Abstract
Significance:
Lymphedema is a common disease that affects hundreds of millions of people worldwide with significant financial and social burdens. Despite increasing prevalence and associated morbidities, the mainstay treatment of lymphedema is largely palliative without an effective cure due to incomplete understanding of the disease.
Recent Advances:
Recent studies have described key histological and pathological processes that contribute to the progression of lymphedema, including lymphatic stasis, inflammation, adipose tissue deposition, and fibrosis. This review aims to highlight cellular and molecular mechanisms involved in each of these pathological processes.
Critical Issues:
Despite recent advances in the understanding of the pathophysiology of lymphedema, cellular and molecular mechanisms underlying the disease remains elusive due to its complex nature.
Future Directions:
Additional research is needed to gain a better insight into the cellular and molecular mechanisms underlying the pathophysiology of lymphedema, which will guide the development of therapeutic strategies that target specific pathology of the disease.
Keywords: lymphedema, pathophysiology, inflammation, adipose deposition, fibrosis

Alex K. Wong, MD, FACS
SCOPE AND SIGNIFICANCE
The prevalence of lymphedema is estimated to be in 250 million people worldwide.1 Primary lymphedema is estimated to affect about 1 in 100,000 individuals. Secondary lymphedema is more prevalent than primary lymphedema. In the United states, secondary lymphedema affects ∼1 in 1,000 people and cancer-related therapies represent the most common cause.2
A systematic review reported the incidence of lymphedema among breast cancer survivors as high as 20%.3 Prospective studies looking at head and neck cancer and gynecological cancer reported even higher incidence rates, 90% and 37%, respectively.2 As life expectancy in cancer survivors is improving, the number of patients who suffer from the disease will also increase. Lymphedema causes physical and functional discomfort to patients, negatively impacting quality of life, as well as a significant burden on psychosocial wellbeing.4
Lymphedema involves a complex sequence of pathology and ideal treatment of lymphedema will involve a multimodal approach. To develop such treatment strategies, understanding the pathophysiology is important. This review explores current understanding of cellular and molecular mechanisms underlying the pathology of lymphedema.
TRANSLATIONAL RELEVANCE
Treatment of lymphedema represents a challenge because of its complex pathophysiology. Current evidence suggests that lymphedema involves multiple mechanisms, including inflammation, immature lymphangiogenesis, adipose tissue deposition, and fibrosis. However, many aspects of the disease remain complicated by several factors. Lymphedema only develops in a subset of patients following lymphatic injury and in a delayed fashion.5 It is also difficult to predict the severity of the disease phenotype in patients who develop lymphedema. Better knowledge of underlying cellular and molecular mechanisms will help understand these discrepancies and improve treatment approaches.
CLINICAL RELEVANCE
Current management of lymphedema, such as manual lymphatic drainage and compression therapy, is largely palliative with the aim to reduce morbidities and control complications. However, these treatments are not effective in stopping the progression of the disease because chronic lymphedema is characterized as inflammation and fibroadipose tissue deposition in addition to accumulation of protein-rich fluid. Understanding the pathology of lymphedema has broad clinical relevance as better insight can help clinicians develop more targeted therapies that aim at curing the underlying pathology.
OVERVIEW OF LYMPHEDEMA
Lymphedema is a chronic and progressive disease caused by lymphatic insufficiency and interstitial edema. It is characterized by the development of soft tissue swelling, fibroadipose tissue deposition, and skin thickening. Lymphedema carries substantial morbidities, including infection, functional loss, movement restriction, and psychosocial impairment.6
Because of a lack of curative treatments for lymphedema, current management of lymphedema is largely palliative and focuses on controlling progression and complications. Lymphedema requires lifelong treatments that include exercises, manual lymphatic drain massage, compression garments, and skin care. For severe and refractory cases, excisional or reconstructive surgeries can be considered for selected patients. Surgical treatment options have become increasingly common due to advances in microsurgical techniques and are aimed at restoring physiologic lymphatic drainage. This includes lymphovenous bypass and vascularized lymph node transfer, which are proven to be efficacious in numerous studies.7,8 However, there are several questions that require further exploration, including but not limited to optimal techniques, timing of intervention, and donor-site lymphedema.9,10
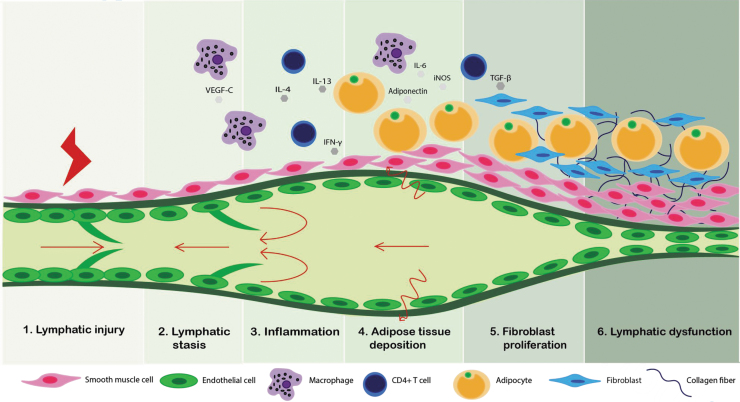
Development of lymphedema is believed to involve multiple pathological steps with lymphatic injury as an inciting event that induces downstream sequela. Lymphatic injury results in a vicious cycle comprising lymphatic fluid stasis, chronic inflammation, adipocyte proliferation, and fibrosis, ultimately leading to a loss of functional lymphatics (Fig. 1). Nevertheless, lymphatic injury is not sufficient for the development of chronic lymphedema. In fact, only a subset of patients develops lymphedema or shows delayed presentation despite similar modes of injury, implying complex pathology of lymphedema.11 Although the pathology of lymphedema remains elusive, recent research has shed light into some of the cellular and molecular mechanisms underlying the disease.
Figure 1.
Schematic diagram summarizing pathological progression of lymphedema. Following initial lymphatic injury, lymphatics undergo a cascade of pathophysiologic events leading to sclerosis and loss of functional lymphatics. Color images are available online.
