Abstract
Objectives:
Dietary factors are important in the prevention and treatment of prediabetes and diabetes mellitus (DM). This study was designed to evaluate the prevalence, associated risk factors, dietary modification, and calories consumption calculated by the food frequency questionnaire and correlate them with the metabolic parameters, namely blood glucose, HbA1c, lipid profile, and cardiovascular parameters as heart rate variability and carotid intima media thickness (CIMT) among the prediabetics.
Methods:
An experimental interventional study was carried out in the Department of Physiology and Medicine at the RUHS College of Medical Sciences and Associated Group of Hospitals. The assessments were done at baseline and after 6 months of post-dietary modification. The total duration of the study was 6 months. A total of 250 prediabetic subjects were enrolled. Study Group A (n = 125) was engaged in dietary modification, whereas Group B (n = 125) was considered as control. The dietary assessment was done by a food frequency questionnaire.
Result:
After dietary modification, a decrease in the body mass index (1.3%), systolic blood pressure (3.1%), diastolic blood pressure (3.1%), blood glucose (2.8%), triglyceride (2.8%), high density lipoprotein (0.9%), HbA1c (2%), cholesterol (1.4%), and low-frequency/high-frequency ratios (1%), carotid intima media thickness (1.6%), as compared to control, was noticed after 6 months of dietary modification.
Conclusion:
This study suggested that prediabetics required health education including nutritional education as diet modification can play an important role to encourage diabetes-onset prevention and its related complications. The health-care providers and workers should increase the awareness about the importance of diet and encourage the prediabetics toward a healthy lifestyle, which may help in the quality of life and appropriate self-care, primary prevention of diabetes and its complications.
CTRI Registration:
CTRI/2017/06/008825.
Keywords: Biochemical parameters, cardiovascular, diet, prediabetes
Introduction
Diabetes mellitus (DM) is a major global health problem. It is primarily attributed to the consequence of a lifestyle change such as unhealthy diet, lack of exercise, overweight, and obesity.[1]
Type 2 diabetes is a characteristic feature of metabolic syndrome, which is represented by insulin resistance, obesity, and a range of cardiovascular risk factors.[2] The prime important risk factors attributed to disability-adjusted life in years (DALYS) in 2016 were increased blood glucose levels, unhealthy diet, obesity, and increased blood pressure.[3] Increased awareness about prediabetes, diabetes, and subsequent dietary intervention and improvement in the attitude and practices lead to better control of the disease.[4]
Prediabetes is demarcated as an intervening stage amongst normal glucose tolerance and type 2 diabetes mellitus. As reported by the American Diabetes Association (ADA), the prediabetes diagnostic criteria are increased fasting blood glucose level (100–125 mg/dL) and an increased level of glucose in the blood subsequent to an oral glucose tolerance test (140–199 mg/dL), 5.7–6.4% value of HbA1c.[5]
The diagnosis of prediabetes is based on the glycaemic parameters above normal but below diabetes thresholds and linked with increased levels of insulin.[6] This hyperinsulinemia might be a result of the compensatory mechanisms by the beta cells for the reduced insulin sensitivity to the tissues and the condition is commonly stated as insulin resistance in prediabetes.[7]
In most cases, prediabetes can be prevented at an early stage with physical activity, dietary modification, and weight loss so that insulin intervention is not required.[8]
The ADA guidelines suggest that the prediabetes subjects should make lifestyle modifications to prevent the onset of diabetes, with a dietary change and increased exercise levels and weight management.[9] Knowler et al.[10] observed that 37% of the prediabetics progress to diabetics within 4 years without lifestyle modification. When prediabetics finish a lifestyle modification programme, the risk of diabetes growing over 4 years is reduced to about 20%.
Dietary interventions assessing the power to increased weight reduction and improvement in the glycaemic control in prediabetics are scarce because the main focus has been on caloric restriction. There is a niche to be filled in understanding the advantages of modifying macronutrients and micronutrients to influence the control of glycaemic parameters with or without weight loss in prediabetics. For instance, previous studies have suggested that reducing caloric intake can cause an improvement in control of glycaemic parameters despite no or modest weight loss.
Raghuram et al.[11] reported in a study that lifestyle modification, through diet and yoga, is a powerful strategy for preventing the progression of prediabetes to diabetes and its complications.
