Abstract
Significance:
Primary lymphedema is a chronic condition without a cure. The lower extremities are more commonly affected than the arms or genitalia. The disease can be syndromic. Morbidity includes decreased self-esteem, infections, and reduced function of the area.
Recent Advances:
Several mutations can cause lymphedema, and new variants continue to be elucidated. A critical determinant that predicts the natural history and morbidity of lymphedema is the patient's body mass index (BMI). Individuals who maintain an active lifestyle with a normal BMI generally have less severe disease compared to subjects who are obese. Because other causes of lower extremity enlargement can be confused with lymphedema, definitive diagnosis requires lymphoscintigraphy.
Critical Issues:
Most patients with primary lymphedema are satisfactorily managed with compression regimens, exercise, and maintenance of a normal body weight. Suction-assisted lipectomy is our preferred operative intervention for symptomatic patients who have failed conservative therapy. Suction-assisted lipectomy effectively removes excess subcutaneous fibro-adipose tissue and can improve underlying lymphatic function.
Future Directions:
Many patients with primary lymphedema do not have an identifiable mutation and thus novel variants will be identified. The mechanisms by which mutations cause lymphedema continue to be studied. In the future, drug therapy for the disease may be developed.
Keywords: extremity, genetic, lymphedema, management, morbidity, mutation

Arin K. Greene, MD
SCOPE OF REVIEW AND SIGNIFICANCE
Primary lymphedema is a rare condition that is poorly understood. Few physicians focus on this disease, which is associated with several myths. Novel causative mutations continue to be elucidated. The purpose of this review is to provide the current understanding of the genetic etiopathogenesis, diagnosis, and treatment of primary lymphedema. This review presents the most current understanding of primary lymphedema since the senior author's previous reviews on this topic.1 The information in this article will facilitate both research and clinical care in the field.
TRANSLATIONAL RELEVANCE
Most patients with primary lymphedema do not have an identifiable mutation and thus the genetic etiology of their disease is unknown. Discovery of additional variants will further our understanding of lymphedema pathogenesis. Once a causative mutation is known, basic and translational researchers then can study how the mutation affects lymphatic function. Deciphering the mechanisms by which mutations cause lymphedema will lead to improved treatments for patients. Drugs for lymphedema do not exist; translation of basic research may result in medications that will protect against developing lymphedema, prevent its progression, or cause it to regress.
CLINICAL RELEVANCE
Primary lymphedema is an uncommon condition and few physicians focus on the disease. Identification of causative mutations enhances diagnosis and management. Recent data show that a major variable that predicts morbidity is the patient's body mass index (BMI). Obese individuals are more likely to suffer infections, have larger extremities, and experience disability. Current evidence illustrates that suction-assisted lipectomy effectively reduces limb volume in patients who have excess subcutaneous adipose tissue. Suction-assisted lipectomy also can improve the subject's underlying lymphatic function.
