Abstract
BACKGROUND
Solitary fibrous tumors are rare neoplasms of mesenchymal origin. They are often of low malignant potential and rarely metastasize. They frequently arise from the pleura and can occur at any soft tissue site in the body. However, these tumors rarely develop in the mesentery, peritoneal cavity or peritoneum.
CASE SUMMARY
We report on a scarce case of solitary fibrous tumor of the rectal mesentery showing sarcomatosis about 4 years after previous tumor resection. This 69-year-old male had no clinical symptoms but was transferred to our hospital because of a suspected tumor recurrence from follow-up abdominal computed tomography. Tumor markers (CEA, CA 19-9 and CA 125) were within the normal range. Open laparotomy showed sarcomatosis, and pathology confirmed its mesenchymal origin and diagnosis as the solitary fibrous tumor. Our case may be the second recurrent mesentery solitary fibrous tumor reported to date, and the only one with progression to sarcomatosis. There has been no evidence of recurrence in follow-up at the 28th mo after extensive intra-operative peritoneal lavage and cytoreductive surgery.
CONCLUSION
Although there are few risk factors of cancer recurrence in this patient, careful long-term follow-up after cytoreductive surgery is necessary.
Keywords: Solitary fibrous tumor of rectum mesentery, Recurrence, Sarcomatosis, Extensive intra-operative peritoneal lavage, Cytoreductive surgery, Case report
Core Tip: Solitary fibrous tumors (SFTs) are mostly benign and they rarely develop in the mesentery and cause sarcomatosis. The favored treatment strategy is whole-tumor excision with continued follow-up. According to a literature review, our patient is the first case report of mesentery SFT with the presentation of postoperative intraperitoneal recurrence and sarcomatosis.
INTRODUCTION
Solitary fibrous tumors (SFTs) are mostly of mesenchymal origin and were first documented by Klemperer and Rabin[1]. SFTs growing from the mesentery are very rare[2]. In this paper, we report a patient with SFT of mesentery origin with postoperative recurrence and sarcomatosis, and subsequently we provide a review of the literature.
CASE PRESENTATION
Chief complaints
According to regular computed tomography (CT) follow-up, a 69-year-old Japanese man was noted to have a suspected SFT recurrence.
History of present illness
This patient received surgical resection of a rectal mesenteric tumor on 13 January 2016. Pathology confirmed a pedunculated rectal mesenteric tumor of mesenchymal origin to be SFT with malignant potential in terms of mildly positive p53 immunoreactivity. In addition to the primary tumor, a white nodule found on the peritoneal cavity was simultaneously resected and was noted with the same pathologic characteristics. He underwent regular follow-up without any symptoms at the hospital, but according to CT images on 1 March 2020, SFT recurrence was suspected. For further management he was referred to Kishiwada Tokushukai Hospital in Osaka Prefecture, Japan.
History of past illness
He had no medical history except for the surgical resection of a rectal mesenteric SFT with malignant potential on 13 January 2016.
Personal and family history
The patient had no family history.
Physical examination
Physical examination revealed no evident abnormalities on admission except for the previous abdominal operation scar.
Laboratory examinations
Routine blood results were normal, including the levels of the tumor marker cancer antigen 19-9 (CA 19-9; < 2.0 U/mL; normal level < 37.0 U/mL), carcinoembryonic antigen (CEA; 1.1 ng/mL; normal level < 5.0 ng/mL), and cancer antigen 125 (CA 125; 9.1 U/mL; normal level < 35.0 U/mL).
Imaging examinations
Contrast-enhanced CT images of the abdomen and pelvis revealed large and small nodules in the peritoneal cavity around the pelvic floor as well as suspected disseminated lesions. No prominent abnormal findings were noted in the liver, biliary system, pancreas, spleen or adrenal glands with no evident ascites (Figure 1).
Figure 1.

Image of abdominal computed tomography. Suspected carcinomatosis or sarcomatosis was noted in the pelvis with no evident ascites.
FINAL DIAGNOSIS
Pathological findings
This patient received cytoreductive surgery and the specimen was examined. Macroscopic examination revealed more than 100 whitish tumor nodules (each measuring 1-3 mm) seeded over the parietal peritoneum and visceral peritoneum of the partial ileum and colon as well as the urinary bladder. The specimens were noted to have relatively clear boundaries, and some were noted to have fat infiltration (Figures 2 and 3).
Figure 2.

Intestine specimen after extended radical right hemicolectomy. Multiple whitish tumor nodules seeding over the visceral peritoneum of the partial ileum and colon.