The importance of dietary intervention in the prevention and treatment of prediabetes and type 2 diabetes is well known. Previous studies have reported the effect of dietary modifications on primary outcomes such as insulin–glucose homeostasis, lipids profile namely triglyceride, cholesterol, high-density lipoprotein vascular functions to blood pressure, coagulation profile, systemic inflammation, and endothelial function, and secondary outcomes such as cardiovascular complications and coronary heart disease. Therefore, the quality and quantity of diet over the longer term are relevant to the management and prevention of prediabetes, diabetes, and its complications through a wide range of physiological and metabolic processes and help the primary care providers and physicians for the management of prediabetes and diabetes. Therefore, this study was designed to evaluate the prevalence and associated risk factors and effect of dietary modifications on metabolic parameters namely blood glucose, HbA1c, lipid profile, and cardiovascular parameters such as heart rate variability and carotid intima media thickness (CIMT) among prediabetics.
Methodology
An experimental interventional study was conducted in the Department of Physiology and Medicine at RUHS College of Medical Sciences and Associated Hospitals, Jaipur, Rajasthan, India.
The prevalence of prediabetes in India is 8%,[7] the estimation of sample size using the relevant sample size formula n = z2pq/d2, n represents sample size, z denotes the statistic corresponding to confidence level, P denotes prevalence, q is (1-p), where P and q were taken as 0.08 and 0.92 to get the sample size with 5% precision and 10% non-response rate. The desired sample size was 125 with a 95% “confidence interval.”
After obtaining Institutional Ethical Committee (EC/P/01/2016) clearance, subjects who fulfilled the inclusion criteria that included the ambulatory participant’s age group between 30 and 50 years who has fasting blood glucose level of “110 to 125 mg/dL” and HbA1c levels according to the ADA criteria “5.7 to 6.4%” and no family history of “coronary heart disease, “cardiovascular disease” were included in the study. A random sample of the population in RUHS College of Medical Sciences and outpatient departments at the institute via hospital-based advertisements, word-of-mouth, face-to-face contact was recruited for screening of fasting blood glucose. After the screening of 2,000 subjects, 250 prediabetics were recruited. Randomization (CTRI Registration: CTRI/2017/06/008825) and allocation were performed using computer-generated random numbers and divided into a dietary intervention group (Group A, n = 125) and a control group (Group B, n = 125) without dietary intervention. After the explanation of the study objectives and the importance of diet and lifestyle modification verbally and in the participation information sheet, subjects who gave “written informed consent” were included in this study. The total duration of the study was 6 months.
The subjects in the dietary modification group participated in a 24-week dietary modification program. Nutritional intervention, including subjective meal plans according to family history of eating patterns and nutritional education, was provided by lectures and handouts to the study population. Subjects were instructed to record their daily food intake in a diary and marked in a checklist. Each subject received a diet plan from a qualified dietician under the dietary guidelines for the prevention of cardiovascular disease in Asian Indians. The dietary modification regimen included 50%–60% complex carbohydrates, total fat intake, should be <30% of calories, saturated fat intake should be <10% of calories, and trans-fatty acids eliminated. Dietary fat should be 10%, 10–15% of monounsaturated and polyunsaturated, 10%–15% of protein, 25–40 g of dietary fiber, and salt intake of less than 5 g. All prediabetics in the study group were encouraged to reduce the intake of chicken, fish, red meat egg, refined grains, and sugar-sweetened drinks for prevention along with the management of prediabetes and diabetes. Compliance was checked by daily message on what’s app group and weekly telephonic conversation with the subjects and family members, subsequent weekly online videos, health education lectures on Google meet during 6 months of the study period.
Anthropometric parameters such as weight, height, body mass index (BMI), waist–hip ratio, and clinical parameters such as blood pressure (BP) pulse, electrocardiogram (ECG) were measured. Central obesity was calculated by BMI, which was calculated using Quetelet’s index (weight in kg/height in m2), stress was measured by Cohen Perceived Stress Scale (PSS).[12]
The FPG, OGTT, and HbA1C were assessed using the following methods: glucose oxidase-peroxidase (GOD), endpoint method,[13] 75 g glucose load,[14] and immunoturbidimetric method.[15] Serum lipid profile triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL)-cholesterol was calculated by enzymatic cholesterol oxidase phenol 4-amino antipyrine peroxidase. (CHOD-PAP),[16] enzymatic glycerol -3 phosphate oxidase and peroxidase method GPO-PAP,[16] HDL-cholesterol using the phosphotungstic acid endpoint method,[17] respectively and low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) were calculated using Friedewald’s formula.[18]
Carotid intima media thickness was measured in the posterior wall of carotid arteries with a B mode Acuson Sequoia ultrasonography device. The scanning duration was 30 min.
