This systematic reviewe and meta-analysis evaluates household secondary attack rates of SARS-CoV-2 by variant and vaccination status.
Key Points
Question
Are viral variants of concern and increased vaccination associated with SARS-CoV-2 household transmission rates?
Findings
In this systemic review and meta-analysis of 135 studies with more than 1.3 million participants in 36 countries, household secondary attack rates increased over time and were higher for Omicron (42.7%), Alpha (36.4%), and Delta (29.7%) variants than previously reported estimates (18.9%). Full vaccination reduced susceptibility and infectiousness, but more so for Alpha than Delta and Omicron.
Meaning
These findings suggest vaccination for SARS-CoV-2 transcends protection of the individual by conferring indirect protection to other household members, but the degree of protection is seemingly lower for emerging variants.
Abstract
Importance
An overall household secondary attack rate (SAR) of 18.9% (95% CI, 16.2%-22.0%) through June 17, 2021 was previously reported for SARS-CoV-2. Emerging variants of concern and increased vaccination have affected transmission rates.
Objective
To evaluate how reported household SARs changed over time and whether SARs varied by viral variant and index case and contact vaccination status.
Data Sources
PubMed and medRxiv from June 18, 2021, through March 8, 2022, and reference lists of eligible articles. Preprints were included.
Study Selection
Articles with original data reporting the number of infected and total number of household contacts. Search terms included SARS-CoV-2, COVID-19, variant, vaccination, secondary attack rate, secondary infection rate, household, index case, family contacts, close contacts, and family transmission.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline was followed. Meta-analyses used generalized linear mixed models to obtain SAR estimates and 95% CIs.
Main Outcomes and Measures
SAR stratified by covariates according to variant, index case and contact vaccination status, and index case identification period. SARs were used to estimate vaccine effectiveness on the basis of the transmission probability for susceptibility to infection (VES,p), infectiousness given infection (VEI,p), and total vaccine effectiveness (VET,p).
Results
Household SARs were higher for 33 studies with midpoints in 2021 to 2022 (37.3%; 95% CI, 32.7% to 42.1%) compared with 63 studies with midpoints through April 2020 (15.5%; 95% CI, 13.2% to 18.2%). Household SARs were 42.7% (95% CI, 35.4% to 50.4%) for Omicron (7 studies), 36.4% (95% CI, 33.4% to 39.5%) for Alpha (11 studies), 29.7% (95% CI, 23.0% to 37.3%) for Delta (16 studies), and 22.5% (95% CI, 18.6% to 26.8%) for Beta (3 studies). For full vaccination, VES,p was 78.6% (95% CI, 76.0% to 80.9%) for Alpha, 56.4% (95% CI, 54.6% to 58.1%) for Delta, and 18.1% (95% CI, −18.3% to 43.3%) for Omicron; VEI,p was 75.3% (95% CI, 69.9% to 79.8%) for Alpha, 21.9% (95% CI, 11.0% to 31.5%) for Delta, and 18.2% (95% CI, 0.6% to 32.6%) for Omicron; and VET,p was 94.7% (95% CI, 93.3% to 95.8%) for Alpha, 64.4% (95% CI, 58.0% to 69.8%) for Delta, and 35.8% (95% CI, 13.0% to 52.6%) for Omicron.
Conclusions and Relevance
These results suggest that emerging SARS-CoV-2 variants of concern have increased transmissibility. Full vaccination was associated with reductions in susceptibility and infectiousness, but more so for Alpha than Delta and Omicron. The changes in estimated vaccine effectiveness underscore the challenges of developing effective vaccines concomitant with viral evolution.
Introduction
A previously published SARS-CoV-2 household transmission meta-analysis1 through June 17, 2021, reported an overall secondary attack rate (SAR) of 18.9% (95% CI, 16.2%-22.0%) for SARS CoV-2. Although COVID-19 vaccines are more widely available to protect household contacts, emerging variants such as Omicron (B.1.1.529) are even more transmissible and are known to evade immunity induced by existing vaccines or natural infections with the original wild type.2 The net impact of emerging variants on household transmission in vaccinated and unvaccinated households is of interest. More importantly, household SAR studies can also yield estimates of vaccine effectiveness (VE), that is, the association between vaccination and susceptibility to infection, infectiousness given infection, and the total direct and indirect benefits associated with vaccinated individuals in vaccinated populations.3,4 In this meta-analysis, we aggregate household contact tracing studies to evaluate SARs for variants and by index case and contact vaccination status.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline using the same definitions and eligibility criteria as our original study.5 We estimated household transmissibility of SARS-CoV-2 by calculating the SAR or the number of new infections among contacts after exposure to an index case divided by the total number of household contacts. Our last review identified studies published through June 17, 2021.1 Herein, we searched PubMed, medRxiv, and reference lists of eligible studies between June 18, 2021, and March 8, 2022, with no restrictions on language, study design, or place of publication. Search terms were SARS-CoV-2, COVID-19, severe acute respiratory syndrome, SARS, SARS-CoV, coronavirus, variant, vaccination, immunization, secondary attack rate, secondary infection rate, household, family contacts, close contacts, index case, contact transmission, contact attack rate, and family transmission (eTable 1 in the Supplement). Preprints were included. Citations were managed in EndNote version 20 (Thomson Reuters).
Articles with original data that reported at least 2 of the following factors were included: number of infected household contacts, total number of household contacts, and household SARs. Studies that reported only infection prevalence, analyzed populations that overlapped with another included study, and tested contacts using antibody tests only or using antibody tests and another test but did not disaggregate SARs by test were excluded. We first screened studies by titles and abstracts to identify potential studies for inclusion. We then evaluated full-text articles and selected those that met the inclusion criteria.
For this study, 1 reviewer (Z.J.M.) extracted the following information: first author, location, index case identification period, number of index cases, index case symptom status, household/family contact type, test used to diagnose contacts, universal/symptomatic testing, number of tests per contact, and follow-up duration. That reviewer also extracted the number of infected household contacts and total number of household contacts and, whenever possible, disaggregated by covariates including viral variant, index case vaccination status, household contact vaccination status, and vaccine type.
Evaluation of Study Quality and Risk of Bias
To assess study quality and risk of bias, we used the same modified version of the Newcastle-Ottawa quality assessment scale used by Fung et al.6 Studies received up to 9 points according to participant selection (4 points), study comparability (1 point), and outcome of interest (4 points). Studies were classified as having high (≤3 points), moderate (4-6 points), and low (≥7 points) risk of bias. When at least 10 studies were available, we also used funnel plots and Begg and Mazumdar rank correlation to evaluate publication bias, with significance set at P < .10.7 If we detected publication bias, we used the trim-and-fill method for adjustment, which consists of imputing missing effect sizes to achieve symmetry.8
Statistical Analysis
To examine temporal patterns, we assessed household SARs by index case identification period midpoint. We restricted this analysis to laboratory-confirmed infections and SARs from unvaccinated index cases to unvaccinated contacts to observe how transmission patterns changed by time independently of vaccination. We then evaluated household SARs by variants that were reported in 2 or more studies regardless of vaccination status and restricted to SARs from unvaccinated index cases to unvaccinated contacts for comparison with SAR estimates from our original analyses of the predominantly wild-type variant. SAR statistical analyses by variant were as previously described.1
We evaluated SARs by index case and household contact vaccination status (unvaccinated, partially vaccinated, fully vaccinated, booster vaccinated, and all) by variant and overall across variants. The resultant SARs were used to estimate vaccine effectiveness for reducing susceptibility (VES,p) and infectiousness (VEI,p) according to the transmission probability p.3,4 We calculated VES,p from the studies included using VES,p = 1 − SAR01 / SAR00 or VES,p = 1 − SAR11 / SAR10, and VE1,p from the studies included using VEI,p = 1 − SAR10 / SAR00 or VEI,p = 1 − SAR11 / SAR01, where SARij represent the SAR associated with vaccine status i (1 = vaccinated, 0 = unvaccinated) for the index case and j for the household contact. Total estimated vaccine effectiveness is defined as VET,p = 1 − (1 − VES,p) × (1 − VEI,p).
For comparing vaccination subgroups, we conducted pairwise analyses using only studies in which SARs were reported from both relevant subgroups. For VE measures, we used generalized linear mixed-effects models to obtain SAR logits and corresponding sampling variances, which were back-transformed to obtain VE summary estimates and 95% CIs. Furthermore, we used generalized linear mixed-effects models to compare SARs by vaccine type for contact vaccination status with study treated as a random effect and vaccine type as a fixed effect moderator. Heterogeneity was measured using the I2 statistic, with thresholds of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. All analyses were performed using the metafor package in R statistical software version 4.1.2 (R Project).9,10 Statistical significance was set at a 2-tailed P ≤ .05.
