To the Editor:
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has led to an unprecedented race to develop vaccines, one of which is the Moderna mRNA-1273 vaccine.1 Liver injury from autoimmune hepatitis (AIH) triggered by the Moderna vaccine has been reported.[2], [3], [4], [5], [6] We present a case of liver injury post-Moderna vaccination, not presenting in an AIH pattern.
A 34-year-old Burmese man presented on 18th June 2021 with a 2-week history of pruritus, fever and jaundice. He received the Moderna vaccine on 2nd June 2021. He was prescribed antihistamines and paracetamol by his family physicians, but symptoms persisted. He denied traditional or over-the-counter medication use. He drank socially and had no significant family history. He had been on SARS-CoV-2 surveillance with fortnightly nasopharyngeal PCR testing; the most recent tests done 3 days before vaccination and 11 days after vaccination. On examination, he was jaundiced with excoriations on his extremities. There were no stigmata of chronic liver disease and abdominal examination was normal.
Investigations showed: haemoglobin 15.4 g/dl, white blood cell 6x10ˆ9/L, eosinophils 0.04x10ˆ9/L, platelets 353x10ˆ9/L. Liver function tests showed bilirubin 132 μmol/L, direct bilirubin 122 μmol/L, alanine aminotransferase 223 U/L, aspartate aminotransferase 111 U/L, gamma-glutamyltransferase 98 U/L, alkaline phosphatase 149 U/L, total protein 84 g/L, albumin 46 g/L, globulin 38 g/L. Coagulation profile was normal: prothrombin time 9.9 seconds and international normalised ratio 0.91.
Extensive investigations performed were normal: this included serologies and PCRs for hepatitis A, B, C and E, cytomegalovirus, Ebstein-Barr virus, and herpes simplex virus. AIH screening included anti-smooth muscle antibody 5 units (ELISA, negative <20 units, positive >20 units), negative anti-liver kidney microsomal antibody (indirect immunofluorescence), anti-nuclear antibody ratio 0.11 (ELISA, positive ≥1), anti-mitochondrial antibody 2 units/ml (ELISA, positive ≥20 units/ml). IgG 14.6 (7.0-16.0 g/L), IgM 0.94 (0.40-2.30 g/L), ferritin 206 (47-452 μg/L) and caeruloplasmin 32.4 (15.0-45.0 mg/dl) were normal. Leptospira IgM and Weil-Felix serology returned non-reactive. Computed tomography of the abdomen and magnetic resonance cholangiopancreatography were unremarkable.
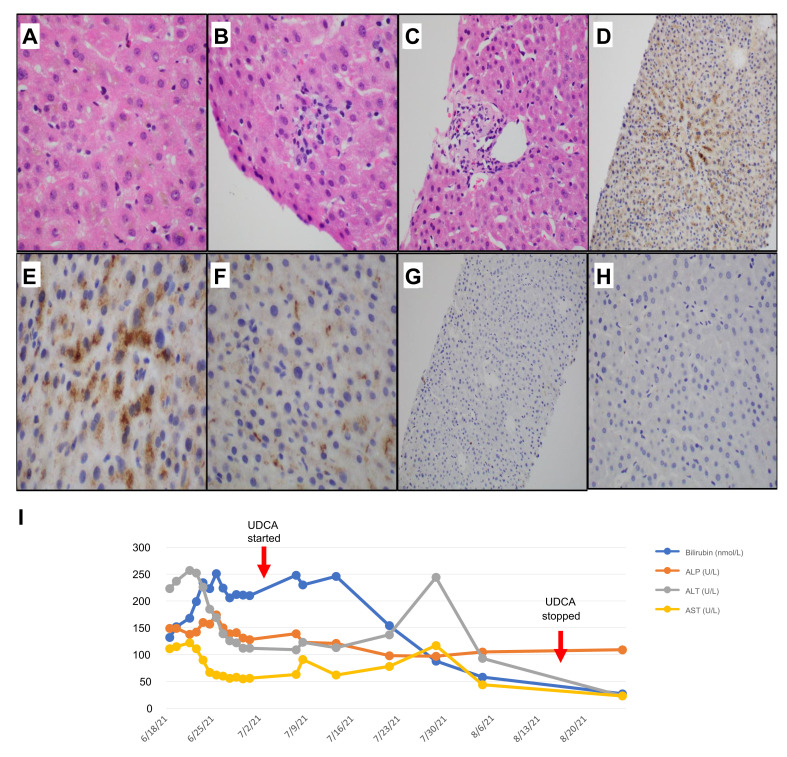
Liver biopsy was performed on day 6 of presentation (day 22 following vaccination) and demonstrated acute lobular hepatitis with cholestasis. There was hepatocytic and canalicular cholestasis, accompanied by predominantly lobular inflammation (Fig. 1 A-B). Few portal tracts showed mixed inflammatory infiltrate with scattered eosinophils (Fig. 1C).
Fig. 1.
Liver core biopsy histology.
Core biopsy showing (A) cholestasis, with scattered foci of (B) lobular inflammation and (C) mild portal inflammation with scattered eosinophils. Immunohistochemical staining with antibodies against SARS-CoV-2 spike protein reveals (D) patchy centrovenular positivity within the cytoplasm of (E) hepatocytes in a granular cytoplasmic pattern, as well as within scattered (F) Kupffer cells in the sinusoids. Immunohistochemical staining with antibodies against SARS-CoV-2 nucleocapsid protein shows negative staining (G and H). (I) Trend in liver function tests. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UDCA, ursodeoxycholic acid. (This figure appears in color on the web.)
