Highlights
-
•
Antibiotic misuse/overuse are a critical public-health issue in Arabic countries.
-
•
Antimicrobial resistance is a growing problem worldwide.
-
•
COVID-19 pandemic uncovers insufficient knowledge and perceptions about antibiotics.
-
•
A comprehensive and effective antibiotic-stewardship program is required.
Keywords: Antibiotic, Arabic Countries, Knowledge, Perceptions
Abbreviations: AR, Antibiotics Resistance; Group A, healthcare professionals; Group B, medical students; Group C, other adults in the community
Abstract
Background
Antibiotics are essential for the treatment of bacterial infections and are considered among the most commonly sold drug classes from the community pharmacy in the developing countries without a prescription in most cases.
Purpose
This study aims to explore the knowledge, practices, and attitudes regarding antibiotic use.
Materials and methods
This study employs a cross-sectional descriptive design that used a pre-validated survey. The participants were classified into three main mutually exclusive groups: healthcare professionals, medical students, and other adults in the community.
Results
Of the 10,226 participants, 1157 (11%) were healthcare professionals; 2322 (23%) were medical students and 6747 (66%) were other adults in community. The majority of participants used antibiotic at least once during the past year. A total of 838 (72.4%) healthcare professionals and 800 (34.5%) medical students had prescribed an antibiotic during the last 6 months.
Almost half of the medical students and adults in the community and almost one-third of healthcare professionals reported that the aim of antibiotics use is for fever. Furthermore, around one-quarter of participants reported that the aim of antibiotics use is for viral infection. Around one-quarter of respondents stated that the antibiotic will always be effective in the treatment of the same infection in the future. Around one-quarter of participants stated that 21 to 50% of antibiotics are considered to be unnecessary or inappropriate prescriptions. Different factors were perceived as being very important causes of antibiotic resistance among the participants.
Conclusions
These findings indicated that this study participants showed unsatisfactory knowledge and perceptions of proper antibiotic use. Therefore, there is a requirement for a comprehensive and effective antibiotic-stewardship program to promote rational antibiotics use, and compensate for knowledge and perceptions gaps to prevent antibiotic resistance development.
1. Introduction
Antibiotics are essential for the treatment of bacterial infections and are considered among the most commonly sold drug classes from the community pharmacy in the developing countries (Cagri Buke et al., 2003) without a prescription in most cases (Shankar et al., 2002). In Jordan, previous researches have shown that the prevalence of self-medication of antibiotics is alarmingly high (Al-Azzam et al., 2007, Sawair et al., 2009). Moreover, different patterns of inappropriate prescription and dispensing of antibiotics in Jordan were detected (Al-Bakri et al., 2005, Al-Azzam et al., 2007, Sawair et al., 2009).
The prescribing practices of physicians are not monitored properly (Al-Momany et al., 2009, Dar-Odeh et al., 2010, Jarab et al., 2018). Despite the existence of regulatory laws that prevent unprescribed dispensing of antibiotics to the adult, these laws are not enforced in community pharmacies (Albsoul-Younes et al., 2010). Appropriate prescribing is essential to help prevent the emergence of resistant bacteria and ultimately to improve patient outcomes (Erb et al., 2007, Boggan et al., 2012, Versporten et al., 2018).
The overuse and misuse of antibiotics result in the increasing emergence of resistant bacterial strains, in addition to adverse reactions and economic burden on national health system (Albrich et al., 2004, Nathwani, 2006, Edlin, 2014, Alsayed et al., 2019, Alsayed et al., 2020) that makes a major public health issue worldwide. There is a well-established temporal relationship between antibiotic use and resistance, both in hospitals and community settings (Austin et al., 1999, López-Lozano et al., 2000, Goossens et al., 2005). Sun and colleagues described a seasonal effect of antibiotic use on antibiotic resistance (AR) (Sun et al., 2012).
AR is a growing problem worldwide, with an often-negative impact on patient outcomes (Cosgrove, 2006, Spellberg et al., 2008). Several studies have strongly supported the relationship between antibiotic use and resistance development (Yagupsky, 2006, Costelloe et al., 2010). Countries with the highest antibiotic consumption per capita have the highest prevalence of resistant organisms (Goossens et al., 2005, van de Sande-Bruinsma et al., 2008).
AR is highly associated with inappropriate antibiotics use. Careful antibiotics use is essential for preserving their clinical effectiveness. Beside the prescribers, the patients are also essential to control of antibiotic use and AR (Ling et al., 2003). Patients’ knowledge, behavior, and beliefs may influence antibiotic prescription (Mangione-Smith et al., 2001, Ling et al., 2003). Most of the strategies for controlling antibiotic resistance, such as guidelines, policies and educational programs, have been focusing on both prescribers and public to promote prudent antibiotic use (Carbon et al., 2002, Ashiru-Oredope and Hopkins, 2015).
The irrational use and overuse of antibiotics arise from several complex contributing factors related to healthcare professionals or patients and/or their parents (Mangione-Smith et al., 2001, Pechere, 2001, Coco et al., 2009). These factors include, among others, demographic data (e.g., age, socio-economic status and education level) in addition to lack of health education, health policies concerning medical insurance, lack of healthcare professionals' concerns about long-term resistance, pharmaceutical marketing and the sale of antibiotics without prescription in some countries. Other factors can include psycho-social aspects, such as behaviors and attitudes (e.g., self-medication, over-the-counter medication, and patients’ expectations).
To preserve the considerable benefit of antibiotics, more comprehensive strategy for better antibiotic use is essential. In particular, there is a clear need to improve the understanding of the factors associated with antibiotic use. To support the proper use of antibiotic, it is important to gain insight into different patterns of its prescriptions and on factors influencing prescribing decisions. However, most of the efforts for controlling the use of antibiotics have been directed toward the prescribers: guidelines, national and international antibiotic policies, and educational programs. Nevertheless, little is known about the importance of patient contribution in antibiotic use and no strategy has been developed for educating the public. As no similar literature exists from Arabic countries, this study aims to explore the knowledge, attitudes and practices regarding antibiotic use.
2. Materials and methods
2.1. Setting and participants
This study was carried out using a cross-sectional descriptive design and was conducted between December 2019 and June 2020 in Arabic countries. A sample of eligible and convenient participants was invited, by 14 voluntary collaborators, to participate in the study from all the Arabic countries through social media (Facebook, WhatsApp).
Inclusion criteria for the participants in the study included: residents in Arabic countries, 18 years of age or older, and they have the ability to speak and write Arabic language. The participants were divided into three main mutually exclusive groups: healthcare professionals, medical students, and other adults in the community. Group A, B, and C are used interchangeably for the aforementioned three main groups, respectively. Common sets of questions were used for each group with some different questions between each group.
This study was approved by the Research Ethics Committee at Faculty of Pharmacy at Applied Science Private University (ASU), Amman, Jordan (Approval No: 2021-PHA-37). The consent to participate was implied by the act of completing and returning the e-survey. The covering letter stressed anonymity and confidentiality and explained the aim and objectives of the study. Participants did not receive any benefits or payments for filling-out the questionnaire.
2.2. Survey instrument and administration
The questionnaire was developed by three experts in questionnaire design and infectious diseases, and after an extensive literature searching for similar published studies (Stephenson, 2002, Wester et al., 2002, Srinivasan et al., 2004, Buke et al., 2005, Chen et al., 2005, Nathwani, 2006, Dellit et al., 2007).
The questionnaire draft was designed to cover the areas of interest in this study and was submitted in a pilot test to 45 pharmacy students and 3 junior doctors (who finished their qualification from medical school but were still in their training years) to check comprehension and clarity of the questions. The term ‘junior doctors’ referred to doctors. Data collected during this pilot part of the study were excluded from the final data analysis. To facilitate data collection, the questionnaire was written in two languages, English and Arabic by three individuals who are fluent in both languages. The translation was validated and followed the standard ‘forward-backward’ procedure. The final version of the questionnaire was further verified for content validity by experts in the filed who gave their constructive suggestions, and positive feedback for the process.
In order to exclude random completion and verify participant’s responses consistency, some couples of similar questions expressed in a different way and some pairs of contradictory questions requiring the opposite answer were included in the questionnaire’s structure. Questionnaires with discordant responses to two or more of these questions were removed.
The questionnaire was initially distributed using a printed copy, however, due to the pandemic of coronavirus 19 disease (COVID-19), the main distribution was using an electronic form and by phone.
2.3. Data collection
The final version of the questionnaire was organized into different sections addressing different topics of interest to answer the aim of the study. This study used qualitative methods to explore the knowledge, perceptions, attitudes and behavior of the participants.
The self-administered questionnaire collected information on demographic data of the respondents such as gender, age, monthly household income, and education level. Data were also collected about the current specialty of the healthcare professionals participated in the study, the frequency of their antibiotic's prescription, and past training in antibiotic prescribing. Additionally, in this study, respondents’ attitudes about antibiotic prescribing, their knowledge of the national prevalence of antibiotic resistance, their perception of the importance of the problem of antibiotic resistance with their beliefs about its causes, and their attitudes about current and potential interventions designed to improve antibiotic prescribing were collected. Most questions about perceptions and attitudes used five-point Likert-style response options, from very unhelpful/unimportant/unconfident, to very helpful/important/ confident. Scores of 1, 2, 3, 4, 5 were assigned to the responses of the aforementioned options.
2.4. Statistical analysis
Data was analyzed using SPSS version 24. Categorical variables are presented as frequencies and percentages. Median (Interquartile range; IQR) and mean (standard deviation; SD) were used to represent the scores of the five-point Likert-style response options. Chi-square test of homogeneity was used to test for the difference for the antibiotics use between the three study‘s groups. P- value of < 0.05 was considered to be statistically significant for all analyses.