PATHOPHYSIOLOGY OF LYMPHEDEMA
Lymphatic injury
Primary lymphedema
Primary lymphedema is rare and caused by developmental malformation of lymphatic system. It can be further divided into three types according to the age of presentation: congenital lymphedema (<2 years of age), lymphedema precox (between 2 and 35 years of age), and lymphedema tarda (>35 years of age). They can be sporadic, familial, or syndrome associated.12
About 30% of primary lymphedema patients are thought to have an identifiable genetic mutation.13 There are more than 20 genes linked to lymphatic malfunction seen in primary lymphedema.14 Mutations seen in familial primary lymphedema are frequently associated with the VEGFC/VEGFR-3 signaling pathway. Several types of lymphedema are known to involve VEGFC, a ligand of VEGFR-3, or downstream proteins or factors, such as CCBE1, PTPN14, GATA2, and FOXC2.14 Mutations in FLT4, which encodes VEGFR-3 and FOXC2, a transcription factor that acts downstream of VEGFR-3, are responsible for Milroy's disease, and lymphedema–distichiasis syndrome, respectively, comprising a major fraction of patients with hereditary lymphedema.15
These genes play an essential role in establishing lymphatic vascular architecture that involves lymphatic endothelial cells, smooth muscle cells (SMCs), and lymphatic valves.10 Mechanical or functional failure of this system leads to interstitial fluid accumulation, triggering downstream effects of inflammation and chronic tissue changes.16
Secondary lymphedema
Secondary lymphedema is acquired as a result of underlying systemic diseases, trauma, infection, malignancy, or malignancy-associated treatments.13 Filariasis infection by Wuchereria bancrofti comprises the majority of secondary lymphedema cases worldwide. These parasitic nematodes migrate to the lymphatics and block the lymphatic flow.
In the developed countries, lymphedema occurs most commonly secondary to cancer-related therapeutic interventions, especially breast, head and neck, and gynecological cancer. Malignancy-related therapies include surgical resection of lymph nodes, directly damaging the lymphatic system, and radiation therapy, contributing to the loss of dermal lymphatics and promoting nodal fibrosis.13 Lymphatic obstruction or damage seen in these processes impairs the transport capacity of the lymphatic system, leading to lymphatic stasis and upregulating various pathological pathways that are discussed below.
Lymphatic stasis
Various causes, including lymphatic malformation and obstruction can lead to the imbalance between lymphatic transport capacity and interstitial fluid production. Upregulation of growth factors/cytokines in addition to mechanical interruption contributes to persistent lymphatic stasis.
Vascular endothelial growth factor-C
Vascular endothelial growth factor-C (VEGF-C) plays a central role in lymphangiogenesis and vascular permeability through VEGFR3 and VEGFR2 signaling pathways, respectively.17 Its role in lymphedema has been extensively studied as a central growth factor of lymphatic endothelial cells (LECs), promoting their survival, proliferation, and migration.18 Mutations in VEGFR-3, such as seen in Milroy disease, comprises a major fraction of patients with hereditary lymphedema.15 Several studies have reported an increase in the level of VEGF-C in both experimental and human clinical lymphedema.19,20
While VEGF-C is important in the regeneration of collateral lymphatic vessels, high levels of VEGF-C is shown to cause blood vessel growth and leakiness, further contributing to edema formation.21–23 In several studies, VEGF-C upregulation in lymphedema leads to lymphatic hyperplasia with less-effective drainage function that results in the accumulation and stasis of interstitial fluid.18,24,25
Gousopoulos et al. showed that overexpression of VEGF-C led to exacerbation of lymphedema with increased immune cell infiltration and vascular leakage while loss of VEGF-C had opposite effects, indicating that VEGF-C actively contributes to edema formation.20 Macrophages are thought to be a major cell type expressing VEGF-C during lymphangiogenesis. Lymphatic injury promotes recruitment of macrophages that upregulates VEGF-C/VEGFR3 signaling pathway and enhances lymphangiogenesis during initial stages of lymphedema.14 Early lymphangiogenesis in the setting of lymphedema is characterized as excessive generation of immature and leaky lymphatic vessels, which contributes to later development of lymphedema.26
Development of chronic lymphedema was historically thought to result from impaired development of collateral lymphatic vessels.27 Therefore, the role of VEGF-C in lymphatic regeneration has promoted multiple studies that investigate VEGF-C as a potential candidate for lymphedema treatment. Several studies have reported that exogenous administration of VEGF-C or VEGF-C gene therapy ameliorates secondary lymphedema of animal models with lymphatic regeneration.28–31
However, other studies demonstrated that exogenous VEGF-C does not improve functionality in the long term and therefore does not have lasting effects on lymphatics despite early lymphatic hyperplasia.32,33 These results indicate both harmful and beneficial roles of VEGF-C in secondary lymphedema. As VEGF-C is considered as a promising therapeutic target for lymphedema, controlling vascular side effects and exploring long-term therapeutic efficacy need to be addressed.34
Upregulation of inflammatory pathway
Inflammation plays a critical role in the pathology of lymphedema. Lymphatic injury and stasis lead to inflammatory cell infiltration around dermis and subcutaneous tissues. Infiltration of inflammatory cells, such as macrophages and CD4+ Th2 lymphocytes and subsequent release of cytokines, including IL-4, IL-13, TGF-β, and IFN-γ, are a hallmark of chronic inflammation in lymphedema.5 These cellular and molecular mechanisms are not only limited to inflammatory pathway but serve multiple functions in the progression of lymphedema.
Macrophages
Several studies have shown that lymphedema results in a significant increase in the number of macrophages.35–37 Macrophages play opposing and complex roles in inflammation, lymphatic function, and fibrosis with different spatial and temporal relationships.38 Macrophages are traditionally classified into M1 and M2 subtypes with pro- and anti-inflammatory functions, respectively.
As previously stated, macrophages are a key regulator of VEGF-C and therefore lymphatic regeneration. Studies have shown increased macrophage infiltration, especially M2 subtype, following lymphatic injury.37,39 Conditional depletion of macrophages after lymphatic injury led to increased CD4+ cell infiltration, decreased VEGF-C expression, impaired lymphatic function, and increased fibrosis.37,40 These results indicate that macrophages have a role in initial lymphangiogenesis to alleviate fluid accumulation by promoting collateral lymphatic formation.
On the contrary, Ogata et al. reported upregulation of VEGF-C from macrophages and formation of poorly functional lymphatic vessels.26 Furthermore, the authors have demonstrated that macrophage-depleted mice showed greatly reduced early lymphangiogenesis and diminished lymphedema development. Other studies also reported macrophages as proinflammatory and profibrotic.41 Macrophages are also known to express iNOS and IL-6, which will be further described in the later sections.27 These results indicate that subpopulations of macrophages may have opposing roles in the progression of the disease. Further studies are needed to elaborate more detailed mechanisms of macrophages in different stages of lymphedema.42
CD4+ T lymphocytes, Th2 subtype
CD4+ T cells, specifically Th2 subtype, play a central role throughout the pathologic processes of lymphedema, including inflammation, impaired lymphangiogenesis, and fibrosis. Several studies have shown an increased number of infiltrating CD4+ T cells, predominantly Th2 subtype, in lymphedematous tissues in animal and human lymphedema.26,35,36,43,44 These studies collectively indicate a critical role of CD4+ Th2 cells in inflammation and contribution to lymphedema development and progression. These studies have shown a positive linear correlation between the number of infiltrating CD4+ cells and severity of disease.
