Abstract
Pathogenic variants in the BRCA1 and BRCA2 (BRCA1/2) genes are associated with elevated cancer risks in men and women. Due to a founder effect, Ashkenazi Jewish individuals are at higher risk for carrying three specific BRCA1/2 pathogenic variants. There have been recent calls for population screening in this population because many carriers do not have family histories suggestive of hereditary cancer. One approach could be to integrate optional BRCA1/2 testing into routinely offered reproductive carrier screening for recessive and X-linked disorders. However, the differing goals of these types of testing (i.e., personal health risks versus family planning) raise questions about the implications for patient education and informed consent. To this end, we aimed to determine interest, attitudes, and preferences regarding integrating such testing by electronically surveying 331 Ashkenazi Jewish participants in JScreen — a national, not-for-profit, at-home carrier screening program focused on genetic risks in Jewish communities. We found that while 41% of participants had plans to pursue BRCA1/2 testing, 93% would have opted for such testing if offered as an add-on to reproductive carrier screening. This was particularly true of those with higher perceived cancer risk and more positive attitudes toward genetic testing. With respect to preferences about delivery of this service, more than 85% of participants preferred remote (telephone, print, or web-based) genetic education rather than traditional genetic counseling. These results suggest that offering optional BRCA1/2 testing within the context of reproductive carrier screening might provide opportunities for cancer prevention without overburdening scarce genetic counseling resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-022-00590-3.
Keywords: BRCA1, BRCA2, Population screening, Ashkenazi Jewish, Carrier screening, Hereditary cancer, Telehealth
Pathogenic variants (PVs) in the BRCA1 and BRCA2 (BRCA1/2) genes are associated with highly elevated risks of cancer, including female breast and ovarian cancer, as well as increased risks for prostate, pancreatic, and male breast cancers (Daly et al. 2020). In women with a BRCA1/2 PV, bilateral salpingo-oophorectomy substantially reduces both the risk of, and mortality from, ovarian cancer and may reduce the risk of breast cancer (Choi et al. 2021; Domchek et al. 2010; Finch et al. 2014). In addition, women with a BRCA1/2 PV can opt for bilateral mastectomy to reduce their risk for breast cancer or enhanced surveillance for early detection (Daly et al. 2020).
Due to a founder effect, approximately 1 in 40 Ashkenazi Jewish individuals (DellaPergola 2017) carries one of three specific BRCA1/2 PVs (Struewing et al. 1997). This frequency is significantly higher than the approximate 1 in 400 carrier rate for one of potentially hundreds of BRCA1/2 PVs in an unselected non-Ashkenazi population (Maxwell et al. 2016). In many instances, carriers are identified only after their first cancer diagnosis because their family histories are not suggestive of hereditary cancer, and they do not meet criteria for genetic testing based on current national guidelines (Daly et al. 2020; Domchek and Robson 2019; King et al. 2014; Metcalfe et al. 2010; US Preventive Services Task Force et al. 2019). Indeed, current testing criteria may fail to identify at least 50% of Ashkenazi Jewish BRCA1/2 carriers (Gabai-Kapara et al. 2014; Manchanda et al. 2015). In addition, even in individuals who meet testing criteria, testing rates for cancer patients and cascade testing in families with an identified BRCA1/2 PV are suboptimal (Childers et al. 2017; Kurian and Katz 2020).
In light of these considerations, some scientists have called for BRCA1/2 population screening in the Ashkenazi and general population (i.e., systematic testing irrespective of personal or family history of cancer) (Guan et al. 2021; King et al. 2014; McBride et al. 2019; Metcalfe et al. 2015; Narod 2018). Internationally, several research programs have begun to assess mechanisms and outcomes of BRCA1/2-population testing (Gronwald et al. 2006; Lieberman et al. 2017; Manchanda et al. 2020; Metcalfe et al. 2010). In the USA, little progress has been made in developing a national public health infrastructure to deliver such widespread BRCA1/2 testing.
One potential approach would be to provide the option of BRCA1/2 testing in the context of routinely offered reproductive carrier screening for recessive and X-linked disorders. National guidelines from ACOG (The American College of Obstetricians and Gynecologists) recommend that information about carrier screening be provided preconceptionally (ideally) or to all pregnant women, and that after counseling, patients may choose to accept or decline any or all screening options (ACOG 2017). Next-generation sequencing panels that assess hundreds of genes simultaneously are increasingly used for preconception and prenatal expanded carrier screening in Jewish and non-Jewish individuals (Akler et al. 2020; Kraft et al. 2019). Thus, it would be potentially feasible and cost-effective to include BRCA1/2 testing as part of routine carrier testing, particularly in the Jewish population.
To this end, we aimed to determine interest, attitudes, and preferences regarding BRCA1/2 genetic testing in the context of carrier screening among Ashkenazi Jewish individuals. To address these questions, we retrospectively surveyed men and women who had participated in consumer-driven carrier screening through JScreen. JScreen is a national not-for-profit public health initiative focused on genetic risks in Jewish communities. The program is based out of the Emory University School of Medicine’s Department of Human Genetics (Atlanta, GA). JScreen provides affordable and accessible at-home expanded carrier screening for reproductive planning, along with complimentary genetic counseling for participants (Grinzaid et al. 2015). Since the program’s inception in 2013, over 23,000 individuals have undergone carrier screening. The vast majority of individuals tested through JScreen have Ashkenazi Jewish background and obtain testing in the preconception period (Grinzaid et al. 2015; Hardy et al. 2018). A 2019 pilot study of Ashkenazi Jewish JScreen participants who did not meet National Comprehensive Cancer Network (NCCN) criteria for BRCA1/2 testing documented high interest in such testing, and a higher rate of BRCA1/2 PVs was detected in this group compared to the general population (Rose et al. 2022). The current survey was designed to complement these results and inform the process of broadening access to BRCA1/2 testing in a manner that meets the needs of the community it serves.
Materials and methods
Participants
Study participants were men (n = 132) and women (n = 199) who had participated in the JScreen carrier screening program from late 2013 to early 2019 and consented to be contacted for future research. We queried the JScreen electronic database to identify men and women who met the study eligibility criteria: age 18 or older, Ashkenazi Jewish ancestry (at least one Ashkenazi Jewish grandparent), and not residing within a Georgia zip code (due to a competing local BRCA1/2 testing protocol). Of the 5507 JScreen participants who potentially met these criteria, we randomly selected 802 to receive study invitations across three waves of recruitment.
Procedures
The study was approved by the Emory University Institutional Review Board (IRB #00107272). Potentially eligible JScreen participants were sent an email that described the study and contained a unique, secure REDCap link to an electronic informed consent and survey along with a $10 electronic gift card incentive. Participants who did not respond within a week of the email were sent two additional emails over the following 2 weeks to invite them to complete the one-time survey. Participants who did not respond by the third attempt were considered passive decliners and were not contacted again.
