Hidden deep within the $2.3 trillion omnibus spending and relief package passed by Congress in December 2020 lies a little-known but powerful provision intended to promote equitable access to clinical trials. Beginning in January 2022, coverage of the “routine costs” associated with clinical trial participation will be guaranteed for all Medicaid beneficiaries for the first time in the program’s history. The absence of federal policy in this area until now has most likely suppressed the representation of low-income and minority populations in the clinical research that underlies therapeutic advances, thereby limiting equitable access to potentially state-of-the-art therapies and compromising the generalizabil- ity of research findings.
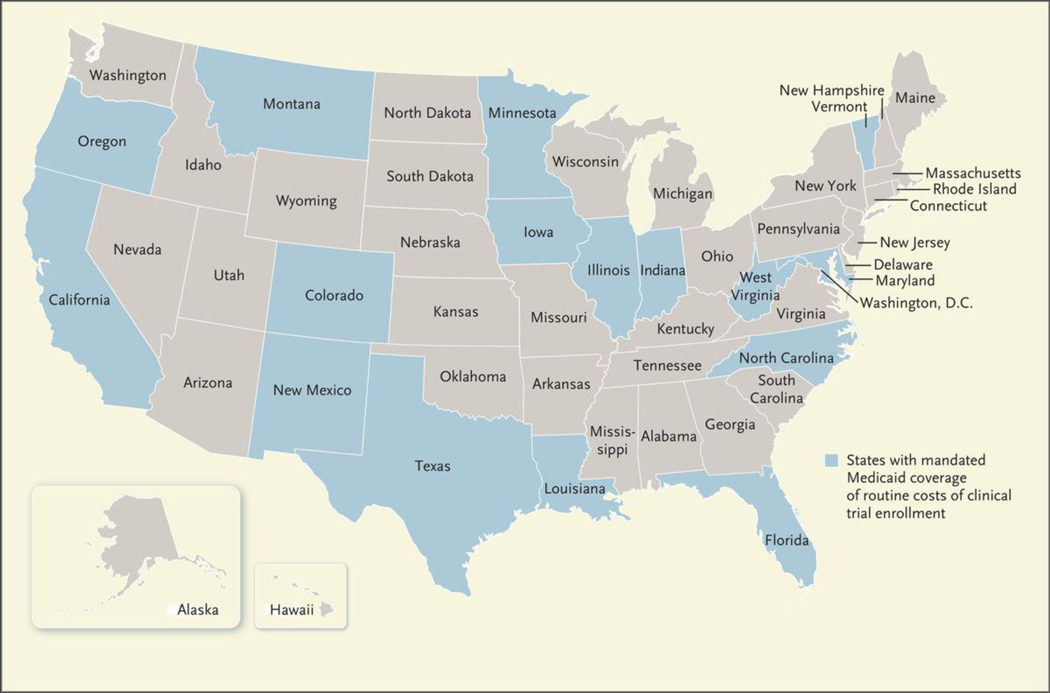
For years, Medicare and private payers have covered the so-called routine costs that accompany clinical trial participation, such as the fees associated with physician visits, hospital stays, diagnostic tests, and other standard clinical services that would have been covered absent the patient’s participation in a trial. A national coverage determination in 2000 mandated coverage of these costs for Medicare beneficiaries. Later, the Affordable Care Act (ACA) extended this mandate to include commercially insured patients (with the exception of those enrolled in grandfathered plans, which were exempted from the requirement). Medicaid beneficiaries, however, were excluded from these federal measures, an omission that left states to legislate their own coverage policies. Consequently, state Medicaid programs vary in the degree to which they cover the routine costs associated with trial participation, with only 15 states mandating coverage (see map).
Map. Standing of State Mandates Requiring Medicaid Coverage of Routine Trial Enrollment Costs, 2020.
Adapted with permission from the Association of Clinical Oncology.
Meanwhile, rates of participation in clinical trials remain low for racial and ethnic minority groups, which results in study samples that don’t accurately reflect the populations that could benefit from the products being studied. For example, non-His-panic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials — a gap that has widened over time.1 Inequities in enrollment have also manifested during the Covid-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of Covid-19 thera-peutics.2
Multiple factors at the patient, clinician, organizational, and structural levels account for these persistent inequities. Low rates of trial participation among non-White patients have been attributed, in part, to cultural and language barriers, distrust of the health care system stemming from a history of mistreatment, inequities in health care access, implicit biases among clinicians and researchers, limited resource availability at hospitals that serve predominantly non-White patients, and restrictive study-eligibility criteria that disproportionately exclude patients who live in marginalized communities.3 Moreover, racial and ethnic minority groups may be especially likely to be affected by financial barriers to study participation, which include the costs associated with more frequent clinic visits, time away from work, and travel-related expenses. Financial concerns — including concerns regarding delays and denials of insurance reimbursement for routine trial- related costs — are frequently cited as deterrents to clinical trial enrollment.