Th2 cells inhibit lymphatic growth and promote fibrosis by increasing collagen production and expression of profibrotic growth factors. Several authors have found that animals lacking all types of T cells (nude mice) or CD4+ T cells (knockout mice) or cytokines produced by CD4+ cells, IL4, or IL13 (neutralizing antibodies), fail to develop lymphedema after injury with decreased tissue fibrosis and improved lymphatic function.35,36,45 This response is specific to CD4+ T cells and was not observed in other cell types such as CD8+ and CD25+ cells.36 These findings were supported by García Nores et al., who demonstrated that in CD4-knockout mice, the adoptive transfer of CD4+ T cells lead to edema, deposition of fibroadipose tissue, and impaired lymphangiogenesis.45
Expression of T cell-derived cytokines, including IL-4 and IL-13, contributes to progression of lymphedema by impairing LEC survival, proliferation, migration, and tubule formation and also downregulates Prox1 and LYVE1.46 Savetsky et al. showed that antilymphangiogenic effects of IL4 and IL13 occurred despite the presence of prolymphangiogenic growth factors such as VEGF-C. These studies suggest that CD4+ T cell activation and Th2 differentiation could be a potential therapeutic target for lymphedema. Gardenier et al. observed that topical tacrolimus, an anti-T cell agent, improved lymphatic function and lymphangiogenesis in animal models of lymphedema. The authors suggested that increased lymphangiogenesis with tacrolimus was promoted by a decrease in antilymphangiogenic mechanisms by T cells and their cytokines.47
Transforming growth factor-beta1
Transforming growth factor-beta1 (TGF-β1), a multifunctional cytokine produced by inflammatory cells, is a well-known regulator of fibrosis in virtually every organ system. TGF-β1 is markedly increased in the lymphedematous limb compared with a normal limb.48 It is involved in various stages of lymphedema progression from suppression of collateral lymphatic vessel formation during acute phase to fibrosis in the chronic phase.49
Similar to IL-4 and IL-13, TGF-β1 has been shown to suppress LEC proliferation, migration, and tubule formation.50,51 These cytokines further decrease responsiveness of LECs to prolymphangiogenic growth factors.46 Inhibition of TGF-β1 improved lymphatic regeneration as well as decreased inflammation and Th2 cell migration after lymphatic ablation.48 Taken together, TGF-β1, similar to Th2 cytokines, exerts both direct and indirect roles in inhibiting lymphangiogenesis.
Interferon-γ
Interferon (IFN)-γ, a key Th1 cytokine, has an antilymphangiogenic role similar to TGF-β in lymphedema. Kataru et al. showed that IFN-γ treatment, through JAK-STAT pathway, downregulates Prox1, LYVE-1, and podoplanin in LECs and thus strongly inhibits lymphangiogenesis.52 Separate studies have supported these findings by showing that neutralization of IFN-γ reduces fibrosis and adipogenesis and increases lymphangiogenesis.26,33
Regulatory T cells
Regulatory T cells (Tregs) are immunoregulatory cells that mediate immune responses through various cytokine signaling pathways. Several authors have noted a significant increase in the infiltration of Tregs and Foxp3, a transcription factor specifically expressed by Treg, in lymphedematous skin of both animals and humans.36,52,53 Gousopoulos et al. showed that manipulating Tregs can control the pathology and external manifestations of inflammation. In the study, depletion of Tregs in mice led to the exacerbation of edema and the increased expression of profibrotic cytokines, such as IL-13, IL-4, IFN-γ, and TGF-β.
On the contrary, Treg amplification before lymphedema surgery resulted in a reduction in mice tail swelling and a decrease in macrophage infiltration.54 Despite its role of limiting lymphedema, Treg is also known to cause suppression of innate and adaptive immune responses. Clinically, this may contribute to recurrent soft tissue infections that are commonly seen in lymphedema patients.53 The experimental reduction of Tregs at the site of lymphatic injury restores, although not completely, these immune responses through significant increases in dendritic cell activation and T cell-mediated responses.53 Taken together, these findings suggest that Tregs have an important role in the homeostatic immune response and the restriction of pathologic changes in lymphedema in contrast to CD4+ Th2 cells.
Induced nitric oxide synthase
The contraction and dilation of lymphatic vessels under normal condition is regulated by nitric oxide (NO) gradients produced by endothelial nitric oxide synthase in LECs.55 NO is produced at specific locations and times, which allows for a robust and fluid contraction cycle. In contrast, inflammatory cells upregulate induced nitric oxide synthase (iNOS) after lymphatic injury.56 While this influx of CD11b+ macrophages that carry iNOS acts as a potential self-protection mechanism from autoreactive responses, it also disrupts the intrinsic NO gradient, thereby inhibiting lymphatic contractility and lymph transport.56
Several animal studies have demonstrated that iNOS inhibition and knockout mice are associated with continued strong lymphatic contractions during inflammatory conditions.56,57 However, although iNOS inhibition increases the frequency of lymphatic pumping and promotes dendritic cell migration, it does not entirely reverse lymphatic defects.57
Leukotriene B4
Leukotriene B4 (LTB4) is a lipid mediator in the innate immune response that promotes pathological inflammation by recruiting neutrophils, macrophages, and CD8+ cytotoxic T cells.58 Increased levels of plasma LTB4 are exhibited in both experimental and human clinical lymphedema.59 Studies have shown that concentrations of lymph LTB4 are in the prolymphangiogenic range immediately following surgery, suggesting its role in lymphatic repair, but subsequently rise to antilymphangiogenic levels.58,59 Tian et al. showed that at the antilymphangiogenic levels, LTB4 inhibits VEGFR3 and Notch signaling pathways, which are important for lymphangiogenesis and lymphatic maintenance, and therefore exacerbates lymphatic dysfunction.