BACKGROUND
Lymphedema is divided into primary and secondary disease.2 Primary lymphedema results from an error in lymphatic development. Secondary lymphedema is caused by injury to a normally developed lymphatic system. Primary lymphedema is rare, affecting ∼1/100,000 children.3 The term “lymphedema” often is used generically to describe an overgrown limb, regardless of the underlying etiology.4,5 Primary lymphedema traditionally has been described according to the age of the patient when the swelling develops: “congenital,” “praecox,” or “tarda.”2 This classification system, however, is not standardized and developmental terminology should be used: onset in infancy, childhood, adolescence, or adulthood.6,7
The lymphatics in primary lymphedema are either hypoplastic/aplastic (89%) or hyperplastic (11%).8 The maldeveloped structures do not have the capacity to return interstitial fluid to the venous circulation, which causes lymphedema. Compensatory lymphaticovenous connections can occur to help return lymph fluid proximally and may be associated with less severe disease.9 Over time, the diseased area enlarges because the interstitial lymphatic fluid causes adipose deposition; fat in an extremity can increase by 73%.9,10 Individuals who maintain a normal BMI have less progression than obese patients.11 Lymphedema worsens through four stages: Stage 0 (no edema, but abnormal lymph transport), Stage 1 (edema that improves with limb elevation), Stage 2 (pitting edema not resolved with elevation), and Stage 3 (fibroadipose deposition).12 Patients with unilateral lower extremity lymphedema have a 9–25% risk of developing the condition in their contralateral limb.6,8
GENETIC ETIOPATHOGENESIS
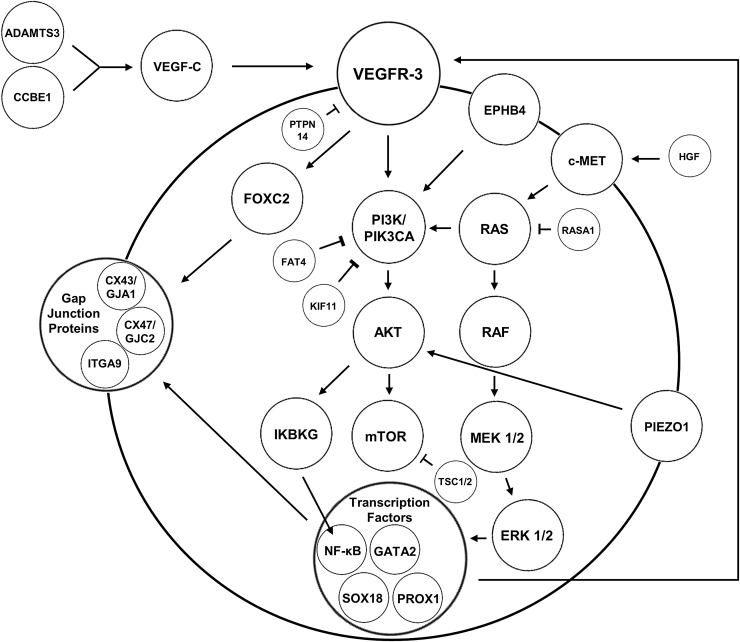
The majority of patients with primary lymphedema have sporadic disease with an unknown mutation. Genetic causes are found in only 36% of patients with familial disease and 8% of patients without a family history.13 More than 20 genes are known to cause primary lymphedema: ADAMTS3, BRAF, CCBE1, EPHB4, FAT4, FLT4/VEGFR3, FOXC2, GATA2, GJA1, GJC2, HGF, IKBKG, KIF11, MAP2K1, MAP2K2, monosomy X, PIEZO1, PIK3CA, PTPN11, PTPN14, RASA, RASA1, RIT1, SOS1, SOX18, TSC1, TSC2, and VEGFC (Table 1; Fig. 1).14,15
Table 1.
Mutations associated with primary lymphedema
| Condition | Gene | Effect of Mutation | Inheritance |
|---|---|---|---|
| Capillary malformation | PIK3CA | Activating missense causing constitutively active PI3K-AKT signaling | Somatic |
| Choanal atresia/lymphedema syndrome | PTPN14 | Loss of function frameshift nonsense in the protein tyrosine phosphatase that inhibits VEGFR3 | Autosomal recessive |
| Cholestasis-lymphedema syndrome (Aagenaes syndrome) | CCBE1 | Loss of function causing decreased activation of the VEGF-C ligand | Autosomal recessive |
| CLOVES syndrome | PIK3CA | Activating missense resulting in constitutively active PI3K-AKT signaling | Somatic |
| CM-AVM/lymphedema |
EPHB4
RASA1 |
Inactivating heterozygous missense in the tyrosine kinase domain of EPHB4 Loss of function in the RAS GTPase-activating protein upregulating RAS/MAPK signaling |
Autosomal dominant |