Before initiating the consent and survey, potential participants completed an eligibility screener. After confirming Ashkenazi Jewish ancestry, we excluded participants who had: (a) a personal diagnosis of breast or ovarian cancer, (b) previously had genetic testing for BRCA1 or BRCA2, or (c) prior breast or ovarian cancer risk-reducing surgery.
Mailings were conducted in early 2019. Of the 802 JScreen participants who were sent a study invitation, 45 were ineligible due to lack of Ashkenazi Jewish ancestry or prior BRCA1/2 genetic testing. Of the 757 potentially eligible participants, 348 (45.9%) consented to study participation and 331 (43.7%) completed the survey.
Survey instrument
We developed a two-part, 25-item electronic survey. Part I used previously developed and tested items to assess sociodemographics, cancer and genetic testing family history, and perceived risks for cancer (Interrante et al. 2017). Part II started with a brief (two-paragraph) description of BRCA1/2, associated cancer risks, and genetic testing. This was followed by face-valid items focused on prior awareness of BRCA1/2 genes, knowledge, perceived risk, testing intentions, and attitudes toward BRCA1/2 genetic testing (Graves et al. 2011). These items were developed via literature review and adapted from our prior work. After reaching team consensus, we pilot-tested these items in nine JScreen participants. Based on their feedback, we revised and finalized the items and added the validated 4-item Perceived Stress Scale (Cohen et al. 1983). The final survey (see Appendix) took approximately 10–15 min to complete.
Background and predictor variables
Sociodemographics
We assessed number of biological children, age, marital status, employment, and educational attainment. Self-identified sex was abstracted from the JScreen registry.
Cancer family history
We assessed personal cancer history, history of cancer in maternal and paternal first- and second-degree relatives, and family history of BRCA1/2 testing.
JScreen carrier screening results
Participants reported whether their prior JScreen carrier testing had indicated that they were a carrier for one or more genetic diseases.
Perceived risk
We measured perceived risk for a BRCA1/2 mutation, breast cancer (women), and prostate cancer (men) using a 0 (“definitely do not have altered gene/definitely will not get breast/prostate cancer”) to 100 (“definitely have altered gene/definitely will get breast/prostate cancer”) scale.
BRCA1/2 knowledge and awareness
We created two short paragraphs containing brief background information about BRCA1/2, which were tailored based on whether individuals self-identified as female or male (see Appendix). We then measured participants’ prior awareness of BRCA1/2 with a single item: “Before participating in this survey, how much had you heard about the BRCA1 and BRCA2 genes associated with hereditary breast and ovarian cancer?” Responses were on a four-point scale ranging from “I had never heard of these genes” to “I knew a great deal about these genes.” For analysis, we dichotomized those with low (never heard/knew little about) vs. high (knew fair amount/great deal) awareness. We measured knowledge with 5 true–false items adapted from our prior studies of BRCA1/2 testing. Based on the distribution of responses, we categorized participants as having high (5 items correct, n = 63) vs. low (0–4 items correct; n = 266) knowledge.
Perceived pros and cons (decisional balance) of BRCA1/2 testing
We measured perceived pros of testing with 11-items and perceived cons with 12-items that we adapted from prior studies (Ladd et al. 2020; Schwartz et al. 2001; Sussner et al. 2009). For each pro and con, participants responded whether it was “Not at all Important,” “Somewhat Important,” or “Very Important.” Total score on pros could range from 0 to 22, and total cons score could range from 0 to 24. Both the pros and cons measures were internally consistent (Cronbach’s alpha = 0.88 and 0.88). Consistent with multiple conceptual models of health behavior change (Janis and Mann 1977; Prochaska and Velicer 1997) and prior studies of cancer screening and genetic testing (Cudjoe et al. 2021; Manne et al. 2009; O’Neill et al. 2006; Schwartz et al. 1999; Tercyak et al. 2011), we created a decisional balance score by subtracting the cons score from the pros score (potential range − 24 to 22). Positive decision balance scores indicate more positive attitudes toward genetic testing, and negative scores indicate more negative attitudes toward testing.
Current BRCA1/2 testing intentions
We assessed current intentions for BRCA1/2 testing with the following question: “Mutations in the BRCA1 and BRCA2 genes increase a woman’s chance of developing breast and ovarian cancers.” (Or, for males: “Mutations in the BRCA1 and BRCA2 genes increase a man’s chance of developing prostate, breast and pancreatic cancers.”) Based on what you know now, “which of the following statements describes you best?” Participants responded on a five-point scale from “I definitely will not get tested” to “I definitely will get tested.” Based on the distribution of this measure, we dichotomized this item into those who reported that they probably or definitely would be tested (N = 136) vs. those who reported that they were unsure about testing or who probably or definitely did not plan to be tested (N = 195).
Outcome variables
Interest in BRCA1/2 testing through JScreen
We asked participants: “If you were participating in JScreen today, and had the option to be tested for BRCA1 and BRCA2 mutations as part of your JScreen carrier screening, what do you think you would choose?” As displayed in Table 1, participants responded on a 4-point scale ranging from “I would definitely be tested as part of JScreen” to “I would definitely not be tested as part of JScreen.” Based on the response distribution, we dichotomized this outcome to compare those who reported they would definitely be tested (n = 183) to all other choices (n = 148).
Table 1.
Study outcomes
| Variable | Frequency | % |
|---|---|---|
| Current intentions for BRCA1/2 testing | ||
| Definitely not | 6 | 1.8% |
| Probably not | 75 | 22.7% |
| Unsure | 114 | 34.4% |
| Probably will | 119 | 36.0% |
| Definitely will | 17 | 5.1% |
| Testing if offered through JScreen | ||
| Definitely not | 3 | 0.9% |
| Probably not | 19 | 5.7% |
| Probably would | 126 | 38.1% |
| Definitely would | 183 | 55.3% |
| How much would you be willing to pay for genetic testing | ||
| $0 | 26 | 7.9% |
| < $20 | 59 | 17.8% |
| $20 to $50 | 115 | 34.7% |
| $51 to $150 | 95 | 28.7% |
| $151 to $300 | 24 | 7.3% |
| $301 + | 12 | 3.6% |
| Preferred genetic counseling/education method | ||
| No pre-test education | 25 | 7.6% |
| Print pre-test education only | 55 | 16.6% |
| Web pre-test education only | 89 | 26.9% |
| Individual pre-test telephone genetic counseling | 83 | 25.1% |
| Individual pre-test video genetic counseling | 32 | 9.7% |
| In-person genetic counseling | 5 | 1.5% |
| In-person physician discussion | 42 | 12.7% |
Willingness to pay for BRCA1/2 testing through JScreen
We assessed willingness to pay for BRCA1/2 testing as an added cost with the following item: “How much would you be willing to pay out of pocket for BRCA1/BRCA2 genetic testing?” As displayed in Table 1, response categories were the following: nothing/ < $20/$20–$50/$51–$150/$151–$300/more than $300. Based on the response distribution, we dichotomized the outcome as close to the median as possible to compare those willing to pay < $50 (N = 200) vs. those willing to pay more than $50 (N = 132).