Results
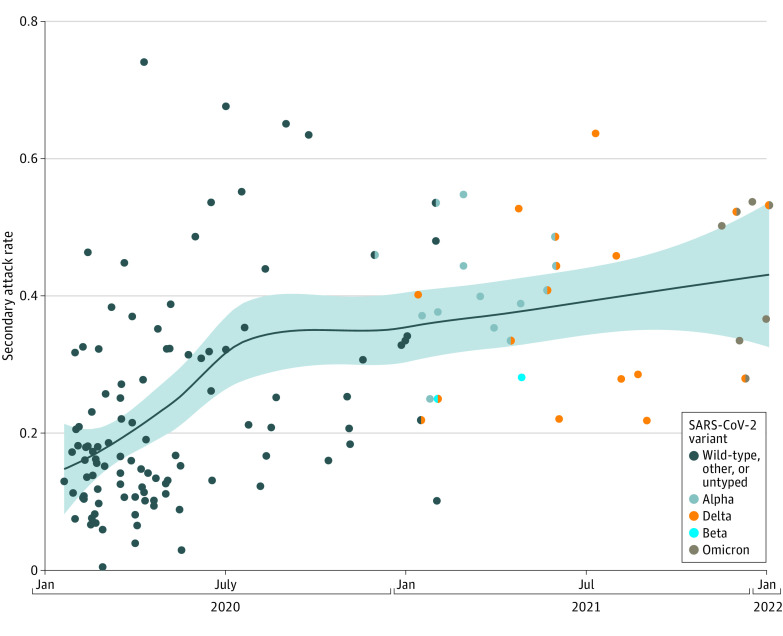
We identified 2097 records (1791 from PubMed, 306 from medRxiv, and 2 from reference lists of eligible articles) published between June 18, 2021, and March 8, 2022 (eFigure 1 in the Supplement). Fifty-eight new studies11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66 (eTable 2 in the Supplement) were combined with 77 studies from our previous review1 (see eTable 3 in the Supplement for references included from our previous review), with Figure 1 showing household SAR by study period, resulting in 135 total studies representing 1 375 806 contacts from 36 countries. Four of the new studies47,48,49,58 were preprints in our previous review that were subsequently published.
Figure 1. Household Secondary Attack Rates Over Time, by Study Midpoint, in 135 Studies of Unvaccinated Index Cases and Unvaccinated Contacts.
Data were restricted to laboratory-confirmed results only. The blue line is a loess smoothing line, and shaded bands are 95% CIs. Bicolored points represent studies with 2 predominant variants.
To assess trends in SAR over time distinct from trends in vaccination, we first restricted attention to unvaccinated index with unvaccinated household contacts. Despite large heterogeneity in SAR estimates over time, particularly during the early stages of the pandemic, an increasing trend is visible in Figure 1. The overall household SAR for 33 studies with midpoints in 2021 or 2022 was 37.3% (95% CI, 32.7%-42.1%), whereas the overall household SAR for 63 studies with midpoints through April 2020 was 15.5% (95% CI, 13.2%-18.2%) (see eTable 3 in the Supplement for references). Begg and Mazumdar rank correlation for publication bias was significant for studies in 2021 to 2022 (P < .001; Kendall τ, 0.664) but not studies through April 2020 (eFigure 2 in the Supplement).
Next, we estimated overall household SARs regardless of index case or contact vaccination status by viral variant. This reflects new variants and changing vaccination coverage. From highest to lowest, the overall household SARs were 42.7% (95% CI, 35.4%-50.4%) for Omicron (7 studies55,61,62,63,64,67,68), 36.4% (95% CI, 33.4%-39.5%) for Alpha (11 studies18,19,21,23,28,33,44,46,49,50,69), 29.7% (95% CI, 23.0%-37.3%) for Delta (16 studies18,20,24,26,35,37,42,46,50,55,57,61,64,65,66,70), and 22.5% (95% CI, 18.6%-26.8%) for Beta (3 studies15,18,50) (Figure 2). High heterogeneity was found among studies for Omicron (I2 = 98.2%; P < .001) and Delta (I2 = 99.1%; P < .001), moderate for Alpha (I2 = 59.6%; P < .001), and low for Beta (I2 = 2.6%; P = .79). Moderate asymmetry was observed in the funnel plot for studies of Alpha, which was significant from Begg and Mazumdar rank correlation (P = .09; Kendall τ, 0.418) (eFigure 3 in the Supplement). We therefore applied the trim-and-fill method, which yielded a mean SAR of 36.1% (95% CI, 33.2%-39.0%) for Alpha. We compared the variant-specific SARs regardless of vaccination status to variant-specific SARs estimated to unvaccinated household contacts only. The mean SAR changed most for Delta (37.0%; 95% CI, 29.7%-44.8%) (12 studies18,20,24,37,42,46,55,57,61,64,65,70) among the variants examined (eFigure 4 in the Supplement).
Figure 2. Household Secondary Attack Rates (SARs) for Omicron (B.1.1.529), Alpha (B.1.1.7), Delta (B.1.617.2), and Beta (B.1.351) Variants.
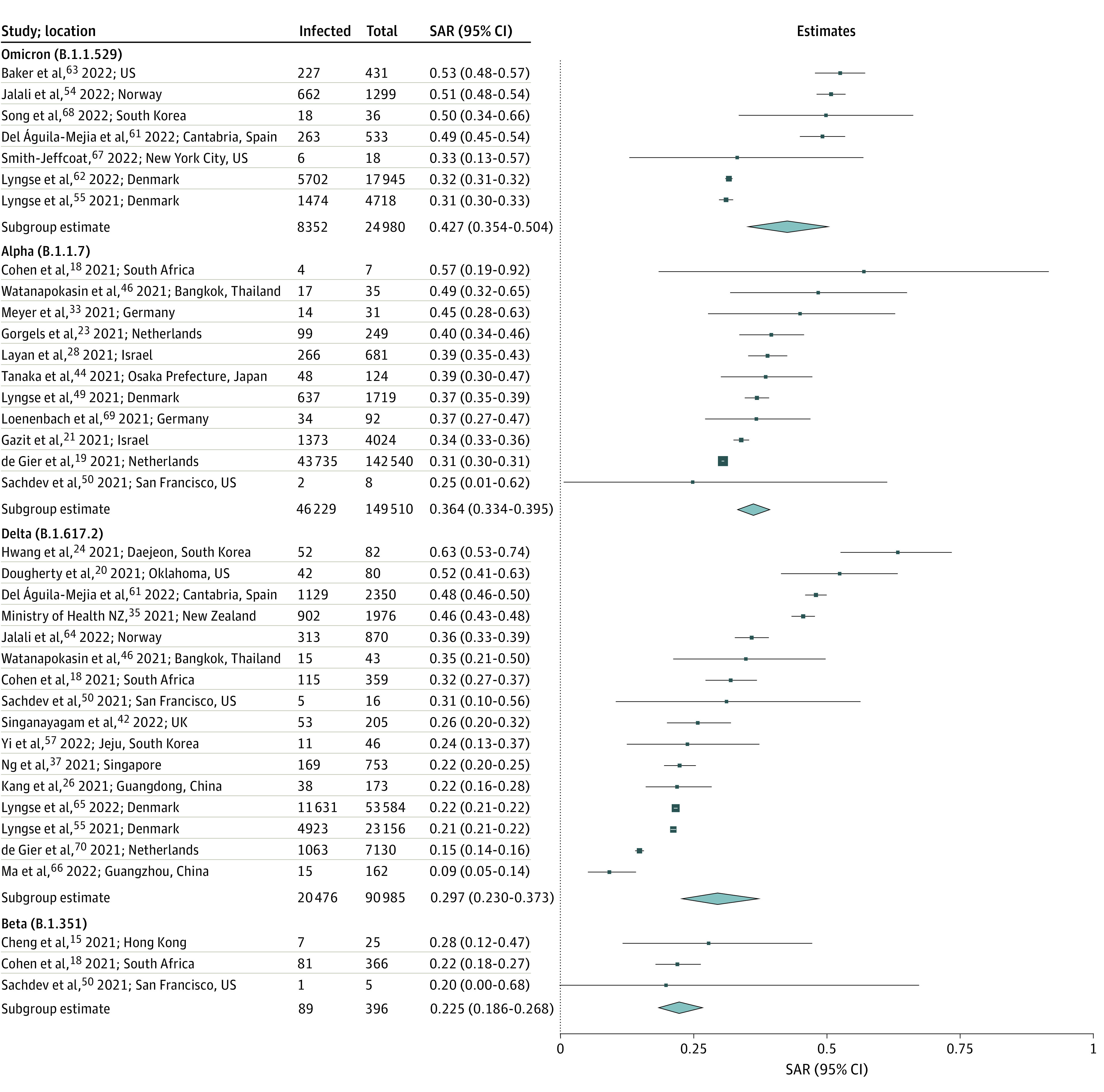
SARs included all index cases and contacts regardless of vaccination status. Point sizes (squares) are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Diamonds represent summary SAR estimates with corresponding 95% CIs. Heterogeneity indexes were as follows: Omicron (I2 = 98.2%), Alpha (I2 = 59.6%), Delta (I2 = 99.1%), and Beta (I2 = 2.6%).