Based on the population size in the Arabic countries, sample size calculation was conducted using a margin of error of 5%, confidence level of 95%, and response distribution of 50%, giving a minimum sample size of 384 participants. A consensus sample of subjects was included in the study. The initial target was 10,000.
3. Results
3.1. Demographic and clinical characteristics
Of the eligible participants, 10,226 (initial target: 10,000) returned questionnaires, 1157 of them (11 %) were healthcare professionals; 2322 (23 %) were medical students and 6747 (66 %) were other adults in community. Around three quarter of participants in the 3 major categories were from Jordan and Iraq (Table 1). Table 1 shows the demographic and clinical characteristics of the respondents. The majority of participants within the three groups were from the age range of 18–25 years and have a bachelor degree. The median number of children the participants have was 2 for groups A and B, while it was 3 for group C. The majority of respondents in the category of medical students and adults in the community (62% and 58%, respectively) were females, and the majority of healthcare professionals' respondents were males (52%).
Table 1.
Demographic and clinical characteristics of the respondents (N = 10,226).
| Health care professionals, n = 1157 (11 %) | Medical students, n = 2322 (23 %) | Adults in community, n = 6747 (66 %) | |
|---|---|---|---|
| Gender | |||
| Male | 600 (52%) | 882 (38%) | 2828 (42%) |
| Female | 557 (48%) | 1440 (62%) | 3919 (58%) |
| Age, years | |||
| 18–25 | 353 (30.5%) | 2192 (94.4%) | 3528 (52.3%) |
| 26–35 | 487 (42.1%) | 130 (5.6%) | 1653 (24.5%) |
| 36–45 | 160 (13.8%) | 0 (0.0%) | 750 (11.1%) |
| 46–55 | 88 (7.6%) | 0 (0.0%) | 549 (8.1%) |
| >55 | 69 (6.0%) | 0 (0.0%) | 267 (4.0%) |
| Nationality | |||
| Jordan | 464 (40.1%) | 825 (35.5%) | 2877 (42.6%) |
| Iraq | 402 (34.7%) | 981 (39.2%) | 1984 (29.4%) |
| Palestine | 92 (8.0%) | 142 (6.1%) | 601 (8.9%) |
| Egypt | 88 (7.6%) | 211 (9.1%) | 516 (7.6%) |
| Syria | 48 (4.1%) | 126 (5.4%) | 387 (5.7%) |
| Lebanon | 15 (1.3%) | 21 (0.9%) | 64 (0.9%) |
| United Arab Emirates | 2 (0.2%) | 5 (0.2%) | 28 (0.4%) |
| Saudi Arabia | 9 (0.8%) | 25 (1.1%) | 127 (1.9%) |
| Qatar | 3 (0.3%) | 4 (0.2%) | 25 (0.4%) |
| Kuwait | 5 (0.4%) | 18 (0.8%) | 67 (1.0%) |
| Others (12 countries) | 29 (2.5%) | 34 (1.5%) | 71 (1.1%) |
| Country of stay | |||
| Jordan | 508 (43.9%) | 1400 (60.3%) | 3181 (47.1%) |
| Iraq | 308 (26.6%) | 422 (18.2%) | 1569 (23.3%) |
| Palestine | 44 (3.8%) | 48 (2.1%) | 196 (2.9%) |
| Egypt | 69 (6.0%) | 165 (7.1%) | 432 (6.4%) |
| Syria | 10 (0.9%) | 28 (1.2%) | 68 (1.0%) |
| Lebanon | 6 (0.5%) | 12 (0.5%) | 38 (0.6%) |
| United Arab Emirates | 62 (5.4%) | 65 (2.8%) | 269 (4.0%) |
| Saudi Arabia | 66 (5.7%) | 74 (3.2%) | 505 (7.5%) |
| Qatar | 31 (2.7%) | 27 (1.2%) | 157 (2.3%) |
| Kuwait | 33 (2.9%) | 50 (2.2%) | 273 (4.0%) |
| Others (12 countries) | 20 (1.7%) | 31 (1.3%) | 59 (0.9%) |
| Education level | |||
| Below University (Bachelor) | 45 (3.9%) | 362 (15.6%) | 1559 (23.1%) |
| University (Bachelor) | 863 (74.6%) | 1906 (82.1%) | 4574 (67.8%) |
| Master or PhD (Medical fields) | 249 (21.5%) | 54 (2.3%) | 132 (2.0%) |
| Master or PhD (Non-medical fields) | 0 (0.0%) | 0 (0.0%) | 482 (7.1%) |
| Monthly income $ | |||
| <1000 $ | 475 (41.1%) | 1040 (44.8%) | 3121 (46.3%) |
| ≥1000 $ | 682 (58.9%) | 1282 (55.2%) | 3626 (53.7%) |
| Children characteristics | |||
| Having children | 2130 (31.6%) | 132 (5.7%) | 440 (38.0 %) |
| Number of children, median (range) | 2 (1->6) | 2 (1->6) | 3 (1->6) |
| Children ages, years | |||
| All older than 18 | 396 (18.6%) | 21 (15.9%) | 64 (14.5%) |
| All younger than 18 | 1226 (57.6%) | 77 (58.3%) | 296 (67.3%) |
| More than half older than 18 | 305 (14.3%) | 18 (13.6%) | 47 (10.7%) |
| More than half younger than 18 | 203 (9.5%) | 16 (12.1%) | 33 (7.5%) |
| Children with a chronic health problem | 287 (13.5%) | 40 (30.3%) | 37 (8.4%) |
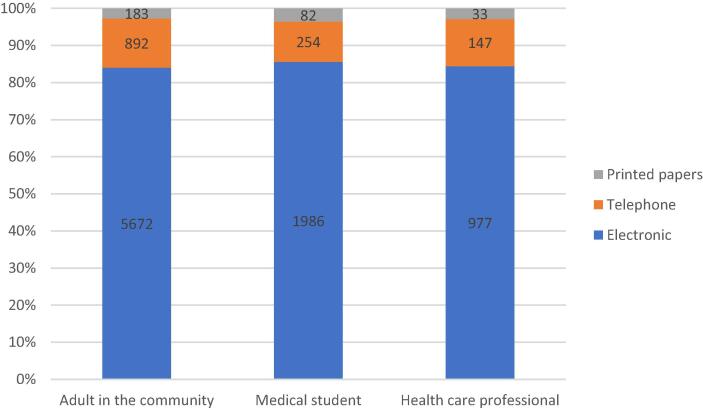
Among the three methods used to collect data from the participants; electronic data collection was the highest used tool; 977 (84.4%) of healthcare professionals, 1986 (85.5%) of medical students, and 5672 (84.1%) of adults in the community. Telephone tool was used in 147 (12.7%) of healthcare professionals, 254 (10.9%) of medical students, and 892 (13.2%) of adults in the community. Printed papers represented the least used tool (Fig. 1).
Fig. 1.
Distribution of the three methods used to collect data from the participants.
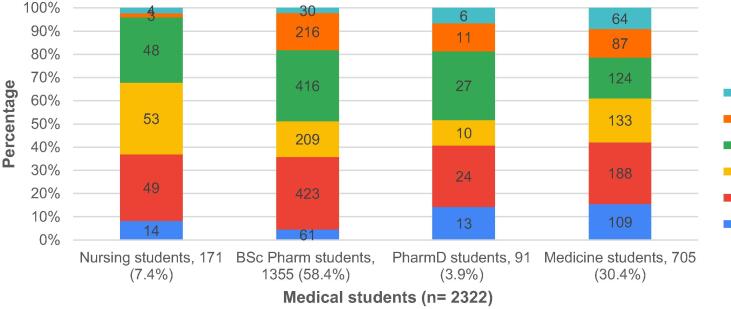
Pharmacists represented the highest percentage among the healthcare professionals' group (n = 503, 43.5%). Of the group A respondents, 391 (33.8%) were working at public hospital and 305 (26.4%) stated that they previously worked on an infectious diseases ward (either as a junior or senior doctor) (Table 2). Fig. 2 represents the distribution of the medical students participated in the study.
Table 2.
Description of the healthcare professionals participated in the study.
| Category | Health care professionals, n = 1157 (11 %) |
|---|---|
| Nurse | 197 (17.0%) |
| BSc Pharm | 503 (43.5%) |
| PharmD | 78 (6.7%) |
| Junior Doctor | 62 (5.4%) |
| General Practitioner (GP) | 93 (8.0%) |
| Senior Doctor | 93 (8.0%) |
| Consultant physicians | 131 (11.3%) |
| Among Senior Doctors and Consultant physicians (n = 224) | |
| Internal Medicine | 51 (22.8%) |
| Surgery | 31 (13.8%) |
| Paediatrics | 16 (7.1%) |
| Anaesthetics | 7 (3.1%) |
| Obstetrics / Gynaecology | 20 (8.9%) |
| Ear, Nose, Throat (ENT) | 11 (4.9%) |
| Oncology | 1 (0.4%) |
| Chest | 7 (3.1%) |
| Others | 80 (35.7%) |
| Location | |
| University hospital | 87 (7.5%) |
| Number of beds (mean) | 211 |
| Public hospital | 391 (33.8%) |
| Number of beds (mean) | 214 |
| Private hospital | 169 (14.6%) |
| Number of beds (mean) | 151 |
| Others | 510 (44.1%) |
| Have you previously worked on an infectious diseases ward (either as a junior or senior doctor)? | 305 (26.4%) |
Fig. 2.
