 | Determinants for low bone mineral density in preschool children: a matched case-control study in Wuhan, China | Between November 2014 and April 2015, a matched case-control study was performed to detect information on growth and development condition and consumption frequency of products of cases with low BMD and controls with normal BMD. | Anthropometric data measurement and blood tests were conducted. Besides, the questionnaires concerning the mentioned information were completed to get relevant determinants. | The results indicated that if children had larger chest circumference (odds ratio [OR]= 0.763), longer duration of breastfeeding (OR=0.899), and lower frequency of eating snacks (OR=0.439), the risk of low BMD would decrease. |
| In total, 88 (28 boys, 60 girls) incident cases (4.15±0.78 years) of low BMD and 88 sex- and age-matched controls of normal BMD were enrolled. | A paired t-test, the McNemar test and univariate and multiple conditional logistic regression models were used to explore the association between these factors and low BMD. | Our findings suggest that preschool children with an association of larger chest circumference, longer duration of breastfeeding, and lower frequency of eating snacks could have lower risk for low BMD. Intended measures to strengthen those protective factors could be effective in reducing the cases of low BMD. | ||
| Audry H Garcia et al.,[26] 2017 | 25-hydroxy vitamin D concentrations during fetal life and bone health in children aged 6 years: a population-based prospective cohort study | In a prospective multiethnic population-based cohort study, embedded within the Generation R 9901 mother-and-child pairs were enrolled and obtained both midpregnancy maternal 25(OH) D concentrations and offspring DXA scans at age 6 years in 4,815 pairs between April 1, 2002, and Jan 1, 2006, for participation in the study. | Maternal D concentrations during fetal D concentrations at birth were measured. Total body bone mineral density, bone mineral content (BMC), area-adjusted BMC, and bone area using DXA in offspring at 6 years of age were measured. associations using multivariable linear regression models, adjusted for lifestyle variables, and for child’s height were examined. | There are inverse associations between 25(OH) D concentrations during fetal life with BMC and bone area in childhood, but these associations were no longer significant after adjustment for childhood 25(OH) D status. Data suggest that 25(OH) D concentrations during childhood might be more relevant for bone outcomes than 25(OH) D concentrations during fetal life. |
In total, out of 2,683 children in the age range of 2 to15 years that were studied in these 13 articles, 1,213 children had bone density problems. Also, 1,109 children showed a correlation between serum vitamin D levels and the incidence of bone density problems. Regarding the diet with vitamin D, in 532 children a significant relationship was observed between the effects of vitamin D supplementation and bone mineral density. In 367 children, a significant relationship was found between vitamin D level and bone mineral density, and in 236 children, a significant relationship was found between vitamin D and lumbar spine bone mineral density. In 265 children, the relationship between the effects of vitamin D on femoral bone mineral density was significant. In 985 children, serum vitamin D levels were found to be associated with children’s age, but no association was found between children’s age and bone density. Lack of sun exposure was observed in 438 children. On the other hand, the results showed that in 272 children, vitamin D levels were not associated with the risk of bone density problems.