Preferred mode of education/genetic counseling
We asked participants: “If you were participating in JScreen today, and were asked to consider BRCA1 and BRCA2 testing as part of your JScreen carrier screening, which of the following educational options would you be most likely to choose?” As displayed in Table 1, response options were the following: “I would not choose to get information or genetic counseling before making a decision/Review only print educational materials/Review only interactive web-based information/Complete individual genetic counseling by telephone/Complete individual genetic counseling by video call/Complete individual in-person genetic counseling outside of JScreen/Talk to my doctor.” For analysis, we compared those who preferred no formal genetic counseling (i.e., no counseling/print information/web information; n = 169) to those who preferred remote or in-person genetic counseling (n = 163).
Statistical analysis
We first characterized the sample on basic demographics, cancer family history, participant attitudes, beliefs, and knowledge regarding BRCA1/2 testing and perceived risk for breast and prostate cancer. In bivariate analyses, we evaluated associations between these variables and our three outcomes: self-reported interest in BRCA1/2 testing as part of JScreen carrier screening, willingness to pay for such testing, and preferred mode of genetic education/counseling. Finally, to identify independent predictors of our outcomes, we conducted multivariate logistic regressions in which we included all variables with significant bivariate associations with the outcome of interest. Our final sample size of n = 331 provided over 90% power to detect correlations as low as r = 0.20.
Results
Sample characteristics
As displayed in Table 2, 60% of participants identified as female, 65% were married or partnered, 70% were employed full time, and 28% had children. The mean age at the time of the survey was just under 30 years (SD = 5.8). Just over 40% of the participants had a family history of breast, ovarian, or prostate cancer, and 22% had at least one relative who had undergone BRCA1/2 testing. Over 70% of participants had received positive carrier screening results through JScreen, indicating that they carried one or more autosomal recessive or X-linked variants related to genetic disease. In terms of awareness, knowledge, and attitudes toward cancer and genetic testing, 54.4% of participants reported that they had never heard of (15.7%) or knew little about (38.7%) BRCA1/2.
Table 2.
Sample characteristics
| Variable | Frequency | % |
|---|---|---|
| Education | ||
| < College graduate | 38 | 11.5 |
| College graduate + | 293 | 88.5% |
| Marital status | ||
| Married/partnered | 216 | 65.3% |
| Unmarried/widowed | 115 | 34.7% |
| Employment | ||
| Employed full time | 230 | 69.7% |
| < Full time | 100 | 30.3% |
| Sex | ||
| Female | 199 | 60.1% |
| Male | 132 | 30.9% |
| Have children | ||
| Yes | 92 | 28.1% |
| No | 230 | 71.9% |
| Relatives with breast, ovarian or prostate cancer | ||
| 0 | 194 | 58.6% |
| 1 | 92 | 27.8% |
| 2 + | 45 | 13.6% |
| Relatives tested for BRCA1/BRCA2 | ||
| No | 159 | 48.2% |
| Yes | 72 | 21.8% |
| Unsure | 99 | 30.0% |
| JScreen results | ||
| Non-carrier | 92 | 27.9% |
| Carrier | 221 | 66.9% |
| Do not recall | 17 | 5.2% |
| Familiarity with BRCA1 and BRCA2 | ||
| Never heard of them | 52 | 15.7% |
| Did not know much about them | 128 | 38.7% |
| Knew a fair amount about them | 108 | 32.6% |
| Knew a great deal about them | 43 | 13.0% |
| Age (mean, SD) | 29.9 (5.8) | |
| Knowledge (mean, SD) | 4.0 (0.69) | |
| Decision balance (mean, SD) | 8.6 (6.0) | |
| Perceived stress (mean, SD) | 5.8 (2.4) | |
| Perceived risk: breast/prostate cancer (mean, SD) | 32.1 (23.0) | |
| Perceived risk: BRCA1/2 mutation (mean, SD) | 21.7 (19.6) | |
Despite the low self-reported familiarity with BRCA1/2, the mean score on our brief knowledge measure was 4.0 (SD = 0.69) and 19.2% of participants correctly answered all five items. Participants had generally positive attitudes toward BRCA1/2 testing with a mean pros score of 15.0 (SD = 5.1) out of 22, and a mean cons score of 6.4 (SD = 5.4) out of 24, for a mean decisional balance score of 8.6 (SD = 6.0). Perceived risk for carrying a BRCA1/2 mutation was 21.7 (SD = 19.6), and perceived risk for developing breast (women) or prostate (men) cancer was 32.1 (SD = 23.0) on a 0 to 100 scale.
Intentions, willingness to pay, and genetic counseling preferences
Intentions
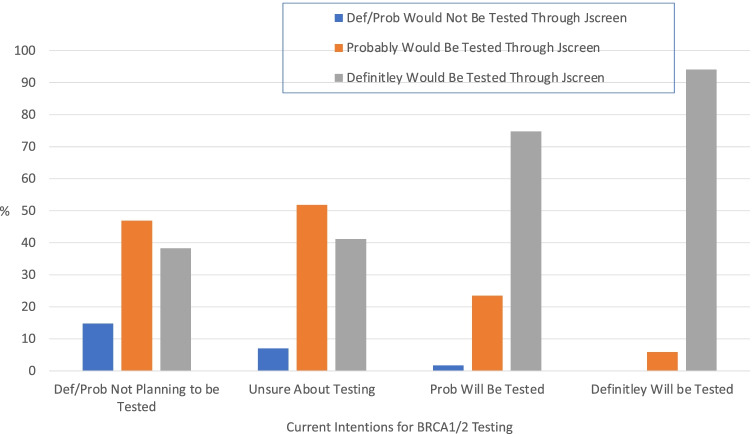
Overall, 93.4% of participants reported that they would have definitely (55.3%) or probably (38.1%) opted to add BRCA1/2 testing to their JScreen carrier testing had it been available. This contrasts with their self-reported current interest in BRCA1/2 testing, in which only 5.1% reported they definitely planned to be tested and 36% reported they would probably be tested. Figure 1 displays participants’ reports of their willingness to have BRCA1/2 testing if integrated into JScreen carrier screening stratified by their current intentions for BRCA1/2 testing. As displayed in Fig. 1, among participants who were not currently planning to pursue BRCA1/2 genetic testing, 85.2% reported that they definitely (38.3%) or probably (46.9%) would have been tested if BRCA1/2 testing were integrated into JScreen carrier screening. Among participants who were currently unsure about whether to pursue BRCA1/2 testing, 93% reported that they would definitely (41.2%) or probably (51.8%) have had BRCA1/2 testing if it was available as an add-on to JScreen carrier screening.