Distribution of the medical students participated in the study.
3.2. Decision making about antibiotics
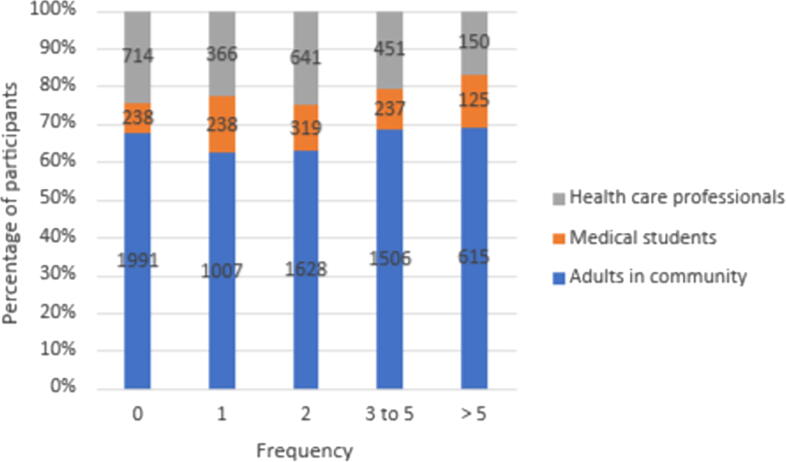
The majority of respondents of the main three categories had used antibiotic at least once during the past year (Fig. 3). Almost half of healthcare professionals (n = 601, 51.9%) used antibiotics three times or more in the past year, while 2121 adults in the community (31.4%) and 362 medical students (15.6%) (p < 0.001). The frequency of antibiotic use by respondents in the past year is shown in Fig. 3.
Fig. 3.
The frequency of antibiotic use in the past year among the study participants.
A total of 838 (72.4%) healthcare professionals and 800 (34.5%) medical students had prescribed an antibiotic within the last 6 months, a statistically significant difference (p < 0.005). A total of 423 (36.6%) healthcare professionals and 778 (33.5%) medical students had received different forms of training on antibiotic prescription in the last year. The majority of healthcare professionals and medical students received the training through attending lectures (n = 236 (55.8%) and n = 386 (49.6%), respectively) and workshops (n = 236 (55.8%) and n = 386 (49.6%), respectively). However, >30% received training via Web-based learning. The most measure rated as the most helpful intervention for guiding antibiotic‘s prescribing or recommending decision was the previous experience, knowledge, or training; 898 (77.6%) and 366 (15.8%) for group A and B, respectively (Table 3).
Table 3.
Antibiotic prescribing, training, and perceptions of the factors influencing the antibiotic prescribing process.
| Health care professionals, n = 1157 (11 %) | Medical students, n = 2322 (23 %) | |
|---|---|---|
| Prescribed/recommended an antibiotic within the past 6 months | ||
| Yes | 838 (72.4%) | 800 (34.5%) |
| Received training in antibiotic prescribing in the last year | ||
| Yes | 423 (36.6) | 778 (33.5) |
| Training delivered by | ||
| Lecture | 273 (64.5) | 642 (82.5) |
| Workshop | 236 (55.8) | 386 (49.6) |
| Informal education in the clinical workplace | 142 (33.6) | 207 (26.6) |
| Web-based learning | 158 (37.4) | 281 (36.1) |
| Self-directed learning | 136 (32.2) | 220 (28.3) |
| Influences that guide your antibiotic‘s prescribing/recommending decision | ||
| Previous experience/knowledge/training | 898 (77.6) | 366 (15.8) |
| Seeking advice from a senior colleague | 368 (31.8) | 251 (10.8) |
| Seeking advice from an infectious diseases’ specialist | 379 (32.8) | 224 (9.6) |
| Seeking advice from a microbiologist | 186 (16.1) | 160 (6.9) |
| Seeking advice from a clinical pharmacist / PharmD | 320 (27.7) | 301 (13.0) |
| Seeking advice from a BSc Pharm | 226 (19.5) | 289 (12.4) |
| Use of local/national guidelines/policies/protocols | 396 (34.2) | 179 (7.7) |
| Unsure | 17 (1.5) | 49 (2.1) |
Confidence levels when prescribing or recommending an antibiotic among healthcare professionals and medical students are relatively low for almost all of the aspects (Table 4).
Table 4.
Confidence level when prescribing/recommending an antibiotic among healthcare professionals (A) and medical students (B).
| 1 | 2 | 3 | 4 | 5 | Mean (SD) | Median (IQR) | |
|---|---|---|---|---|---|---|---|
| |||||||
| A | 137 (11.8) | 159 (13.7) | 319 (27.6) | 403 (34.8) | 151 (13.1) | 3.21 (1.18) | 3 (2) |
| B | 166 (7.1) | 220 (9.5) | 1522 (65.5) | 316 (13.6) | 98 (4.2) | 2.98)0.83( | 3 (0) |
| |||||||
| A | 108 (9.3) | 159 (13.7) | 319 (27.6) | 406 (35.1) | 165 (14.3) | 3.31 (1.16) | 3 (1) |
| B | 128 (5.5) | 193 (8.3) | 1522 (65.5) | 358 (15.4) | 121 (5.2) | 3.07 (0.81( | 3 (0) |
| |||||||
| A | 119 (10.3) | 137 (11.8) | 319 (27.6) | 393 (34.0) | 189 (16.3) | 3.34 (1.19) | 4 (1) |
| B | 129 (5.6) | 208 (9.0) | 1522 (65.5) | 341 (14.7) | 122 (5.3) | 3.05 (0.82( | 3 (0) |
| |||||||
| A | 115 (9.9) | 170 (14.7) | 319 (27.6) | 382 (33.0) | 171 (14.8) | 3.28 (1.18) | 3 (1) |
| B | 120 (5.2) | 228 (9.8) | 1522 (65.5) | 336 (14.5) | 116 (5.0) | 3.04)0.80( | 3 (0) |
| |||||||
| A | 161 (13.9) | 261 (22.6) | 319 (27.6) | 313 (27.1) | 103 (8.9) | 2.94 (1.19) | 3 (2) |
| B | 201 (8.7) | 322 (13.9) | 1522 (65.5) | 220 (9.5) | 57 (2.5) | 2.83 (0.81( | 3 (0) |
| |||||||
| A | 113 (9.8) | 172 (14.9) | 319 (27.6) | 378 (32.7) | 175 (15.1) | 3.29 (1.18) | 3 (1) |
| B | 12 (5.5) | 220 (9.5) | 1522 (65.5) | 355 (15.3) | 98 (4.2) | 3.14 (0.66( | 3 (0) |
| |||||||
| A | 142 (12.3) | 236 (20.4) | 319 (27.6) | 341 (29.5) | 119 (10.3) | 3.05 (1.18) | 3 (2) |
| B | 167 (7.2) | 276 (11.9) | 1522 (65.5) | 288 (12.4) | 69 (3.0) | 2.92 (0.80( | 3 (0) |
| |||||||
| A | 141 (12.2) | 201 (17.4) | 319 (27.6) | 369 (31.9) | 127 (11.0) | 3.12 (1.19) | 3 (2) |
| B | 156 (6.7) | 241 (10.4) | 1522 (65.5) | 315 (13.6) | 88 (3.8) | 2.97 (0.81( | 3 (0) |
1: very unconfident; 2: unconfident; 3: unsure; 4: confident; 5: very confident.
Almost half of the medical students and adults in the community and almost one-third of healthcare professionals reported that the aim of antibiotics use is for fever. Furthermore, 16.1%, 29.5%, and 39.4% of Group A, B, C, respectively reported that the aim of antibiotics use is for viral infection (Table 5). Around one quarter of respondents stated that the antibiotic will always be effective in the treatment of same infection in the future. Ninety percent of healthcare professionals and medical students were not prescribing or recommending an antibiotic if they are not sure about their diagnosis (Table 5).
Table 5.
Respondents’ opinions about statements evaluating indication and efficacy of antibiotics.
| Statements evaluating indication and efficacy of antibiotics | Health care professionals, n = 1157 (11 %) | Medical students, n = 2322 (23 %) | Adults in community, n = 6747 (66 %) |
|---|---|---|---|
| (A) The aim of antibiotic use: | |||
| 1- Fever | 405 (35.0) | 1023 (44.1) | 3257 (48.3) |
| 2- Viral infection | 186 (16.1) | 686 (29.5) | 2656 (39.4) |
| 3- Bacterial infectionة | 1078 (93.2) | 2006 (86.4) | 4575 (67.8) |
| 4- Parasitic infection | 480 (41.5) | 1119 (48.2) | 2621 (38.8) |
| 5- Bacterial infection with fever | 1076 (93.0) | 1946 (83.8) | 4450 (66.0) |
| 6- Viral infection with fever | 301 (26.0) | 835 (36.0) | 2813 (41.7) |
| 7- Common cold, cough and nasal congestion | 265 (22.9) | 1034 (44.5) | 3887 (57.6) |
| 8- Stomach ache | 245 (21.2) | 650 (28.0) | 1851 (27.4) |
| 9-Treatment of sore throat | 449 (38.8) | 1209 (52.1) | 3964 (58.8) |
| (B) An antibiotic will always be effective in the treatment of same infection in the future | 263 (22.7) | 540 (23.3) | 1954 (29.0) |
| (C) Not prescribing an antibiotic if you are not sure about your diagnosis | 1043 (90.1) | 2091 (90.1) | NA |
3.3. Knowledge and perceptions regarding antibiotics use for children
Table 6 shows the respondents believes about the antibiotics use for children. Slightly more than one quarter of respondents reported that antibiotics could be unsafe for children. More than half of the respondents believed that the physicians prescribe the antibiotic when they feel that the benefit of the medicine would outweigh the risks of using it (Table 6).