Figure 3.

Image of an ileum specimen. Multiple whitish tumor nodules seeding over the visceral peritoneum of the distal ileum.
Histopathologically, this tumor had a heterogeneous cell population comprising mainly spindle cells with proliferated fibrous collagen and diverse groups of cells exhibiting patternless or storiform growth. No tumor necrosis, nuclear polymorphism or cellular atypia was noted. The nuclear mitotic index was 4 mitoses/50 high-power fields (Figure 4A and B).
Figure 4.
Microscopic features. The heterogeneous cell population comprised of mostly spindle cells with fibrous collagen proliferation as well as various other cell populations exhibiting patternless or storiform growth. No tumor necrosis, nuclear polymorphism, or cellular atypia was noted. The nuclear mitotic index was 4 mitoses/50 high-power fields (A: × 100 original magnification; B: × 400 original magnification).
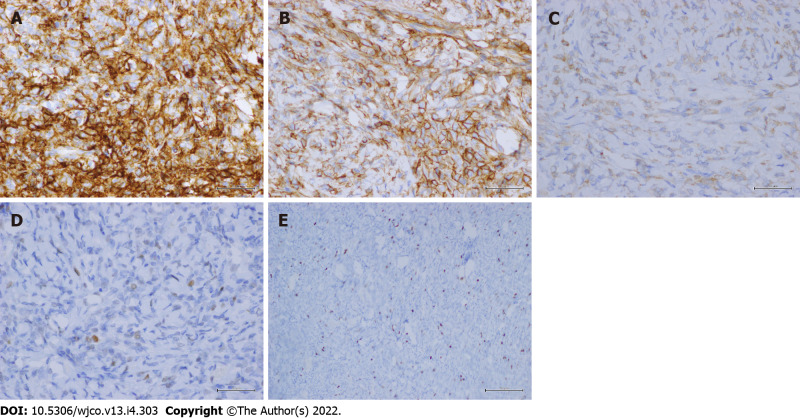
Immunohistochemically, the tumor showed diffuse strong immunoreactivity against CD34 (Figure 5A), CD99 (Figure 5B) and Bcl-2 (Figure 5C). The patient was diagnosed with SFT. Immunoreactivity for p53 was mildly positive (Figure 5D). The mitotic proliferative index for Ki-67 immunostaining was fewer than 4 mitoses/HP, representing a Ki-67 mitotic proliferative index of approximately 5% (Figure 5E). However, the tumor stained negatively for S100, HMB45, actin, desmin, CD10, cytokeratin, cytokeratin 7, CD31, EMA and CD68.
Figure 5.
Immunohistochemical staining. A-E: The lesion showed diffuse strong staining for CD34 (× 400 original magnification) (A); CD99 (× 400 original magnification) (B); Bcl-2 (× 400 original magnification) (C); mildly positive immunoreactivity for p53 (× 400 original magnification) (D); and a Ki-67 mitotic proliferative index of approximately 5% (× 100 original magnification) (E).
Grossly, pathology examination indicated that our patient’s tumor fitted both the histopathological and immunohistochemical characteristics of SFT. Moreover, the washing cytology of the pelvis did not reveal any suspicious malignant cells.
TREATMENT
Operative intervention
A midline laparotomy was performed from the xiphoid to the pubis. The old abdominal incision was also excised. Nearly 100 whitish tumor nodules (each measuring 1–3 mm) were seeded over the parietal peritoneum and visceral peritoneum of the partial ileum and colon as well as the urinary bladder, with no ascites in the pelvis (Figure 6). The intraoperative peritoneal carcinomatosis index was 19 (2-0-0-0-2-2-2-2-2-1-2-2-2).
Figure 6.

Image of the pelvis during operation. Multiple whitish nodules were noted to be seeded over the parietal peritoneum and visceral peritoneum of the partial ileum and colon as well as the urinary bladder, with no ascites in the pelvis.
The laparotomy procedure was initiated after extensive intraoperative peritoneal lavage (EIPL) with 10 L of saline in the abdominopelvic cavity. Cytoreductive surgery (CRS) with electroevaporation was performed, which included adhesiolysis, umbilical and falciform ligament resection, total anterior parietal peritonectomy, bilateral subphrenic peritonectomy, complete pelvic peritonectomy (Figure 7), greater and lesser omentectomy, cholecystectomy, stripping of the tumor from Glisson’s capsule and hepatoduodenal ligament, stripping of the floor of the omental bursa, circumferential resection of hepatogastric ligaments and extended radical right hemicolectomy. Subsequently, EIPL was performed with 10 L of saline in the abdominopelvic cavity. At the end of the surgery, primary ileo-transverse colon anastomosis was performed without stoma establishment. The overall cancer resection score was 0 and the operative duration was 285 minutes. Total blood loss was 1180 mL, and blood component transfusion (total packed red blood cells = 4 units, fresh frozen plasma = 8 units) was required.