| Ectodermal dysplasia, anhidrotic, immunodeficiency, osteoporosis, and lymphedema | IKBKG | Hypomorphic decreasing IKBKG activation of NF-κB | X linked |
| Fetal hydrops | EPHB4 | Inactivating heterozygous missense in the tyrosine kinase domain of EPHB4 | Autosomal dominant |
| Hennekam syndrome type 1 | CCBE1 | Homozygous or compound heterozygous in the calcium-binding EGF domain inhibiting activation of the VEGFC ligand | Autosomal recessive |
| Hennekam syndrome type 2 | FAT4 | Loss of function homozygous or compound heterozygous | Autosomal recessive |
| Hennekam syndrome type 3 | ADAMTS3 | Loss of function bi-allelic missense in the prodomain and the peptidase domain of ADAMTS inhibit activation of the VEGFC ligand | Autosomal recessive |
| Hereditary lymphedema type 3/generalized lymphatic dysplasia of Fotiou | PIEZO1 | Activating or inactivating homozygous or compound heterozygous missense, nonsense, and splice site in the mechanosensitive ion channel PIEZO1 | Autosomal recessive |
| Hypotrichosis-lymphedema-telangiectasia | SOX18 | Loss of function homozygous missense or heterozygous nonsense affecting the alpha-helix of the DNA-binding domain of the transcription factor SOX18 | Autosomal recessive |
| Klippel–Trenaunay syndrome | PIK3CA | Activating missense results in constitutively active PI3K-AKT signaling | Somatic |
| Lymphedema-distichiasis syndrome | FOXC2 | Loss of function heterozygous in the transcription factor FOXC2 | Autosomal dominant |
| Lymphedema-lymphangiectasia | HGF | Possible loss of function results in decreased activation of the c-MET receptor tyrosine kinase | Autosomal dominant |
| Meige disease | GJC2 | Heterozygous missense affecting gap junction protein connexin 47 | Autosomal dominant |
| Microcephaly-chorioretinopathy-lymphedema | KIF11 | Loss of function variable types result in dysfunctional EG5, a kinesin-type motor protein, and activation of PI3K-AKT signaling | Autosomal dominant |
| Milroy-like disease | VEGFC | Loss of function frameshift results in truncated inactive VEGFR-3 ligand | Autosomal dominant |
| Noonan syndrome |
BRAF
MAP2K1 MAP2K2 PTPN11 RIT1 SOS1 |
Variable types result in RAS-MAPK pathway dysregulation | Autosomal dominant |
| Oculodentodigital dysplasia/lymphedema syndrome | GJA1 | Missense affects gap junction protein connexin 43 | Autosomal dominant |
| Parkes-Weber syndrome |
EPHB4
RASA1 |
Inactivating heterozygous missense in the tyrosine kinase domain of EPHB4 Loss of function in the RAS GTPase-activating protein upregulating RAS/MAPK signaling |
Autosomal dominant |
| Primary congenital lymphedema (Milroy disease) | VEGFR-3 | Inactivating missense in the tyrosine kinase domain of VEGFR-3 resulting in decreased downstream signal transduction | Autosomal dominant |
| Primary lymphedema with myelodysplasia (Emberger syndrome) | GATA2 | Loss of function heterozygous truncating in the transcription factor GATA2 | Autosomal dominant |
| Tuberous sclerosis |
TSC1
TSC2 |
Loss of function resulting in constitutive activation of mTOR | Autosomal dominant |
| Turner syndrome | Monosomy X | Chromosomal aneuploidy | X linked |
Figure 1.
Signaling pathways and mutations associated with primary lymphedema.