Table 6.
Knowledge and Perceptions of the respondents regarding antibiotics use for children.
| Health care professionals, n = 1157 (11 %) | Medical students, n = 2322 (23 %) | Adults in community, n = 6747 (66 %) | Total (N = 10,226) | |
|---|---|---|---|---|
| In your opinion, how safe are antibiotics that are used for children? | ||||
| Extremely safe | 52 (4.5) | 117 (5.0) | 401 (5.9) | 570 (5.8) |
| Safe | 609 (52.6) | 945 (40.7) | 2532 (37.5) | 4086 (40) |
| Unsafe | 362 (31.3) | 781 (33.6) | 1964 (29.1) | 3107 (30.4) |
| Extremely unsafe | 48 (4.1) | 91 (3.9) | 374 (5.5) | 513 (5.0) |
| I don't know | 83 (7.2) | 388 (16.7) | 1476 (21.9) | 1947 (19.0) |
| All adult antibiotics go through a testing process in adults before they are given a license and put on the market. Do you think that all antibiotics prescribed for children have gone through a similar testing and licensing process for use in children before they are prescribed/used for children? | ||||
| Yes | 478 (41.3) | 879 (37.9) | 2433 (36.1) | 3790 (37.1) |
| No | 401 (34.7) | 749 (32.3) | 1476 (21.9) | 2617 (25.6) |
| I don't know | 278 (24.0) | 694 (29.9) | 2838 (42.1) | 3810 (37.3) |
| Although it may come as a surprise, the actual situation is that some antibiotics that are routinely prescribed for children have not been fully studied or licensed for use in children. Also, sometimes the dose used has to be increased beyond that which has been recommended by the manufacturer. Have you ever read or heard about this? | ||||
| Yes | 678 (58.6) | 1096 (47.2) | 2185(32.4) | 3959 (38.7) |
| No | 479 (41.4) | 1226 (52.8) | 4562(67.6) | 6267 (61.3) |
| Do you think that parents should be told every time their child is prescribed an antibiotic that has not been fully tested for use in children? | ||||
| Yes | 714 (61.7) | 1745 (75.2) | 5537 (82.1) | 7996 (78.2) |
| No | 443 (37.4) | 577 (24.8) | 1810 (26.8) | 2830 (27.7) |
| Why do you think a doctor would prescribe an antibiotic that has not been fully tested for use in children? (More than one answer could be selected) | ||||
| The doctor felt that the benefit of the medicine would outweigh the risks of using it | 867 (74.9) | 1526 (65.7) | 3805 (56.4) | 6198 (60.6) |
| Doctors have enough experience to prescribe all medicines | 409 (35.4) | 913 (39.3) | 2550 (37.8) | 3872 (37.9) |
| There is no alternative fully tested medicine for children | 555 (48.0) | 1127 (48.5) | 2258 (33.5) | 3940 (38.5) |
3.4. Antibiotics resistance
More than half respondents perceived antibiotic resistance as a global and national problem with a higher percentage reported among healthcare professionals followed by medical students, but only 47.0% believed that it was a problem in their clinical practice (Table 7). Around one quarter of participants stated that 21 to 50% of antibiotics are considered to be unnecessary or inappropriate prescriptions, and this percentage range was the highest among others.
Table 7.
Perceived importance of the problem of antibiotic resistance.
| Health care professionals, n = 1157 (11 %) | Medical students, n = 2322 (23 %) | Adults in community, n = 6747 (66 %) | Total (N = 10,226) | |
|---|---|---|---|---|
| Do you think that antibiotic resistance is a global problem? | ||||
| Yes | 976 (84.4) | 1695(73.0) | 3677(54.5) | 6348 (62.1) |
| No | 103 (9.0) | 241 (10.4) | 757 (11.2) | 1101 (10.8) |
| Unsure | 786 (6.7) | 386 (16.6) | 2313 (34.3) | 3485 (34.1) |
| Do you think that antibiotic resistance is a problem in your country? | ||||
| Yes | 900 (77.8) | 1429 (61.5) | 3014 (44.7) | 5343 (52.2) |
| No | 143 (12.4) | 426 (18.3) | 1251 (18.5) | 1820 (17.8) |
| Unsure | 114 (9.9) | 467 (20.1) | 2483 (36.8) | 3064 (30.0) |
| Do you think that antibiotic resistance is a problem in your clinical practice? | ||||
| Yes | 627 (54.2) | – | – | – |
| No | 327 (28.3) | – | – | – |
| Unsure | 203 (17.5) | – | – | – |
| What percentage of antibiotics do you consider to be unnecessary or inappropriate prescriptions in your country / clinical practice? | ||||
| <10% | 80 (7.0) | 219 (9.4) | 705 (10.4) | 1004 (9.8) |
| 11–20% | 239 (20.7) | 479 (20.6) | 1181 (17.5) | 1899 (18.6) |
| 21–50% | 416 (36.0) | 669 (28.8) | 1450 (21.5) | 2535 (24.8) |
| >50% | 308 (26.6) | 404 (17.4) | 848 (12.6) | 1560 (15.3) |
| Unsure | 114 (9.9) | 551 (23.7) | 2563 (38.0) | 3228 (31.6) |
Two factors were perceived as being very important causes of antibiotic resistance among healthcare professionals: using antibiotics when they are not necessary and prescription of too many antibiotics. Whereas, three factors were perceived as being very important causes of antibiotic resistance among medical students: using antibiotics when they are not necessary, not completing the full course of antibiotic, and using antibiotic without physician or pharmacist supervision (self-medication) (Table 8).
Table 8.
The potential causes for resistance in the opinion of the participants.
| 1 | 2 | 3 | 4 | 5 | Mean (SD) | Median (IQR) | |
|---|---|---|---|---|---|---|---|
| 1. Using antibiotics when they are not necessary | |||||||
| A | 45 (3.9) | 33 (2.9) | 72 (6.2) | 174 (15.1) | 833 (72.0) | 4.39 (0.89( | 5 (1) |
| B | 202 (8.7) | 176 (7.6) | 250 (10.8) | 324 (14.0) | 1370 (59.0) | 4.07 (1.33( | 5 (2) |
| C | 617 (9.1) | 571 (8.5) | 1038 (15.4) | 1277 (18.9) | 3244 (48.1) | 3.88 (1.34) | 4 (2) |
| 2. Too many antibiotic prescriptions | |||||||
| A | 27 (2.3) | 49 (4.2) | 103 (8.9) | 333 (28.8) | 64 (55.7) | 4.43 (0.93( | 5 (1) |
| B | 117 (5.0) | 164 (7.1) | 356 (15.3) | 658 (28.3) | 1027 (44.2) | 4.00 (1.15) | 4 (2) |
| C | 307 (4.6) | 614 (9.1) | 1351 (20.0) | 1990 (29.5) | 2485 (36.8) | 3.85 (1.15) | 4 (2) |
| 3. Too many broad-spectrum antibiotics used | |||||||
| A | 27 (2.3) | 38 (3.3) | 118 (10.2) | 331 (28.6) | 643 (55.6) | 3.89 (1.02( | 4 (2) |
| B | 58 (2.5) | 138 (5.9) | 488 (21.0) | 679 (29.2) | 959 (41.3) | 4.01 (1.04) | 4 (2) |
| C | 200 (3.0) | 433 (6.4) | 1697 (25.2) | 2088 (31.0) | 2329 (34.5) | 3.88 (1.05) | 4 (2) |
| 4. Not completing the full course of antibiotic | |||||||
| A | 21 (1.8) | 31 (2.7) | 102 (8.8) | 327 (28.3) | 676 (58.4) | 3.85 (1.05( | 4 (2) |
| B | 91 (3.9) | 136 (5.9) | 389 (16.8) | 508 (21.9) | 1198 (51.6) | 4.11 (1.12) | 5 (2) |
| C | 217 (3.2) | 365 (5.6) | 1447 (21.4) | 1899 (28.1) | 2789 (41.3) | 3.99 (1.07) | 4 (2) |
| 5. Using antibiotic without physician or pharmacist supervision (self-medication). | |||||||
| A | 32 (2.8) | 23 (2.0) | 94 (8.1) | 278 (24.1) | 730 (63.1) | 3.81 (1.17( | 4 (2) |
| B | 157 (6.8) | 112 (4.8) | 327 (14.1) | 523 (22.5) | 1203 (51.8) | 4.08 (1.21) | 5 (2) |
| C | 450 (6.7) | 392 (5.8) | 1300 (19.3) | 1672 (24.8) | 2933 (43.5) | 3.93 (1.21) | 4 (2) |
| 6. Over the phone prescription | |||||||
| A | 33 (2.9) | 65 (5.6) | 283 (24.5) | 388 (33.6) | 388 (33.5) | 4.15 (0.98( | 4 (1) |
| B | 115 (5.0) | 202 (8.7) | 710 (30.6) | 695 (29.9) | 600 (25.8) | 3.63 (1.11) | 4 (2) |
| C | 343 (5.1) | 590 (8.7) | 2314 (34.3) | 1867 (27.7) | 1633 (24.2) | 3.57 (1.10) | 4 (1) |
| 7. Taking antibiotic with another drug (Drug-Drug interaction) | |||||||
| A | 38 (3.3) | 86 (7.4) | 258 (22.3) | 407 (35.2) | 368 (31.8) | 3.96 (1.03( | 4 (2) |