Fig. 1.
Willingness to have add-on BRCA1/2 testing as part of JScreen by current BRCA1/2 testing intentions
As displayed in Table 3, bivariate predictors of JScreen BRCA1/2 testing intentions were as follows: younger age, being married, having children, male sex, more positive decision balance, and higher perceived risks for cancer and for carrying a BRCA1/2 mutation.
Table 3.
Bivariate predictors of study outcomes
| Variable | JScreen BRCA1/2 testing intentions | Willingness to pay | Counseling preference | ||||
|---|---|---|---|---|---|---|---|
| Definitely yes N (%) |
Prob yes/prob no/definitely no N (%) |
< $50 N (%) |
$51 + N (%) |
Web/print/none | Remote | In-person | |
| Education | |||||||
| < College grad | 163 (55.6) | 130 (44.4) | 28 (73.7) | 10 (26.3) | 20 (52.6) | 10 (26.3) | 8 (21.1) |
| College grad + | 20 (52.6) | 18 (47.4) | 172 (58.7) | 121 (41.3) + | 149 (50.9) | 105 (35.8) | 39 (13.3) |
| Marital status | |||||||
| Married/partnered | 128 (59.3) | 88 (40.7)* | 124 (57.4) | 92 (42.6) | 107 (49.5) | 78 (36.1) | 31 (14.4) |
| Unmarried | 55 (47.8) | 60 (52.2) | 76 (66.1) | 39 (33.9) | 62 (53.9) | 37 (32.2) | 16 (13.9) |
| Employment | |||||||
| Full time | 128 (55.7) | 102 (44.3) | 136 (59.1) | 94 (40.9) | 114 (49.6) | 84 (36.5) | 32 (13.9) |
| < Full time | 54 (54.0) | 46 (46.0) | 63 (63) | 37 (37) | 54 (54.0) | 31 (31.0) | 15 (15.0) |
| Sex | |||||||
| Female | 101 (55.2) | 82 (44.8) | 114 (57.3) | 85 (42.7) | 90 (45.2) | 77 (38.7) | 32 (16.1) |
| Male | 98 (66.2) | 50 (33.8)* | 86 (65.2) | 46 (34.8) | 79 (59.9) | 38 (33.0) | 15 (11.4)* |
| Have children | |||||||
| Yes | 58 (63.0) | 34 (37.0) + | 54 (58.7) | 38 (41.3) | 42 (45.7) | 37 (40.2) | 13 (14.1) |
| No | 122 (51.7) | 114 (48.3) | 144 (61.0) | 92 (39.0) | 125 (53.0) | 77 (32.6) | 34 (14.4) |
| Cancer Fam Hx | |||||||
| 0 or 1 | 153 (53.4) | 133 (46.6) | 111 (57.2) | 83 (42.8) | 98 (50.5) | 66 (34.0) | 30 (15.5) |
| 2 + | 30 (66.7) | 15 (33.3) | 89 (65.0) | 48 (35.0) | 71 (51.8) | 49 (35.8) | 17 (12.4) |
| Relatives tested BRCA1/2 | |||||||
| No/unsure | 139 (53.9) | 119 (46.1) | 158 (61.2) | 100 (38.8) | 126 (48.8) | 93 (36.1) | 39 (15.1) |
| Yes | 44 (61.1) | 28 (38.9) | 42 (58.3) | 30 (41.7) | 42 (58.3) | 22 (30.6) | 8 (11.1) |
| JScreen results | |||||||
| Neg/do not recall | 63 (57.8) | 46 (42.2) | 73 (67.0) | 36 (33.0) | 52 (47.7) | 33 (30.3) | 24 (22.0) |
| Carrier | 120 (54.3) | 101 (45.7) | 127 (57.5) | 94 (42.5) + | 117 (52.9) | 81 (36.6) | 23 (10.4)* |
| Familiarity BRCA1/2 | |||||||
| None/not much | 100 (55.6) | 80 (44.4) | 110 (61.1) | 70 (38.9) | 86 (47.8) | 61 (33.9) | 33 (18.3) |
| Fair/great deal | 83 (55.0) | 68 (45.0) | 90 (59.6) | 61 (40.4) | 83 (55.0) | 54 (35.8) | 14 (9.3) |
| BRCA knowledge | |||||||
| Low | 146 (54.9) | 120 (45.1) | 170 (63.9) | 96 (36.1) | 132 (49.6) | 94 (35.3) | 40 (15.0) |
| High | 35 (55.6) | 28 (44.4) | 29 (46.0) | 34 (54.0) | 36 (57.1) | 20 (31.8) | 7 (11.1) |
| Age (mean, SD) | 29.1 (5.6) | 30.6 (5.8)* | 29.3 (5.7) | 30.9 (5.8)** | 29.8 (5.6) | 30.4 (6.1) | 29.2 (5.5) |
| Decisional balance | 10.2 (6.0) | 6.5 (5.5)*** | 7.5 (5.8) | 10.3 (5.9)*** | 9.0 (6.0) | 8.2 (5.7) | 7.9 (6.9) |
| Perceived stress | 5.9 (2.5) | 5.7 (2.4) | 6.0 (2.6) | 5.5 (2.2)* | 5.8 (2.5) | 6.0 (2.4) | 5.6 (2.1) |
| Perceived cancer risk | 34.8 (24.0) | 28.7 (21.2)* | 30.1 (23.1) | 36.1 (22.5) + | 32.3 (21.9) | 33.0 (25.2) | 29.2 (21.2) |
| Perceived BRCA risk | 25.4 (21.7) | 17.0 (15.7)*** | 20.3 (19.9) | 23.7 (19.2) | 20.1 (18.6) | 24.3 (21.0) | 20.9 (19.5) |
+p < .10; *p < .05; **p < .01; ***p < .001
As displayed in Table 4, when we included all variables with significant bivariate associations with JScreen BRCA1/2 testing intentions in a logistic regression model, decisional balance and perceived mutation risk independently predicted testing interest. Participants with more positive attitudes toward testing and those with a higher perceived risk for a BRCA1/2 PV were more likely to report that they would definitely have been tested if it had been offered as part of JScreen. For each half-standard deviation increase in decisional balance and perceived PV risk, the odds of testing increased by 39% and 22%, respectively.
Table 4.