To determine whether there are differences in infectiousness depending on the vaccination status of the index case, we conducted pairwise analyses of studies in which SARs were reported from both subgroups (eg, fully vaccinated vs unvaccinated index cases). Twelve studies19,21,28,33,37,42,48,61,63,64,65,70 reported SARs by index case vaccination status to all household contacts regardless of vaccination status (eFigure 5 in the Supplement), 11 of which were at low risk of bias, and 1 of which was moderate (eTable 4 in the Supplement). Estimated mean SAR for all variants combined was not significantly different from fully vaccinated index cases (22.8%; 95% CI, 15.3%-32.7%) than from unvaccinated (35.5%; 95% CI, 27.3%-44.6%; P = .05) (11 study pairs19,21,28,33,37,42,61,63,64,65,70), from partially vaccinated (26.2%; 95% CI, 11.5%-49.2%) than from unvaccinated (28.0%; 95% CI, 17.3%-42.0%; P = .12) (7 study pairs19,37,42,48,63,64,70), and from fully vaccinated (24.9%; 95% CI, 14.6%-39.2%) than from partially vaccinated (31.7%; 95% CI, 15.0%-55.0%; P = .62) (6 study pairs19,37,42,63,64,70) to all contacts regardless of vaccination status (eTable 5 in the Supplement). No significant publication bias was observed for studies of fully vaccinated or unvaccinated index cases (eFigure 6 in the Supplement). For 4 studies19,21,28,33 of Alpha variant, estimated mean SAR was significantly higher from unvaccinated index cases (36.3%; 95% CI, 31.3%-41.6%) than from fully vaccinated index cases (10.7%; 95% CI, 9.0%-12.8%; P < .001) (Figure 3). We found no significant difference in SARs by index case vaccination status for Delta and Omicron variants, but few studies were included in those subanalyses (Figure 3). Restricting to unvaccinated household contacts (eFigure 7 in the Supplement), estimated mean SAR was also significantly higher from unvaccinated index cases (30.9%, 95% CI, 23.9%-38.8%) than from fully vaccinated index cases (12.0%, 95% CI, 10.0%-14.2%; P < .001) in 4 paired studies,19,21,42,56 2 of which19,21 included Alpha and 2 of which42,70 included Delta variants.
Figure 3. Household Secondary Attack Rates (SARs) by Index Case Vaccination Status.
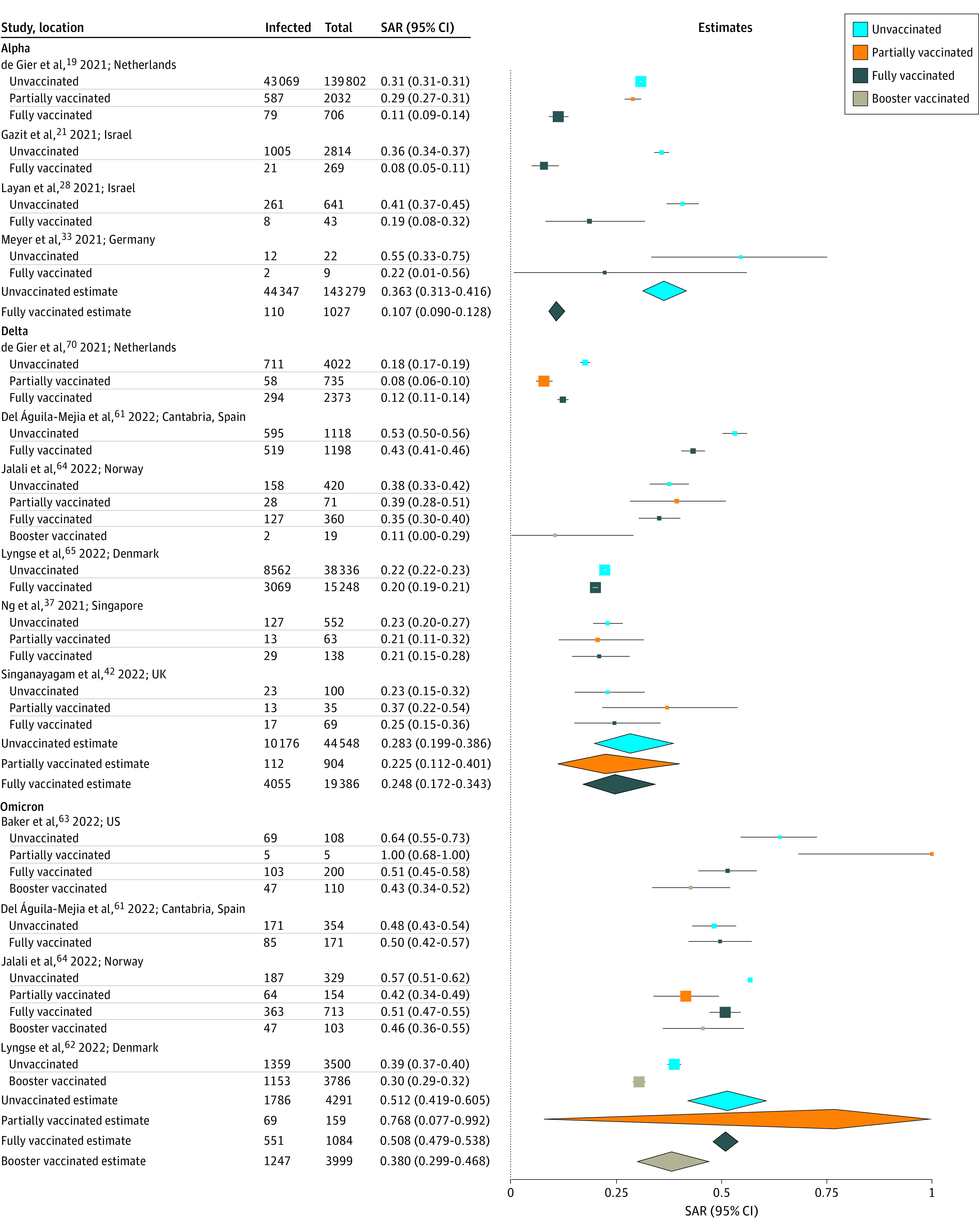
All contacts are included regardless of vaccination status. For Harris et al,48 most of the vaccinated index cases (93%) had received only the first dose of vaccine and SARs were not disaggregated by dose. Point sizes (squares) are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Diamonds represent summary SAR estimates with corresponding 95% CIs. Heterogeneity indexes are as follows: Alpha (unvaccinated: I2 = 94.6%; fully vaccinated: I2 = 52.7%), Delta (unvaccinated: I2 = 99.1%; partially vaccinated: I2 = 91.7%; fully vaccinated: I2 = 98.6%), and Omicron (unvaccinated: I2 = 93.5%; partially vaccinated: I2 = 70.5%; fully vaccinated: I2 = 0.4%; booster vaccinated: I2 = 78.5%).
We then evaluated whether there were differences in susceptibility to SARS-CoV-2 infection depending on household contact vaccination status, again restricting to pairwise comparisons of studies reporting SARs for both relevant subgroups. eFigure 8 in the Supplement summarizes 12 studies19,21,28,32,37,42,50,57,63,64,65,70 reporting household SARs by contact vaccination status regardless of index case vaccination status, 10 of which were at low risk of bias and 2 of which were moderate. In 12 study pairs,19,21,28,32,37,42,50,57,63,64,65,70 estimated mean SAR for all variants combined was significantly higher for unvaccinated contacts (36.5%; 95% CI, 30.5%-43.0%) than for fully vaccinated contacts (18.8%; 95% CI, 12.6%-27.1%; P < .001). In 8 study pairs32,37,42,50,57,63,64,70 reporting SAR to partially vaccinated contacts (27.8%; 95% CI, 20.0%-37.1%), estimated mean SAR was not significantly different than to unvaccinated contacts (39.6%; 95% CI, 32.3%-47.4%; P = .08) or to fully vaccinated contacts (23.9%; 95% CI, 14.7%-36.4%; P = .66) (eTable 6 in the Supplement). Begg test did not show significant evidence of publication bias for studies of fully vaccinated or unvaccinated contact status (eFigure 9 in the Supplement). SARs were consistent when restricting to unvaccinated index cases only (eFigure 10 in the Supplement). When examining SARs by viral variant, estimated mean SARs were significantly higher for unvaccinated contacts for Alpha (38.4%; 95% CI, 34.4%-42.5%; P < .001) (3 studies19,21,28) and Delta (30.1%; 95% CI, 23.2%-38.1%; P = .01) (6 studies37,42,57,64,65,70) than for fully vaccinated contacts (Alpha: 10.5%; 95% CI, 7.9%-13.8%; Delta: 17.1%; 95% CI, 11.6%-24.6%) (Figure 4). For 2 studies55,64 of Delta, estimated mean SAR was also significantly higher for unvaccinated contacts (36.1%; 95% CI, 24.2%-50.0%) than for booster-vaccinated contacts (11.3%; 95% CI, 9.8%-13.0%; P < .001). For 4 studies55,62,63,64 of Omicron, SARs were not significantly different for unvaccinated contacts (43.9%; 95% CI, 32.2%-56.2%) than booster-vaccinated contacts (32.7%; 95% CI, 24.5%-42.2%; P = .16). SARs were generally lower for fully vaccinated contacts regardless of index case vaccination status (eFigure 11 in the Supplement).