| B | 102 (4.4) | 214 (9.2) | 644 (27.7) | 690 (29.7) | 672 (28.9) | 3.70 (1.11) | 4 (2) |
| C | 307 (4.6) | 614 (9.1) | 1351 (20.0) | 1990 (29.5) | 2485 (36.8) | 3.85 (1.15) | 4 (2) |
| 8. Using the same antibiotic with different names | |||||||
| A | 64 (5.5) | 104 (9.0) | 225 (19.4) | 363 (31.4) | 401 (34.7) | 3.70 (1.15( | 4 (2) |
| B | 120 (5.2) | 264 (11.4) | 646 (27.8) | 656 (28.3) | 636 (27.3) | 3.64 (1.17) | 4 (2) |
| C | 334 (5.0) | 589 (8.7) | 2053 (30.4) | 1963 (29.1) | 1779 (26.4) | 3.64 (1.11) | 4 (2) |
| 9. Too long durations of antibiotic treatment | |||||||
| A | 31 (2.7) | 46 (4.0) | 161 (13.9) | 402 (34.8) | 517 (44.7) | 3.47 (1.23( | 4 (2) |
| B | 90 (3.9) | 161 (6.9) | 470 (20.2) | 639 (27.5) | 962 (41.4) | 3.96 (1.11) | 4 (2) |
| C | 322 (4.8) | 483 (7.2) | 1663 (24.6) | 1934 (28.7) | 2345 (34.8) | 3.81 (1.13) | 4 (2) |
| 10. Too low dose of antibiotics | |||||||
| A | 25 (2.2) | 89 (7.7) | 221 (19.1) | 391 (33.8) | 431 (37.3) | 3.68 (1.16( | 4 (2) |
| B | 69 (3.0) | 252 (10.9) | 660 (28.4) | 669 (28.8) | 672 (28.9) | 3.70 (1.09) | 4 (2) |
| C | 215 (3.2) | 569 (8.4) | 2029 (30.1) | 2199 (32.6) | 1735 (25.7) | 3.32 (1.12) | 4 (2) |
| 11. Excessive use of antibiotics in livestock | |||||||
| A | 64 (5.5) | 91 (7.9) | 317 (27.4) | 316 (27.3) | 349 (30.2) | 3.92 (1.03( | 4 (2) |
| B | 139 (6.0) | 256 (11.0) | 677 (29.2) | 572 (24.6) | 678 (29.2) | 3.60 (1.18) | 4 (2) |
| C | 493 (7.3) | 661 (9.8) | 2145 (31.8) | 1586 (23.5) | 1862 (27.6) | 3.54 (1.20) | 4 (2) |
| 12. Poor hand hygiene | |||||||
| A | 100 (8.6) | 140 (12.1) | 330 (28.5) | 295 (25.5) | 292 (25.2) | 4.18 (0.99( | 4 (1) |
| B | 205 (8.8) | 301 (13.0) | 694 (29.9) | 532 (22.9) | 590 (25.4) | 3.43 (1.24) | 3 (2) |
| C | 514 (7.6) | 611 (9.1) | 1874 (27.8) | 1699 (25.2) | 2048 (30.4) | 3.62 (1.22) | 4 (2) |
| 13. Presence of a foreign body (e.g. medical devices or catheters) | |||||||
| A | 70 (6.1) | 108 (9.3) | 288 (24.9) | 352 (30.4) | 340 (29.4) | 3.98 (1.08) | 4 (2) |
| B | 106 (4.6) | 236 (10.2) | 724 (31.2) | 633 (27.3) | 623 (26.8) | 3.62 (1.12) | 4 (2) |
| C | 263 (3.9) | 499 (7.4) | 2156 (32.0) | 1889 (28.0) | 1940 (28.8) | 3.70 (1.08) | 4 (2) |
| 14.Paying too much attention to pharmaceutical representatives / advertising | |||||||
| A | 31 (2.7) | 83 (7.2) | 227 (19.6) | 423 (36.6) | 393 (34.0) | 4.08 (0.98) | 4 (2) |
| B | 107 (4.6) | 189 (8.1) | 632 (27.2) | 701 (30.2) | 693 (29.8) | 3.73 (1.11) | 4 (2) |
| C | 360 (5.3) | 530 (7.9) | 1965 (25.6) | 1996 (29.6) | 1896 (28.1) | 3.67 (1.12) | 4 (2) |
| 15. Lacking the advice from a clinical pharmacist / PharmD | |||||||
| A | 30 (2.6) | 52 (4.5) | 141 (12.2) | 387 (33.4) | 547 (47.3) | 4.18 (0.99) | 4 (2) |
| B | 81 (3.5) | 124 (5.3) | 414 (17.8) | 688 (29.6) | 1015 (43.7) | 4.05 (1.07) | 4 (2) |
| C | 246 (3.6) | 384 (5.7) | 1474 (21.8) | 2045 (30.3) | 2598 (38.5) | 3.94 (1.08) | 4 (2) |
1: very unimportant; 2: unimportant; 3: neutral; 4: important; 5: very important.
3.5. Perceptions of the benefit of potential interventions to improve antibiotic prescription
For healthcare professionals' group, the eight measures rated as the most helpful interventions for improving antibiotic prescribing were educational sessions, availability of guidelines, availability of national resistance data, availability of microbiological expert, infectious diseases specialist, clinical pharmacist / PharmD and BSc Pharm advice, restriction of certain antibiotics prescription, in addition to regular audit and feedback on antibiotic prescribing from the clinical practice. Regarding the medical students‘ group: availability of national resistance data, Computer-aided prescribing, and availability of microbiological, clinical pharmacist / PharmD and BSc Pharm advice rated as the most helpful interventions. Regular audit and feedback on antibiotic prescribing from the clinical practice was the most helpful intervention from the adults in community group perception. Similarly, availability of microbiological team advice was another most helpful intervention (Table 9).
Table 9.
Possible interventions to improve antibiotic prescribing according to the participants perspective.
| 1 | 2 | 3 | 4 | 5 | Mean (SD) | Median (IQR) | |
|---|---|---|---|---|---|---|---|
| 1-Educational sessions on antibiotics‘ prescribing | |||||||
| A | 2 (0.2) | 40 (3.5) | 98 (8.5) | 315 (27.2) | 702 (60.7) | 4.39 (0.77) | 5 (1) |
| B | 18 (0.8) | 62 (2.7) | 359 (15.5) | 813 (35.0) | 1070 (46.1) | 4.21 (0.84) | 4 (0) |
| C | 38 (0.6) | 77 (1.1) | 564 (8.4) | 1933 (28.6) | 4135 (61.3) | 3.51 (1.16) | 4 (1) |
| 2-Availability of local / national guidelines / policies / protocols | |||||||
| A | 11 (1.0) | 15 (1.3) | 100 (8.6) | 400 (34.6) | 631 (54.5) | 4.41 (0.79) | 5 (1) |
| B | 18 (0.8) | 52 (2.2) | 349 (15.0) | 833 (35.9) | 1070 (46.1) | 4.31 (0.80) | 4 (0) |
| C | 28 (0.4) | 88 (1.3) | 710 (10.5) | 2346 (34.8) | 3575 (53.0) | 3.51 (1.16) | 4 (1) |
| 3-Availability of local/national resistance data | |||||||
| A | 6 (0.5) | 21 (1.8) | 100 (8.6) | 432 (37.2) | 598 (51.7) | 4.36 (0.78) | 5 (1) |
| B | 8 (0.3) | 62 (2.7) | 359 (15.5) | 813 (35.0) | 1080 (46.5) | 4.36 (0.80) | 5 (0) |
| C | 25 (0.4) | 102 (1.5) | 794 (11.8) | 2328 (34.5) | 3498 (51.8) | 4.17 (0.91) | 4 (1) |
| 4-Computer-aided prescribing | |||||||
| A | 5 (0.4) | 40 (3.5) | 88 (7.6) | 325 (28.1) | 699 (60.4) | 3.44 (1.27) | 4 (1) |
| B | 11 (0.5) | 45 (1.9) | 255 (11.0) | 774 (33.4) | 1237 (53.3) | 4.34 (0.82) | 5 (1) |
| C | 187 (2.8) | 487 (7.2) | 1976 (29.2) | 2120 (31.4) | 1977 (29.3) | 4.06 (0.98) | 4 (2) |
| 5-Presence of an antimicrobial management team | |||||||
| A | 11 (1.0) | 15 (1.3) | 126 (10.9) | 400 (34.6) | 605 (52.3) | 3.84 (1.17) | 4 (1) |
| B | 18 (0.8) | 52 (2.2) | 369 (15.9) | 813 (35.0) | 1070 (46.1) | 3.89 (1.03) | 4 (1) |
| C | 48 (0.7) | 159 (2.4) | 1178 (17.5) | 2488 (36.9) | 2874 (42.6) | 4.32 (0.84) | 4 (1) |
| 6-Readily accessible advice from infection control team | |||||||
| A | 6 (0.5) | 31 (2.7) | 125 (10.8) | 432 (37.2) | 563 (48.7) | 3.44 (1.27) | 4 (1) |
| B | 9 (0.4) | 68 (2.9) | 332 (14.3) | 840 (36.2) | 1073 (46.2) | 3.53 (1.21) | 4 (1) |
| C | 50 (0.7) | 163 (2.4) | 951 (14.1) | 2603 (38.6) | 2980 (44.2) | 3.49 (1.19) | 4 (1) |
| 7-Readily accessible microbiological advice | |||||||
| A | 9 (0.8) | 28 (2.4) | 151 (13.1) | 425 (36.7) | 544 (47.1) | 4.44 (0.82) | 5 (1) |
| B | 11 (0.5) | 51 (2.2) | 396 (17.1) | 836 (36.0) | 1028 (44.3) | 4.25 (0.92) | 5 (1) |
| C | 60 (0.9) | 176 (2.6) | 1218 (18.1) | 2564 (36.9) | 2729 (40.4) | 4.23 (0.98) | 5 (1) |
| 8-Readily accessible advice from an infectious diseases’ physician | |||||||
| A | 8 (0.7) | 17 (1.5) | 110 (9.5) | 404 (34.9) | 618 (53.5) | 4.39 (0.95) | 5 (1) |
| B | 11 (0.5) | 44 (1.9) | 296 (12.7) | 835 (36.0) | 1136 (48.9) | 4.07 (0.99) | 4 (1) |
| C | 48 (0.7) | 164 (2.4) | 897 (13.3) | 2445 (36.3) | 3193 (47.3) | 4.31 (0.95) | 4 (1) |
| 9-Readily accessible advice from a clinical pharmacist / PharmD | |||||||
| A | 11 (1.0) | 19 (1.6) | 98 (8.5) | 384 (33.2) | 645 (55.8) | 4.41 (0.79) | 5 (1) |
| B | 11 (0.5) | 45 (1.9) | 275 (11.8) | 754 (32.5) | 1237 (53.3) | 4.36 (0.99) | 5 (1) |