Figure 7.

Peritonectomy during cytoreductive surgery. Total anterior parietal peritonectomy, bilateral subphrenic peritonectomy, and complete pelvic peritonectomy (including the visceral peritoneum covering the urinary bladder) were performed.
OUTCOME AND FOLLOW-UP
The patient experienced an uneventful recovery with no postoperative complications and was discharged 13 d after surgery. Neither chemotherapy nor radiation therapy was administered. Repeat CT imaging was performed for every 4 mo during the first 2 years of follow-up. He has been in good general condition without evidence of recurrence or metastasis 28 mo after our surgical management. Subsequent CT imaging every 6 mo through the subsequent 5 years is planned.
DISCUSSION
SFT was first described by Klemperer and Rabin in 1931[1] and was originally an exclusive diagnosis of pleural neoplasms[3]. Later, some SFTs were recognized as originating in various extra-thoracic sites[4]. They are predominantly localized in the pleura, followed by the head and neck, and are seldom present in the abdomen or pelvis[1,5-7]. To date, fewer than 1,000 cases of SFT in the pleura[8], as well as fewer than 100 cases of SFT in the abdomen or pelvis[9] have been reported. SFT of mesentery origin is extremely rare. We searched the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) and reviewed the relevant papers published. Of these reports of SFTs originating from the mesentery retrieved from the literature, we only found 13 cases (Table 1)[2,10-20].
Table 1.
Patients with mesentery solitary fibrous tumors
|
Patient
|
Age
|
Sex
|
Symptom
|
Tumor location
|
Tumor size in cm
|
Management
|
Follow-up (F/U) in mo
|
Recurrence during F/U
|
Ref.
|
| 1 | 33 | M | NM | Mesentery | NM | Operation | NM | NM | [10] |
| 2 | 68 | M | Abdominal pain | Sigmoid mesentery | 18 | Operation | NM | NM | [11] |
| 3 | 53 | M | Abdominal pain | Ileum mesentery | 22 | Operation | 1 | NM | [12] |
| 4 | 73 | M | Abdominal pain | Mesentery | 25 | Operation | NM | NM | [13] |
| 5 | 71 | M | Abdominal mass | Mesentery | 16 | Operation | 12 | No | [14] |
| 6 | 41 | M | Abdominal pain | Mesentery | 23 | Operation | 7 | No | [15] |
| 7 | 26 | M | Abdominal distension | Ileum mesentery | 12 | Operation | 18 | No | [16] |
| 8 | 36 | M | Abdominal pain | Rectum mesentery | 16 | Pre-op radiotherapy → operation | NM | NM | [17] |
| 9 | 59 | F | Abdominal pain | Mesentery | 21 | Operation | 9 | Yes | [18] |
| 10 | 61 | M | No symptom | Jejunum mesentery | 3 | Operation | 2 | NM | [19] |
| 11 | 32 | M | Abdominal mass | Sigmoid mesentery | 13 | Operation | 252 | No | [19] |
| 12 | 41 | M | Abdominal mass | Ileum mesentery | 10 | Operation | NM | NM | [20] |
| 13 | 65 | M | Abdominal pain | Ileum mesentery | 26 | Operation | 12 | No | [2] |
| 14 | 69 | M | No symptom | Sarcomatosis (rectum mesentery recurrence related) | Multiple | Operation (cytoreductive) | 28 | No | Our patient |
F: Female; NM: Not mentioned; M: Male.
SFT is predominant in the sixth and seventh decades of life with no difference in sex distribution[5,21]. To date, no definite causative/contributing etiology or genetic predilection exists for this tumor[8].
Patients with SFT may be asymptomatic at presentation[8]. However, extra-pleural lesions may cause clinically related symptoms to the tumor site. Systemic symptoms such as hypoglycemia (caused by insulin-like growth factor II secretion from the tumor[22]), arthralgia, osteoarthritis and clubbing have been documented[21]. These symptoms usually resolve upon tumor removal. In the present case, recurrent SFT was incidentally detected during regular follow-up and the patient did not exhibit any symptoms.