Logistic regression models
| Variable | Odds ratio | 95% confidence interval |
|---|---|---|
| Interest in testing if offered as part of JScreen (definitely test vs. probably/probably not/definitely not) | ||
| Sex | 1.53 | 0.93–2.50 |
| Marital status | 1.34 | 0.75–2.38 |
| Children | 1.13 | 0.60–2.12 |
| Age# | 1.06 | 0.92–1.23 |
| Decision balance# | 1.39*** | 1.22–1.58 |
| Perceived risk breast/prostate cancer# | 1.02 | 0.88–1.18 |
| Perceived risk BRCA1/2 mutation# | 1.22** | 1.05–1.42 |
| Willingness to pay (< $50 vs. $50 +) | ||
| Age+ | 1.19** | 1.04–1.36 |
| Education | 1.26 | 0.54–2.96 |
| Identified as carrier in JScreen | 0.86 | 0.71–1.04 |
| Decision balance# | 1.25*** | 1.10–1.42 |
| Perceived stress# | 0.98 | 0.86–1.10 |
| Perceived risk breast/prostate cancer# | 1.11 | 0.99–1.26 |
| Knowledge | 2.28** | 1.23–4.23 |
#Odds ratio and 95% CI reflect a change of 0.5 SDs on continuous predictors
**p < .01; ***p < .001
Willingness to pay for JScreen BRCA1/2 testing
Despite the high interest in testing through JScreen, 60.2% of participants reported that they would be unwilling to pay more than $50 and only 10.8% reported that they would be willing to pay more than $150.
As displayed in Table 3, bivariate predictors of willingness to pay more than $50 to add BRCA1/2 testing were the following: being a college graduate, being identified as a carrier through JScreen, having more positive attitudes toward genetic testing, lower perceived stress, and higher cancer perceived risk.
Table 4 displays the logistic regression model predicting willingness to pay for BRCA1/2 testing as part of JScreen. Older age, more positive decisional balance and greater knowledge were independently associated with willingness to pay more than $50. Participants who correctly answered all five knowledge items were more than twice as likely to be willing to pay more than $50. Half-standard deviation increases in age and decisional balance were associated with 19% and 25% increased odds of being willing to pay greater than $50.
Genetic counseling preferences
Only 14.5% of participants reported that they preferred in-person genetic counseling (from a genetic counselor or physician) and 34.5% preferred remote counseling delivered by a genetics professional. The majority of participants (51%) preferred either no genetic education (7.6%), education through print materials (16.6%), or education through web-based materials (26.8%). In bivariate analyses (Table 3), men were less likely to favor either remote or in-person genetic counseling, and carriers of one or more recessive/X-linked disease variants were less likely to favor in-person genetic counseling. Since only two variables exhibited significant bivariate associations with genetic counseling preference, we did not conduct a logistic regression on this outcome.
Discussion
The results of this study provide support for making BRCA1/2 testing more accessible to Ashkenazi Jewish individuals who are pursuing reproductive carrier screening through JScreen, most of whom do not meet current NCCN guidelines for cancer genetic testing. While fewer than half of participants reported definite or probable plans to pursue BRCA1/2 gene testing, this percentage more than doubled in a scenario in which BRCA1/2 testing would be offered as an add-on to JScreen’s carrier testing panel. Those who reported that they would definitely be tested if offered as a JScreen add-on had more positive attitudes toward genetic testing and perceived themselves to be at higher risk for a BRCA1/2 PV. This suggests that while interest in pursuing genetic testing for BRCA1/2 may be influenced by accessibility and convenience, testing intentions remained aligned with participant values and preferences.
These results also suggest that timing of the genetic testing offer may be important. Participants may have considered that learning their BRCA1/2 status preconceptionally could affect their reproductive plans. For example, decisions about whether or when to have children could be influenced by learning positive BRCA1/2 results (Dean and Rauscher 2017; Werner-Lin 2008). Moreover, carriers may be interested in pursuing prenatal testing or preimplantation genetic testing to select embryos without the BRCA1/2 PV (Chan et al. 2017; Gietel-Habets et al. 2017; Julian-Reynier et al. 2012). Conversely, unwed individuals in some populations may have reduced interest in pursuing personal health-related genetic testing due to its potential effect on marriageability (Rose et al. 2016). We did not ask participants whether they would have wanted the choice of opting into or out of BRCA1/2 testing if it was offered along with the standard carrier testing. However, it is interesting to consider whether providing this choice is even feasible within clinic-based or population testing programs (Gbur et al. 2021).
With respect to preferences about genetic education to inform testing decisions, although most participants preferred some form of pre-test education, under half chose genetic counseling as their preferred modality, and only 15% preferred in-person genetic counseling. These data suggest that most participants prefer streamlined pre-test education. This is not surprising because the surveyed population had previously completed JScreen, which incorporates streamlined pre-test genetic education through video and electronic communication (Hardy et al. 2018). A streamlined approach is also consistent with models of population-based testing that have been implemented in multiple countries (Gronwald et al. 2006; Lieberman et al. 2017; Manchanda et al. 2020; Metcalfe et al. 2015) and with recent studies suggesting the feasibility and efficacy of streamlining genetic education and support without adverse effects on the patient experience (Brown et al. 2021; Hardy et al. 2018; Interrante et al. 2017; Schwartz et al. 2014). Furthermore, since these data were collected prior to the COVID-19 pandemic, the responses do not reflect the COVID-19-driven pivot to telehealth. Given the shift to telehealth over the last 2 years, it is likely that patients and providers are increasingly comfortable with this mode of delivery (Mann et al. 2020; Uhlmann et al. 2021).
Despite the majority of participants reporting that they would opt for add-on testing through JScreen, most participants would be unwilling to pay over $50 for testing. Participants willing to pay over $50 were older, had greater genetic knowledge, and had more positive attitudes toward testing. The finding that willingness to pay may be at least partially driven by cognitive and attitudinal factors suggests that pre-test education may favorably impact people’s receptivity to higher testing costs. However, these factors may be associated with socioeconomic status which could also impact willingness to pay for genetic testing (Blouin-Bougie et al. 2018; Bowen et al. 2010; Guo et al. 2020). Furthermore, our focus on theoretical testing intentions cannot account for the potential affective drivers of genetic testing decisions.