| C | 56 (0.8) | 131 (1.9) | 806 (11.9) | 2411 (35.8) | 3343 (49.5) | 4.31 (0.96) | 4 (1) |
| 10-Readily accessible advice from a BSc Pharm | |||||||
| A | 7 (0.6) | 20 (1.7) | 115 (9.9) | 418 (36.1) | 594 (51.3) | 4.36 (0.96) | 5 (1) |
| B | 10 (0.4) | 58 (2.5) | 292 (12.6) | 735 (31.7) | 1227 (52.8) | 4.34 (0.85) | 5 (1) |
| C | 39 (0.6) | 160 (2.4) | 873 (12.9) | 2435 (36.1) | 3240 (48.0) | 4.29 (0.94) | 4 (2) |
| 11-Advice from senior colleagues | |||||||
| A | 87 (7.5) | 59 (5.1) | 204 (17.6) | 406 (35.1) | 401 (34.7) | 3.84 (1.02) | 4 (2) |
| B | 52 (2.2) | 188 (8.1) | 510 (22.0) | 795 (34.2) | 777 (33.5) | 3.89 (1.07) | 4 (2) |
| C | 217 (3.2) | 506 (7.5) | 1863 (27.6) | 2219 (32.9) | 1942 (28.8) | 3.77 (1.03) | 4 (1) |
| 12-Speaking to a pharmaceutical representative | |||||||
| A | 122 (10.5) | 127 (11.0) | 315 (27.2) | 301 (26.0) | 292 (25.2) | 3.44 (1.12) | 4 (2) |
| B | 178 (7.7) | 255 (11.0) | 674 (29.0) | 590 (25.4) | 625 (27.0) | 3.53 (1.14) | 4 (2) |
| C | 428 (6.3) | 773 (11.5) | 2090 (31.0) | 1856 (27.5) | 1600 (23.7) | 3.51 (1.20) | 4 (1) |
| 13-Restriction of prescription of certain antibiotics | |||||||
| A | 8 (0.7) | 34 (2.9) | 98 (8.5) | 315 (27.2) | 702 (60.7) | 4.44 (1.19) | 5 (2) |
| B | 31 (1.3) | 69 (3.0) | 361 (15.5) | 686 (29.5) | 1175 (50.6) | 4.07 (0.88) | 4 (1) |
| C | 81 (1.2) | 228 (3.4) | 1132 (16.8) | 2323 (34.5) | 2983 (44.2) | 4.06 (0.76) | 4 (1) |
| 14-Restriction of prescription of all antibiotics | |||||||
| A | 11 (1.0) | 60 (5.9) | 164 (14.2) | 333 (28.8) | 531 (45.9) | 4.19 (0.94) | 4 (1) |
| B | 34 (1.5) | 126 (5.4) | 488 (21.0) | 674 (29.0) | 1000 (40.1) | 3.49 (1.16) | 4 (2) |
| C | 111 (1.6) | 344 (5.1) | 1341 (19.9) | 2171 (32.2) | 2780 (41.2) | 3.51 (1.18) | 4 (2) |
| 15-Regular audit and feedback on antibiotic prescribing on your clinical practice | |||||||
| A | 6 (0.3) | 15 (1.3) | 142 (6.1) | 336 (29.0) | 658 (56.9) | 4.40 (0.76) | 5 (1) |
| B | 18 (0.8) | 52 (2.2) | 379 (16.3) | 813 (35.0) | 1060 (45.7) | 4.23 (0.87) | 4 (1) |
| C | 43 (0.6) | 158 (2.3) | 889 (13.2) | 2164 (32.1) | 3493 (51.8) | 4.32 (0.95) | 5 (1) |
1: very uunhelpful; 2: uunhelpful; 3: neutral; 4: helpful; 5: very helpful.
4. Discussion
This study purpose was to explore the knowledge, practices, and attitudes regarding antibiotics use. Only few studies have performed surveys similar to this one in Arab countries, so there was a need for more comprehensive study. Around half of the medical students and adults in the community and almost one-third of healthcare professionals reported that the aim of antibiotics use is for fever. Furthermore, 16.1%, 29.5%, and 39.4% of the groups A, B, and C, respectively reported that the aim of antibiotics use is for viral infection. Around one-quarter of respondents stated that the antibiotic will be the effective treatment for the same infection at any time in the future. In agreement with the current results, a recent study has demonstrated that 36% of the students inaccurately thought that antibiotics are used to treat viral infections and 26% believed that antibiotics help them to recover faster from common cold (Zaidi et al., 2020). According to a previous study in Jordan, 67% of participants thought that antibiotics treat common cold and around one-quarter misused antibiotics as analgesics, while half of the participants misused antibiotics based on their relative advice (Shehadeh et al., 2012).
AR is a vital public-health and patient-safety issue, especially with the shortage of new classes of antibiotics (Finch, 2007, Boucher et al., 2009). Several factors were documented to be related to AR, including antimicrobial consumption (Albrich et al., 2004, Goossens et al., 2005, Hillier et al., 2007, Costelloe et al., 2010). This was recognized in a meta-analysis that reported a high link between AR in microorganisms causing skin, urinary, and respiratory infections with the antibiotics prescription in primary care, especially with increasing the number and/or duration of antibiotic courses received in the last one year (Costelloe et al., 2010). In this current study, the majority of participants used antibiotics at least once during the past year. This finding is consistent with a previous study done in Kuwait that reported around three-quarters of participants used antibiotics during the past year, and over a quarter had not completed the antibiotic course (Awad and Aboud 2015).
In our study, a total of 838 (72.4%) healthcare professionals and 800 (34.5%) medical students had prescribed an antibiotic during the previous 6 months. Confidence levels when prescribing or recommending an antibiotic among them are relatively low for almost all of the aspects. In a recent study, only 39% of the students were aware of AR phenomena and how it relates to the overuse of antibiotics (Zaidi et al., 2020). In a prior study, it was shown that around half of participants lacked general information of antibiotics, and that increased knowledge was a predictor of a positive attitude toward antibiotics (Awad and Aboud 2015). The university students in the United Arab Emirates, in a recent study, reported a high rate of antibiotic self-medication with an average knowledge, attitude, practice score of 56% (95% CI 55%–57%) (Jairoun et al., 2019). All of these findings reflect a gap in medical curricula in many medical colleges and emphasize the need for additional efforts in updating the healthcare professionals and medical students regarding antibiotic usage and AR.
Antibiotics are often used for the upper respiratory tract infections, particularly in children, even though viruses cause most of these infections (Earnshaw et al., 2014). This inappropriate practice is an important contributing factor to the AR development (Ling et al., 2006, Harnden et al., 2007). Factors leading to antibiotics overuse in pediatrics are complex, involving: parental knowledge and attitude, in addition to physician practice (Mangione-Smith et al., 2001, Pechere, 2001, Coco et al., 2009). There’s an alarming observation that the antibiotics used for adults can be safely used for children as reported by more than one-quarter of this study respondents. Empirical antibiotics are frequently prescribed to infants in intensive care units (Clark et al., 2006). Broad-spectrum and long duration of antibiotics can be associated with a high risk of some complications like invasive candidiasis, necrotizing enterocolitis, sepsis, and death (Clark et al., 2006, Cotten et al., 2009).
Around one-quarter of participants, in this study, stated that 21 to 50% of antibiotics are considered to be unnecessary or inappropriate prescriptions, and this was the highest reported percentage range. Similarly, previous data have shown that between 20% and 50% of antibiotic use is either unnecessary or inappropriate (Mora et al., 2002, Dellit et al., 2007, Pulcini et al., 2007, Davey et al., 2013) leading to raised AR, patients‘ length of stay in the hospital and subsequently increased economic burden on patients and national health system. Thus, decreasing this inappropriate use is a crucial first step to reduce AR and its detrimental consequences.