Dietary assessment was done using a food frequency questionnaire[19] every month until 6 months. Food Frequency Questionnaire (FFQ) was used to obtain the frequency and portion size of food and beverage consumption. A list of food items was prepared on the basis of food items consumed in India according to the Indian Council of Medical Research (ICMR)’s dietary guidelines for Indians.[20] FFQ contains 92 food items. All 92 food items were categorized under nine groups. To calculate, the nutrient scoring values were computed from the nutrient database provided by the National Institute of Nutrition guidelines, Hyderabad.[21,22]
Statistical Analysis: The collected data were entered in Microsoft Excel 2007 and statistical analysis was done using SSPS version 19.0. Socio-demographic, morbidity profile, and risk factors were observed using descriptive statistics such as mean and standard deviation and frequencies. A table of prediabetic risk factors was prepared. The correlations between dietary modification and dependent variables such as blood glucose, cholesterol, triglycerides, HDL, and CIMT were analyzed by linear regression weights analysis.
Results
All data variables were quantitative. A total of 2,000 study population was screened, out of which 250 were prediabetic and the prevalence rate was 12.5%.
There were 250 subjects in this study in the 30–40 years age group, out of 100 subjects males and females were 35 and 65 re and in the age group 41-50 years out of 150 subjects, male and females were 50 and 100 respectively. The mean age of total subjects was 42.14 ± 4.90 years, male was 42.00 ± 4.95 years, and female was 42.22 ± 4.87 years.
Table 1 was constructed showing ascending order of these percentages, P values, and inference. Results showed that prescription of the characteristics in this study was as mentioned in [Table 1], such as dietary habits, that is, consumption of fish, egg, chicken, and red meat more than once a week, deep-fried snacks, and Fruit lesser than once a time in a week, green leafy vegetable less than three times a day, and physical inactivity was highly significant (P-value <0.0001). The family history of diabetics (P-value = 0.0034), and BMI (P-value = 0.0086) showed a statistically significant association with prediabetes. Prediabetes was more commonly found in tobacco users and this finding was statistically significant (P = 0.013). A P value <0.05 was significant.
Table 1.
Prescription of characteristics of the study group for Prediabetes
| Prescription of Characteristic | Total | Prediabetes | Percentage | P |
|---|---|---|---|---|
| Chicken, fish, and egg, red meat greater than once time in a week | 800 | 40 | 5 | ** |
| Deep fried snacks | 1000 | 72 | 7.2 | ** |
| Tobacco consumption | 500 | 40 | 8.0 | * |
| Fruit less than one time in a week | 1600 | 152 | 9.5 | ** |
| Green leafy vegetable less than three times a day | 1800 | 178 | 9.9 | ** |
| Alcohol use | 540 | 60 | 11.1 | NS |
| Physical inactivity | 1800 | 200 | 11.1 | ** |
| Sweet greater than three time per week, refined grains, and carbohydrates | 720 | 80 | 11.1 | NS |
| BMI greater than 25 | 1600 | 178 | 11.1 | ** |
| Age group between 30 to 50 years | 2000 | 250 | 12.5 | 1.0000 |
| Perceived stress (assessed by Cohen Perceived Stress Scale) | 1600 | 200 | 12.5 | ** |
| Vegetables less than two servings a day | 1600 | 200 | 12.5 | NS |
| Central obesity | 1592 | 202 | 12.7 | ** |
| Bakery items greater once time a week | 400 | 60 | “15.0” | NS |
| Family history of diabetes | 800 | 130 | “16.3” | ** |
| Carbonated drink greater than once a week | 120 | 22 | “18.3” | NS |
P<0.001: highly significant**
Table 2 depicts calories measured by the food frequency questionnaire (FFQ) after dietary modification correlated negatively and significantly with body mass index, systolic blood pressure, diastolic blood pressure, blood glucose, HbA1c, lipid profile, cholesterol, triglyceride, HDL, heart rate variability, and CIMT. These results show that a change in calories intake by dietary modification helps in significantly decreasing BMI, SBP, and DBP, blood glucose, HbA1c, triglycerides, heart rate variability, and carotid intima-media thickness.
Table 2.