Although these results provide preliminary support for the notion of bundling BRCA1/2 testing with expanded carrier testing, there are some considerations that may impact its feasibility. If BRCA1/2 or broader multigene cancer genetic testing is combined with expanded reproductive carrier screening, it would be important for pre-test education to address the implications, including benefits and limitations, of both types of testing. Given the limited size of the current genetic counseling workforce and its capability to handle increasing demand for services and expertise (Hoskovec et al. 2018), it is unlikely that offering universal pre-test genetic counseling would be feasible. Indeed, individuals already have the option of obtaining limited carrier screening and testing for disease predispositions (including the three BRCA1/2 Ashkenazi founder mutations) through direct-to-consumer companies such as 23andMe (Roberts et al. 2017; Wynn and Chung 2017). However, hybrid models of genetic testing have emerged recently in which consumers can request bundled health tests (e.g., for cancer, cardiac diseases, and pharmacogenomics) offered through clinical labs and ordered by a clinician (Brodwin 2019; Phillips et al. 2019). In this context, only post-test genetic counseling is offered through the testing laboratory and is included in the cost of testing.
JScreen’s streamlined model for patient education does not include in-person or pre-test genetic counseling, and that model could continue if BRCA1/2 were bundled with reproductive carrier screening. A prior study to assess JScreen’s patient education strategy showed that knowledge scores improved significantly following educational interventions, and patient satisfaction was very high at 98% (Hardy et al 2018). In a scenario where testing was bundled, pre-test education could be developed to incorporate the relevant pre-test information for both BRCA1/2 and reproductive carrier screening. This approach would allow genetic counselors to focus their time and energy on results disclosure, especially for individuals who test positive.
Limitations
This study sampled prior JScreen patients retrospectively, asking about their theoretical intentions had they been offered a cancer genetic test at the time of their JScreen reproductive carrier testing. Their answers on this survey may not match their actual choices had they been offered the option to bundle testing. Given our 43% participation rate, it is possible that study participants are not representative of the broader JScreen population or those who are offered testing by a gynecologist/obstetrician. It is certainly possible that those who were most satisfied with JScreen’s process were most likely to participate in this survey. Thus, even among the JScreen population, testing intentions may be lower than in our study sample.
In addition, JScreen’s patient population is mostly Ashkenazi Jewish and tends to be more engaged in health-related screening, more interested in testing through a Jewish-based organization, and more knowledgeable about testing for Jewish-related diseases than the general population. Because all had previously participated in JScreen’s reproductive carrier screening, and most had a positive result demonstrating carrier status for at least one condition, they may have greater familiarity with, and less fear of, obtaining a positive genetic testing result than other populations. Relative to the general population, this highly educated and knowledgeable group of participants may also be more amenable to obtaining streamlined information prior to testing and to receiving education and genetic counseling through telehealth. Finally, since individuals of Ashkenazi Jewish background have a higher risk of carrying certain variants in cancer-related genes, there may be a heightened desire for health-related information and genetic testing in this population. As a result, we cannot determine if the results of this study are generalizable to other populations.
Conclusion and future directions
The results of this study suggest that adding BRCA1/2 testing to JScreen’s platform of expanded carrier screening could be an efficient and desirable way to expand access to cancer predisposition testing. Invariably, a substantial number of BRCA1/2 carriers would be identified who would not have otherwise come forward for cancer-risk testing or who do not meet current criteria for such testing. Thus, the inclusion of these and potentially other genes on testing panels presents an opportunity to provide risk information and cancer risk management recommendations before individuals develop cancer. Given that the goals of reproductive carrier screening and BRCA1/2 testing are different (i.e., for family planning versus personal health risks and management), some form of pre-test education and counseling, however streamlined, will still be important to ensure understanding, informed consent, and autonomous decision making. It would also be important to determine what types of education and genetic counseling adjuncts may be needed for individuals who test positive given that they may be highly interested in resources that provide support around reproductive decision-making. This added support could be essential as many would also learn that they are carriers for potentially severe recessive or X-linked diseases that present in infancy or childhood. Although tested individuals would likely face many personal health decisions related to their testing experience, it would also be important to assess psychological effects of this bundled testing and how professionals can facilitate the process of family communication about positive test results that encompasses reproductive and personal disease risks.
Despite the initial call for BRCA1/2 population testing in 2014, it has not been widely implemented. Integrating such testing into JScreen’s existing, successful, and safe platform is a reasonable approach to address this need in a population that is at higher risk for PVs in the BRCA1/2 genes. Lessons learned from this initial step could pave the way to broadening the platform even further, to include testing for other potentially actionable gene variants that are also associated with very high risks of cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to the study conception, design, and data collection. Data analysis was performed by Marc D. Schwartz. The first draft of the manuscript was written by Marc D. Schwartz, Melanie W. Hardy, Beth N. Peshkin, and Karen A. Grinzaid. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Cancer Institute Grant P30 CA051008 and by the Jess and Mildred Fisher Center for Hereditary Cancer and Clinical Genomics Research.
Declarations
Human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- ACOG Committee opinion no. 691: carrier screening for genetic conditions. Obstet Gynecol. 2017;129(3):e41–e55. doi: 10.1097/AOG.0000000000001952. [DOI] [PubMed] [Google Scholar]