A total of 423 (36.6%) healthcare professionals and 778 (33.5%) medical students had received different forms of training in the last year about antibiotics prescription. Most of them received the training through attending lectures (n = 236 (55.8%) and n = 386 (49.6%), respectively) and workshops (n = 236 (55.8%) and n = 386 (49.6%), respectively). However, >30% received training via Web-based learning. The most measure rated as the most helpful intervention for guiding antibiotic‘s prescribing or recommending decision was the previous experience, knowledge, or training; 898 (77.6%) and 366 (15.8%) for group A and B, respectively. It is imperative to expose healthcare professionals for refresher courses on the safety of using antibiotics for children and adults equally. This observation has been enforced by the feedback from healthcare professionals and students that they prefer the intervention of the pharmacists when it comes to prescribing antibiotics and considered this as highly helpful from the clinical practice point of view.
The antibiotics use in animals also contributes to the increasing rates of AR even without antimicrobial consumption (Bartoloni et al., 2004), despite unclear percentage of human resistance that is attributed to antibiotics use in livestock (Singer et al., 2003). In this study, the majority of participants stated that excessive use of antibiotics in livestock can be a potential cause of AR.
Beside the prescribers, the patients are also essential to control the antibiotic use and AR (Ling et al., 2003). Patients’ knowledge, behavior, and beliefs may influence antibiotic prescription (Mangione-Smith et al., 2001, Ling et al., 2003).
Several methods have been used to reduce improper antibiotic use (Berild et al., 2001, Divanon et al., 2001, Gross et al., 2001, Lemmen et al., 2001). Controlling AR requires a multi-faceted approach. While many studies have focused on the number and cost of antibiotics, few have looked at the appropriateness or accuracy of antibiotic use. So, monitoring antibiotic prescribing should remain a priority. An antimicrobial stewardship is an approach that is used to control AR and improve the antibiotics prescription. A very recent study stated that community pharmacists have a suitable knowledge about antimicrobial stewardship, despite not being extremely familiar with its concept. However, pharmacists encouraged incorporating antimicrobial stewardship within community pharmacy level to control AR globally (Saleh et al., 2021). Careful antibiotics use is essential for preserving their clinical effectiveness, while the reduction of unnecessary use will decrease AR.
5. Conclusions
Antibiotic misuse and overuse are a critical public-health issue, influenced by different associated factors related to patients, their parents and/or prescribers. These factors include demographic characteristics or psycho-social aspects, such as behaviors and attitudes. Other factors, for example lack of health education, may also be related to the antibiotics‘ misuse/overuse. A reliable and valid measurement scale is needed to measure these factors and to explore suitable interventions to promote rational use of antibiotics.
Our findings indicated that this study participants showed unsatisfactory knowledge and perceptions of proper antibiotic use. Therefore, there is a requirement for a comprehensive and effective antibiotic-stewardship program to promote rational antibiotics use, and compensate for knowledge and perceptions gaps to prevent AR development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
A very special thank you goes out to the pharmacy students and healthcare professionals who contribute in sharing this study questionnaires, with a special thanks to Yazan Qashou.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Azzam S., Al-Husein B.A., Alzoubi F., et al. Self-medication with antibiotics in Jordanian population. Int J Occup Med Environ Health. 2007;20(4) doi: 10.2478/v10001-007-0038-9. [DOI] [PubMed] [Google Scholar]
- Al-Bakri A.G., Bustanji Y., Yousef A.-M. Community consumption of antibacterial drugs within the Jordanian population: sources, patterns and appropriateness. Int J Antimicrob Agents. 2005;26(5):389–395. doi: 10.1016/j.ijantimicag.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Al-Momany N.H., Al-Bakri A.G., Makahleh Z.M., Wazaify M.M.B. Adherence to international antimicrobial prophylaxis guidelines in cardiac surgery: a Jordanian study demonstrates need for quality improvement. J Manag Care Pharm. 2009;15(3):262–271. doi: 10.18553/jmcp.2009.15.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich W.C., Monnet D.L., Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis. 2004;10(3):514–517. doi: 10.3201/eid1003.030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albsoul-Younes A., Wazaify M., Yousef A.-M., Tahaineh L. Abuse and misuse of prescription and nonprescription drugs sold in community pharmacies in Jordan. Subst Use Misuse. 2010;45(9):1319–1329. doi: 10.3109/10826080802490683. [DOI] [PubMed] [Google Scholar]
- Alsayed A., Al-Doori A., Al-Dulaimi A., Alnaseri A., Abuhashish J., Aliasin K., Alfayoumi I. Influences of bovine colostrum on nasal swab microbiome and viral upper respiratory tract infections–A case report. Respiratory medicine case reports. 2020;31:101189. doi: 10.1016/j.rmcr.2020.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsayed A.R., Alzihlif M., Al Ramahi J.A.W. Relevance of vancomycin suceptibility on patients outcome infected with Staphylococcus aureus. Int Ar J Ant Ag. 2019;9(1) doi: 10.3823/830. [DOI] [Google Scholar]
- Ashiru-Oredope D., Hopkins S. Antimicrobial resistance: moving from professional engagement to public action. J Antimicrob Chemother. 2015;70(11):2927–2930. doi: 10.1093/jac/dkv297. [DOI] [PubMed] [Google Scholar]
- Austin D.J., Kristinsson K.G., Anderson R.M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci U S A. 1999;96(3):1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad A.I., Aboud E.A., Singer A.C. Knowledge, attitude and practice towards antibiotic use among the public in Kuwait. PLoS One. 2015;10(2):e0117910. doi: 10.1371/journal.pone.0117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoloni A., Bartalesi F., Mantella A., Dell’Amico E., Roselli M., Strohmeyer M., Barahona H., Barrón V., Paradisi F., Rossolini G. High prevalence of acquired antimicrobial resistance unrelated to heavy antimicrobial consumption. J Infect Dis. 2004;189(7):1291–1294. doi: 10.1086/382191. [DOI] [PubMed] [Google Scholar]
- Berild D., Ringertz S.H., Lelek M., et al. Antibiotic guidelines lead to reductions in the use and cost of antibiotics in a university hospital. Scand J Infect Dis. 2001;33:63–67. doi: 10.1080/003655401750064103. [DOI] [PubMed] [Google Scholar]
- Boggan J.C., Navar-Boggan A.M., Jhaveri R. Pediatric-specific antimicrobial susceptibility data and empiric antibiotic selection. Pediatrics. 2012;130:e615–e622. doi: 10.1542/peds.2012-0563. [DOI] [PubMed] [Google Scholar]
- Boucher H., Talbot G., Bradley J., Edwards J., Gilbert D., Rice L., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Buke C., Hosgorlimoncu M., Ermertcan S., Ciceklioglu M., Tuncel M., Kose T., Eren S. Irrational use of antibiotics among university students. J Infect. 2005;51(2):135–139. doi: 10.1016/j.jinf.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Cagri Buke A., Ermertcan S., Hosgor-Limoncu M., Ciceklioglu M., Eren S. Rational antibiotic use and academic staff. Int J Antimicrob Agents. 2003;21(1):63–66. doi: 10.1016/s0924-8579(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Carbon C., Cars O., Christiansen K.J.C.M., et al. Moving from recommendation to implementation and audit: Part 1. Current recommendations and programs: a critical commentary. Clin Microbiol Infect. 2002;8:92–106. doi: 10.1046/j.1469-0691.8.s.2.8.x. [DOI] [PubMed] [Google Scholar]
- Chen C., Chen Y.-M., Hwang K.-L., et al. Behavior, attitudes and knowledge about antibiotic usage among residents of Changhua. Taiwan. J Infect. 2005;38:53–59. [PubMed] [Google Scholar]
- Clark R.H., Bloom B.T., Spitzer A.R., Gerstmann D.R. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- Coco A.S., Horst M.A., Gambler A.S. Trends in broad-spectrum antibiotic prescribing for children with acute otitis media in the United States, 1998–2004. BMC Pediatr. 2009;9:1–10. doi: 10.1186/1471-2431-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove, S. E., 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 42, S82-S89. [DOI] [PubMed]
- Costelloe, C., C. Metcalfe, A. Lovering, et al., 2010. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 340, c2096. https://doi.org/10.1136/bmj.c2096 [DOI] [PubMed]
- Cotten C.M., Taylor S., Stoll B., Goldberg R.N., Hansen N.I., Sánchez P.J., Ambalavanan N., Benjamin D.K. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Odeh N.S., Abu-Hammad O.A., Al-Omiri M.K., et al. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag. 2010;6:301. doi: 10.2147/tcrm.s9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey P., Brown E., Charani E., et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. CD003543. 2013 doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- Dellit, T. H., R. C. Owens, J. E. McGowan, Jr., et al., 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 44, 159-177. https://doi.org/10.1086/510393 [DOI] [PubMed]