Genotype–phenotype associations in primary lymphedema can occur. Milroy disease presents at birth and is caused by mutations in VEGFR3.13 Lymphedema-distichiasis syndrome results from inactivating mutations in FOXC2 and is associated with an extra row of eyelashes, eyelid ptosis, and/or yellow nails.16 Hennekam lymphangiectasia-lymphedema syndrome is an autosomal recessive disease with general lymphatic dysplasia, developmental delay, flat faces, hypertelorism, and a broad nasal bridge caused by mutations in CCBE1, FAT4 and ADAMTS3.17–19 Microcephaly-chorioretinopathy-lymphedema syndrome results from variants in KIF11; patients have central nervous system, lymphatic, and ocular abnormalities.20 Meige disease is associated with missense mutations in GJC2 and refers to familial lymphedema that manifests during adolescence.21,22 Hypotrichosis-lymphedema-telangiectasia (SOX18 mutation) causes sparse hair and telangiectasias. Patients with Turner (XO) syndrome have a 57% risk of lymphedema.23 Noonan syndrome (PTPN11/SOS1) may manifest with generalized lymphedema, intestinal lymphangiectasis, and/or fetal hydrops; patients have a 3% risk of lymphedema.24 Noonan syndrome also has been associated with mutations in BRAF, KRAS, MAP2K1, MAP2K2, and RIT1.15 Patients with tuberous sclerosis (TSC1 and TSC2) have a 4% risk of lymphedema.25
Autosomal dominant lymphatic-related fetal hydrops (EPHB4) is a generalized lymphatic dysplasia causing fetal hydrops.26 Primary lymphedema associated with choanal atresia results from a frameshift mutation in PTPN14.27 Lymphedema-cholestasis syndrome (CCBE1) is characterized by neonatal intrahepatic cholestasis and lymphedema.28 Mutations in IKBKG are associated with the X-linked syndrome anhidrotic ectodermal dysplasia with immunodeficiency, osteoporosis, and lymphedema.14 Hereditary lymphedema type 3/general lymphatic dysplasia of Fotiou (PIEZO1) is characterized by lymphedema of all four limbs, genitalia, and face and fetal hydrops.29
Mutations in HGF can cause lymphedema and visceral lymphangiectasia.30 Oculodentodigital syndrome (abnormalities of the face, eyes, dentition, and digits, including hypotelorism, hypoplastic alae nasi, microphthalmia, microcornea, microdontia, and syndactyly) is associated with lymphedema and caused by a mutation in GJA1.31 Variants in GATA2 result in lymphedema and acute myeloid leukemia (Emberger syndrome).32 Primary lymphedema also can occur in combination with other vascular anomalies and overgrowth conditions: (i) capillary malformation (PIK3CA mutation),33,34 (ii) Klippel–Trenaunay syndrome (PIK3CA mutation), (iii) CLOVES syndrome (PIK3CA mutation), and (iv) Parkes-Weber syndrome (RASA and EPHB4 mutations).
MANAGEMENT
Clinical Features
In the pediatric population, onset occurs in infancy (49%), childhood (10%), or adolescence (41%) (Fig. 2).6 Males are more likely to present in infancy (68%), while females commonly develop the disease during adolescence (55%).6 The lower extremities are affected in 92% of patients; 50% have unilateral lymphedema and 50% have bilateral disease.6 Bilateral lower extremity lymphedema is more common in patients presenting in infancy (63%), compared to adolescence (30%).6 Eighteen percent have genital lymphedema, which is usually associated with lower extremity lymphedema (4% have isolated genital involvement). Adult-onset primary lymphedema (>21 years) occurs in 10% of patients, typically affects one lower extremity, is not associated with systemic lymphatic anomalies, and familial transmission is rare.35 Ten percent of patients have upper extremity lymphedema; 50% have other lymphatic anomalies, including lower extremity lymphedema, and familial transmission is rare.36
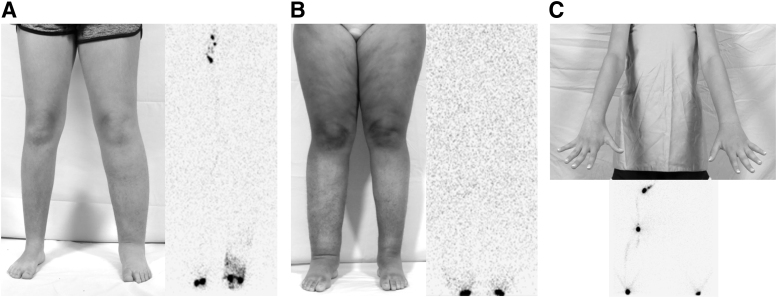
Figure 2.