SFTs do not display any tumor markers. Fluorodeoxyglucose positron emission tomography (FDG-PET) manifestations of SFT have been sparsely reported in the literature and clinical use of FDG-PET imaging for SFT detection remains unclear[23,24]. Cardillo et al[24] showed a weak association between malignancy and FDG-PET uptake in their eight-case study. However, we could acquire important information about the lesions or detect postoperative recurrence according to the radiological examinations, such as type-B ultrasonic, CT and magnetic resonance imaging scans[25,26].
Type-B ultrasonic examination could show intraperitoneal SFT as a hypoechoic but sometimes as a heterogeneous lesion. Besides, this lesion might exhibit flow during Doppler imaging due to its characteristic of high vascularity[23].
On CT imaging, we could note that some well-circumscribed SFT lesions compress adjacent tissues and organs and even cause colon obstruction, urinary retention or bilateral hydronephrosis[27,28]. After contrast use, larger lesions would present with scattered intratumoral foci of hypoenhancement or non-enhancement in the necrosis, hemorrhage or cystic change regions. On the contrary, the homogeneous enhancement would be typically demonstrated in the smaller lesions[29]. Besides, it could provide information on the local extent of disease and the presence of distant metastases. However, the malignancy potential could not be decided according to this radiological distinction.
T1 weighted signal enhancement of MRI imaging could identify subacute hemorrhage of the lesion with the characteristic of intermediate heterogeneous signal intensity. On T2 weighted images, flow voids could also be noticed as areas of heterogeneous low-signal intensity[30]. Besides, intense heterogeneous enhancement of the lesion is noticed in the arterial phase of Gadolinium-enhanced, fat-suppressed T1 weighted images and progressive enhancement in the venous phase[29].
Definite diagnosis of SFT is confirmed through histopathology and immunohistochemical staining. Histopathologically, most SFTs have a patternless or storiform architecture characterized by the coexistence of hypo- and hypercellular areas separated by fibrous stroma, with hemangiopericytoma-like branching blood vessels[31]. Studies of immunohistochemical and electron microscopic aspects have proven that SFTs grow from fibroblastic or myofibroblastic cells of the mesothelium[32,33]. Although differential diagnosis includes other spindle cell tumors, SFT has a unique staining pattern (positive for STAT6, CD34, CD99 and Bcl-2). Among these immunohistochemical markers, STAT6 is probably the most sensitive and specific marker of SFT, because most SFTs have an NAB2-STAT6 fusion gene, which is specific to this tumor type[34]. By contrast, SFTs generally exhibit negative reactivity to cytokeratin, alpha-SMA, S-100, CD31 and c-kit[35]. In our patient, intense diffuse strong staining for CD34, CD99 and Bcl-2 was detected, and SFT was confirmed, although the STAT6 marker was not examined.
SFTs have historically been considered indolent tumors that rarely metastasize in the literature. However, their behavior is unpredictable, with a broad spectrum of biologic behavior. Most SFTs behave benignly after complete surgical resection; however, some have been reported to behave aggressively either through local recurrence or distant metastasis[35]. The 2013 World Health Organization classification of SFT defines malignant forms as having a large tumor size (longer than 5 or 10 cm), sessile lesions, hypercellularity, and increased mitotic index (> 4 mitoses/10 high-power fields)[36,37], with cytological atypia, nuclear pleomorphism, tumor necrosis, infiltrative margins or hemorrhage[38]. However, discrepancies of morphological malignancy and clinical malignancy have been observed for SFT[3]. Some experts have advocated that malignant transformation with dedifferentiation of tumor cells might contribute to such discrepancies[39]. Malignant transformation is of two types: malignant or high-grade SFT and the de novo occurrence of malignancy[21]. In our case, malignancy may be the recurrence of the previous tumor.
Immunohistochemistry for some proteins may clinically provide hints of SFT malignancy[3]. Takizawa et al[40] noted no tumor recurrence in cases with positive immunostaining for both CD34 and Bcl-2. Deprivation of CD34 and Bcl-2 immunoreactivity was observed in the component with malignant transformation[39,41]. In benign SFTs, p53 immunoreactivity was not detected. However, p53 immunoreactivity has been confirmed in morphologically and clinically malignant SFT[3,41]. According to these criteria, our present case had borderline benign (diffuse strong immunostaining for CD34, CD99 and Bcl-2) and malignant (mildly positive p53 immunoreactivity) pathologic characteristics. However, due to the clinical presentation of intraperitoneal sarcomatosis, recurrence remains a concern despite the complete removal of the seeding tumors achieved for our patient.