- Akler G, Birch AH, Schreiber-Agus N, Cai X, Cai G, Shi L, Yu C, Larmore AM, Mendiratta-Vij G, Elkhoury L, Dillon MW, Zhu J, Mclellan AS, Suer FE, Webb BD, Schadt EE, Kornreich R, Edelmann L. Lessons learned from expanded reproductive carrier screening in self-reported Ashkenazi, Sephardi, and Mizrahi Jewish patients. Mol Genet Genomic Med. 2020;8(2):e1053. doi: 10.1002/mgg3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin-Bougie J, Amara N, Bouchard K, Simard J, Dorval M. Disentangling the determinants of interest and willingness-to-pay for breast cancer susceptibility testing in the general population: a cross-sectional Web-based survey among women of Québec (Canada) BMJ Open. 2018;8(2):e016662. doi: 10.1136/bmjopen-2017-016662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DJ, Harris J, Jorgensen CM, Myers MF, Kuniyuki A. Socioeconomic influences on the effects of a genetic testing direct-to-consumer marketing campaign. Public Health Genomics. 2010;13(3):131–142. doi: 10.1159/000231722. [DOI] [PubMed] [Google Scholar]
- Brodwin E (2019) A genetic testing company you’ve never heard of could become a household name as it takes on 23andMe. Business Insider. https://www.businessinsider.com/invitae-genetics-will-offer-personal-genetic-testing-23andme-2019-1. Accessed 26 March 2022
- Brown EM, Grinzaid KA, Ali N, Mehta N, Hardy MW. Evaluating the experiences of individuals with personal health risks identified through expanded carrier screening. J Genet Couns. 2021 doi: 10.1002/jgc4.1527. [DOI] [PubMed] [Google Scholar]
- Chan JL, Johnson LNC, Sammel MD, DiGiovanni L, Voong C, Domchek SM, Gracia CR. Reproductive decision-making in women with BRCA1/2 mutations. J Genet Couns. 2017;26(3):594–603. doi: 10.1007/s10897-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2017;35(34):3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-H, Terry MB, Daly MB, MacInnis RJ, Hopper JL, Colonna S, Buys SS, Andrulis IL, John EM, Kurian AW, Briollais L. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7(4):585–592. doi: 10.1001/jamaoncol.2020.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cudjoe J, Budhathoki C, Roter D, Gallo JJ, Sharps P, Han H-R. Exploring health literacy and the correlates of pap testing among African immigrant women: findings from the AfroPap study. Journal of Cancer Education: the Official Journal of the American Association for Cancer Education. 2021;36(3):441–451. doi: 10.1007/s13187-020-01755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, Domchek SM, Elkhanany A, Friedman S, Garber JE, Goggins M, Hutton ML, Khan S, Klein C, Kohlmann W, Kurian AW, Laronga C, Litton JK, Mak JS, … Darlow SD (2020) NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. Journal of the National Comprehensive Cancer Network: JNCCN, 18(4): 380–391. 10.6004/jnccn.2020.0017 [DOI] [PubMed]
- Dean M, Rauscher EA. “It was an emotional baby”: previvors’ family planning decision-making styles about hereditary breast and ovarian cancer risk. J Genet Couns. 2017;26(6):1301–1313. doi: 10.1007/s10897-017-0069-8. [DOI] [PubMed] [Google Scholar]
- DellaPergola S (2017) World Jewish Population, 2016. In: Dashefsky A & Sheskin IM (Eds.) American Jewish year book 2016: the annual record of North American Jewish communities (pp. 253–332). Springer International Publishing. 10.1007/978-3-319-46122-9_17
- Domchek S, Robson M. Broadening criteria for BRCA1/2 evaluation: placing the USPSTF recommendation in context. JAMA. 2019;322(7):619–621. doi: 10.1001/jama.2019.9688. [DOI] [PubMed] [Google Scholar]
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t’veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, … Rebbeck TR (2010) Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 304(9): 967–975. 10.1001/jama.2010.1237 [DOI] [PMC free article] [PubMed]
- Finch APM, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, Huzarski T, Eisen A, Foulkes WD, Kim-Sing C, Ainsworth P, Tung N, Lynch HT, Neuhausen S, Metcalfe KA, … Narod SA (2014) Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 32(15): 1547–1553. 10.1200/JCO.2013.53.2820 [DOI] [PMC free article] [PubMed]
- Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, Beeri R, Gal M, Grinshpun-Cohen J, Djemal K, Mandell JB, Lee MK, Beller U, Catane R, King M-C, Levy-Lahad E. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA. 2014;111(39):14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbur S, Mauney L, Gray KJ, Wilkins-Haug L, Guseh S. Counseling for personal health implications identified during reproductive genetic carrier screening. Prenat Diagn. 2021;41(11):1460–1466. doi: 10.1002/pd.6033. [DOI] [PubMed] [Google Scholar]
- Gietel-Habets JJG, de Die-Smulders CEM, Derks-Smeets IAP, Tibben A, Tjan-Heijnen VCG, van Golde R, Gomez-Garcia E, Kets CM, van Osch LADM. Awareness and attitude regarding reproductive options of persons carrying a BRCA mutation and their partners. Human Reproduction (Oxford, England) 2017;32(3):588–597. doi: 10.1093/humrep/dew352. [DOI] [PubMed] [Google Scholar]
- Graves KD, Peshkin BN, Luta G, Tuong W, Schwartz MD. Interest in genetic testing for modest changes in breast cancer risk: implications for SNP testing. Public Health Genomics. 2011;14(3):178–189. doi: 10.1159/000324703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinzaid KA, Page PZ, Denton JJ, Ginsberg J. Creation of a national, at-home model for Ashkenazi Jewish carrier screening. J Genet Couns. 2015;24(3):381–387. doi: 10.1007/s10897-014-9800-x. [DOI] [PubMed] [Google Scholar]
- Gronwald J, Huzarski T, Byrski T, Debniak T, Metcalfe K, Narod SA, Lubiński J. Direct-to-patient BRCA1 testing: the Twoj Styl experience. Breast Cancer Res Treat. 2006;100(3):239–245. doi: 10.1007/s10549-006-9261-5. [DOI] [PubMed] [Google Scholar]
- Guan Y, McBride CM, Rogers H, Zhao J, Allen CG, Escoffery C. Initiatives to scale up and expand reach of cancer genomic services outside of specialty clinical settings: a systematic review. Am J Prev Med. 2021;60(2):e85–e94. doi: 10.1016/j.amepre.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Hirth JM, Fuchs EL, Cofie LE, Brown V, Kuo Y-F, Fernandez ME, Berenson AB. Knowledge, attitudes, willingness to pay, and patient preferences about genetic testing and subsequent risk management for cancer prevention. Journal of Cancer Education: The Official Journal of the American Association for Cancer Education. 2020 doi: 10.1007/s13187-020-01823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MW, Kener HJ, Grinzaid KA. Implementation of a carrier screening program in a high-risk undergraduate student population using digital marketing, online education, and telehealth. Public Health Genomics. 2018;21(1–2):67–76. doi: 10.1159/000493971. [DOI] [PubMed] [Google Scholar]
- Hoskovec JM, Bennett RL, Carey ME, DaVanzo JE, Dougherty M, Hahn SE, LeRoy BS, O’Neal S, Richardson JG, Wicklund CA. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018;27(1):16–20. doi: 10.1007/s10897-017-0158-8. [DOI] [PubMed] [Google Scholar]
- Interrante MK, Segal H, Peshkin BN, Valdimarsdottir HB, Nusbaum R, Similuk M, DeMarco T, Hooker G, Graves K, Isaacs C, Wood M, McKinnon W, Garber J, McCormick S, Heinzmann J, Kinney AY, Schwartz MD. Randomized noninferiority trial of telephone vs in-person genetic counseling for hereditary breast and ovarian cancer: a 12-month follow-up. JNCI Cancer Spectrum. 2017;1(1):pkx002. doi: 10.1093/jncics/pkx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis IL, Mann L. Decision making: a psychological analysis of conflict, choice, and commitment. Free Press; 1977. [Google Scholar]