Medicaid programs are well positioned to mitigate inequities in clinical trial enrollment by improving access to care and directly addressing patient-level financial barriers to participation. The ACA expanded Medicaid to millions of Americans who previously were not eligible for the program, which led to dramatic improvements in coverage for low-income people and racial and ethnic minority groups. Medicaid expansion has been associated with better access to care, increased financial protection, and improved health outcomes. More recent evidence suggests that state Medicaid programs have provided a robust safety net amid widespread pandemic-related joblessness and health insurance loss. Medicaid expansion — combined with the new policy mandating coverage of clinical trial-related costs — has the potential to increase rates of trial participation and reduce inequities in enrollment, both by extending coverage of routine costs and, more generally, by enhancing access to medical services for people who were previously uninsured.
The question now is how to leverage the coverage mandate to address ongoing barriers to trial participation and reduce persistent inequities in trial enrollment. We believe there are several important steps that state and federal policymakers should take to ensure the success of this provision.
First, states and provider organizations could use Medicaid’s nonemergency medical transportation (NEMT) benefit to ensure affordable access to appointments associated with clinical trials. Although the NEMT benefit is most commonly used to increase access to behavioral and preventive health services and care for chronic conditions, it could play an important role in facilitating trial participation for underserved groups that disproportionately report transportation-related challenges, including low-income, elderly, and racial and ethnic minority populations. Some states, including Indiana and Iowa, have limited the NEMT benefit as part of Medicaid-expansion demonstration projects; early evidence suggests that these waivers probably had an adverse effect on access to care. Going forward, we believe the Centers for Medicare and Medicaid Services should deny waivers that limit access to the NEMT benefit, given the prevalence of transportation-related barriers to care in the Medicaid population. In addition, states could work to increase awareness of this benefit, reduce the administrative burdens for clinicians and patients associated with Medicaid-covered NEMT services, and avoid imposing unnecessary restrictions on the use of these services, including restrictions on the number of rides, type of ride, and ride distance.
Second, states could use Medicaid funding to invest in patientnavigation programs to help people gain entry to clinical trials and negotiate the complex trial participation process. Patient navigators assist people in addressing health and social needs and have been shown to improve patients’ access to care and outcomes for a range of clinical conditions. Navigator programs have the potential to increase and maintain accrual of minority patients in clinical trials.4 Serving as an important bridge between the community and academic medical centers, patient navigators are well positioned not only to improve health care access and the availability of social supports, but also to engender trust in health care systems among marginalized populations — particularly those that have been subject to historical neglect or mistreatment by providers and institutions.
Finally, in addition to calling for standardized reporting and enhanced transparency regarding participation of marginalized populations in clinical trials, we believe that stronger accountability measures are necessary. Although agencies such as the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) have outlined strategies for increasing representation of minority groups in research, these strategies lack meaningful enforcement measures and fall short of ensuring equitable access to clinical trials. A recent study examining 230 trials leading to FDA approvals of oncology drugs found that data on participants’ race was reported in only 63% of trials; in studies that did report data on race and ethnic group, Black and Hispanic patients were underrepresented relative to distributions of people with cancer in the United States.5 The NIH and FDA could pilot and evaluate new mechanisms for promoting accountability — for example, withholding a portion of funding from National Cancer Institute- designated cancer centers that fail to demonstrate equitable access to and participation in clinical trials. If successful, such a policy could be generalized to institutional funding for clinical research in other disease areas. To protect marginalized populations against the potential for coercion or mis-treatment while enhancing access to valuable opportunities, data and safety monitoring boards should be charged with evaluating equity in trial recruitment and retention, in addition to ensuring the safety of members of these groups in trials.
Extending federally mandated coverage of routine trial-related costs to Medicaid beneficiaries could help ameliorate long-standing barriers that have suppressed enrollment of low-income and non-White patients in research in the United States. It is now up to federal and state policymakers, working together with health systems and researchers, to ensure that the new legislation achieves its full promise of reducing inequities in clinical trial participation — inequities that compromise both the scientific process and access to new therapeutics for all people who could benefit from them.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
References
- 1.Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract 2018;14(1):e1–e10. [DOI] [PubMed] [Google Scholar]
- 2.Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young HN. Racial disproportionality in Covid clinical trials. N Engl J Med 2020;383(9):e59. [DOI] [PubMed] [Google Scholar]
- 3.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
- 4.Ghebre RG, Jones LA, Wenzel JA, Martin MY, Durant RW, Ford JG. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer 2014;120:Suppl 7:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 2019;5(10):e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]