Phenotypes of primary lymphedema. (A) Eleven-year-old female with left lower extremity disease. Her lymphoscintigram showed absence of tracer uptake into the left inguinal nodes as well as dermal backflow. (B) Eighteen-year-old female with lymphedema affecting both legs. Her lymphoscintigram illustrated no inguinal node tracer uptake bilaterally. (C) Thirteen-year-old female with left arm lymphedema and absence of tracer uptake into her left axillary nodes.
The most common problem caused by lymphedema is psychosocial morbidity because the involved area does not look normal. A lymphedematous extremity has an increased risk of cellulitis compared to the nonaffected limb.37 Fifteen percent of patients will develop cutaneous problems such as bleeding from vesicles, fungal toenail lesions, hyperkeratosis, lymphorrhea, and verrucous changes.6 Lymphangiosarcoma has been described in patients with primary lymphedema and prognosis is poor.38
Diagnosis
Ninety percent of patients with primary lymphedema can be diagnosed by history and physical examination. Patients are queried about a family history of extremity edema and infections in the limb. Individuals are asked about potential variables that might be associated with lymphedema (e.g., Turner syndrome and Noonan syndrome). Lymphedema almost always affects the distal extremity and the Stemmer sign is 92% sensitive and 57% specific for the disease.39 If the examiner is unable to pinch the skin on the dorsum of the hand or foot (positive Stemmer sign), then it is likely the patient has lymphedema.
Clinically, the severity of lymphedema can be categorized as mild (<20% increase in extremity volume), moderate (20–40% increase in extremity volume), or severe (>40% increase in extremity volume).12 Lymphoscintigraphy is the definitive test to diagnose lymphedema and is 100% specific and 96% sensitive for the condition.40 A radiolabeled colloid is injected into the dorsum of the hand or foot and is only taken up by lymphatic vessels. Delayed transit time to the regional lymph nodes, dermal back-flow, and collateral lymphatic channels represent abnormal lymphatic function.41
Treatment
The most important variable that determines the morbidity of lymphedema is the patient's BMI. Obese subjects with lymphedema have an increased risk of infection and larger extremities.11 Patients are counseled to exercise and maintain a normal body weight. Individuals with >3 episodes of cellulitis each year are given chronic suppressive antibiotic therapy. The mainstay of treatment for lymphedema is compression stockings. In the pediatric population, we often recommend commercially available socks instead of medical-grade garments to increase compliance. Pneumatic compression delivers intermittent pressure through a power source and inflatable sleeve. In children, we only prescribe pneumatic compression for adolescents or younger children with severe disease. Massage and bandaging regimens are difficult in the pediatric population and we prefer stockings and pneumatic compression.
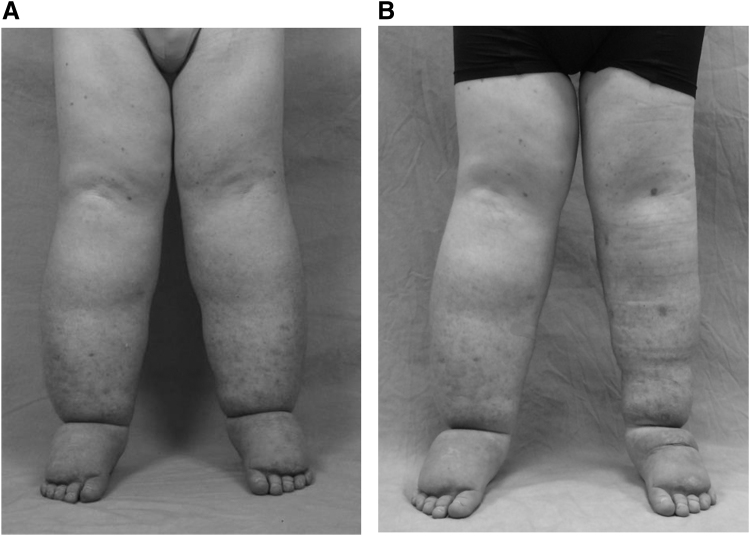
Operative intervention is rarely indicated for patients with primary lymphedema. Individuals are considered for a surgical procedure if they have significant morbidity, despite conservative interventions. The role of lymphatic-venous anastomosis (LVA) or lymph node transfer is less clear in patients with primary lymphedema compared to secondary disease because they have hypoplastic/aplastic lymphatics. Patients with primary lymphedema also have an increased risk of developing donor site lymphedema from the harvest of lymph nodes because of their underlying genetic abnormality. Suction-assisted lipectomy (liposuction) is our first-line operative intervention for extremity lymphedema because of its efficacy, consistent results, and low morbidity (Fig. 3).42 Liposuction increases cutaneous blood flow, reduces the risk of cellulitis, and improves quality of life.9 The procedure also can provide physiological benefit by improving lymph flow.43 Skin and subcutaneous adipose excision are required for severe lymphedema with significant skin excess and for genital lymphedema.