Several authors have demonstrated that LTB4 antagonism, specifically with ketoprofen, in experimental murine lymphedema can ameliorate inflammation and reverse fibrosis.59,60 Ketoprofen is a nonsteroidal anti-inflammatory agent (NSAID) that inhibits the 5-lipoxygenase (5-LO) pathway from which LTB4 is formed, thus reversing edema and promoting VEGF-C-mediated lymphangiogenesis.60 Clinical pilot studies by Rockson et al. showed potential therapeutic benefits of ketoprofen in humans with both primary and secondary lymphedema. Targeted anti-inflammatory therapy with ketoprofen in the trial resulted in a reduction in skin thickness and an improvement in histopathology, including dermal thickness, intracellular mucin deposits, collagen accumulation, and perivascular inflammation.61 LTB4 antagonist represents a promising approach to treating lymphedema.
Adipocyte differentiation and proliferation
Adipose tissue deposition has been observed in both experimental and clinical lymphedema, which contribute to the swollen appearance of limbs.62,63 The pathology of adipose tissue mimics that seen in obesity, where adipocytes undergo hypertrophy or hyperplasia due to an imbalance in regulatory cytokines.62
Macrophage
Following initial lymphatic injury, the subsequent imbalance of cellular and inflammatory components of adipose tissue exacerbates the natural response to lymphedema. One significant feature of lymphedema is infiltration of immune cells, including macrophages. Macrophages are generally classified as M1 and M2 subtypes, which represent pro- and anti-inflammatory, respectively. M1 and M2 are thought to represent two extremes of phenotypes that macrophages assume. M2 macrophages are generally more prevalent in lean adipose tissue, whereas M1 macrophages are detected to a greater degree in obese adipose tissue and low-grade chronic inflammation.64
Lymphedematous tissues express a lower number of M2 macrophages compared with M1, and this imbalance may contribute to impaired immune function in lymphedema.63 Macrophages interact with adipose tissue and contribute to adipose tissue inflammation by producing inflammatory mediators, including hypoxia-inducible factor 1-alpha (HIF-1α), interleukin-6 (IL-6), and adiponectin.65 The interaction of these tissue dependent signals and their relationship to lymphedema will be described in the following sections.
Hypoxia-inducible factor-1α
The development of hypoxia and subsequent activation of HIF-1α initiates immune cell migration and fibrosis, further contributing to pathological changes seen in lymphedema.63,66 HIF-1α, a transcription factor involved in angiogenesis and tumor growth, has been largely targeted by oncological studies.67
While its role in lymphangiogenesis has been less extensively studied, a lymphatic stasis in mouse model exhibited the expression HIF-1α by macrophages in the early inflammatory response. Inhibition of HIF-1α resulted in decreased signs of lymphatic regeneration during the first 6 weeks of wound healing.68 However, the role of HIF-1α in clinical lymphedema requires further investigation. In a study of lymphedematous tissue of nine patients, HIF-1α was only expressed at similar levels as in normal tissue.69
While animal studies established the role of HIF-1α in the early lymphatic response, this transcription factor may not necessarily be expressed at later stages of lymphedema, which presents clinically as a chronic condition. Large-scale studies may be required due to the heterogeneous nature of clinical studies compared with controlled animal studies. Perhaps detection of HIF-1α may be used as a supplemental tool to confirm early stages of lymphedema.
Interleukin-6
Another key regulatory hormone of adipose homeostasis is IL-6, which has been observed to be elevated in lymphedema. Similar to the previously described inflammatory mediator HIF-1α, IL-6 was also shown to be primarily expressed by macrophages while regulated by CD4+ T cells.42,70
In patients with lymphedema and animal models, levels of IL-6 as well as its downstream mediators were significantly increased.42 Loss of IL-6 resulted in increased adipose deposition, which suggests that the mechanism of IL-6 in response to inflammation serves to decrease adipose deposition.42 In a recent study, IL-6 was measured in adipose tissues of mice to demonstrate that inflammation precedes adipogenesis, whereas the latter begins 3 weeks after lymphatic injury.71 These findings elucidate a modulatory role of IL-6 in response to inflammation and lymphedema-associated adipose deposition.
Adiponectin, PPAR-γ, and CEBP-α
Several studies noted that the lymphatic structures interact with surrounding adipose tissue. In animal models, lymphatic obstruction and stasis results in subcutaneous fat deposition with increased number of adipocytes and lipid accumulation.65,72 Several studies have shown that lymphatic fluid stasis is associated with increased expression of adiponectin and adipogenic transcription factors, including proliferator-activated receptor gamma (PPAR-γ) and CCAAT/enhancer-binding protein-alpha (CEBP-α).
One significant transcription factor is adiponectin, an endocrine hormone primarily secreted from adipose tissue that exerts organ-specific oxidative and anti-inflammatory effects.73 In the context of lymphatic biology, adiponectin is a late marker of activated adipocytes and elevated concentrations were observed in both animal lymphedematous tissue and serum of patients with lymphedema.72,74 In a study with both mouse tail and axillary lymph node dissection lymphedema models, adiponectin expression was characterized by a gradient pattern, with greatest degree of expression observed just distal to the site of lymphatic injury.72 In a study of one hundred patients with chronic lymphedema, adiponectin was detected at nearly two-fold.74
While adiponectin may be a marker specific to adipocytes, an adiponectin-knockout mouse lymphedema model demonstrated that supplemental adiponectin resulted in reduced thickness of injured tails and increased lymphatic endothelial cell viability through the AMP-activated protein kinase pathway.75 In this study, the authors suggested that adiponectin functions to promote lymphangiogenic cellular responses to lymphedema. The proliferation of adipose tissue in response to lymphatic injury suggest a pivotal role for adipocyte involvement in wound healing and lymphatic regeneration.
Fibrosis and sclerosis of lymphatic vessels
Fibrosis is one of the hallmarks of secondary lymphedema and has a significant impact on long-term outcomes due to its disfiguring and irreversible effects. Lymphedema has been described as a fibroproliferative disorder because of its highly variable sequelae and progressive nature.76 In addition, once fibrosis has initiated, the excessive deposition of extracellular matrix components will continuously progress.