Discussion
In our systematic review, it was found that providing adequate nutrition with vitamin D has a beneficial effect on reducing the complications associated with vitamin D deficiency, including the low bone mineral density of the hip, spine, and arm bones. The effect of supplementation on the arm bone was more than other points, which was also seen in similar clinical studies.[21,22,24]
Another important issue that was addressed in this study, was paying attention to vitamin D levels in children before taking a vitamin D supplement. Many healthy children are deficient in vitamin D; a parallel systematic review has shown that targeting children and adolescents with vitamin D deficiency could lead to clinically significant improvements in their bone density, however, taking vitamin D supplements to improve bone density in healthy children and adolescents with normal vitamin levels is generally not recommended.[27]
Examination of vitamin D levels in children has shown the role of this vitamin in the better development of children. This vitamin affects many important hormones and proteins in the body of children. Also, the amount of free vitamin D is affected by various factors such as different seasons, so that in children over 6 years of age the amount of vitamin D was higher in spring and summer compared with autumn and winter.[28]
In two of the studies, the role of the sun in increasing vitamin D in children was mentioned. A number of risk factors for VDD have been identified, many of which are related to reduced UVB exposure and limited skin production of cholecalciferol. In high-latitude countries, seasonal variation is so influential in serum 25(OH) D status of individuals that it reduces to minimum levels in late winter and maximizes in midsummer.[29,30,31] In addition, the effect of latitude on 25(OH) D status is clearly observable in the United Kingdom, since the children and postmenopausal women living in the north of England and Scotland have lower levels of 25(OH) D, in comparison with those living in the south of England.[32]
A study conducted in Japan showed that in the first survey, the number of children with vitamin D deficiency was 183 per year. The overall annual incidence of vitamin D deficiency in children under the age of 15 was estimated at 1.1 per 100,000. In the second survey of 89 patients with symptomatic vitamin D deficiency under the age of 5, the overall annual incidence of symptomatic vitamin D deficiency in children under the age of 5 was estimated at 3.5 per 100,000. Regarding the symptoms and causes of deficiency in these children, 83% had bent legs, 88% exclusive breastfeeding, 49% limited or unbalanced diet, and 31% insufficient sun exposure. The researchers found that elucidating the frequency and characteristics of vitamin D deficiency in children is useful for developing prevention strategies and increasing children’s general health.[16]
Intestinal health in children plays an important role in the absorption of vitamin D. In one of the studies in children with chronic intestinal diseases, it was found that children with intestinal diseases showed lower levels of vitamin D in their blood serum than healthy children, and their bone mineral density was significantly reduced. Also, unhealthy children had complications in the L2-L4 vertebrae of the lumbar spine compared with healthy children and showed a lower frequency of physical activity.[18,33]
In another study, one of the influential factors determining body composition and bone health in children was the children’s body mass index. Vitamin D normal levels were higher in children with appropriate body mass index and the apparent density of the lumbar spine bone mineral was significantly different in the children studied.[14] A study also showed that the use of warfarin in children reduces bone density and affects vitamin D levels. Thus, this rate is in the desired range in healthy children. Vitamin deficiency in children taking warfarin has affected bone health, especially in their spine.[20,34,35,36]
One of the points mentioned in one of the studies is the level of vitamin D in newborns and its effect on the growth of children in childhood.[37] Studies by Sharma et al.[38] have shown that newborns with vitamin D deficiency were smaller and had a calcium deficiency. They stated that vitamin D deficiency would certainly affect growth disorders in the future.[39] The results showed that there was an inverse relationship between vitamin D concentration during fetal life and BMC and bone growth in childhood, but these correlations were no longer significant after adjusting vitamin D levels in childhood. The data also showed that regulating the concentration of vitamin D in childhood could have a positive effect on bone complications during the life of children and that vitamin D supply in childhood is very important.[26] Thus, another study found that children with larger chest circumference, longer duration of breastfeeding during infancy, and lower frequency of eating fast foods and snacks, have a lower risk of bone density problems in adolescence.[25] Paying attention to vitamin D levels from the prenatal period to adolescence is very important.[5,6,25,40] Measures to boost vitamin D and protective factors could be effective in reducing cases of low BMD in children.[41]
The present systematic review demonstrates that one of the potential strategies to reduce bone problems in children is to improve vitamin D levels in them. Consumption of foods containing vitamin D and exposure to sunlight are effective in improving vitamin D levels in healthy children and promote better bone growth in them.