Pearson correlation analysis in pre and post in study group (BMI, SBP, DBP, glucose, and HbA1C, TG, cholesterol, HDL, LF/HF ratio, CIMT, FFQ
| Study Pre/FFQ (kcal) | Post/FFQ (k cal) | |||
|---|---|---|---|---|
|
|
|
|||
| Pearson Correlation | Significance (2-tailed) | Pearson Correlation | Significance (2-tailed) | |
| Pre/BMI (Kg/m2) | 0.027 | 0.769 | -0.043 | 0.05 |
| Post/BMI (Kg/m2) | -0.055 | 0.544 | -0.037 | 0.03 |
| Pre/SBP (mm Hg) | -0.005 | 0.955 | 0.014 | 0.876 |
| Post/SBP (mm Hg) | -0.90 | 0.318 | .-.045 | 0.045 |
| Pre/DBP (mm Hg) | -0.028 | 0.760 | 0.020 | 0.489 |
| Post/DBP (mm Hg) | 0.013 | 0.889 | .-.016 | 0.05 |
| pre/glucose | -0.080 | 0.377 | -0.106 | 0.239 |
| post/glucose | 0.032 | 0.725 | -0.028 | 0.02 |
| Pre HbA1C | -0.085 | 0.345 | -0.142 | 0.115 |
| Post HbA1C | 0.093 | 0.301 | -0.086 | 0.05 |
| Pre TG | 0.003 | 0.992 | 0.002 | 0.712 |
| Post TG | 0.046 | 0.608 | .-.097 | 0.01 |
| Pre-cholesterol | -0.133 | 0.141 | -0.058 | 0.518 |
| Post-cholesterol | -0.071 | 0.431 | .-.008 | 0.05 |
| Pre HDL | 0.123 | 0.170 | 0.038 | 0.01 |
| Post HDL | 0.113 | 0.209 | -0.209 | 0.07 |
| Pre LF/HF ratio | -0.112 | 0.214 | -0.013 | 0.882 |
| Post LF/HF ratio | -0.057 | 0.528 | -0.047 | 0.599 |
| Pre CIMT | -0.079 | 0.384 | -0.066 | 0.196 |
| Post CIMT | .-.142 | 0.002 | -0.086 | 0.000 |
Post Dietary Modification Path Analysis of Study Parameters in Study Group:
Figure 1 shows the relationship between differences in calorie intake measured by FFQ and study parameters after dietary modification in prediabetics. This diagram presents multiple regression coefficients between dependent and independent variables.
Figure 1.
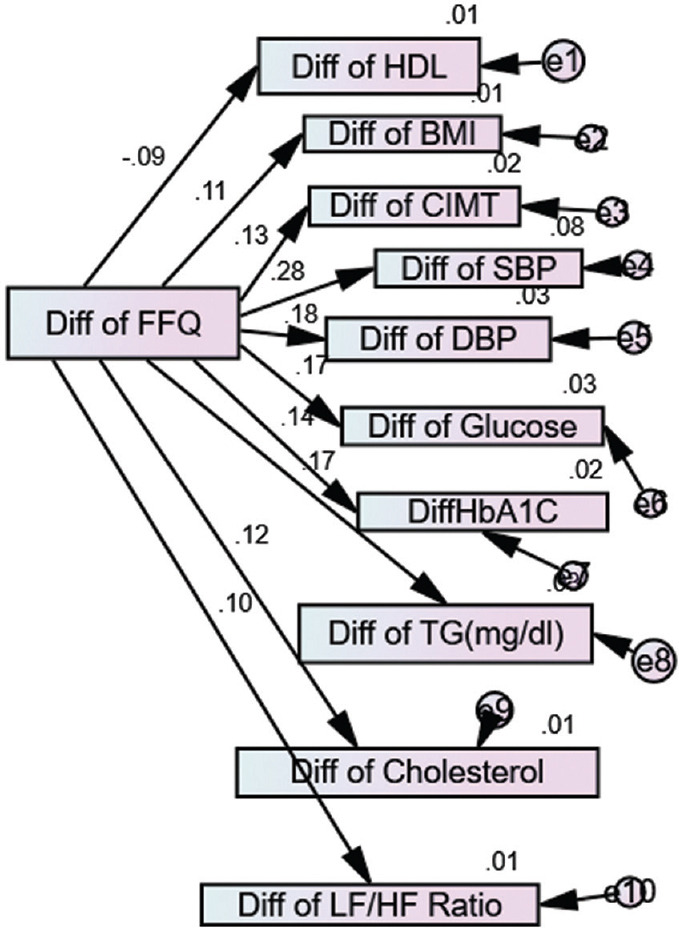
Post-dietary modification path analysis of study parameters in the study group (AMOS software)
Table 3 depicts that linear regression weight analysis for post dietary modification measured by food frequency questionnaire, which is significantly correlated with BMI, systolic and diastolic blood pressure (DBP), blood glucose, HbA1c, triglyceride (TG), CIMT because P value was found to be less than 0.05.
Table 3.