- Julian-Reynier C, Fabre R, Coupier I, Stoppa-Lyonnet D, Lasset C, Caron O, Mouret-Fourme E, Berthet P, Faivre L, Frenay M, Gesta P, Gladieff L, Bouhnik A-D, Protière C, Noguès C. BRCA1/2 carriers: their childbearing plans and theoretical intentions about having preimplantation genetic diagnosis and prenatal diagnosis. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2012;14(5):527–534. doi: 10.1038/gim.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M-C, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312(11):1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- Kraft SA, Duenas D, Wilfond BS, Goddard KAB. The evolving landscape of expanded carrier screening: challenges and opportunities. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2019;21(4):790–797. doi: 10.1038/s41436-018-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian AW, Katz SJ. Emerging opportunity of cascade genetic testing for population-wide cancer prevention and control. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2020;38(13):1371–1374. doi: 10.1200/JCO.20.00140. [DOI] [PubMed] [Google Scholar]
- Ladd MK, Peshkin BN, Senter L, Baldinger S, Isaacs C, Segal H, Philip S, Phillips C, Shane K, Martin A, Weinstein V, Pilarski R, Jeter J, Sweet K, Hatten B, Wurtmann EJ, Phippen S, Bro D, Schwartz MD. Predictors of risk-reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer. Transl Behav Med. 2020;10(2):337–346. doi: 10.1093/tbm/iby101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman S, Tomer A, Ben-Chetrit A, Olsha O, Strano S, Beeri R, Koka S, Fridman H, Djemal K, Glick I, Zalut T, Segev S, Sklair M, Kaufman B, Lahad A, Raz A, Levy-Lahad E. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2017;19(7):754–762. doi: 10.1038/gim.2016.182. [DOI] [PubMed] [Google Scholar]
- Manchanda R, Loggenberg K, Sanderson S, Burnell M, Wardle J, Gessler S, Side L, Balogun N, Desai R, Kumar A, Dorkins H, Wallis Y, Chapman C, Taylor R, Jacobs C, Tomlinson I, McGuire A, Beller U, Menon U, Jacobs I. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: a randomized controlled trial. J Natl Cancer Inst. 2015;107(1):379. doi: 10.1093/jnci/dju379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, Side L, Sanderson S, Loggenberg K, Brady AF, Dorkins H, Wallis Y, Chapman C, Jacobs C, Legood R, Beller U, Tomlinson I, Menon U, Jacobs I. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG: An International Journal of Obstetrics and Gynaecology. 2020;127(3):364–375. doi: 10.1111/1471-0528.15905. [DOI] [PubMed] [Google Scholar]
- Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. Journal of the American Medical Informatics Association: JAMIA. 2020;27(7):1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, Jandorf L, Peterson SK, Lesko S, Pilipshen S, Winkel G. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2009;37(2):207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2016;34(34):4183–4185. doi: 10.1200/JCO.2016.67.0554. [DOI] [PubMed] [Google Scholar]
- McBride CM, Guan Y, Hay JL. Regarding the yin and yang of precision cancer- screening and treatment: are we creating a neglected majority? Int J Environ Res Public Health. 2019;16(21):E4168. doi: 10.3390/ijerph16214168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe KA, Poll A, Royer R, Llacuachaqui M, Tulman A, Sun P, Narod SA. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2010;28(3):387–391. doi: 10.1200/JCO.2009.25.0712. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Eisen A, Lerner-Ellis J, Narod SA. Is it time to offer BRCA1 and BRCA2 testing to all Jewish women? Curr Oncol. 2015;22(4):e233–236. doi: 10.3747/co.22.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod SA. Personalised medicine and population health: breast and ovarian cancer. Hum Genet. 2018;137(10):769–778. doi: 10.1007/s00439-018-1944-6. [DOI] [PubMed] [Google Scholar]
- O’Neill SM, Peters JA, Vogel VG, Feingold E, Rubinstein WS. Referral to cancer genetic counseling: are there stages of readiness? Am J Med Genet C Semin Med Genet. 2006;142C(4):221–231. doi: 10.1002/ajmg.c.30109. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Trosman JR, Douglas MP. Emergence of hybrid models of genetic testing beyond direct-to-consumer or traditional labs. JAMA. 2019;321(24):2403–2404. doi: 10.1001/jama.2019.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot: AJHP. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Roberts MC, Wood EM, Gaieski JB, Bradbury AR. Possible barriers for genetic counselors returning actionable genetic research results across state lines. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2017;19(11):1202–1204. doi: 10.1038/gim.2017.34. [DOI] [PubMed] [Google Scholar]
- Rose E, Schreiber-Agus N, Bajaj K, Klugman S, Goldwaser T. Challenges of pre- and post-test counseling for Orthodox Jewish individuals in the premarital phase. J Genet Couns. 2016;25(1):18–24. doi: 10.1007/s10897-015-9880-2. [DOI] [PubMed] [Google Scholar]
- Rose E, Hardy MW, Gates R, Stanislaw C, Meisel J and Grinzaid KA (2022) Evaluating the effectiveness of a telehealth cancer genetics program: A BRCA pilot study. [Paper Submitted for Publication] [DOI] [PubMed]
- Schwartz MD, Taylor KL, Willard KS, Siegel JE, Lamdan RM, Moran K. Distress, personality, and mammography utilization among women with a family history of breast cancer. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 1999;18(4):327–332. doi: 10.1037//0278-6133.18.4.327. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Benkendorf J, Lerman C, Isaacs C, Ryan-Robertson A, Johnson L. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer. 2001;92(4):932–940. doi: 10.1002/1097-0142(20010815)92:4<932::aid-cncr1403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang A-T, Chang Y, Graves K, Isaacs C, Wood M, McKinnon W, Garber J, McCormick S, Kinney AY, Luta G, Kelleher S, Leventhal K-G, Vegella P, Tong A, King L. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32(7):618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- Sussner KM, Thompson HS, Jandorf L, Edwards TA, Forman A, Brown K, Kapil-Pair N, Bovbjerg DH, Schwartz MD, Valdimarsdottir HB. The influence of acculturation and breast cancer-specific distress on perceived barriers to genetic testing for breast cancer among women of African descent. Psychooncology. 2009;18(9):945–955. doi: 10.1002/pon.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercyak KP, Hensley Alford S, Emmons KM, Lipkus IM, Wilfond BS, McBride CM. Parents’ attitudes toward pediatric genetic testing for common disease risk. Pediatrics. 2011;127(5):e1288–1295. doi: 10.1542/peds.2010-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann WR, McKeon AJ, Wang C. Genetic counseling, virtual visits, and equity in the era of COVID-19 and beyond. J Genet Couns. 2021;30(4):1038–1045. doi: 10.1002/jgc4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Doubeni CA, Epling JW, Kubik M, Landefeld CS, Mangione CM, Pbert L, Silverstein M, Simon MA, Tseng C-W, Wong JB. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322(7):652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- Werner-Lin A. Beating the biological clock: the compressed family life cycle of young women with BRCA gene alterations. Soc Work Health Care. 2008;47(4):416–437. doi: 10.1080/00981380802173509. [DOI] [PubMed] [Google Scholar]
- Wynn J, Chung WK. 23andMe paves the way for direct-to-consumer genetic health risk tests of limited clinical utility. Ann Intern Med. 2017;167(2):125–126. doi: 10.7326/M17-1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.