Conclusion
In general, the present systematic review properly shows the role of vitamin D on bone density in children, so that in many cases, complications related to low bone mineral density were observed. The study of the frequency and characteristics of vitamin D deficiency in children is useful for the development of prevention strategies and increasing the general health of children. The role of the sun is important in increasing vitamin D levels in children, also, providing proper nutrition containing vitamin D has a beneficial effect in reducing the complications associated with vitamin D deficiency. Intestinal health and body mass index in children play an important role in the absorption of vitamin D. The use of warfarin in children reduces bone density and the consumption of this medication is not recommended. Vitamin D concentrations throughout fetal life are associated with BMC and bone growth in childhood, and adequate levels of vitamin D in childhood could have a positive effect on bone problems during childhood and adolescence. Finally, conduction of more clinical and longitudinal studies is suggested, to further understand the role of vitamin D in bone health in children and to accurately measure the best concentration of vitamin D for bone growth and density in healthy children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Moon RJ, Davies JH, Cooper C, Harvey NC. Vitamin D, and maternal and child health. Calcif Tissue Int. 2020;106:30–46. doi: 10.1007/s00223-019-00560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006944.pub2. Cd006944. doi:10.1002/14651858.CD006944.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Fahimi D, Delavar MA, Karahoudi M, Honarmand M, Eghbalkhah A. Comparison of two intravenous fluid maintenance therapy with different sodium concentrations in hospitalized children:A randomized trial study. J Pediatr Nephrol. 2014;2:110–5. [Google Scholar]
- 4.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, et al. 25(OH) D2 half-life is shorter than 25(OH) D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–81. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96:E436–46. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atapattu N, Shaw N, Högler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res. 2013;74:552–6. doi: 10.1038/pr.2013.139. [DOI] [PubMed] [Google Scholar]
- 7.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy:double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133:e143–53. doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- 9.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 10.Rockell JE, Skeaff CM, Williams SM, Green TJ. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int. 2006;17:1382–9. doi: 10.1007/s00198-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 11.Ma D, Jones G. The association between bone mineral density, metacarpal morphometry, and upper limb fractures in children:A population-based case-control study. J Clin Endocrinol Metab. 2003;88:1486–91. doi: 10.1210/jc.2002-021682. [DOI] [PubMed] [Google Scholar]
- 12.Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand:A consensus statement. Med J Aust. 2006;185:268–72. doi: 10.5694/j.1326-5377.2006.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, Lyytikäinen A, Kröger H, Lamberg-Allardt C, Alén M, Koistinen A, et al. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10-12-y-old girls:A 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–26. doi: 10.1093/ajcn/82.5.1115. [DOI] [PubMed] [Google Scholar]
- 14.White Z, White S, Dalvie T, Kruger MC, Van Zyl A, Becker P. Bone Health, body composition, and vitamin D status of black preadolescent children in South Africa. Nutrients. 2019;11:1243. doi: 10.3390/nu11061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen R, Mølgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J, et al. Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark:A randomised double-blinded placebo-controlled intervention study. Br J Nutr. 2008;100:197–207. doi: 10.1017/S000711450789430X. [DOI] [PubMed] [Google Scholar]
- 16.Kubota T, Nakayama H, Kitaoka T, Nakamura Y, Fukumoto S, Fujiwara I, et al. Incidence rate and characteristics of symptomatic vitamin D deficiency in children:a nationwide survey in Japan. Endocr J. 2018;65:593–9. doi: 10.1507/endocrj.EJ18-0008. [DOI] [PubMed] [Google Scholar]
- 17.Karimian P, Yaghini O, Nasr Azadani H, Mohammadizadeh M, Arabzadeh SAM, Adibi A, et al. Prevalence, characteristics, and one-year follow-up of congenital cytomegalovirus infection in Isfahan City, Iran. Interdiscip Perspect Infect Dis. 2016;2016:7812106. doi: 10.1155/2016/7812106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvammen JA, Thomassen RA, Kjeserud CN, Sæland C, Godang K, Bollerslev J, et al. Bone mineral density and vitamin D in paediatric intestinal failure patients receiving home parenteral nutrition. Clin Nutr ESPEN. 2020;39:234–41. doi: 10.1016/j.clnesp.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Mager DR, Qiao J, Turner J. Vitamin D and K status influences bone mineral density and bone accrual in children and adolescents with celiac disease. Eur J Clin Nutr. 2012;66:488–95. doi: 10.1038/ejcn.2011.176. [DOI] [PubMed] [Google Scholar]