Figure 3.
Operative management of primary lymphedema. (A) Adolescent male with bilateral lower extremity lymphedema. (B) Improved volume of his left leg following suction-assisted lipectomy.
Challenges and Future Interventions
Although most patients who maintain a normal BMI and active lifestyle do not have significant worsening of their disease, primary lymphedema remains an incurable and progressive condition. The disease commonly is confused with other conditions and one-fourth of patients diagnosed with “lymphedema” have another disease.4 The differential diagnosis of primary lymphedema includes other vascular anomalies and overgrowth syndromes, venous stasis, systemic conditions (cardiac, hepatic, renal, and autoimmune), orthopedic and rheumatologic disorders, lipedema, and obesity. It is important to accurately diagnose the patient with lymphedema, typically using lymphoscintigraphy, because the prognosis and treatment of the condition is different than other diseases in its differential diagnosis.
The most common microsurgical procedures used to treat secondary lymphedema are LVA and vascularized lymph node transfer (VLNT). These techniques attempt to improve lymph flow and are best indicated in early lymphedema before significant fibroadipose tissue has developed. Microsurgical procedures for primary lymphedema, however, are less clear because patients have hypoplastic or absent lymphatic vessels.8 Individuals also are at increased risk of donor site lymphedema from VLNT because they have an underlying mutation affecting their lymphatics and are at risk for developing lymphedema at other anatomical sites. As new causative mutations are identified and our understanding of these variants improves, targeted therapy may prove to prevent the onset of lymphedema. For example, drugs may block abnormal signaling pathways or stimulate lymphangiogenesis. Topical, injectable, or systemic pharmacotherapy also may be able to prevent the worsening of lymphedema by preventing fibroadipose deposition.
Take-Home Messages
Although many mutations and syndromes are associated with primary lymphedema, the majority of patients do not have an identifiable disease-causing variant.
Most patients who maintain an active lifestyle and normal BMI have minimal progression of their disease.
Children are encouraged to engage in all sports and wear compression stockings as much as possible.
Operative intervention is indicated in a minority of patients and we favor suction-assisted lipectomy. As the pathophysiology of primary lymphedema continues to be elucidated, improved therapies will be developed.
AUTHORS' CONTRIBUTIONS
Both authors made substantial contributions to the work, drafted and revised the work, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Abbreviations and Acronyms
- BMI
body mass index
- LVA
lymphatic venous anastomosis
- VLNT
vascularized lymph node transfer
ACKNOWLEDGMENTS AND FUNDING SOURCES
None declared. No funding was received for this article.
AUTHOR DISCLOSURE AND GHOSTWRITING
No competing financial interests exist. No ghostwriters were used to write this article.
ABOUT THE AUTHORS
Arin K. Greene, MD, MMSc, is the Vascular Anomalies and Pediatric Plastic Surgery Endowed Chair, Director of the Lymphedema Program, and Director of the Plastic and Oral Surgery Laboratory at Boston Children's Hospital. He is Professor of Surgery at Harvard Medical School. Christopher L. Sudduth, MD, is a Postdoctoral Research Fellow in the Department of Plastic and Oral Surgery at Boston Children's Hospital and Harvard Medical School.