No consensus has been reached for the treatment guidelines for SFTs in this particular location (rectal mesentery) because of their scarcity and the confusion regarding their pathological confirmation[14]. However, complete surgical resection is the standard and mainstay treatment for most SFTs, including abdominopelvic SFTs and those that arise in other organs, regardless of histologic subtype[38]. The most essential prognostic factor is surgical resectability because complete resection of the tumor is curative in more than 90% of cases[1,42]. Poor prognosis is observed in patients with incomplete resection[43]. However, SFTs are hypervascular, complicating surgical resection[9] regardless of the tumor location.
Local recurrence or metastasis develops in 12%-22% of cases[44]. Patients with extra-thoracic SFTs are statistically more likely to develop the metastatic disease than those with thoracic SFTs[45]. Adjuvant chemoradiotherapy is not widely practiced or accepted as the standard of care[46]. Some experts have suggested radiotherapy or adjuvant chemotherapy, although poor outcomes have been reported for these treatments[43]. Some experts have used antiangiogenic agents as a therapeutic strategy in recent years. However, similar to the finding of the low response rate in standard chemotherapy, progression-free survival appeared similar between cytotoxic chemotherapy and antiangiogenic agents[47]. Thus, improved systemic therapies are required for metastatic or unresectable diseases.
To date, no standard therapy has been established for inoperable SFTs[4]. SFTs are generally regarded as chemoresistant tumors[48]. Our patient's Ki-67 mitotic proliferative index was only approximately 5%, suggesting that it was a type of low-grade sarcoma; we proposed that chemotherapy would have little effect. Although radiotherapy was another option, radiotherapy was not conducted for our patient because of the side effect of radiation injury on the small bowel related to the proposed large area of radiation and adhesion after two episodes of laparotomy. Instead, we sincerely believed that EIPL could effectively lower the amount of intra-abdominal free cancer cells, and it is a preventative strategy for further peritoneal recurrence. Moreover, we performed complete en bloc surgical resection with negative margins through CRS for this “inoperable” patient, which is of paramount importance.
Predicting the aggressive clinical behavior of SFTs is difficult[21,35]. Late recurrence or metastasis may develop even when the SFT has been identified as benign[49]. While surgical resection continues to be the initial modality for treating malignant SFTs, the utility of adjuvant chemotherapy or radiation is still unknown given that most data is based on small case series. Thus, no guidelines exist for determining the modality and frequency of post-treatment surveillance[31]. Long-term and regular surveillance is mandatory[50]. Some experts have suggested that long follow-up periods (≥ 15 years) should be maintained with closer follow-ups during the first 2 years[49], particularly for patients with high-risk features.
CONCLUSION
In conclusion, this article reported the first case of rectal mesentery SFT with postoperative recurrence and sarcomatosis. Instead of adjuvant chemotherapy or radiotherapy, we performed EIPL and CRS, and no evidence of recurrence was found during the 28-mo follow-up period.
Footnotes
Informed consent statement: Written informed consent for the publication of this case report was obtained from the patient.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 25, 2021
First decision: January 12, 2022
Article in press: March 27, 2022
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dragoni G, Italy; Zhang X, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
Contributor Information
Chong-Chi Chiu, Department of General Surgery, E-Da Cancer Hospital, Kaohsiung 82445, Taiwan; School of Medicine, College of Medicine, I-Shou University, Kaohsiung 82445, Taiwan; Department of Medical Education and Research, E-Da Cancer Hospital, Kaohsiung 82445, Taiwan.
Haruaki Ishibashi, Peritoneal Surface Malignancy Center, Kishiwada Tokushukai Hospital, Osaka 596-8522, Japan.
Satoshi Wakama, Peritoneal Surface Malignancy Center, Kishiwada Tokushukai Hospital, Osaka 596-8522, Japan.
Yang Liu, Peritoneal Surface Malignancy Center, Kishiwada Tokushukai Hospital, Osaka 596-8522, Japan.
Yuan Hao, Department of General Surgery, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Chao-Ming Hung, Department of General Surgery, E-Da Cancer Hospital, Kaohsiung 82445, Taiwan; School of Medicine, College of Medicine, I-Shou University, Kaohsiung 82445, Taiwan.
Po-Huang Lee, Department of General Surgery, E-Da Hospital, Kaohsiung 82445, Taiwan; College of Medicine, I-Shou University, Kaohsiung 82445, Taiwan.