- 20.Barnes C, Newall F, Ignjatovic V, Wong P, Cameron F, Jones G, et al. Reduced bone density in children on long-term warfarin. Pediatr Res. 2005;57:578–81. doi: 10.1203/01.PDR.0000155943.07244.04. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, et al. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10-12 years in Beijing. Br J Nutr. 2004;92:159–68. doi: 10.1079/BJN20041118. [DOI] [PubMed] [Google Scholar]
- 22.El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children:A randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 23.Ong YL, Quah PL, Tint MT, Aris IM, Chen LW, van Dam RM, et al. The association of maternal vitamin D status with infant birth outcomes, postnatal growth and adiposity in the first 2 years of life in a multi-ethnic Asian population:The Growing Up in Singapore towards healthy outcomes (GUSTO) cohort study. Br J Nutr. 2016;116:621–31. doi: 10.1017/S0007114516000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, et al. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls:A double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res. 2006;21:836–44. doi: 10.1359/jbmr.060302. [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Xi X, Hu D, Shang W, Peng S, Fan L, et al. Determinants for low bone mineral density in pre-school children:A matched case-control study in Wuhan, China. J Pediatr Endocrinol Metab. 2019;32:739–48. doi: 10.1515/jpem-2018-0554. [DOI] [PubMed] [Google Scholar]
- 26.Garcia AH, Erler NS, Jaddoe VWV, Tiemeier H, van den Hooven EH, Franco OH, et al. 25-hydroxyvitamin D concentrations during fetal life and bone health in children aged 6 years:A population-based prospective cohort study. Lancet Diabetes Endocrinol. 2017;5:367–76. doi: 10.1016/S2213-8587(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 27.Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children:Systematic review and meta-analysis. BMJ. 2011;342:c7254. doi: 10.1136/bmj.c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantecón L, Alonso MA, Moya V, Andrés AG, Avello N, Martínez-Morillo E, et al. Marker of vitamin D status in healthy children:Free or total 25-hydroxyvitamin D? PLoS One. 2018;13:e0202237. doi: 10.1371/journal.pone.0202237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolppanen AM, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-hydroxyvitamin D₃ and D₂ concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab. 2012;97:1202–10. doi: 10.1210/jc.2011-2516. [DOI] [PubMed] [Google Scholar]
- 30.Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HM, et al. Tracking of 25-hydroxyvitamin D status during pregnancy:The importance of vitamin D supplementation. Am J Clin Nutr. 2015;102:1081–7. doi: 10.3945/ajcn.115.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimi H, Karimian P. RT-PCR urine pool test, the probable method for congenital cyto-megalovirus infection diagnosis and screening in the near future;An observational study and literature review. J Crit Rev. 2020;7:284–90. [Google Scholar]
- 32.Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N. Prevalence and predictors of vitamin D insufficiency in children:A Great Britain population based study. PLoS One. 2011;6:e22179. doi: 10.1371/journal.pone.0022179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab:A meta-analysis. The lancet oncology. 2009;10:559–68. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimi HK, Sohrabi S, Ashtiyani FZ, Hafize F, Esmaeilian S, Jafarnejad S. Effect of simulation-based CPR education on the knowledge and performance of neonatal intensive care unit nurses. J Crit Rev. 2020;7:1135–40. [Google Scholar]
- 35.Ebrahimi HK, Jafarnejad S, Sohrabi S, Abbasi A, Esmaeilian S. Effect of simulation-based suction education on the knowledge and performance of pediatric intensive care unit nurses. J Crit Rev. 2020;7:685–94. [Google Scholar]
- 36.Delavar MA, Soheilirad Z. Drug and herbal medicine-induced nephrotoxicity in children;Review of the mechanisms. J Renal Inj Prev. 2020;9:e21. [Google Scholar]
- 37.Jafarnejad S, Ebrahimi HK. Clinical guidelines on pediatric asthma exacerbation in emergency department, A narrative review. Eur J Transl Myol. 2020;30:8682. doi: 10.4081/ejtm.2019.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma S, Khan N, Khadri A, Julies P, Gnanasambandam S, Saroey S, et al. Vitamin D in pregnancy-time for action:A paediatric audit. BJOG. 2009;116:1678–82. doi: 10.1111/j.1471-0528.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahimi HK, Jafarnejad S, Esmaeilian S, Amirmohamadi M, Sohrabi S. Examining the effect of massage on preterm infants'pain caused by invasive procedures in neonatal intensive care unit. J Complement Med Res. 2020;11:99–105. [Google Scholar]
- 40.Zandieh F, Mirsaed GB, Izadi A, Gharegozlu M, Aghajani M, Sheikh M. Papillon Lefevre syndrome and footprints of mycobacterium tuberculosis Iran. J Allergy Asthma Immunol. 2014;13:286–9. [PubMed] [Google Scholar]
- 41.Shoar S, Naderan M, Aghajani M, Sahimi-Izadian E, Hosseini-Araghi N, Khorgami Z. Prevalence and determinants of depression and anxiety symptoms in surgical patients. Oman Med J. 2016;31:176–81. doi: 10.5001/omj.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