Figure 4. Household Secondary Attack Rates (SARs) by Contact Vaccination Status.
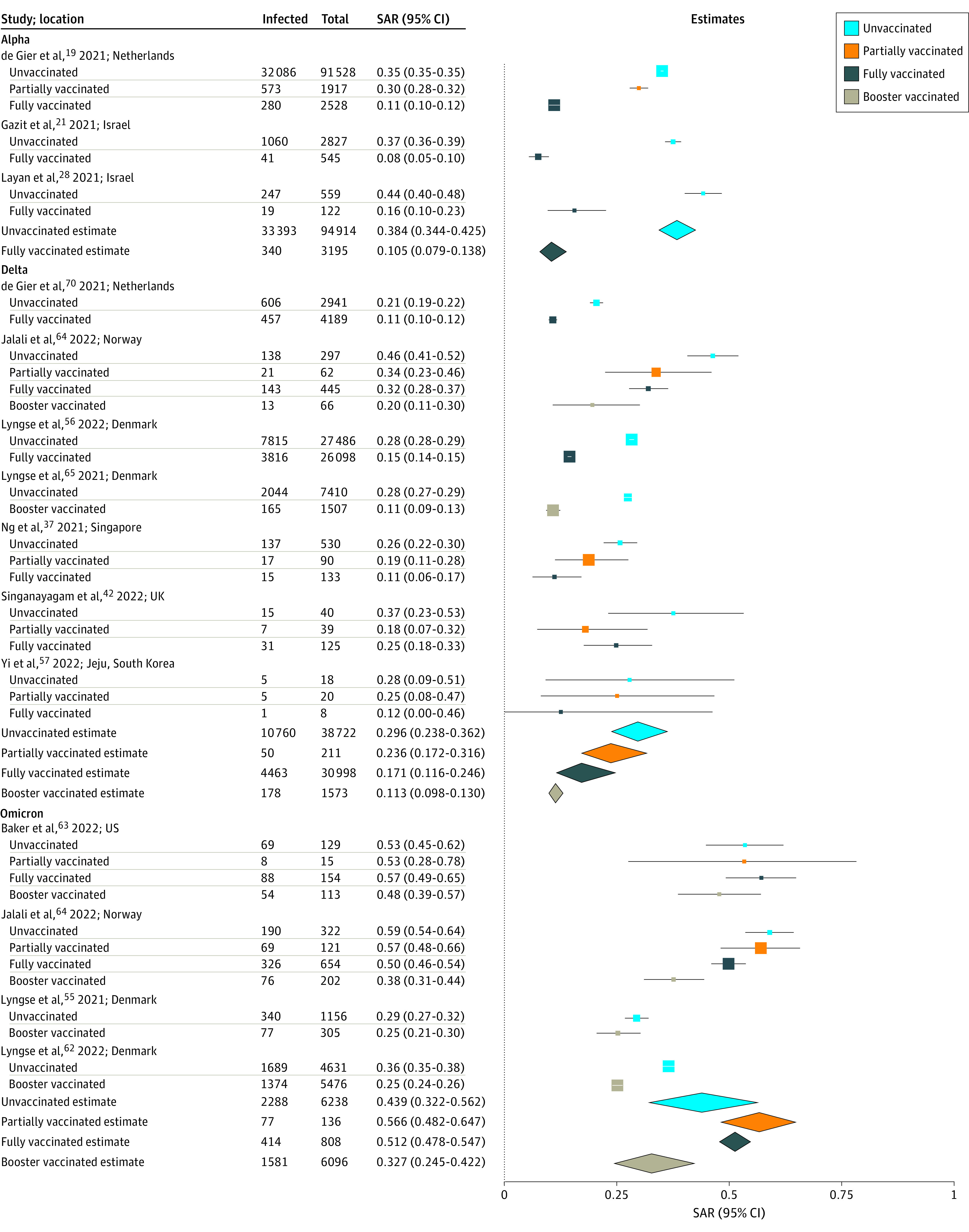
All index cases are included regardless of vaccination status. Point sizes (squares) are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Diamonds represent summary SAR estimates with corresponding 95% CIs. Heterogeneity indexes are as follows: Alpha (unvaccinated: I2 = 92.5%; fully vaccinated: I2 = 69.5%), Delta (unvaccinated: I2 = 95.4%; partially vaccinated: I2 = 30.4%; fully vaccinated: I2 = 96.8%; booster vaccinated: I2 = 76.5%), and Omicron (unvaccinated: I2 = 97.1%; partially vaccinated: I2 = 0.3%; fully vaccinated: I2 = 60.1%; booster vaccinated: I2 = 92.5%).
We also examined SARs by vaccine type and contact vaccination status regardless of index case vaccination status where reported. In 4 study pairs,19,32,50,65 estimated mean SARs for household contacts fully vaccinated with Ad26.COV2.S (Janssen) (1 dose) (34.2%, 95% CI, 14.4%-61.5%) or BNT162b2 (Pfizer-BioNTech) (2 doses) (15.2%, 95% CI, 14.6%-16.0%) were significantly higher than those for contacts fully vaccinated with mRNA-1273 (Moderna) (2 doses) (9.5%, 95% CI, 8.6%-10.6%; P < .001) (eTable 7 in the Supplement). In 2 study pairs,32,70 estimated mean SAR was higher for contacts partially vaccinated with ChAdOx1 (AstraZeneca) (29.5%; 95% CI, 24.0%-35.7%) than contacts partially vaccinated with mRNA-1273 (17.5%; 95% CI, 13.7%-22.3%; P = .008). There was no significant difference in SAR for contacts fully vaccinated with ChAdOx1 and BNT162b2, Ad26.COV2.S, or RNA-1273; or for contacts partially vaccinated with BNT162b2 and RNA-1273 or ChAdOx1.
We also estimated vaccine effectiveness based on the SARs without considering vaccine type (Table). For full vaccination, estimated VES,p (vaccine effectiveness for susceptibility) was 78.6% (95% CI, 76.0% to 80.9%) for Alpha, 56.4% (95% CI, 54.6% to 58.1%) for Delta, and 18.1% (95% CI, −18.3% to 43.3%) for Omicron; estimated VEI,p (vaccine effectiveness for infectiousness) was 75.3% (95% CI, 69.9% to 79.8%) for Alpha, 21.9% (95% CI, 11.0% to 31.5%) for Delta, and 18.2% (95% CI, 0.6% to 32.6%) for Omicron; and estimated VET,p (the combined effect of direct vaccine protection and indirect vaccine effectiveness) was 94.7% (95% CI, 93.3% to 95.8%) for Alpha, 64.4% (95% CI, 58.0% to 69.8%) for Delta, and 35.8% (95% CI, 13.0% to 52.6%) for Omicron. Estimated VES,p was also higher for Delta (68.0%; 95% CI, 62.3% to 72.8%) than Omicron (40.8%; 95% CI, 35.9% to 45.3%) for booster vaccination. Estimated VES,p was highest for booster vaccination, followed by full vaccination and then partial vaccination, for Delta and Omicron. Including studies of all variants, for full vaccination estimated VES,p was 61.4% (95% CI, 45.6% to 72.6%), VEI,p was 44.2% (95% CI, 20.7% to 60.8%), and VET,p was 78.5% (95% CI, 64.8% to 86.8%).