- Divanon F., Hazera P., El Baroudi N., et al. Economic impact of rationalized antibiotic therapy in a general hospital. Rev Med Interne. 2001;22:737–744. doi: 10.1016/s0248-8663(01)00419-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw S., Mancarella G., Mendez A., Todorova B., Magiorakos A.P., Possenti E., Stryk M., Gilbro S., Goossens H., Albiger B., Monnet D.L. European Antibiotic Awareness Day Technical Advisory CommitteeEuropean Antibiotic Awareness Day Collaborative Group. European Antibiotic Awareness Day: a five-year perspective of Europe-wide actions to promote prudent use of antibiotics. Euro Surveill. 2014;19(41) doi: 10.2807/1560-7917.ES2014.19.41.20928. [DOI] [PubMed] [Google Scholar]
- Edlin, R. S. and H. L. J. T. a. i. u. Copp, 2014. Antibiotic resistance in pediatric urology. Ther Adv Urol. 6, 54-61. https://doi.org/10.1177/1756287213511508 [DOI] [PMC free article] [PubMed]
- Erb A., Stürmer T., Marre R., Brenner H. Prevalence of antibiotic resistance in Escherichia coli: overview of geographical, temporal, and methodological variations. Eur J Clin Microbiol Infect Dis. 2007;26(2):83–90. doi: 10.1007/s10096-006-0248-2. [DOI] [PubMed] [Google Scholar]
- Finch, R., 2007. Innovation—drugs and diagnostics. J Antimicrob Chemother. 60, i79-i82. [DOI] [PubMed]
- Goossens H., Ferech M., Vander Stichele R., Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Gross R., Morgan A., Kinky D., Weiner M., Gibson G., Fishman N. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. 2001;33(3):289–295. doi: 10.1086/321880. [DOI] [PubMed] [Google Scholar]
- Harnden A., Perera R., Brueggemann A.B., Mayon-White R., Crook D.W., Thomson A., Mant D. Respiratory infections for which general practitioners consider prescribing an antibiotic: a prospective study. Arch Dis Child. 2007;92(7):594–597. doi: 10.1136/adc.2007.116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier, S., Z. Roberts, F. Dunstan, et al., 2007. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case–control study. J Antimicrob Chemother. 60, 92-99. [DOI] [PubMed]
- Jairoun A., Hassan N., Ali A., et al. University students’ knowledge, attitudes, and practice regarding antibiotic use and associated factors: a cross-sectional study in the United Arab Emirates. Int J Gen Med. 2019;12:235. doi: 10.2147/IJGM.S200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarab A.S., Mukattash T.L., Nusairat B., Shawaqfeh M., Farha R.A. Patterns of antibiotic use and administration in hospitalized patients in Jordan. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2018;26(6):764–770. doi: 10.1016/j.jsps.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmen S.W., Becker G., Frank U., et al. Influence of an infectious disease consulting service on quality and costs of antibiotic prescriptions in a university hospital. Scand J Infect Dis. 2001;33:219–221. doi: 10.1080/00365540151060923. [DOI] [PubMed] [Google Scholar]
- Ling J., Lam A., Chan E., et al. What have we learnt from community-acquired infections in Hong Kong? J Antimicrob Chemother. 2003;51:895–904. doi: 10.1093/jac/dkg167. [DOI] [PubMed] [Google Scholar]
- Ling T.K.W., Xiong J., Yu Y., Lee C.C., Ye H., Hawkey P.M. Multicenter antimicrobial susceptibility survey of gram-negative bacteria isolated from patients with community-acquired infections in the People's Republic of China. Antimicrob Agents Chemother. 2006;50(1):374–378. doi: 10.1128/AAC.50.1.374-378.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Lozano J.-M., Monnet D.L., Yagüe A., Burgos A., Gonzalo N., Campillos P., Saez M. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: a time series analysis. Int J Antimicrob Agents. 2000;14(1):21–31. doi: 10.1016/s0924-8579(99)00135-1. [DOI] [PubMed] [Google Scholar]
- Mangione-Smith R., McGlynn E.A., Elliott M.N., McDonald L., Franz C.E., Kravitz R.L. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800. doi: 10.1001/archpedi.155.7.800. [DOI] [PubMed] [Google Scholar]
- Mora Y., Avila-Agüero M.L., Umaña M.A., Jiménez A.L., París M.M., Faingezicht I. Epidemiologic observations of the judicious use of antibiotics in a pediatric teaching hospital. Int J Infect Dis. 2002;6(1):74–77. doi: 10.1016/s1201-9712(02)90141-4. [DOI] [PubMed] [Google Scholar]
- Nathwani D. Antimicrobial prescribing policy and practice in Scotland: recommendations for good antimicrobial practice in acute hospitals. J Antimicrob Chemother. 2006;57:1189–1196. doi: 10.1093/jac/dkl137. [DOI] [PubMed] [Google Scholar]
- Pechere, J. C. J. C. i. d., 2001. Patients' interviews and misuse of antibiotics. Clin Infect Dis. 33, S170-S173. https://doi.org/10.1086/321844 [DOI] [PubMed]
- Pulcini C., Cua E., Lieutier F., Landraud L., Dellamonica P., Roger P.M. Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280. doi: 10.1007/s10096-007-0277-5. [DOI] [PubMed] [Google Scholar]
- Saleh, D., R. Abu Farha and F. Darwish El-Hajji, 2021. Antimicrobial stewardship in community pharmacies in Jordan: assessing current status. J Pharm Health Serv Res. 12, 181-187.
- Sawair F.A., Baqain Z.H., Abu Karaky A., Abu Eid R. Assessment of self-medication of antibiotics in a Jordanian population. Med Princ Pract. 2009;18(1):21–25. doi: 10.1159/000163041. [DOI] [PubMed] [Google Scholar]
- Shankar, P., P. Partha and N. J. B. f. p. Shenoy, 2002. Self-medication and non-doctor prescription practices in Pokhara valley, Western Nepal: a questionnaire-based study. BMC Fam Pract. 3, 1-7. https://doi.org/10.1186/1471-2296-3-17 [DOI] [PMC free article] [PubMed]
- Shehadeh M., Suaifan G., Darwish R.M., Wazaify M., Zaru L., Alja’fari S. Knowledge, attitudes and behavior regarding antibiotics use and misuse among adults in the community of Jordan. A pilot study. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2012;20(2):125–133. doi: 10.1016/j.jsps.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R.S., Finch R., Wegener H.C., Bywater R., Walters J., Lipsitch M. Antibiotic resistance—the interplay between antibiotic use in animals and human beings. Lancet Infect Dis. 2003;3(1):47–51. doi: 10.1016/s1473-3099(03)00490-0. [DOI] [PubMed] [Google Scholar]
- Spellberg B., Guidos R., Gilbert D., Bradley J., Boucher H.W., Scheld W.M., Bartlett J.G., Edwards J. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Song X., Richards A., Sinkowitz-Cochran R., Cardo D., Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451. doi: 10.1001/archinte.164.13.1451. [DOI] [PubMed] [Google Scholar]
- Stephenson J. CDC campaign targets antimicrobial resistance in hospitals. JAMA. 2002;287(18):2351. doi: 10.1001/jama.287.18.2351-JMN0508-2-1. [DOI] [PubMed] [Google Scholar]
- Sun, L., E. Y. Klein and R. Laxminarayan, 2012. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis. 55, 687-694. [DOI] [PubMed]
- van de Sande-Bruinsma N., Grundmann H., Verloo D., Tiemersma E., Monen J., Goossens H., Ferech M. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versporten A., Zarb P., Caniaux I., Gros M.-F., Drapier N., Miller M., Jarlier V., Nathwani D., Goossens H., Koraqi A., Hoxha I., Tafaj S., Lacej D., Hojman M., Quiros R.E., Ghazaryan L., Cairns K.A., Cheng A., Horne K.C., Doukas F.F., Gottlieb T., Alsalman J., Magerman K., Marielle G.YT., Ljubovic A.D., Coelho A.A.M., Gales A.C., Keuleyan E., Sabuda D., Boswell J.L., Conly J.M., Rojas A., Carvajal C., Labarca J., Solano A., Valverde C.R., Villalobos-Vindas J.M., Pristas I., Plecko V., Paphitou N., Shaqiri E., Rummukainen M.-L., Pagava K., Korinteli I., Brandt T., Messler S., Enimil A., Iosifidis E., Roilides E., Sow M.S., Sengupta S., George J.V., Poojary A., Patil P., Soltani J., Jafarpour Z., Ameen H., Fitzgerald D., Maor Y., Chowers M., Temkin E., Esposito S., Arnoldo L., Brusaferro S., Gu Y., El-Hajji F.D., Kim N.J., Kambaralieva B., Pavare J., Zarakauska L., Usonis V., Burokiene S., Ivaskeviciene I., Mijovic G., Duborija-Kovacevic N., Bondesio K., Iregbu K., Oduyebo O., Raka D., Raka L., Rachina S., Enani M.A., Al Shehri M., Carevic B., Dragovac G., Obradovic D., Stojadinovic A., Radulovic L., Wu J.EN., Wei Teng Chung G., Chen H.H., Tambyah P.A., Lye D., Tan S.H., Ng T.M., Tay H.L., Ling M.L., Chlebicki M.P., Kwa A.L., Lee W., Beović B., Dramowski A., Finlayson H., Taljaard J., Ojeda-Burgos G., Retamar P., Lucas J., Pot W., Verduin C., Kluytmans J., Scott M., Aldeyab M.A., McCullagh B., Gormley C., Sharpe D., Gilchrist M., Whitney L., Laundy M., Lockwood D., Drysdale S.B., Boudreaux J., Septimus E.J., Greer N., Gawrys G., Rios E., May S. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- Wester C.W., Durairaj L., Evans A.T., Schwartz D.N., Husain S., Martinez E. Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210. doi: 10.1001/archinte.162.19.2210. [DOI] [PubMed] [Google Scholar]
- Yagupsky, P. J. T. P. i. d. j., 2006. Selection of antibiotic-resistant pathogens in the community. Pediatr Infect Dis J. 25, 974-976. https://doi.org/10.1097/01.inf.0000239270.33190.71 [DOI] [PubMed]
- Zaidi S.F., Alotaibi R., Nagro A., Alsalmi M., Almansouri H., Khan M.A., Khan A., Memon I. Knowledge and attitude towards antibiotic usage: a questionnaire-based survey among pre-professional students at King Saud bin Abdulaziz University for health sciences on Jeddah Campus, Saudi Arabia. Pharmacy (Basel, Switzerland). 2020;8(1):5. doi: 10.3390/pharmacy8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]