Linear regression weights analysis after post dietary modification in study group
| Parameters | Linear regression | FFQ | Estimate | S.E. | C.R. | P |
|---|---|---|---|---|---|---|
| Diff HDL | <--- | Diff FFQ | -0.001 | 0.001 | -1.327 | 0.185 |
| Diff BMI | <--- | Diff FFQ | 0.005 | 0.003 | 1.606 | 0.001 |
| Diff CIMT | <--- | Diff FFQ | 0.000 | 0.000 | 1.802 | 0.032 |
| Diff SBP | <--- | Diff FFQ | 0.009 | 0.002 | 4.083 | 0.000 |
| Diff DBP | <--- | Diff FFQ | 0.003 | 0.001 | 2.533 | 0.011 |
| Diff Glucose | <--- | Diff FFQ | 0.004 | 0.002 | 2.400 | 0.016 |
| Diff HbA1C | <--- | Diff FFQ | 0.000 | 0.000 | 2.031 | 0.042 |
| Diff TG | <--- | Diff FFQ | 0.005 | 0.002 | 2.411 | 0.016 |
| Diff Cholesterol | <--- | Diff FFQ | 0.003 | 0.002 | 1.688 | 0.091 |
| Diff LFHF ratio | <--- | Diff FFQ | 0.000 | 0.000 | 1.430 | 0.153 |
Table 4 reveals the strength of the relationship between calories consumption after dietary modification, BMI, HDL, CIMT, SBP and DBP, glucose and TG. Positive beta values of BMI, CIMT, SBP and DBP, glucose, HbA1c, TG, and cholesterol, indicated a change in these parameters in identical direction with calories consumption, which was measured by FFQ, and negative beta coefficient value of HDL represented a change in the opposite direction of calories consumption. In a nutshell, if calories consumption decreased, the HDL values increased and BMI, SBP, DBP, glucose, HbA1c, TG, and cholesterol were decreased.
Table 4.
Standardized linear regression weights analysis after post dietary modification in study group: computation of standardized regression weights
| Parameters | Linear regression | FFQ | Beta Value |
|---|---|---|---|
| Diff HDL | <--- | Diff FFQ | -0.094 |
| Diff BMI | <--- | Diff FFQ | 0.113 |
| Diff CIMT | <--- | Diff FFQ | 0.127 |
| Diff SBP | <--- | Diff FFQ | 0.278 |
| Diff DBP | <--- | Diff FFQ | 0.177 |
| Diff Glucose | <--- | Diff FFQ | 0.168 |
| DiffHbA1C | <--- | Diff FFQ | 0.143 |
| Diff TG | <--- | Diff FFQ | 0.168 |
| Diff cholesterol | <--- | Diff FFQ | 0.119 |
| Diff LFHF ratio | <--- | Diff FFQ | 0.101 |
Table 5 depicts if TG is reduced by 1 unit, then its 2.8% variation is explained by dietary modification and the same results were observed for blood glucose in target subjects. If SBP and DBP are reduced by 1 unit, then SBP shows 7.7% variation and DBP shows 3.1% variations were explained by dietary modification in BMI’s variation of 1.3%, HDL (0.9%), CIMT (1.6%), HbA1C (2%), cholesterol (1.4%), and low-frequency/high-frequency (LF/HF) ratios, 1% variation was explained by dietary modification.
Table 5.
Squared multiple correlations in study group after post dietary modification
| Parameters | Beta coefficient |
|---|---|
| Diff LFHF ratio | 0.010 |
| Diff cholesterol | 0.014 |
| Diff TG | 0.028 |
| Diff HbA1C | 0.020 |
| Diff Glucose | 0.028 |
| Diff DBP | 0.031 |
| Diff SBP | 0.077 |
| Diff CIMT | 0.016 |
| Diff BMI | 0.013 |
| Diff HDL | 0.009 |
Discussion
The objectives of the present study were to observe the prevalence and prediabetic risk factors and the effect of dietary modification on metabolic parameters among prediabetes over a period of 6 months. Food intake includes composition, quality, and quantity of food; these factors were strongly linked with weight gain, obesity, insulin resistance, and metabolic syndrome.[23]
Despite large, well-conducted studies in diabetes, nutrition therapy interventions, trials continue to lag far behind. Reports of national data indicate that prediabetic and diabetic populations do not educate themselves on diabetic nutrition.[24] Dietary modifications that include the development of a diet plan designed for the management of weight, blood glucose, HbA1c, lipid profile, and blood pressure were important in the treatment of prediabetes and type 2 diabetes and lower the risk of coronary heart disease, cardiovascular disease, and stroke.[25] Strategies to improve diabetes nutrition therapy education via person-to-person or information technology integrated with medical management, physician, primary and community health workers, and peer coaches to provide ongoing support, culturally appropriate behavior, and clinically linked care are required.[26]
In this study, the consumption of dietary fiber greater than 25–40 g/day significantly improved blood glucose, HbA1c, lipid profile, and physical health status, with positive effects on the gastrointestinal tract that helped in weight reduction and improved carbohydrate and fat metabolism.[27] Meyer et al.,[28] Schulze et al.,[29] and Hu et al.[30] have reported that consumption of a high-fibre diet helps in the control and prevention of prediabetes and diabetes, similar to the present study. The function of dietary fiber is it inhibits the absorption of macronutrients, increases micronutrient absorption, stabilizes blood glucose, has a beneficial effect on weight control, improves insulin resistance and homeostasis of glucose and lipid metabolism, and control various inflammatory markers.[31]
In this study, the consumption of eggs more than once a week showed a significant relationship with an increase in prediabetes. Adamopoulos et al.,[32] Feskens et al.,[33] and Hu et al.[30] reported a positive association between dietary cholesterol and fasting glucose. These findings were consistent with the present study. This can be caused by saturated fats and high cholesterol in the yolk that increased the risk of coronary heart diseases.[28,29,30] On the contrary, Mutungi et al.[34] reported that utilization of three eggs per day, in a carbohydrate-restricted diet, consumption had no effects on fasting blood glucose compared to without egg consumption.