Table. Estimated Vaccine Effectiveness From Household Secondary Attack Rates.
| Variant and Vaccination Type | Estimated vaccine effectiveness | ||||
|---|---|---|---|---|---|
| VEI,p, % (95% CI) | Studies | VES,p, % (95% CI) | Studies | VET,p, % (95% CI) | |
| All | |||||
| Booster vaccination | 31.8 (27.1 to 36.2) | Lyngse et al,62 2022; Baker et al,63 2022; Jalali et al,642022 | 49.5 (37.7 to 59.1) | Lyngse et al,55 2021; Lyngse et al,62 2022; Baker et al,63 2022; Jalali et al,642022 | 65.4 (55.7 to 74.9) |
| Full vaccination | 44.2 (20.7 to 60.8) | de Gier et al,19 2021; Gazit et al,21 2021; Layan et al,28 2021; Meyer et al,33 2021; Ng et al,37 2021; Singanayagam et al,42 2022; Águila-Mejía et al,61 2022; Baker et al,63 2022; Jalali et al,64 2022; Lyngse et al,65 2022; de Gier et al702021 | 61.4 (45.6 to 72.6) | de Gier et al,19 2021; Gazit et al,21 2021; Layan et al,28 2021; Martínez-Baz et al,32 2021; Ng et al,37 2021; Singanayagam et al,42 2022; Sachdev et al,50 2021; Yi et al,57 2022; Baker et al,63 2022; Jalali et al,64 2022; Lyngse et al,65 2022; de Gier et al702021 | 78.5 (64.8 to 86.8) |
| Partial vaccination | 23.6 (−6.0 to 44.9) | de Gier et al,19 2021; Ng et al,37 2021; Singanayagam et al,42 2022; Harris et al,48 2021; Baker et al,63 2022; Jalali et al,64 2022; de Gier et al702021 | 37.2 (16.4 to 53.0) | de Gier et al,19 2021; Martínez-Baz et al,32 2021; Ng et al,37 2021; Singanayagam et al,42 2022; Sachdev et al,50 2021; Baker et al,63 2022; Jalali et al642022 | 52.1 (27.7 to 68.8) |
| Alpha | |||||
| Full vaccination | 75.3 (69.9 to 79.8) | de Gier et al,19 2021; Gazit et al,21 2021; Layan et al,28 2021; Meyer et al332021 | 78.6 (76.0 to 80.9) | de Gier et al,19 2021; Gazit et al,21 2021; Layan et al282021 | 94.7 (93.3 to 95.8) |
| Delta | |||||
| Booster vaccination | NA | NA | 68.0 (62.3 to 72.8) | Lyngse et al55 2021; Jalali et al642022 | NA |
| Full vaccination | 21.9 (11.0 to 31.5) | Ng et al,37 2021; Singanayagam et al,42 2022; Águila-Mejía et al,612022; Jalali et al,642022; Lyngse et al,65 2022; de Gier et al702021 | 56.4 (54.6 to 58.1) | Ng et al,37 2021; Singanayagam et al,42 2022; Yi et al,57 2022; Jalali et al,64 2022; Lyngse et al,65 2022; de Gier et al702021 | 64.4 (58.0 to 69.8) |
| Partial vaccination | 16.0 (−46.9 to 51.9) | Ng et al,37 2021; Singanayagam et al,42 2022; Jalali et al,64 2022; de Gier et al702021 | 37.8 (12.0 to 56.0) | Ng et al,37 2021; Singanayagam et al,42 2022; Yi et al,57 2022; Jalali et al642022 | 51.2 (6.1 to 74.6) |
| Omicron | |||||
| Booster vaccination | 32.3 (25.6 to 38.3) | Lyngse et al,62 2022; Baker et al,63 2022; and Jalali et al642022 | 40.8 (35.9 to 45.3) | Lyngse et al,55 2021; Lyngse et al,62 2022; Baker et al,63 2022; and Jalali et al642022 | 59.8 (54.7 to 64.5) |
| Full vaccination | 18.2 (0.6 to 32.6) | Águila-Mejía et al,61 2022; Baker et al,63 2022; Jalali et al642022 | 18.1 (−18.3 to 43.3) | Baker et al63 2022; Jalali et al642022 | 35.8 (13.0 to 52.6) |
| Partial vaccination | NA | NA | 6.9 (−38.0 to 37.2) | Baker et al63 2022; Jalali et al642022 | NA |
Abbreviations: NA, not applicable because not reported in at least 2 studies; VEI,p, vaccine effectiveness for infectiousness based on the transmission probability p; VES,p, vaccine effectiveness for susceptibility; VET,p, total vaccine effectiveness.
Discussion
We aggregated household studies to examine how variants of concern and vaccination were associated with SARS-CoV-2 household transmission rates. Full vaccination was shown to not only reduce susceptibility to infection, but also reduce transmissibility to other household contacts, albeit more so for Alpha than Delta or Omicron. SARs for Omicron, Delta, and Alpha were significantly higher than estimates for the original wild-type variant.
We found evidence of reduced infectiousness from breakthrough cases among fully vaccinated index cases compared with unvaccinated, though the level of protection conferred for Delta (VEI,p = 21.9%) and Omicron (18.2%) was lower than for Alpha (75.3%). These findings are consistent with a cohort study71 in England, which demonstrated a reduction in estimated vaccine effectiveness against onward transmission for Omicron compared with Delta in household and nonhousehold settings. That study did show, however, that infectiousness was reduced from booster-vaccinated individuals for both Delta and Omicron cases, but less so for Omicron. An observational cohort study72 from England, which included contacts outside the household, also reported that 2 doses of BNT162b2 or ChAdOx1 reduced onward transmission of Delta less than Alpha, and the protection of vaccination against onward transmission waned over time. Our 2-dose VEI,p estimate of 75.3% for Alpha was similar to the VEI of 72.1% (95% CI, 36.6%-89.3%) based on adjusted odds ratios reported by Hayek et al73 during a period in which the Alpha variant was dominant. Potential mechanisms for reduced infectiousness following vaccination for Alpha include decreases in the respiratory tract viral load, duration of infection, and severity of symptoms.74 Our overall 2-dose VEI,p estimate of 44.2% was within the lower range reported for VEI (41%-79%) from a modeling study that used household data from Israel before Delta or Omicron became widespread.75
Fully vaccinated contacts were generally less susceptible to infection with Alpha and Delta than unvaccinated contacts, and individuals who were booster vaccinated were less susceptible to Omicron. Our 3-dose VES,p estimate of 40.8% for Omicron is closer to the greater than 60-day postbooster estimate (47.4%; 95% CI, 40.5%–53.5%) than the 14- to 60-day estimate (71.6%; 95% CI, 69.7%-73.4%) reported in a test-negative design study76 of mRNA-1273, which adjusted for age, sex, race and ethnicity, and specimen collection date. Our 2-dose VES,p estimate of 56.4% for Delta is also lower than the 61.3% (95% CI, 55.1%-66.7%) at greater than 270 days.76 Booster doses of either BNT162b2 or mRNA-1273 increased direct protection against mild Omicron infection, but that protection waned over time.77 Lower protection against susceptibility for Omicron may be attributed to variations in the spike glycoprotein and its ability to evade immune responses.78 Less severe symptoms for Omicron may also lead to reduced household vigilance in maintaining isolation of the infected individual. One study62 included in this analysis reported higher susceptibility to BA.2 compared with BA.1 among unvaccinated, fully vaccinated, and booster-vaccinated individuals, which may be attributed to higher viral load. Other observational studies conducted before the emergence of Omicron demonstrated reduced susceptibility to infection among high-risk or household contacts vaccinated with BNT162b2 or ChAdOx1 in Scotland,79 BNT162b2 in Sweden,80 and BNT162b2 or mRNA-1273 in Belgium.81 Studies have reported that full vaccination with mRNA vaccines or ChAdOx1 effectively prevent infection against the original wild-type, Alpha, and Beta variants, but are less protective against infection from Delta.82,83 Additionally, there is a combined net protective effect from both the index cases and contacts being fully vaccinated as demonstrated by our overall estimate of VET,p (78.5%). Our overall estimates for full vaccination of VES,p (61.4%) and VET,p were lower than the age-adjusted VES (80.5%, 95% CI, 78.9%-82.1%) and VET (88.5%, 95% CI, 82.3%-94.8%) reported by Prunas et al.84
SAR estimates for Omicron (42.7%), Alpha (36.4%), and Delta (29.7%) variants were higher than the overall SARs previously reported (18.9%)1 for study periods earlier in the pandemic when the wild-type variant was prevalent. Public Health England (PHE), which tracks SARs for variants of concern and variants of interest regardless of vaccination status for index cases and household contacts, found that almost all current household transmission is from Omicron BA.2 and BA.1 with increasing prevalence of BA.2.85 PHE had previously reported similar SARs for Alpha (10.2%; 95% CI, 10.1%-10.3%) and Delta (10.4%; 95% CI, 10.4%-10.5%) variants,86 noting however that direct comparisons between variants are not valid, as vaccination levels and social restrictions in England have varied over this period.