Transforming growth factor-β
TGF-β1 exhibits a wide range of biological functions, including the upregulation of fibrosis by promoting extracellular matrix synthesis.77–79 As previously noted, TGF-β1 is secreted by many cell types, including macrophages and Th2 cells. TGF-β1 promotes fibrosis through various pathological mechanisms.78 Activated TGF-β1 can directly upregulate the expression of profibrotic cytokines, such as connective tissue growth factor.78,80 TGF-β1 can also form a complex with fibroblasts by binding to type II and III receptors and then phosphorylate its downstream effector, Smad.79 Lastly, TGF-β1 can inhibit fibroblast expression of matrix metalloproteinase-1 through Smad3 and Smad4, which prevents the degradation of collagen.81
Mast cell
Although it is well known that the inflammatory response is integral to understanding fibrosis, Di et al. proposed a mechanism that is centered around mast cells and chymase-mediated TGF-β1 activation. The authors elucidated the role of mast cells in fibrosis by analyzing skin biopsies of patients with stage II or III lymphedema in the lower limb. Lymphedematous limbs demonstrated an increased number of mast cells near blood and lymphatic vessels compared with that of control limbs.82
In addition, biochemical analyses revealed that there were significantly higher levels of both chymase, a chymotrypsin serine protease released by mast cells, and TGF-β1, a key mediator of tissue fibrosis.82 The authors speculated that fibrosis observed in the lymphedematous limbs could be due to the biochemical pathways involving chymase and TGF-β1. Previous studies have demonstrated that mast cells initially release an inactive complex containing mature TGF-β1, latency-associated peptide TGF-β1 (LAP TGF-β1), and latent TGF-β-binding protein (LTβP).83,84 Chymase subsequently disrupts the noncovalent bonds between mature TGF-β1 and LAP TGF-β1, which then releases the biologically active TGF-β1. Taken together, these findings suggest that mast cells and its associated enzyme, chymase, play a crucial role in promoting TGF-β1-mediated fibrosis.
Loss of functional lymphatics
The lymphatic system is responsible for maintaining fluid homeostasis in the body by collecting interstitial fluid and draining it to the central circulation. Various intrinsic and extrinsic mechanisms work collectively to achieve tissue fluid homeostasis. Extrinsic factors originate from surrounding tissues, ranging from pulsatile arterial blood flow to skeletal muscle contractions.85 Extrinsic forces are variable throughout the body and less reliable than intrinsic forces. The intrinsic mechanism involves a sophisticated system of myogenic and valvular activities that actively respond to local physical stimuli and modulate lymph transport.86 The main components of the intrinsic mechanism are intraluminal valves and phasic contractions of SMCs, which are fundamental for maintaining forward propulsion of lymph.87
Lymphatic valves
Lymphatic valves serve to prevent backflow of lymph and break up the total hydrostatic pressure into a series of smaller pressure gradients to aid lymphatic contractions.85 Following lymphatic injury, accumulation of lymphatic fluid and ineffective clearance result in dilation of lymphatic vessels and exacerbation of lymphatic stasis, contributing to valvular incompetence. Various lymphatic imaging modalities, including lymphoscintigraphy have demonstrated increased vessel diameter and dermal backflow in patients with lymphedema, further supporting valvular incompetence in lymphedema.88
Several in vitro and in vivo studies have demonstrated a crucial role of shear stress from unidirectional lymph flow in the development of lymphatic valves.89–92 These studies suggest that mechanotransduction in response to lymph flow as well as upregulation of transcriptional factors, such as Prox1 and Foxc2, cooperatively regulate the formation and maintenance of lymphatic valves.91 Interestingly, several studies have identified single nucleotide polymorphisms in these genes in patients who developed cancer-related lymphedema, suggesting a potential role for genetic predisposition in clinical lymphedema development.92
As the progression of lymphedema is characterized by markedly reduced or absent lymph flow, impaired mechanical stimuli from altered lymph flow may contribute to degeneration and apoptosis of lymphatic valves.85 However, studies that assess valvular defects or regression in clinical lymphedema are lacking and thus, further investigation in lymphedema patients is necessary.
Smooth muscle cells
As lymphedema progresses, contractile function of SMCs is progressively lost and tissue remodeling occurs. As mentioned previously, inflammatory response in lymphedema is associated with an increased gradient of iNOS, which produces NO, a potent inhibitor of lymphatic contractions. In addition, lymphatic injury produces increased outflow resistance in the downstream lumen. Modi et al. used lymphoscintigraphy to quantify lymphatic contractility in patients with breast cancer-related lymphedema and observed that lymphatic contractile force is weakened proportional to the degree of edema. Similar to cardiac failure, increase in lymphatic afterload leads to the failure of lymphatic contractility.94 Furthermore, studies have shown that SMCs transform to a synthetic form, losing their inherent contractile ability and instead contributing to collagen fiber synthesis.87,95 These phenotypic changes of SMCs are followed by buildup of collagen fibers and remodeling of the surrounding tissue. In the end stage, the buildup of fibrotic components leads to the narrowing or even complete occlusion, “lymphaticosclerosis” of lymphatic vessels.95
SUMMARY
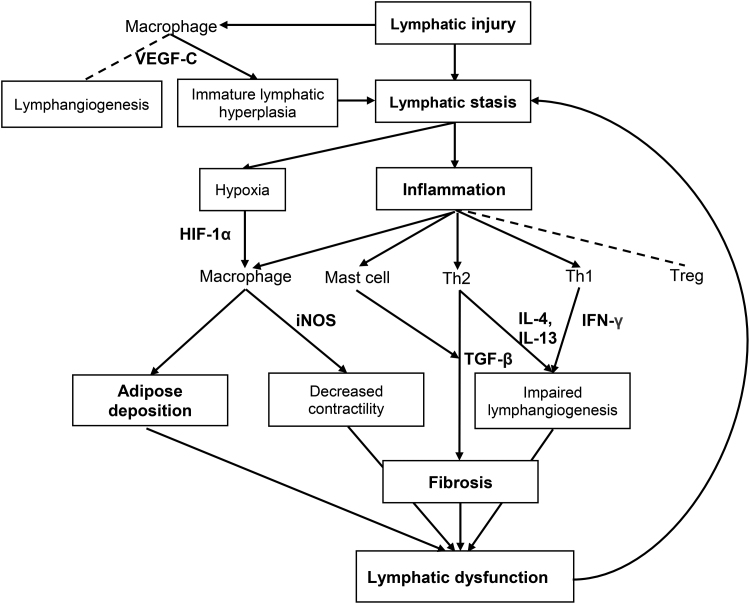
Recent research advances have shed light into cellular and molecular mechanisms involved in the pathophysiology of lymphedema (Fig. 2). Although the exact sequence remains to be further elucidated, lymphedema occurs with lymphatic injury as an inciting event with a series of downstream events, including lymphatic stasis, inflammation, adipose tissue deposition, and fibrosis (Table 1). These changes further exacerbate lymphatic stasis, setting up a vicious cycle.
Figure 2.