Results of the present study observed that consumption of deep-fried fish greater than a time in a week leads to an increased risk of weight gain, obesity, prediabetes, and diabetes. Kaushik et al.,[35] Djousse et al.,[36] and Wallin et al.[37] reported that shell and fried fish consumption was linked to increased risk of type 2 diabetes, similar to the present study. The reason is that during frying, fat particles are converted to more energy-dense foods, increasing the overall fat intake and reducing omega-3 fatty acids and increased in other fatty acids, inducing the formation of AGEs glycation and mutagenic end products.[38]
Results of the present study observed that the consumption of red meat, chicken, and high fat intake greater than once times/week was significantly associated with prediabetes. Feskens et al.[39] and Pan et al.[40] reported a correlation between fat intake and increased risk of developing type 2 diabetes mellitus; these findings were similar to those of the present study. On the contrary, the study done by Villegas et al.[41] showed no association between the consumption of red meat and the risk of diabetes.
Excess fat is released into the bloodstream and sequestered in the liver and skeletal muscles increasing lipotoxicity that accelerates the progression of impaired glucose tolerance, insulin resistance, and prediabetes.[39-40]
In this study, a significant inverse correlation was found with the consumption of fruit and vegetable and prediabetes, similar to the study done by Carter et al.,[42] Cooper et al.,[43] Hamer et al.,[44] and Kacker et al.[45] Fruits and vegetables are a good source of fibers, flavonoids, antioxidant compounds (vitamins C and E, carotenoid), potassium, and folate, which explained the protective effect on the progression of diabetes.[41,42,43]
Results of the present study reported that after dietary modification, a significant decrease in BMI, SBP, and DBP, and metabolic parameters such as blood glucose, HbA1c, triglycerides, cholesterol, LF/HF ratio, and CIMT.
In this study, we recommended the subjects to:
Maintain a healthy weight, which was the main core of the treatment strategy. Weight loss was associated with improvements in blood glucose levels, SBP and DBP, and lipid profile and prevented cardiovascular complications.[44] Portion control of food was also recommended to subjects for limiting calories consumption.
An ideal recommendation was 55–60% of energy from carbohydrates. Carbohydrate intake should be focused on complex nutrient-dense carbohydrate sources, high in fibers, such as “unpolished cereals,” “millets,” peas, beans, legumes, barley, oats, and “low glycemic index fruits” as well as dairy products. Prediabetics were advised to avoid sugar-sweetened beverages.
Proteins recommendation was 10–15% of the total energy intake in prediabetes. Proteins from vegetable sources such as soya, pulses grams, as well as milk, curds, lean meats, fish were recommended. The combination of pulses and cereals was (1:4 ratio i.e., Idli, dosa, Missi roti, Khichdi, Dhokla, Khandvi). Decrease the intake of red meats, chicken, fish, and egg, refined grains to control weight, blood glucose to reduce the risk for cardiovascular disease and fatty liver.
Fats should provide 20–30% of total energy intake in prediabetics. Recommendations of saturated fats (SFA) and polyunsaturated fats (PUFA) less than 10% of energy and monounsaturated fatty acids (MUFA) 10–15% energy and trans fats less than 1% energy. Monounsaturated fatty oils such as rice bran, mustard, groundnut, and sesame may benefit from glycemic control and cardiovascular risk factors.
Fibre recommendation was 25–40 g/day. A diet high in leafy vegetables, fruit intake was recommended that included three “servings of vegetables” and two “serving of fruits” a day per individual. Fruits such as guava, pears, papaya, apples, oranges, mosambi can be taken in moderation.