Limitations
This study has limitations that should be addressed. There was large heterogeneity in SARs over time, which may be attributed to variations in study methods, environmental factors, and contact patterns. Myriad factors preclude our ability to make direct comparisons of vaccine effectiveness across studies, including differences in the study population (eg, age, comorbidities, and serostatus), location, diagnostic procedures and tools, definition of vaccination status (eg, time elapsed since vaccination or dosage) (eTable 8 in the Supplement), follow-up duration, viral variants, vaccine types and coverage rates, intensity of the epidemic, community behavior, and use of nonpharmaceutical interventions (masks and social distancing).87 For example, in this analysis Singanayagam et al42 included households of any size with contacts 5 or more years, whereas Gazit et al21 restricted to households with only 1 contact other than the index case. Moreover, Ng et al37 in Singapore reported that all identified close contacts were placed under a legally binding quarantine for 14 days during which they were not allowed to leave their homes, whereas contacts in other studies may have had a higher risk of infection outside the household. Few studies disaggregated SARs by both vaccination status of the index cases and contacts. We were unable to calculate VET,p where both the index cases and contacts were fully vaccinated compared with those where both the index cases and contacts were unvaccinated (VET,p = 1 − SAR11 / SAR00) as there were too few studies that reported this information. The studies included in this review are from contact tracing investigations, which are more likely to identify symptomatic index cases than asymptomatic individuals and which could inflate the crude SAR. This may also underestimate the reduction in transmission from vaccination for people infected with Delta.88 There were insufficient data to evaluate Omicron subvariants BA.1 and BA.2 separately and to determine vaccine effectiveness for specific subgroups (eg, by age group).
Conclusions
This meta-analysis of 135 studies suggests that there is increased transmissibility of emerging SARS-CoV-2 variants of concern in the confines of the household where there is prolonged close contact between household members and index cases. Full vaccination reduced susceptibility and infectiousness, but more so for Alpha than Delta and Omicron. The changes in estimated vaccine effectiveness underscore the challenges of developing effective vaccines concomitant with viral evolution.
eFigure 1. PRISMA Flow Diagram
eFigure 2. Funnel Plots of Studies Reporting Household Secondary Attack Rates With Midpoints in 2021 and Through April 2020
eFigure 3. Funnel Plots of Studies Reporting Household Secondary Attack Rates for Alpha (B.1.1.7) and Delta (B.1.617.2) Variants
eFigure 4. Household Secondary Attack Rates for Omicron (B.1.1.529), Alpha (B.1.1.7), Delta (B.1.617.2), and Beta (B.1.351) Variants From Unvaccinated Index Cases to Unvaccinated Household Contacts
eFigure 5. Household Secondary Attack Rates by Index Case Vaccination Status With All Contacts Are Included Regardless of Vaccination Status
eFigure 6. Funnel Plots of Studies Reporting Household Secondary Attack Rates From Unvaccinated or Fully Vaccinated Index Cases to All Contacts Regardless of Vaccination Status
eFigure 7. Household Secondary Attack Rates by Index Case Vaccination Status With Only Unvaccinated Contacts Included
eFigure 8. Household Secondary Attack Rates by Contact Vaccination Status With All Index Cases Included Regardless of Vaccination Status
eFigure 9. Funnel Plots of Studies Reporting Household Secondary Attack Rates to Unvaccinated or Fully Vaccinated Contacts From All Index Cases Regardless of Vaccination Status
eFigure 10. Household Secondary Attack Rates by Contact Vaccination Status With Only Unvaccinated Index Cases Included
eFigure 11. Household Secondary Attack Rates by Vaccination Status of the Index Cases and Contacts
eTable 1. Electronic Databases and Search Strategy for Household Secondary Attack Rate of SARS-CoV-2
eTable 2. Description of Studies Identified From June 18, 2021 to March 8, 2022
eTable 3. References for Studies Included in Figure 1 of SAR Over Time
eTable 4. Risk of Bias Assessment for Studies Included in Review of Household Transmissibility of SARS-CoV-2 Using the Same Modified Version of the Newcastle–Ottawa Quality Assessment Scale for Observational Studies Used by Fung et al
eTable 5. Pairwise Analyses of Index Case Vaccination Status Using Only Studies in Which SARs Were Reported From Both Relevant Subgroups
eTable 6. Pairwise Analyses of Household Contact Vaccination Status Using Only Studies in Which SARs Were Reported From Both Relevant Subgroups
eTable 7. Household Secondary Attack Rates by Vaccine Type and Contact Vaccination Status With All Index Cases Included Regardless of Vaccination Status
eTable 8. Vaccination Status Definitions for Studies That Reported Household Secondary Attack Rates by Vaccination Status of Index Cases or Contacts
eReferences
References
- 1.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Factors associated with household transmission of SARS-CoV-2: an updated systematic review and meta-analysis. JAMA Netw Open. 2021;4(8):e2122240. doi: 10.1001/jamanetworkopen.2021.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaassen F, Chitwood MH, Cohen T, et al. Population immunity to pre-Omicron and Omicron SARS-CoV-2 variants in US states and counties through December 1, 2021. medRxiv. Preprint posted online March 1, 2022.
- 3.Halloran ME, Longini IM, Struchiner CJ, Longini IM. Design and Analysis of Vaccine Studies. Vol 18. Springer; 2010. doi: 10.1007/978-0-387-68636-3 [DOI] [Google Scholar]
- 4.Halloran ME, Préziosi MP, Chu H. Estimating vaccine efficacy from secondary attack rates. J Am Stat Assoc. 2003;98(461):38-46. doi: 10.1198/016214503388619076 [DOI] [Google Scholar]
- 5.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung HF, Martinez L, Alarid-Escudero F, et al. ; Stanford-CIDE Coronavirus Simulation Model (SC-COSMO) Modeling Group . The household secondary attack rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a rapid review. Clin Infect Dis. 2021;73(2)(suppl):S138-S145. doi: 10.1093/cid/ciaa1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 8.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 9.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 10.R Core Team . R: a language and environment for statistical computing. Accessed December 7, 2021. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- 11.Afonso ET, Marques SM, Costa LD, et al. Secondary household transmission of SARS-CoV-2 among children and adolescents: clinical and epidemiological aspects. Pediatr Pulmonol. 2022;57(1):162-175.doi: 10.1002/ppul.25711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bistaraki A, Roussos S, Tsiodras S, Sypsa V. Age-dependent effects on infectivity and susceptibility to SARS-CoV-2 infection: results from nationwide contact tracing data in Greece. Infect Dis. 2022;54(3):186-195.doi: 10.1080/23744235.2021.1995627 [DOI] [PubMed] [Google Scholar]
- 13.Burke RM, Calderwood L, Killerby ME, et al. ; COVID-19 Case Investigation Form Working Group . Patterns of virus exposure and presumed household transmission among persons with coronavirus disease, United States, January-April 2020. Emerg Infect Dis. 2021;27(9):2323-2332. doi: 10.3201/eid2709.204577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvani M, Cantiello G, Cavani M, et al. Reasons for SARS-CoV-2 infection in children and their role in the transmission of infection according to age: a case-control study. Ital J Pediatr. 2021;47(1):193. doi: 10.1186/s13052-021-01141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng VC-C, Siu GK-H, Wong S-C, et al. Complementation of contact tracing by mass testing for successful containment of Beta COVID-19 variant (SARS-CoV-2 VOC B.1.351) epidemic in Hong Kong. Lancet Reg Health West Pac. 2021;17:100281. doi: 10.1016/j.lanwpc.2021.100281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu VT, Yousaf AR, Chang K, et al. ; Georgia Camp Investigation Team . Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med. 2021;385(10):954-956. doi: 10.1056/NEJMc2031915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford S, Waight P, Hackman J, et al. Effectiveness of BNT162b2 and ChAdOx1 against SARS-CoV-2 household transmission: a prospective cohort study in England. medRxiv. Preprint posted online November 25, 2021. doi: 10.1101/2021.11.24.21266401 [DOI] [PMC free article] [PubMed]
- 18.Cohen C, Kleynhans J, von Gottberg A, et al. SARS-CoV-2 incidence, transmission and reinfection in a rural and an urban setting: results of the PHIRST-C cohort study, South Africa, 2020-2021. medRxiv. Preprint posted online December 4, 2021. doi: 10.1101/2021.07.20.21260855 [DOI] [PMC free article] [PubMed]
- 19.de Gier B, Andeweg S, Joosten R, et al. ; RIVM COVID-19 surveillance and epidemiology team 1; Members of the RIVM COVID-19 surveillance and epidemiology team . Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill. 2021;26(31):2100640. doi: 10.2807/1560-7917.ES.2021.26.31.2100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS-CoV-2 B. 1.617. 2 (Delta) variant COVID-19 outbreak associated with a gymnastics facility—Oklahoma, April–May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(28):1004-1007. doi: 10.15585/mmwr.mm7028e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazit S, Mizrahi B, Kalkstein N, et al. BNT162b2 mRNA vaccine effectiveness given confirmed exposure: analysis of household members of COVID-19 patients. Clin Infect Dis. 2021;ciab973. doi: 10.1093/cid/ciab973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Martinez L, Sun S, et al. COVID-19 transmission dynamics among close contacts of index patients with COVID-19: a population-based cohort study in Zhejiang province, China. JAMA Intern Med. 2021;181(10):1343-1350. doi: 10.1001/jamainternmed.2021.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorgels K, Alphen L, van der Veer BMJW, et al. Increased transmissibility of SARS-CoV-2 Alpha variant (B.1.1.7) in children: three large primary school outbreaks revealed by whole genome sequencing in the Netherlands. Research Square. Preprint posted online December 8, 2021. doi: 10.21203/rs.3.rs-1107495/v1 [DOI] [PMC free article] [PubMed]