Kun-Ming Rau, School of Medicine, College of Medicine, I-Shou University, Kaohsiung 82445, Taiwan; Department of Hematology & Oncology, E-Da Cancer Hospital, Kaohsiung 82445, Taiwan.
Hui-Ming Lee, Department of General Surgery, E-Da Cancer Hospital, Kaohsiung 82445, Taiwan; College of Medicine, I-Shou University, Kaohsiung 82445, Taiwan.
Yutaka Yonemura, Peritoneal Surface Malignancy Center, Kishiwada Tokushukai Hospital, Osaka 596-8522, Japan. y.yonemura@coda.ocn.ne.jp.
References
- 1.Yokoyama Y, Hata K, Kanazawa T, Yamaguchi H, Ishihara S, Sunami E, Kitayama J, Watanabe T. Giant solitary fibrous tumor of the pelvis successfully treated with preoperative embolization and surgical resection: a case report. World J Surg Oncol. 2015;13:164. doi: 10.1186/s12957-015-0578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida K, Ubukata H, Konishi S, Shimazaki J, Yano Y, Morishita Y, Tabuchi T. A giant solitary fibrous tumor of the mesentery: a case report and literature review. World J Surg Oncol. 2015;13:17. doi: 10.1186/s12957-014-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maki T, Fujino S, Misu K, Kaneko H, Inomata H, Omi M, Tateno M, Nihei K. Integrally calcified solitary fibrous tumor in the retroperitoneum: a case report and review of the literature. Surg Case Rep. 2016;2:14. doi: 10.1186/s40792-016-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zong L, Chen P, Wang GY, Zhu QS. Giant solitary fibrous tumor arising from greater omentum. World J Gastroenterol. 2012;18:6515–6520. doi: 10.3748/wjg.v18.i44.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li XM, Reng J, Zhou P, Cao Y, Cheng ZZ, Xiao Y, Xu GH. Solitary fibrous tumors in abdomen and pelvis: imaging characteristics and radiologic-pathologic correlation. World J Gastroenterol. 2014;20:5066–5073. doi: 10.3748/wjg.v20.i17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramythiotis D, Kofina K, Bangeas P, Tsiompanou F, Karayannopoulou G, Basdanis G. Solitary fibrous tumor of the pancreas: Case report and review of the literature. World J Gastrointest Surg. 2016;8:461–466. doi: 10.4240/wjgs.v8.i6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakob M, Schneider M, Hoeller I, Laffer U, Kaderli R. Malignant solitary fibrous tumor involving the liver. World J Gastroenterol. 2013;19:3354–3357. doi: 10.3748/wjg.v19.i21.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boe J, Chimpiri AR, Liu CZ. Solitary fibrous tumor originating in the pelvis: a case report. J Radiol Case Rep. 2010;4:21–28. doi: 10.3941/jrcr.v4i7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez A, Conrad M, Gill RM, Choi WT, Kumar V, Behr S. Solitary fibrous tumor in the abdomen and pelvis: A case series with radiological findings and treatment recommendations. Clin Imaging. 2018;48:48–54. doi: 10.1016/j.clinimag.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Hardisson D, Limeres MA, Jimenez-Heffernan JA, De la Rosa P, Burgos E. Solitary fibrous tumor of the mesentery. Am J Gastroenterol. 1996;91:810–811. [PubMed] [Google Scholar]
- 11.Balaji R, Ramachandran K, Somanathan T. A rare case of solitary fibrous tumour of the sigmoid mesocolon: imaging features and review of literature. Cancer Imaging. 2009;9:67–69. doi: 10.1102/1470-7330.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau MI, Foo FJ, Sissons MC, Kiruparan P. Solitary fibrous tumor of small bowel mesentery: a case report and review of the literature. Tumori. 2010;96:1035–1039. [PubMed] [Google Scholar]
- 13.Muroni M, Mezzetti G, Noto S. Malignant solitary fibrous tumor originating from the mesentery. Gastroenterology. 2012;142:12–13, 187. doi: 10.1053/j.gastro.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Bouhabel S, Leblanc G, Ferreira J, Leclerc YE, Dubé P, Sidéris L. Solitary fibrous tumor arising in the mesentery: a case report. World J Surg Oncol. 2011;9:140. doi: 10.1186/1477-7819-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudva R, Monappa V, Rao A. Giant solitary fibrous tumor of the mesentery: a rare case. J Cancer Res Ther. 2011;7:376–378. doi: 10.4103/0973-1482.87021. [DOI] [PubMed] [Google Scholar]