Sodium intake should be less than 5 g/day. All processed and packaged foods such as chutneys, pickles, namkeen, biscuits, and sauces should be avoided.
Limitations
The findings of the present study need to be conducted in a larger sample size that assesses the long-term effect of dietary modification on metabolic profile among prediabetics. Moreover, we recommend a further multicentric study to detect the effect of lifestyle modifications among prediabetics.
Conclusion
Among the prediabetic population, dietary intervention plays an important role in controlling the progression of prediabetes to diabetes and related complications. Prediabetics should, also, know about the disease and the importance of effective dietary intervention to prevent the onset of prediabetes, diabetes, and its complications. The physicians, primary health care providers, and health workers “should encourage” prediabetics to understand the importance of a diet that may help in the primary prevention of “disease management, appropriate self-care, and improvement in quality of life.”
The novelty of this study was to increase awareness about dietary intervention among the prediabetic population because it plays an important role in controlling the progression of prediabetes to diabetes and related complications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Ethics approval
Yes.
Consent to participate
I understand that my identity shall be kept confidential and My involvement in the study is purely voluntary. The procedures regarding confidentiality have been clearly explained to me. (participant language)
Consent for publication
I understand my rights and my responsibilities as a participant in this study. I permit the investigator to utilize the information provided by me and the results obtained from this study for presentation and publication. (participant language).
Authors’ contributions
Sudhanshu Kacker: Designing the study, specifying the research question, editing and checking of the manuscripts.
Neha Saboo: obtaining ethics committee approval, and study participants screening, collecting, analyzing and interpreting data writing, reading, checking and editing in final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
All study participants and staff members of the department of physiology and study participants.
References
- 1.Naeem Z. Burden of Diabetes Mellitus in Saudi Arabia. Int J Health Sci (Qassim) 2015;9:5–6. doi: 10.12816/0024690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci (Qassim) 2007;1:5–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood FM. Prevalence and prevention of lifestyle-related diseases in Saudi Arabia. Int J Health Sci (Qassim) 2018;12:1–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus:A review. Int J Health Sci (Qassim) 2017;11:65–71. [PMC free article] [PubMed] [Google Scholar]
- 5.American diabetes association diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62. doi: 10.2337/dc11-S062. doi:10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephen LA, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation:Beyond insulin and glucagon diabetes. Spectrum. 2004;17:183–90. [Google Scholar]
- 7.Barrett KE, Barman SM, Boitano S, Brook HL, Weitz M, Kearns BP, et al. 23rd ed. New York: McGraw Hill Education; 2016. Ganong's Review of Medical Physiology; pp. 315–25. [Google Scholar]
- 8.Guyton AC, Hall JE. 11th ed. Philadelphia: Elsevier Saunders; 2006. Textbook of Medical Physiology; pp. 961–71. [Google Scholar]
- 9.American diabetes Association. Standards of Medical Care in diabetes-2017 Abridged for primary care providers. Clin Diabetes. 2017;35:5–26. doi: 10.2337/cd16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghuram N, Ram V, Majumdar V, Sk R, Singh A, Patil S, et al. Effectiveness of a Yoga-Based Lifestyle Protocol (YLP) in preventing diabetes in a high-risk Indian cohort:A multicenter cluster-randomized controlled trial (NMB-Trial) Front Endocrinol. 2021;12:664657. doi: 10.3389/fendo.2021.664657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of psychological stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 13.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem. 1969;6:2434. [Google Scholar]
- 14.Eyth E, Basit H, Smith CJ. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Last accessed on 2021 Aug 11]. Glucose Tolerance Test. Available from:https://www.ncbi.nlm.nih.gov/books/NBK532915/ [Google Scholar]
- 15.Metus P, Ruzzante N, Bonvicini P, Meneghetti M, Zaninotto M, Plebani M. Immunoturbidimetric assay of glycated hemoglobin. J Clin Lab Anal. 1999;13:5–8. doi: 10.1002/(SICI)1098-2825(1999)13:1<5::AID-JCLA2>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:4705. [PubMed] [Google Scholar]
- 17.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:207780. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Pope SK, Kritchevsky SB, Morris MC, Block G, Tylavsky FA, Lee JS, et al. Cognitive ability is associated with suspecting errors on food frequency questionnaire. J Nutr Health Aging. 2007;11:55–8. [PubMed] [Google Scholar]
- 20. Available from:https://main.icmr.nic.in/sites/default/files/guidelines/ICMR_GuidelinesType 2diabetes2018_0.pdf .