Cellular and molecular mechanisms involved in the pathology of lymphedema. Lymphatic stasis promotes upregulation of inflammatory response and adipose deposition. Inflammatory cells, especially macrophages and CD4+ T cells, and their cytokines contribute to lymphatic leakiness, lymphatic dysfunction, and fibrosis. Arrow indicates mechanisms that contribute to the progression of chronic lymphedema. Dotted line indicates mechanisms that counteract the progression of the disease.
Table 1.
Summary of pathologic events and associated cellular and molecular factors in lymphedema
| Pathologic Events | Cellular or Molecular Factors | Pathology | References |
|---|---|---|---|
| Lymphatic stasis | VEGF-C | Promote excessive generation of immature and leaky lymphatics during initial stages of lymphedema | 20,24,26,32,96 |
| Promote vascular permeability | 21–23 | ||
| Upregulation of inflammatory pathway | Macrophage | Promote VEGF-C expression, antifibrotic | 37 |
| Proinflammatory, profibrotic | 41 | ||
| CD4+ Th2 cell | Promote chronic inflammatory response | 26,35,36,44 | |
| Antilymphangiogenic | 46 | ||
| TGF-β1 | Antilymphangiogenic | 48–51 | |
| IFN-γ | Antilymphangiogenic | 33 | |
| iNOS | Impair contractile function of lymphatics | 56,57,97 | |
| LTB4 | Inhibit VEGFR3, Antilymphangiogenic | 58–61 | |
| Treg | Ameliorate inflammation, fibrosis, and lymphatic function | 53,54 | |
| Adipocyte differentiation and proliferation | Macrophage | Promote adipose tissue Inflammation |
36,63,73,98 |
| IL-6 | Reduce adipose tissue deposition | 42 | |
| HIF-1 | Promote inflammation | 99 | |
| Fibrosis | TGF-β | Profibrotic | 48–51 |
| Mast cell | Profibrotic through chymase | 55 | |
| Loss of functional lymphatics | Lymphatic valve | Dysfunction and degeneration | 85,87,100 |
| SMC | Loss of contractile function and tissue remodeling | 87,95,100 |
Inflammation mediated by macrophages and Th2 cells is considered a critical event in chronic lymphedema development. Macrophages are involved in various stages of lymphedema: promoting lymphangiogenesis through VEGF-C; impairing contractile capacity of lymphatics through iNOS; and contributing to adipose tissue inflammation through HIF-1α, adiponectin, and IL-6.
Although VEGF-C shows great potential to improve lymphedema by promoting lymphangiogenesis, this mechanism has undesirable effects of development of immature and leaky collateral lymphatics during early stages of lymphedema. Another important regulator of inflammation, CD4+ T lymphocytes produce cytokines, IL-4, IL-13, IFN-γ, and TGF-β1, and contribute to antilymphangiogenesis and fibrosis. Several studies have attempted to explore other types of cells or cytokines that may contribute to this process, including a recent study by Di et al., which elucidated the role of mast cells in TGF-β1-mediated fibrosis. Resulting lymphatic stasis and inflammation drives adipocyte differentiation and proliferation. The deposition of adipose tissue is known to contribute to tissue swelling. Interestingly, activated adipocytes and adipocyte-specific marker, adiponectin, may be involved in lymphatic regeneration and therefore wound healing. The intrinsic function of lymphatics is also altered with the progression of lymphedema, including valvular and contractile functions.
Despite recent advances in the knowledge, our current understanding of pathological events in lymphedema is still limited.
Further investigation is warranted to determine how various inflammatory responses interact with each other in the pathogenesis of this debilitating disease. The cells and molecules described in this review have been explored as potential therapeutic targets for lymphedema. Nevertheless, determining the best treatment strategy remains a challenge given complex pathophysiological changes and various clinical manifestations in each patient. Given its complex nature, understanding various mechanisms underlying the pathology will facilitate the development of novel therapeutic approaches for lymphedema.
TAKE-HOME MESSAGES
The pathology of lymphedema involves a complex sequence of events involving lymphatic stasis, leaky lymphatic vessel formation, chronic inflammation, adipose tissue deposition, fibrosis, and loss of functional lymphatics.
Chronic inflammation mediated by macrophages and CD4+ Th2 cells, and their cytokines plays a central role in the pathology of lymphedema.
Lymphatic stasis and inflammation result in valvular dysfunction, impaired contractility, and tissue remodeling.
A better understanding of the pathology will lead to the development of effective therapeutic strategies.
Abbreviations and Acronyms
- HIF-1
hypoxia-inducible factor-1
- IFN-γ
interferon-gamma
- IL-13
interleukin-13
- IL-4
interleukin-4
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- LEC
lymphatic endothelial cell
- LTB4
leukotriene B4
- SMC
smooth muscle cell
- TGF-β1
transforming growth factor-beta1
- Th1
T helper 1
- Th2
T helper 2
- Tregs
T regulatory cells
- VEGF-C
vascular endothelial growth factor C
- VEGFR2
vascular endothelial growth factor receptor 2
- VEGFR3
vascular endothelial growth factor receptor 3
AUTHOR CONFIRMATION
Conceptualization: A.K.W., C.J.S.
Methodology: A.K.W., C.J.S.
Project Administration and Supervision: A.K.W., C.J.S.
Literature Search and Review: C.J.S., S.W., J.F.H., R.P.Y.
Writing – Original Draft Preparation: C.J.S., S.W., J.F.H., R.P.Y.
Writing – Review & Editing: C.J.S., S.W., J.F.H., R.P.Y., A.K.W.
AUTHOR DISCLOSURE AND GHOSTWRITING
No competing financial interests exist for any of the authors listed. The content of this article was expressly written by these authors. No ghostwriters were used to write this article. Cynthia J. Sung, Sarah Wang, Jerry F. Hsu, Roy P. Yu, and Alex K. Wong declare that they have no conflicts of interest.
FUNDING STATEMENT
Research reported in this publication was supported by the National Institutes of Health R01HL157626, R03HL154300, and K08HL132110; and the American Society for Reconstructive Microsurgery (ASRM) and Lymphatic Education and Research Network's (LE&RN) Combined Pilot Lymphedema Research Grant.
ABOUT THE AUTHORS
Cynthia J. Sung, BS is a third-year medical student at the Keck School of Medicine of USC and is interested in a career in plastic and reconstructive surgery. Sarah Wang is an undergraduate student at the University of Southern California (USC) and is interested in a career in medicine. Jerry F. Hsu, BS is a second-year medical student at Case Western Reserve University School of Medicine and is currently a research fellow in Dr. Wong's laboratory at the Keck School of Medicine of USC. Roy P. Yu, BS is a first-year medical student at the Keck School of Medicine of USC, who conducts research in Dr. Wong's laboratory. Alex K. Wong, MD is a Professor and Chief of the Division of Plastic Surgery, Department of Surgery, City of Hope National Medical Center. Dr. Wong is also an Adjunct Clinical Professor of Surgery in the Division of Plastic and Reconstructive Surgery at Keck School of Medicine of USC.