Immunohistochemistry (IHC) was performed. Tissue sections were cut onto Bond Plus slides (Leica Biosystems, Richmond) and heated at 60oC for 20 min. Tissue slides underwent deparaffinization, rehydration and heat-induced epitope retrieval with Bond Epitope Retrieval Solutions using a Leica Bond Max autostainer (Leica Biosystems, Germany) before endogenous peroxidase blocking (Leica Biosystems, Newcastle). Slides were labelled with antibodies targeting the SARS-CoV-2 spike protein (GeneTex, Cat#GTX632604, 1A9) at 1:8,000 dilution (ER2, pH 9.0, #AR9640) and SARS-CoV-2 nucleocapsid (Novus Biologicals, Cat#NB100-56576) at 1:250 dilution (ER1, pH 6.0, #AR9961). Positive and negative controls were included. Indirect IHC was performed using secondary antibody amplification with post-primary antibody and Bond Polymer Refine Detection (DS9800). Nuclei were counterstained with haematoxylin. To support the positive staining, antibodies used were previously tested in a separate cohort where tissues were obtained prior to 2019 to rule out non-specific staining.7
IHC showed patchy centrovenular granular positivity for the SARS-CoV-2 spike protein, within the cytoplasms of both hepatocytes and Kupffer cells (Fig. 1D-F). There was negative staining for SARS-CoV-2 nucleocapsid protein (Fig. 1G-H). With these histological findings, absence of alternative causes and proximity of vaccination to jaundice onset, we postulate this might be related to the vaccine triggering an immune-mediated liver injury.
The patient was started on ursodeoxycholic acid. Bilirubin remained stable around 200-250 μmol/L for about 4 weeks and improved with normalisation (Fig. 1J) 5 weeks later (10 weeks after receiving the vaccination). He subsequently received a full course of Sinovac vaccination with no ill-effects.
Unique to this case is the demonstration of anti-SARS-CoV-2 spike protein within the liver parenchyma. A crucial negative to validate our hypothesis is the exclusion of concurrent COVID-19 infection. The patient had negative nasal PCRs before and after Moderna vaccination but unfortunately such tests can be false negative.8 , 9 Temporally flanking negative nasal PCRs and a negative SARS-CoV-2 nucleocapsid staining (on the same biopsy sample that demonstrated SARS-CoV-2 spike protein) reasonably exclude concurrent COVID-19 infection.
The exact mechanism by which SARS-CoV-2 spike protein reached the liver is unknown. It was likely produced by skeletal myocytes at the site of administration, gained access to the blood stream10 and travelled to the liver. An alternative explanation is phagocytosis of SARS-CoV-2 spike protein by local macrophages or other antigen-presenting cells which migrated to the liver. Unfortunately, there is no published data on the presence or absence of mRNA-induced SARS-CoV-2 spike protein in liver tissue in asymptomatic patients. Our patient tolerated the inactivated Sinovac vaccine suggesting the immunological processes may be unique to mRNA vaccination.
To our knowledge, this is the first published report on de novo liver injury developing post-Moderna vaccination with supportive histology of a possible direct effect from anti-SARS-CoV-2 spike protein antibodies, without features of AIH.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
CMN and ZHSL contributed to writing of the manuscript and collection of data. WQL and PSJY contributed to the pathology slides staining and technical aspects of immunohistochemistry. WQL, PSJY and GHH contributed to writing and final editing of the manuscript. GHH conceptualised the manuscript. All authors were involved in the final approval of the submitted manuscript.
Conflicts of interest
GHH has received speaker honorarium from Johnson & Johnson and conference financial support from Gilead Sciences. All other authors had no conflict of interest or financial disclosures to declare.
Acknowledgements
We would like to acknowledge Ms Hilmy Maryam Hazly and Mr Joseph Craig Ryan for their valuable help in the preparation and staining of the biopsy specimens and immunohistochemistry. We would also like to acknowledge Dr Thinesh Lee Krishnamoorthy for his valuable opinion and vetting of this letter.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.03.041.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. for the COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Londono M.C., Gratacos-Gines J., Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination. Still casualty? J Hepatol. 2021;75(5):1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123:102706. doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McShane C., Kiat C., Rigby J., Crosbie O. The mRNA COVID-19 vaccine - a rare trigger of autoimmune hepatitis? J Hepatol. 2021;75(5):1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 Vaccination: true causality or mere association? J Hepatol. 2021;75(5):1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tun G.S.Z., Gleeson D., Dube A., Al-Joudeh A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.09.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung C.C.L., Goh D., Lim X., Tien T.Z., Lim J.C.T., Lee J.N., et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2021 doi: 10.1136/gutjnl-2021-324280. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Kanji J.N., Zelyas N., Macdonald C., Pabbaraju K., Khan M.N., Prasad A., et al. False negative rate of COVID019 PCR testing: a discordant testing analysis. Virol J. 2021;18(13) doi: 10.1186/s12985-021-01489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins T.S., Wu A.W., Ying J.Y. SARS-CoV-2 nasopharyngeal swab testing – false-negative results from a pervasive anatomical misconception. JAMA Otolaryngol Head Neck Surg. 2020;146(11):993–994. doi: 10.1001/jamaoto.2020.2946. [DOI] [PubMed] [Google Scholar]
- 10.Ogata A.F., Cheng C., Desjardins M., Senussi Y., Sherman A.C., Powell M., et al. Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.