- 24.Hwang H, Lim J-S, Song S-A, et al. Transmission dynamics of the Delta variant of SARS-CoV-2 infections in South Korea. J Infect Dis. 2021;225(5):793-799. doi: 10.1093/infdis/jiab586 [DOI] [PubMed] [Google Scholar]
- 25.Jagdale GR, Parande MA, Borle P, et al. Secondary attack rate among the contacts of COVID-19 patients at the beginning of the pandemic in Pune City of Western Maharashtra, India. J Commun Dis. 2021;53(3):51-59. [Google Scholar]
- 26.Kang M, Xin H, Yuan J, et al. Transmission dynamics and epidemiological characteristics of Delta variant infections in China. medRxiv. Preprint posted online August 13, 2021. doi: 10.1101/2021.08.12.21261991 [DOI]
- 27.Karumanagoundar K, Raju M, Ponnaiah M, et al. Secondary attack rate of COVID-19 among contacts and risk factors, Tamil Nadu, March-May 2020: a retrospective cohort study. BMJ Open. 2021;11(11):e051491. doi: 10.1136/bmjopen-2021-051491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layan M, Gilboa M, Gonen T, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. medRxiv. Preprint posted online July 16, 2021. doi: 10.1101/2021.07.12.21260377 [DOI] [PMC free article] [PubMed]
- 29.Li Y, Liu J, Yang Z, et al. Transmission of severe acute respiratory syndrome coronavirus 2 to close contacts, China, January-February 2020. Emerg Infect Dis. 2021;27(9):2288-2293. doi: 10.3201/eid2709.202035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu PY, Gragnani CM, Timmerman J, et al. Pediatric household transmission of severe acute respiratory coronavirus-2 infection-Los Angeles County, December 2020 to February 2021. Pediatr Infect Dis J. 2021;40(10):e379-e381. doi: 10.1097/INF.0000000000003251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez DA, Klein EY, Parent C, et al. Latino household transmission of severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2021;ciab753. doi: 10.1093/cid/ciab753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Baz I, Trobajo-Sanmartín C, Miqueleiz A, et al. ; Working Group for the Study of COVID-19 in Navarre; Investigators, other members of the Working Group for the Study of COVID-19 in Navarre . Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021;26(39):2100894. doi: 10.2807/1560-7917.ES.2021.26.39.2100894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer ED, Sandfort M, Bender J, et al. Two doses of the mRNA BNT162b2 vaccine reduce severe outcomes, viral load and secondary attack rate: evidence from a SARS-CoV-2 Alpha outbreak in a nursing home in Germany, January-March 2021. medRxiv. Preprint posted online September 23, 2021. doi: 10.1101/2021.09.13.21262519 [DOI]
- 34.Miller E, Waight PA, Andrews NJ, et al. Transmission of SARS-CoV-2 in the household setting: a prospective cohort study in children and adults in England. J Infect. 2021;83(4):483-489. doi: 10.1016/j.jinf.2021.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Health New Zealand . COVID-19 variants update. December 3, 2021. Accessed December 3, 2021. https://www.health.govt.nz/system/files/documents/pages/22-november-2021-variants-update-summary.pdf
- 36.Musa S, Kissling E, Valenciano M, et al. Household transmission of SARS-CoV-2: a prospective observational study in Bosnia and Herzegovina, August-December 2020. Int J Infect Dis. 2021;112:352-361. doi: 10.1016/j.ijid.2021.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng OT, Koh V, Chiew CJ, et al. Impact of Delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Lancet Reg Health West Pac. 2021;17:100299. doi: 10.1016/j.lanwpc.2021.100299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng DCE, Tan KK, Chin L, et al. Risk factors associated with household transmission of SARS-CoV-2 in Negeri Sembilan, Malaysia. J Paediatr Child Health. 2021. doi: 10.1111/jpc.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata T, Irie F, Ogawa E, et al. Secondary attack rate among non-spousal household contacts of coronavirus disease 2019 in Tsuchiura, Japan, August 2020-February 2021. Int J Environ Res Public Health. 2021;18(17):8921. doi: 10.3390/ijerph18178921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajmohan P, Jose P, Thodi JBA, et al. Dynamics of transmission of COVID-19 cases and household contacts: a prospective cohort study. J Acute Dis. 2021;10(4):162-168. doi: 10.4103/2221-6189.321590 [DOI] [Google Scholar]
- 41.Ratovoson R, Razafimahatratra R, Randriamanantsoa L, et al. Household transmission of COVID-19 among the earliest cases in Antananarivo, Madagascar. Influenza Other Respir Viruses. 2022;16(1):48-55. doi: 10.1111/irv.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singanayagam A, Hakki S, Dunning J, et al. ; ATACCC Study Investigators . Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183-195. doi: 10.1016/S1473-3099(21)00648-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriano-Arandes A, Gatell A, Serrano P, et al. ; COVID-19 Pediatric Disease in Catalonia Research Group . Household severe acute respiratory syndrome coronavirus 2 transmission and children: a network prospective study. Clin Infect Dis. 2021;73(6):e1261-e1269. doi: 10.1093/cid/ciab228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka H, Hirayama A, Nagai H, et al. Increased transmissibility of the SARS-CoV-2 Alpha variant in a Japanese population. Int J Environ Res Public Health. 2021;18(15):7752. doi: 10.3390/ijerph18157752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman SU, Qaisrani M, Abbasi S, et al. COVID-19 outbreak in Islamabad resulting from a travel-associated primary case: a case series. Glob Biosecur. 2021;3(1). [Google Scholar]
- 46.Watanapokasin N, Siripongboonsitti T, Ungtrakul T, et al. Transmissibility of SARS-CoV-2 variants as a secondary attack in Thai households: a retrospective study. IJID Reg. 2021;1:1-2. doi: 10.1016/j.ijregi.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerami C, Popkin-Hall ZR, Rapp T, et al. Household transmission of SARS-CoV-2 in the United States: living density, viral load, and disproportionate impact on communities of color. Clin Infect Dis. 2021;ciab701. doi: 10.1093/cid/ciab701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385(8):759-760. doi: 10.1056/NEJMc2107717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyngse FP, Mølbak K, Skov RL, et al. ; Danish Covid-19 Genome Consortium . Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load. Nat Commun. 2021;12(1):7251. doi: 10.1038/s41467-021-27202-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachdev DD, Chew Ng R, Sankaran M, et al. Contact tracing outcomes among household contacts of fully vaccinated COVID-19 patients - San Francisco, California, January 29-July 2, 2021. Clin Infect Dis. 2021;ciab1042. doi: 10.1093/cid/ciab1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dub T, Nohynek H, Hagberg L, et al. High secondary attack rate and persistence of SARS-CoV-2 antibodies in household transmission study participants, Finland 2020. medRxiv. Preprint posted online July 27, 2021. doi: 10.2139/ssrn.3892117 [DOI] [PMC free article] [PubMed]
- 52.Montecucco A, Dini G, Rahmani A, et al. Investigating SARS-CoV-2 transmission among co-workers in a University of Northern Italy during COVID-19 pandemic: an observational study. Med Lav. 2021;112(6):429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remón-Berrade M, Guillen-Aguinaga S, Sarrate-Adot I, et al. Risk of secondary household transmission of COVID-19 from health care workers in a hospital in Spain. Epidemiologia. 2022;3(1):1-10. doi: 10.3390/epidemiologia3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loss J, Wurm J, Varnaccia G, et al. Transmission of SARS-CoV-2 among children and staff in German daycare centers: results from the COALA study. medRxiv. Preprint posted online December 27, 2021. doi: 10.1101/2021.12.21.21268157 [DOI]
- 55.Lyngse FP, Mortensen LH, Denwood MJ, et al. SARS-CoV-2 Omicron VOC transmission in Danish households. medRxiv. Preprint posted online December 27, 2021. doi: 10.1101/2021.12.27.21268278 [DOI]
- 56.de Gier B, Andeweg S, Backer JA, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), August-September 2021, the Netherlands. medRxiv. Preprint posted online October 14, 2021. doi: 10.2807/1560-7917.ES.2021.26.44.2100977 [DOI] [PMC free article] [PubMed]
- 57.Yi S, Kim JM, Choe YJ, et al. SARS-CoV-2 Delta variant breakthrough infection and onward secondary transmission in household. J Korean Med Sci. 2022;37(1):e12. doi: 10.3346/jkms.2022.37.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka ML, Marentes Ruiz CJ, Malhotra S, et al. SARS-CoV-2 transmission dynamics in households with children, Los Angeles, California. Front Pediatr. 2022;9:752993. doi: 10.3389/fped.2021.752993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meena MS, Priya S, Thirukumaran R, Gowrilakshmi M, Essakiraja K, Madhumitha MS. Factors influencing the acquisition of COVID infection among high-risk contacts of COVID-19 patients in Madurai district-a case control study. J Family Med Prim Care. 2022;11(1):182-189. doi: 10.4103/jfmpc.jfmpc_355_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedman-Klabanoff DJ, Fitzpatrick MC, Deming ME, et al. Risk of severe acute respiratory syndrome coronavirus 2 acquisition is associated with individual exposure but not community-level transmission. J Infect Dis. 2022;jiac029. doi: 10.1093/infdis/jiac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Águila-Mejía JD, Wallmann R, Calvo-Montes J, Rodríguez-Lozano J, Valle-Madrazo T, Aginagalde-Llorente A. Secondary attack rates, transmission, incubation and serial interval periods of first SARS-CoV-2 Omicron variant cases in a northern region of Spain. Research Square. Preprint posted online January 20, 2022.