- 21.Gopalan C, Rama Sastri BV, Balasubramanian SC. National Institute of Nutrition. Hyderabad, India: ICMR; 2012. Nutritive Value of Indian Foods. Available from:https://www.nin.res.in/downloads/DietaryGuidelinesforNINwebsite.pdf . [Google Scholar]
- 22.Pasricha S, Rebello LM. National Institute of Nutrition. Hyderabad, India: ICMR; 2011. Some COMMON INDIAN RECIPES and their Nutritive Value. Available from:https://main.icmr.nic.in/price, publications/1353?title=andfield_select_category_tid=Allandpage=1 78 . [Google Scholar]
- 23.Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, et al. Academy of Nutrition and Dietetics Nutrition practice guideline for type 1 and type 2 diabetes in adults:Systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet. 2017;117:1659–79. doi: 10.1016/j.jand.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn B. Prediabetes in Youth. JAMA. 2020;323:302. doi: 10.1001/jama.2019.21122. [DOI] [PubMed] [Google Scholar]
- 25.Lam K, Lee SJ. Prediabetes—A risk factor twice removed. JAMA Intern Med. 2021;181:520–1. doi: 10.1001/jamainternmed.2020.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marincic PZ, Hardin A, Salazar MV, Scott S, Fan SX, Gaillard PR. Diabetes self-management education and medical nutrition therapy improve patient outcomes:A pilot study documenting the efficacy of registered dietitian nutritionist interventions through retrospective chart review. J Acad Nutr Diet. 2017;117:1254–64. doi: 10.1016/j.jand.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Amin TT, Al-Sultan AI, Ali A. Overweight and obesity and their association with dietary habits, and sociodemographic characteristics among male primary school children in Al-Hassa, Kingdom of Saudi Arabia. Indian J Community Med. 2008;33:172–81. doi: 10.4103/0970-0218.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer KA, Kushi LH, Jacobs DR, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–30. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 29.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle–aged women. Am J Clin Nutr. 2004;80:348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 31.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–89. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamopoulos PN, Papamichael CM, Zampelas A, Moulopoulos SD. Cholesterol and unsaturated fat diets influence lipid and glucose concentrations in rats. Comp Biochem Physiol B Biochem Mol Biol. 1996;13:659–63. doi: 10.1016/0305-0491(95)02078-0. [DOI] [PubMed] [Google Scholar]
- 33.Feskens EJ, Kromhout D. Habitual dietary intake and glucose tolerance in euglycaemic men:The Zutphen Study. Int J Epidemiol. 1990;19:953–9. doi: 10.1093/ije/19.4.953. [DOI] [PubMed] [Google Scholar]
- 34.Mutungi G, Ratliff J, Puglisi M, Torres-Gonzalez M, Vaishnav U, Leite JO, et al. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J Nutr. 2008;138:272–6. doi: 10.1093/jn/138.2.272. [DOI] [PubMed] [Google Scholar]
- 35.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:613–20. doi: 10.3945/ajcn.2008.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djousse L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011;93:143–50. doi: 10.3945/ajcn.110.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallin A, Di Giuseppe D, Orsini N, Åkesson A, Forouhi NG, Wolk A. Fish consumption and frying of fish in relation to type 2 diabetes incidence:A prospective cohort study of Swedish men. Eur J Nutr. 2017;56:843–52. doi: 10.1007/s00394-015-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mark AB, Poulsen MW, Andersen S, Andersen JM, Bak MJ, Ritz C, et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Car. 2014;37:88–95. doi: 10.2337/dc13-0842. [DOI] [PubMed] [Google Scholar]
- 39.Feskens EJ, Sluik D, van Woudenbergh GJ. Meat consumption, diabetes, and its complications. Curr Diab Rep. 2013;13:298–306. doi: 10.1007/s11892-013-0365-0. [DOI] [PubMed] [Google Scholar]
- 40.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, et al. Red meat consumption and risk of type 2 diabetes:3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–96. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villegas R, Shu XO, Gao YT, Yang G, Cai H, Li H, et al. The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci. 2006;3:152–9. doi: 10.7150/ijms.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus:Systematic review and meta-analysis. BMJ. 2010;18:341–c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, et al. Fruit and vegetable intake and type 2 diabetes:EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012;66:1082–92. doi: 10.1038/ejcn.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes:Systematic review and meta-analysis. J Hypertens. 2007;25:2361–9. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- 45.Kacker S, Saboo N, Sharma S, Sorout J. Quasi prospective comparative study on effect of yoga among prediabetics on progression of cardiovascular risk factors. Int J Yoga. 2019;12:114–9. doi: 10.4103/ijoy.IJOY_49_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