- 16.Cantarella F, Graziosi L, Cavazzoni E, Donini A. Small bowel mesentery solitary fibrous tumor. A rare neoplasia in a young male. G Chir. 2012;33:271–273. [PubMed] [Google Scholar]
- 17.Nishikawa T, Takei Y, Teshima S, Deie K, Takano Y, Tsuno NH, Maeda M. A hypervascular malignant solitary fibrous tumor in the mesentery of the rectum with good response to preoperative radiation therapy. Int Canc Conf J. 2013;2:30–35. [Google Scholar]
- 18.Liu Y, Ishibashi H, Sako S, Takeshita K, Li Y, Elnemr A, Yonemura Y. A giant mesentery malignant solitary fibrous tumor recurring as dedifferentiated liposarcoma- a report of a very rare case and literature review. Gan To Kagaku Ryoho. 2013;40:2466–2469. [PubMed] [Google Scholar]
- 19.Val-Bernal JF, Mayorga M, Fernández F, Parra A, Crespo J, García-Polavieja M. Solitary fibrous tumor arising from the mesentery of adult patients. Report of two cases and review of the literature. Rom J Morphol Embryol. 2014;55:203–207. [PubMed] [Google Scholar]
- 20.Zhang GJ, Li RT, Zhou Y, Huang F, Zhao ZC, Li WD, Fu WH. Solitary fibrous tumor of small bowel mesentery with postoperative bowel obstruction: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:11691–11697. [PMC free article] [PubMed] [Google Scholar]
- 21.Gao C, Zhang Y, Li YY, Yu YH, Qu W, Gao YS, Zhang S. Postoperative recurrence solitary fibrous tumor of the pelvic with malignant transformation. Int J Clin Exp Med. 2015;8:16827–16833. [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda S, Sugita M, Sagawa M, Ueda Y, Sakuma T. Solitary fibrous tumor of the pleura suddenly induced hypoglycemia before surgical treatment. Ann Thorac Cardiovasc Surg. 2011;17:293–296. doi: 10.5761/atcs.cr.10.01554. [DOI] [PubMed] [Google Scholar]
- 23.Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. 2011;196:487–495. doi: 10.2214/AJR.10.4948. [DOI] [PubMed] [Google Scholar]
- 24.Cardillo G, Carbone L, Carleo F, Masala N, Graziano P, Bray A, Martelli M. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg. 2009;88:1632–1637. doi: 10.1016/j.athoracsur.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Enon S, Kilic D, Yuksel C, Kayi Cangir A, Percinel S, Sak SD, Gungor A, Kavukcu S, Okten I. Benign localized fibrous tumor of the pleura: report of 25 new cases. Thorac Cardiovasc Surg. 2012;60:468–473. doi: 10.1055/s-0031-1295519. [DOI] [PubMed] [Google Scholar]
- 26.Ge W, Yu DC, Jiang CP, Ding YT. Giant solitary fibrous tumor of the diaphragm: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:9044–9049. [PMC free article] [PubMed] [Google Scholar]
- 27.Yi B, Bewtra C, Yussef K, Silva E. Giant pelvic solitary fibrous tumor obstructing intestinal and urinary tract: a case report and literature review. Am Surg. 2007;73:478–480. [PubMed] [Google Scholar]
- 28.Ando R, Kobayashi D, Naiki T, Kawai N, Sasaki S, Kohri K. Pelvic solitary fibrous tumor originally diagnosed as prostatic in origin. Clin Imaging. 2012;36:243–245. doi: 10.1016/j.clinimag.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. Radiographics. 2011;31:393–408. doi: 10.1148/rg.312105080. [DOI] [PubMed] [Google Scholar]
- 30.Wat SY, Sur M, Dhamanaskar K. Solitary fibrous tumor (SFT) of the pelvis. Clin Imaging. 2008;32:152–156. doi: 10.1016/j.clinimag.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhanlong M, Haibin S, Xiangshan F, Jiacheng S, Yicheng N. Variable Solitary Fibrous Tumor Locations: CT and MR Imaging Features. Medicine (Baltimore) 2016;95:e3031. doi: 10.1097/MD.0000000000003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Said JW, Nash G, Banks-Schlegel S, Sassoon AF, Shintaku IP. Localized fibrous mesothelioma: an immunohistochemical and electron microscopic study. Hum Pathol. 1984;15:440–443. doi: 10.1016/s0046-8177(84)80077-5. [DOI] [PubMed] [Google Scholar]