REFERENCES
- 1. Greene AK. Primary lymphedema. In: Greene AK, Slavin SA, Brorson H, eds. Lymphedema: Presentation, Diagnosis, and Management. New York: Springer, 2015;59–78. [Google Scholar]
- 2. Allen EV. Lymphedema of the extremities: classification, etiology and differential diagnosis: a study of three hundred cases. Arch Intern Med 1934;54:606–624. [Google Scholar]
- 3. Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow-up study and review. Pediatrics 1985;76:206–218. [PubMed] [Google Scholar]
- 4. Schook CC, Mulliken JB, Fishman SJ, Alomari AI, Grant FD, Greene AK. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast Reconstr Surg 2011;127:1571–1581. [DOI] [PubMed] [Google Scholar]
- 5. Sudduth CL, Maclellan RA, Greene AK. Study of 700 referrals to a lymphedema program. Lymphat Res Biol 2020 [Epub ahead of print]; DOI: 10.1089/lrb.2019.0086. [DOI] [PubMed] [Google Scholar]
- 6. Schook CC, Mulliken JB, Fishman SJ, Grant FD, Zurakowski D, Greene AK. Primary lymphedema: clinical features and management in 138 pediatric patients. Plast Reconstr Surg 2011;127:2419–2431. [DOI] [PubMed] [Google Scholar]
- 7. Greene AK, Schook CC. Primary lymphedema: definition of onset based on developmental age. Plast Reconstr Surg 2012;129:221e–222e. [DOI] [PubMed] [Google Scholar]
- 8. Wolfe JHN, Kinmonth JB. The prognosis of primary lymphedema of the lower limbs. Arch Surg 1981;116:1157–1160. [DOI] [PubMed] [Google Scholar]
- 9. Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998;102:1058–1068. [PubMed] [Google Scholar]
- 10. Brorson H, Ohlin K, Olsson G, Karlsson MK. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol 2009;7:3–10. [DOI] [PubMed] [Google Scholar]
- 11. Greene AK, Zurakowski D, Goss JA. Body mass index and lymphedema morbidity: comparison of obese versus normal-weight patients. Plast Reconstr Surg 2020;146:402–407. [DOI] [PubMed] [Google Scholar]
- 12. Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology 2016;49:170–184. [PubMed] [Google Scholar]
- 13. Mendola A, Schlögel MJ, Ghalamkarpour A, et al. Mutations in the VEGFR3 signaling pathway explain 36% of familial lymphedema. Mol Syndromol 2013;4:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest 2014;124:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mansour S, Martin-Almedina S, Ostergaard P. Genetic disorders of the lymphatic system. In: Pyeritz R, Korf B, Grody W, eds. Emery and Rimoin's Principles and Practice of Medical Genetics and Genomics: Cardiovascular, Respiratory, and Gastrointestinal Disorders. Cambridge, MA: Elsevier, 2019:231–249. [Google Scholar]
- 16. Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet 2002;39:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet 2009;41:1272–1274. [DOI] [PubMed] [Google Scholar]
- 18. Brouillard P, Dupont L, Helaers R, et al. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum Mol Genet 2017;26:4095–4104. [DOI] [PubMed] [Google Scholar]
- 19. Alders M, Al-Gazali L, Cordeiro I, et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum Genet 2014;133:1161–1167. [DOI] [PubMed] [Google Scholar]
- 20. Jones GE, Ostergaard P, Moore AT, et al. Microcephaly with or without chorioretinopathy, lymphoedema, or mental retardation (MCLMR): review of phenotype associated with KIF11 mutations. Eur J Hum Genet 2014;22:881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrell RE, Baty CJ, Kimak MA, et al. GJC2 missense mutations cause human lymphedema. Am J Hum Genet 2010;86:943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ostergaard P, Simpson MA, Brice G, et al. Rapid identification of mutations in GJC2 in primary lymphoedema using whole exome sequencing combined with linkage analysis with delineation of the phenotype. J Med Genet 2011;48:251–255. [DOI] [PubMed] [Google Scholar]