REFERENCES
- 1. Schulze H, Nacke M, Gutenbrunner C, Hadamitzky C. Worldwide assessment of healthcare personnel dealing with lymphoedema. Health Econ Rev 2018;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sleigh BC, Manna B. Lymphedema. [Updated 2021 Jun 4]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. [Google Scholar]
- 3. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 4. Bowman C, Piedalue K-A, Baydoun M, Carlson L-E. The quality of life and psychosocial implications of cancer-related lower-extremity lymphedema: a systematic review of the literature. J Clin Med 2020;9:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ly CL, Kataru RP, Mehrara BJ. Inflammatory manifestations of lymphedema. Int J Mol Sci 2017;18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol 2009;7:97–100. [DOI] [PubMed] [Google Scholar]
- 7. Garza R III, Skoracki R, Hock K, Povoski SP. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017;17:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mihara M, Hara H, Tange S, et al. Multisite lymphaticovenular bypass using supermicrosurgery technique for lymphedema management in lower lymphedema cases. Plast Reconstr Surg 2016;138:262–272. [DOI] [PubMed] [Google Scholar]
- 9. Hanson SE, Chang EI, Schaverien MV, Chu C, Selber JC, Hanasono MM. Controversies in surgical management of lymphedema. Plast Reconstr Surg Glob Open 2020;8:e2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tavian D, Missaglia S, Maltese PE, et al. FOXC2 disease-mutations identified in lymphedema-distichiasis patients cause both loss and gain of protein function. Oncotarget 2016;7:54228–54239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schaverien MV, Aldrich MB. New and emerging treatments for lymphedema. Semin Plast Surg 2018;32:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis, and treatment guidelines. J Am Acad Dermatol 2008;59:324–331. [DOI] [PubMed] [Google Scholar]
- 13. Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol 2017;77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 14. Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest 2014;124:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rauniyar K, Jha SK, Jeltsch M. Biology of vascular endothelial growth factor C in the morphogenesis of lymphatic vessels. Front Bioeng Biotechnol 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mellor RH, Hubert CE, Stanton AW, et al. Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation 2010;17:281–296. [DOI] [PubMed] [Google Scholar]
- 17. Heinolainen K, Karaman S, D'Amico G, et al. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ Res 2017;120:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997;276:1423–1425. [DOI] [PubMed] [Google Scholar]
- 19. Jensen MR, Simonsen L, Karlsmark T, Lanng C, Bülow J. Higher vascular endothelial growth factor-C concentration in plasma is associated with increased forearm capillary filtration capacity in breast cancer-related lymphedema. Physiol Rep 2015;3:e12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gousopoulos E, Proulx ST, Bachmann SB, et al. An important role of VEGF-C in promoting lymphedema development. J Invest Dermatol 2017;137:1995–2004. [DOI] [PubMed] [Google Scholar]
- 21. Saaristo A, Veikkola T, Tammela T, et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 2002;196:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saaristo A, Veikkola T, Enholm B, et al. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J 2002;16:1041–1049. [DOI] [PubMed] [Google Scholar]
- 23. Enholm B, Karpanen T, Jeltsch M, et al. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res 2001;88:623–629. [DOI] [PubMed] [Google Scholar]
- 24. Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 2006;72:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 2001;20:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogata F, Fujiu K, Matsumoto S, et al. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4(+) T Cells Drives the Pathogenesis of Lymphedema. J Invest Dermatol 2016;136:706–714. [DOI] [PubMed] [Google Scholar]
- 27. Kataru RP, Baik JE, Park HJ, et al. Regulation of immune function by the lymphatic system in lymphedema. Front Immunol 2019;10:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin DP, An A, Liu J, Nakamura K, Rockson SG. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol 2009;7:47–57. [DOI] [PubMed] [Google Scholar]
- 29. Yoon YS, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 2003;111:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szuba A, Skobe M, Karkkainen MJ, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 2002;16:1985–1987. [DOI] [PubMed] [Google Scholar]
- 31. Visuri MT, Honkonen KM, Hartiala P, et al. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis 2015;18:313–326. [DOI] [PubMed] [Google Scholar]
- 32. Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res 2005;96:1193–1199. [DOI] [PubMed] [Google Scholar]
- 33. Zampell JC, Avraham T, Yoder N, et al. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am J Physiol Cell Physiol 2012;302:C392–C404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartiala P, Suominen S, Suominen E, et al. Phase 1 Lymfactin. J Plast Reconstr Aesthet Surg 2020;73:1612–1621. [DOI] [PubMed] [Google Scholar]
- 35. Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 2013;27:1114–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 2012;7:e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghanta S, Cuzzone DA, Torrisi JS, et al. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol 2015;308:H1065–H1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zampell JC, Elhadad S, Avraham T, et al. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol 2012;302:C709–C719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gardenier JC, Hespe GE, Kataru RP, et al. Diphtheria toxin-mediated ablation of lymphatic endothelial cells results in progressive lymphedema. JCI Insight 2016;1:e84095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 2010;30:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cuzzone DA, Weitman ES, Albano NJ, et al. IL-6 regulates adipose deposition and homeostasis in lymphedema. Am J Physiol Heart Circ Physiol 2014;306:H1426–H1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li CY, Kataru RP, Mehrara BJ. Histopathologic features of lymphedema: a molecular review. Int J Mol Sci 2020;21:2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gousopoulos E, Proulx ST, Scholl J, Uecker M, Detmar M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am J Pathol 2016;186:2193–2203. [DOI] [PubMed] [Google Scholar]
- 45. García Nores GD, Ly CL, Cuzzone DA, et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat Commun 2018;9:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Savetsky IL, Ghanta S, Gardenier JC, et al. Th2 cytokines inhibit lymphangiogenesis. PLoS One 2015;10:e0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gardenier JC, Kataru RP, Hespe GE, et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat Commun 2017;8:14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Avraham T, Daluvoy S, Zampell J, et al. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 2010;177:3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sano M, Hirakawa S, Suzuki M, et al. Potential role of transforming growth factor-beta 1/Smad signaling in secondary lymphedema after cancer surgery. Cancer Sci 2020;111:2620–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clavin NW, Avraham T, Fernandez J, et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 2008;295:H2113–H2127. [DOI] [PubMed] [Google Scholar]