- 62.Lyngse FP, Kirkeby CT, Denwood M, et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: evidence from Danish households. medRxiv. Preprint posted online January 30, 2022. doi: 10.1101/2022.01.28.22270044 [DOI]
- 63.Baker JM, Nakayama JY, O’Hegarty M, et al. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households—four U.S. jurisdictions, November 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):341-346. doi: 10.15585/mmwr.mm7109e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jalali N, Brustad HK, Frigessi A, et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron variant compared to the Delta variant: evidence from Norwegian contact tracing and vaccination data. medRxiv. Preprint posted online February 18, 2022.
- 65.Lyngse FP, Mølbak K, Denwood M, et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta VOC. medRxiv. Preprint posted online January 6, 2022. doi: 10.1101/2022.01.06.22268841 [DOI]
- 66.Ma X, Wu K, Li Y, et al. Contact tracing period and epidemiological characteristics of an outbreak of the SARS-CoV-2 Delta variant in Guangzhou. Int J Infect Dis. 2022;117:18-23. doi: 10.1016/j.ijid.2022.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith-Jeffcoat SE, Pomeroy MA, Sleweon S, et al. Multistate outbreak of SARS-CoV-2 B. 1.1. 529 (Omicron) variant infections among persons in a social network attending a convention—New York City, November 18–December 20, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(7):238-242. doi: 10.15585/mmwr.mm7107a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song JS, Lee J, Kim M, et al. Serial intervals and household transmission of SARS-CoV-2 Omicron variant, South Korea, 2021. Emerg Infect Dis. 2022;28(3):756-759. doi: 10.3201/eid2803.212607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loenenbach A, Markus I, Lehfeld A-S, et al. SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill. 2021;26(21):2100433. doi: 10.2807/1560-7917.ES.2021.26.21.2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Gier B, Andeweg S, Backer JA, et al. ; RIVM COVID-19 Surveillance and Epidemiology Team . Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Euro Surveill. 2021;26(44):2100977. doi: 10.2807/1560-7917.ES.2021.26.44.2100977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen H, Tessier E, Turner C, et al. Comparative transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants and the impact of vaccination: national cohort study, England. medRxiv. Preprint posted online February 17, 2022. doi: 10.1101/2022.02.15.22271001 [DOI] [PMC free article] [PubMed]
- 72.Eyre DW, Taylor D, Purver M, et al. The impact of SARS-CoV-2 vaccination on Alpha and Delta variant transmission. medRxiv. Preprint posted online February 17, 2021. doi: 10.1101/2021.09.28.21264260 [DOI]
- 73.Hayek S, Shaham G, Ben-Shlomo Y, et al. Indirect protection of children from SARS-CoV-2 infection through parental vaccination. Science. 2022;375(6585):1155-1159. doi: 10.1126/science.abm3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richterman A, Meyerowitz EA, Cevik M. Indirect protection by reducing transmission: ending the pandemic with SARS-CoV-2 vaccination. Open Forum Infect Dis. 2021;9(2):ofab259. doi: 10.1093/ofid/ofab259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science. 2022;375(6585):1151-1154. doi: 10.1126/science.abl4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022. doi: 10.1038/s41591-022-01753-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022. doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dejnirattisai W, Shaw RH, Supasa P, et al. ; Com-COV2 study group . Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399(10321):234-236. doi: 10.1016/S0140-6736(21)02844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med. 2021;385(18):1718-1720. doi: 10.1056/NEJMc2106757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Björk J, Inghammar M, Moghaddassi M, Rasmussen M, Malmqvist U, Kahn F. High level of protection against COVID-19 after two doses of BNT162b2 vaccine in the working age population—first results from a cohort study in Southern Sweden. Infect Dis (Lond). 2021;54(2):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braeye T, Cornelissen L, Catteau L, et al. Vaccine effectiveness against infection and onwards transmission of COVID-19: analysis of Belgian contact tracing data, January-June 2021. Vaccine. 2021;39(39):5456-5460. doi: 10.1016/j.vaccine.2021.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiolet T, Kherabi Y, MacDonald C-J, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202-221. doi: 10.1016/j.cmi.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seppälä E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26(35):2100793. doi: 10.2807/1560-7917.ES.2021.26.35.2100793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. Preprint posted online December 20, 2021. doi: 10.1101/2021.07.13.21260393 [DOI] [PMC free article] [PubMed]
- 85.Public Health England . SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 37. February 11, 2022. Accessed March 2, 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1057359/Technical-Briefing-37-25February2022.pdf
- 86.Public Health England . SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 23. September 17, 2021. Accessed December 12, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018547/Technical_Briefing_23_21_09_16.pdf
- 87.Madewell ZJ, Dean NE, Berlin JA, et al. Challenges of evaluating and modelling vaccination in emerging infectious diseases. Epidemics. 2021;37:100506. doi: 10.1016/j.epidem.2021.100506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyerowitz E, Richterman A. SARS-CoV-2 transmission and prevention in the era of the Delta variant. SSRN. Preprint posted online November 18, 2021. doi: 10.2139/ssrn.3964247 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. Funnel Plots of Studies Reporting Household Secondary Attack Rates With Midpoints in 2021 and Through April 2020
eFigure 3. Funnel Plots of Studies Reporting Household Secondary Attack Rates for Alpha (B.1.1.7) and Delta (B.1.617.2) Variants
eFigure 4. Household Secondary Attack Rates for Omicron (B.1.1.529), Alpha (B.1.1.7), Delta (B.1.617.2), and Beta (B.1.351) Variants From Unvaccinated Index Cases to Unvaccinated Household Contacts
eFigure 5. Household Secondary Attack Rates by Index Case Vaccination Status With All Contacts Are Included Regardless of Vaccination Status
eFigure 6. Funnel Plots of Studies Reporting Household Secondary Attack Rates From Unvaccinated or Fully Vaccinated Index Cases to All Contacts Regardless of Vaccination Status
eFigure 7. Household Secondary Attack Rates by Index Case Vaccination Status With Only Unvaccinated Contacts Included
eFigure 8. Household Secondary Attack Rates by Contact Vaccination Status With All Index Cases Included Regardless of Vaccination Status
eFigure 9. Funnel Plots of Studies Reporting Household Secondary Attack Rates to Unvaccinated or Fully Vaccinated Contacts From All Index Cases Regardless of Vaccination Status
eFigure 10. Household Secondary Attack Rates by Contact Vaccination Status With Only Unvaccinated Index Cases Included
eFigure 11. Household Secondary Attack Rates by Vaccination Status of the Index Cases and Contacts
eTable 1. Electronic Databases and Search Strategy for Household Secondary Attack Rate of SARS-CoV-2
eTable 2. Description of Studies Identified From June 18, 2021 to March 8, 2022
eTable 3. References for Studies Included in Figure 1 of SAR Over Time
eTable 4. Risk of Bias Assessment for Studies Included in Review of Household Transmissibility of SARS-CoV-2 Using the Same Modified Version of the Newcastle–Ottawa Quality Assessment Scale for Observational Studies Used by Fung et al
eTable 5. Pairwise Analyses of Index Case Vaccination Status Using Only Studies in Which SARs Were Reported From Both Relevant Subgroups
eTable 6. Pairwise Analyses of Household Contact Vaccination Status Using Only Studies in Which SARs Were Reported From Both Relevant Subgroups
eTable 7. Household Secondary Attack Rates by Vaccine Type and Contact Vaccination Status With All Index Cases Included Regardless of Vaccination Status
eTable 8. Vaccination Status Definitions for Studies That Reported Household Secondary Attack Rates by Vaccination Status of Index Cases or Contacts
eReferences