- 23. Welsh J, Todd M. Incidence and characteristics of lymphedema in Turner's syndrome. Lymphology 2006;39:152–153. [PubMed] [Google Scholar]
- 24. Shaw AC, Kalidas K, Crosby AH, Jeffery S, Patton MA. The natural history of Noonan syndrome: a long-term follow-up study. Arch Dis Child 2007;92:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geffrey AL, Shinnick JE, Staley BA, Boronat S, Thiele EA. Lymphedema in tuberous sclerosis complex. Am J Med Genet Part A 2014;164:1438–1442. [DOI] [PubMed] [Google Scholar]
- 26. Martin-Almedina S, Martinez-Corral I, Holdhus R, et al. EPHB4 kinase-inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. J Clin Invest 2016;126:3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Au AC, Hernandez PA, Lieber E, et al. Protein tyrosine phosphatase PTPN14 Is a regulator of lymphatic function and choanal development in humans. Am J Hum Genet 2010;87:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah S, Conlin LK, Gomez L, et al. CCBE1 mutation in two siblings, one manifesting lymphedema-cholestasis syndrome, and the other, fetal hydrops. PLoS One 2013;8:e75770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fotiou E, Martin-Almedina S, Simpson MA, et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat Commun 2015;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finegold DN, Schacht V, Kimak MA, et al. HGF and MET mutations in primary and secondary lymphedema. Lymphat Res Biol 2008;6:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brice G, Ostergaard P, Jeffery S, Gordon K, Mortimer P, Mansour S. A novel mutation in GJA1 causing oculodentodigital syndrome and primary lymphoedema in a three generation family. Clin Genet 2013;84:378–381. [DOI] [PubMed] [Google Scholar]
- 32. Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 2011;43:929–931. [DOI] [PubMed] [Google Scholar]
- 33. Goss JA, Konczyk DJ, Smits P, et al. Diffuse capillary malformation with overgrowth contains somatic PIK3CA variants. Clin Genet 2020;97:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maclellan RA, Chaudry G, Greene AK. Combined lymphedema and capillary malformation of the lower extremity. Plast Reconstr Surg Glob Open 2016;4:e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goss JA, Maclellan RA, Greene AK. Adult-onset primary lymphedema: a clinical-lymphoscintigraphic study of 26 patients. Lymphat Res Biol 2019;17:620–623. [DOI] [PubMed] [Google Scholar]
- 36. Goss JA, MacLellan RA, Greene AK. Primary lymphedema of the upper extremities: clinical and lymphoscintigraphic features in 23 patients. Lymphat Res Biol 2019;17:40–44. [DOI] [PubMed] [Google Scholar]
- 37. Quere I, Nagot N, Vikkula M. Incidence of cellulitis among children with primary lymphedema. N Engl J Med 2018;378:2047–2048. [DOI] [PubMed] [Google Scholar]
- 38. Sharma A, Schwartz RA. Stewart-Treves syndrome: pathogenesis and management. J Am Acad Dermatol 2012;67:1342–1348. [DOI] [PubMed] [Google Scholar]
- 39. Goss JA, Greene AK. Sensitivity and specificity of the stemmer sign for lymphedema. Plast Reconstr Surg Glob Open 2019;7:e2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hassanein AH, Maclellan RA, Grant FD, Greene AK. Diagnostic accuracy of lymphoscintigraphy for lymphedema and analysis of false-negative tests. Plast Reconstr Surg Glob Open 2017;5:e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maclellan RA, Zurakowski D, Voss S, Greene AK. Correlation between lymphedema disease severity and lymphoscintigraphic findings: a clinical-radiologic study. J Am Coll Surg 2017;225:366–370. [DOI] [PubMed] [Google Scholar]
- 42. Greene AK, Maclellan RA. Operative treatment of lymphedema using suction-assisted lipectomy. Ann Plast Surg 2016;77:337–340. [DOI] [PubMed] [Google Scholar]
- 43. Greene AK, Voss SD, Maclellan RA. Liposuction for swelling in patients with lymphedema. N Engl J Med 2017;377:1788–1789. [DOI] [PubMed] [Google Scholar]