- 51. Oka M, Iwata C, Suzuki HI, et al. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 2008;111:4571–4579. [DOI] [PubMed] [Google Scholar]
- 52. Kataru RP, Kim H, Jang C, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011;34:96–107. [DOI] [PubMed] [Google Scholar]
- 53. García Nores GD, Ly CL, Savetsky IL, et al. Regulatory T cells mediate local immunosuppression in lymphedema. J Invest Dermatol 2018;138:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gousopoulos E, Proulx ST, Bachmann SB, et al. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 2016;1:e89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dayan JH, Ly CL, Kataru RP, Mehrara BJ. Lymphedema: pathogenesis and novel therapies. Annu Rev Med 2018;69:263–276. [DOI] [PubMed] [Google Scholar]
- 56. Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A 2011;108:18784–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Torrisi JS, Hespe GE, Cuzzone DA, et al. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep 2016;6:19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang X, Nicolls MR, Tian W, Rockson SG. Lymphatic dysfunction, leukotrienes, and lymphedema. Annu Rev Physiol 2018;80:49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tian W, Rockson SG, Jiang X, et al. Leukotriene B. Sci Transl Med 2017;9:389. [DOI] [PubMed] [Google Scholar]
- 60. Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One 2009;4:e8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rockson SG, Tian W, Jiang X, et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018;3:e123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Azhar SH, Lim HY, Tan B-K, Angeli V. The unresolved pathophysiology of lymphedema. Front Physiol 2020;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tashiro K, Feng J, Wu S-H, et al. Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol 2017;177:158–167. [DOI] [PubMed] [Google Scholar]
- 64. Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009;58:2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg 2012;129:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Buechler C, Krautbauer S, Eisinger K. Adipose tissue fibrosis. World J Diabetes 2015;6:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 2007;26:281–290. [DOI] [PubMed] [Google Scholar]
- 68. Zampell JC, Yan A, Avraham T, Daluvoy S, Weitman ES, Mehrara BJ. HIF-1alpha coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J 2012;26:1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Couto RA, Kulungowski AM, Chawla AS, Fishman SJ, Greene AK. Expression of angiogenic and vasculogenic factors in human lymphedematous tissue. Lymphat Res Biol 2011;9:143–149. [DOI] [PubMed] [Google Scholar]
- 70. Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 1993;54:1–78. [DOI] [PubMed] [Google Scholar]
- 71. Cuadrado GA, de Andrade MFC, Ariga SK, de Lima TM, Souza HP. Inflammation precedes fat deposition in an experimental model of lymphedema. Lymphat Res Biol 2021;19:116–125. [DOI] [PubMed] [Google Scholar]
- 72. Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot Andrade M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg 2012;129:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol 2018;8:1031–1063. [DOI] [PubMed] [Google Scholar]
- 74. Zaleska MT, Olszewski WL. Serum immune proteins in limb lymphedema reflecting tissue processes caused by lymph stasis and chronic dermato-lymphangio-adenitis (Cellulitis). Lymphat Res Biol 2017;15:246–251. [DOI] [PubMed] [Google Scholar]
- 75. Shimizu Y, Shibata R, Ishii M, et al. Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J Am Heart Assoc 2013;2:e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kataru RP, Wiser I, Baik JE, et al. Fibrosis and secondary lymphedema: chicken or egg? Transl Res 2019;209:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol 2002;14:681–685. [DOI] [PubMed] [Google Scholar]
- 78. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors 2011;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–338. [DOI] [PubMed] [Google Scholar]
- 80. Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J 1999;13:1774–1786. [PubMed] [Google Scholar]
- 81. Yuan W, Varga J. Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem 2001;276:38502–38510. [DOI] [PubMed] [Google Scholar]
- 82. Di S, Ziyou Y, Liu NF. Pathological changes of lymphedematous skin: increased mast cells, related proteases, and activated transforming growth factor-beta1. Lymphat Res Biol 2016;14:162–171. [DOI] [PubMed] [Google Scholar]
- 83. Lindstedt KA, Wang Y, Shiota N, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J 2001;15:1377–1388. [DOI] [PubMed] [Google Scholar]
- 84. Khalil N. TGF-beta: from latent to active. Microbes Infect 1999;1:1255–1263. [DOI] [PubMed] [Google Scholar]
- 85. Iyer D, Jannaway M, Yang Y, Scallan JP. Lymphatic valves and lymph flow in cancer-related lymphedema. Cancers (Basel) 2020;12:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Solari E, Marcozzi C, Negrini D, Moriondo A. Lymphatic vessels and their surroundings: how local physical factors affect lymph flow. Biology (Basel) 2020;9:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012;7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O'Donnell TF, Rasmussen JC, Sevick-Muraca EM. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord 2017;5:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sweet DT, Jiménez JM, Chang J, et al. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest 2015;125:2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sabine A, Agalarov Y, Maby-El Hajjami H, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 2012;22:430–445. [DOI] [PubMed] [Google Scholar]
- 91. Sabine A, Bovay E, Demir CS, et al. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 2015;125:3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang Y, Cha B, Motawe ZY, Srinivasan RS, Scallan JP. VE-cadherin is required for lymphatic valve formation and maintenance. Cell Rep 2019;28:2397–2412.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Visser J, van Geel M, Cornelissen AJM, van der Hulst RRWJ, Qiu SS. Breast cancer-related lymphedema and genetic predisposition: a systematic review of the literature. Lymphat Res Biol 2019;17:288–293. [DOI] [PubMed] [Google Scholar]
- 94. Modi S, Stanton AW, Svensson WE, Peters AM, Mortimer PS, Levick JR. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 2007;583(Pt 1):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ogata F, Fujiu K, Koshima I, Nagai R, Manabe I. Phenotypic modulation of smooth muscle cells in lymphoedema. Br J Dermatol 2015;172:1286–1293. [DOI] [PubMed] [Google Scholar]
- 96. Tammela T, Petrova TV, Alitalo K. Molecular lymphangiogenesis: new players. Trends Cell Biol 2005;15:434–441. [DOI] [PubMed] [Google Scholar]
- 97. Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 2013;591:2139–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zampell JC, Aschen S, Weitman ES, et al. Regulation of adipogenesis by lymphatic fluid stasis: part I. Adipogenesis, fibrosis, and inflammation. Plast Reconstr Surg 2012;129:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zampell JC, Yan A, Avraham T, Daluvoy S, Weitman ES, Mehrara BJ. HIF-1α coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J 2012;26:1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 2016;594:5749–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]