- 33.Dervan PA, Tobin B, O'Connor M. Solitary (localized) fibrous mesothelioma: evidence against mesothelial cell origin. Histopathology. 1986;10:867–875. doi: 10.1111/j.1365-2559.1986.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Liao Q, Liao X, Wen G, Li Z, Lin C, Zhao L. A huge malignant solitary fibrous tumor of kidney: case report and review of the literature. Diagn Pathol. 2014;9:13. doi: 10.1186/1746-1596-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng L, Liu Y, Ai Y, Liu Z, He Y, Liu Q. Skull base metastases from a malignant solitary fibrous tumor of the liver. A case report and literature review. Diagn Pathol. 2011;6:127. doi: 10.1186/1746-1596-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Liu J, Chen W, Mao S, Guo Y. Primary solitary fibrous tumors of liver: a case report and literature review. Diagn Pathol. 2013;8:195. doi: 10.1186/1746-1596-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeVito N, Henderson E, Han G, Reed D, Bui MM, Lavey R, Robinson L, Zager JS, Gonzalez RJ, Sondak VK, Letson GD, Conley A. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS One. 2015;10:e0140362. doi: 10.1371/journal.pone.0140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component--is this dedifferentiated SFT? Am J Surg Pathol. 2009;33:1314–1321. doi: 10.1097/pas.0b013e3181a6cd33. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa I, Saito T, Kitamura Y, Arai K, Kawaguchi M, Takahashi K, Hara N. Primary solitary fibrous tumor (SFT) in the retroperitoneum. Urol Oncol. 2008;26:254–259. doi: 10.1016/j.urolonc.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Yokoi T, Tsuzuki T, Yatabe Y, Suzuki M, Kurumaya H, Koshikawa T, Kuhara H, Kuroda M, Nakamura N, Nakatani Y, Kakudo K. Solitary fibrous tumour: significance of p53 and CD34 immunoreactivity in its malignant transformation. Histopathology. 1998;32:423–432. doi: 10.1046/j.1365-2559.1998.00412.x. [DOI] [PubMed] [Google Scholar]
- 42.Ge W, Yu DC, Chen G, Ding YT. Clinical analysis of 47 cases of solitary fibrous tumor. Oncol Lett. 2016;12:2475–2480. doi: 10.3892/ol.2016.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez Cruz MI, Hernández Sánchez JE, Blázquez BS, Prieto Nogal SB, Gómez Tejeda LM. Malignant solitary fibrous kidney tumor with peritoneal disease: a case report. Case Rep Nephrol Urol. 2014;4:70–74. doi: 10.1159/000362539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johannet P, Kamaya A, Gayer G. Radiological findings in pelvic solitary fibrous tumour. BJR Case Rep. 2016;2:20150373. doi: 10.1259/bjrcr.20150373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neill AC, Tirumani SH, Do WS, Keraliya AR, Hornick JL, Shinagare AB, Ramaiya NH. Metastatic Patterns of Solitary Fibrous Tumors: A Single-Institution Experience. AJR Am J Roentgenol. 2017;208:2–9. doi: 10.2214/AJR.16.16662. [DOI] [PubMed] [Google Scholar]
- 46.Thway K, Ng W, Noujaim J, Jones RL, Fisher C. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int J Surg Pathol. 2016;24:281–292. doi: 10.1177/1066896915627485. [DOI] [PubMed] [Google Scholar]
- 47.Levard A, Derbel O, Méeus P, Ranchère D, Ray-Coquard I, Blay JY, Cassier PA. Outcome of patients with advanced solitary fibrous tumors: the Centre Léon Bérard experience. BMC Cancer. 2013;13:109. doi: 10.1186/1471-2407-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park MS, Ravi V, Conley A, Patel SR, Trent JC, Lev DC, Lazar AJ, Wang WL, Benjamin RS, Araujo DM. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res. 2013;3:7. doi: 10.1186/2045-3329-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Chen P, Zhao W, Shi L, Gu X, Xu Q. Clinicopathological findings in a case series of abdominopelvic solitary fibrous tumors. Oncol Lett. 2014;7:1067–1072. doi: 10.3892/ol.2014.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bi X, Zhai J, Chun CD. Solitary fibrous tumor of the abdominal wall re-surfacing as unilateral pleural effusion and mass: A case report and review of the literature. Respir Med Case Rep. 2018;23:4–7. doi: 10.1016/j.rmcr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]