Abstract
Objective
To assess the diagnostic value of fluorine 18 (18F)-labeled prostate-specific membrane antigen (PSMA)-1007 Positron emission tomography/Magnetic resonance imaging (PET/MRI) and compared with that of biparametric MRI (bpMRI) for the detection of prostate cancer (PCa).
Materials and methods
The study enrolled 29 patients with suspected PCa preoperatively who underwent 18F-PSMA-1007 PET/MRI and subsequent targeted biopsy for suspected PCa lesions. Two readers independently assessed the images of each suspected PCa lesion and determined their overall assessment category on bpMRI and 18F-PSMA-1007 PET/MRI. By using biopsy histopathology as the reference standard, the accuracies of 18F-PSMA-1007 PET/MRI and bpMRI for the detection of PCa lesion were determined. Furthermore, the receiver-operating characteristic (ROC) curves of their semi-quantitative parameters of the optimal standardized uptake value (SUVmax) and apparent diffusion coefficient (ADC) for detecting PCa lesions were derived, and their correlations with the International Society of Urological Pathology (ISUP) grade were reported.
Results
Of the 48 suspected PCa lesions in 29 patients, 38 were pathologically diagnosed with clinically significant PCa and 10 with nonprostate cancer (non-PCa) lesions. Compared with the pathological results, 18F-PSMA-1007 PET/MRI demonstrated much greater diagnostic accuracy (area under the curve, AUC), sensitivity, specificity, positive predictive value, and negative predictive value than bpMRI: 0.974 versus 0.711, 94.74% versus 92.11%, 100% versus 50%, 100% versus 87.50%, and 83.33% versus 62.50%, respectively. The semi-quantitative parameters of SUVmax demonstrated a higher AUC of 0.874 than that of ADC with 0.776 for detecting PCa. The ISUP grade was positively associated with SUVmax at spearman’s rho correlation coefficient (Rho) = 0.539, p = 0), but not associated with ADC (Rho = −0.105, p = 0.529).
Conclusion
The diagnostic value of 18F-PSMA-1007 PET/MRI for the detection of PCa is better than that of bpMRI, and a high SUVmax may indicate a lesion with a high ISUP grade.
Keywords: 18F-PSMA-1007, PET/MRI, bpMRI, Prostate cancer, Diagnostic value
Abbreviations: ADC, Apparent diffusion coefficient; AUC, Area under the curve; bpMRI, Biparametric MRI; CI, Confidence interval; DCE, Dynamic contrast-enhanced; DWI, Diffusion-weighted imaging; 18F-PSMA-1007, Fluorine 18-labeled prostate-specific membrane antigen-1007; ISUP, International Society of Urological Pathology; mpMRI, Multiparametric magnetic resonance imaging; NPV, Negative predictive value; PCa, Prostate cancer; PET/MRI, Positron emission tomography/Magnetic resonance imaging; PI-RADS, Prostate imaging reporting and data system; PPV, Positive predictive value; PSA, Prostate-specific antigen; ROC, Receiver-operating characteristic; ROI, Region of interest; SUVmax, Optimal standardized uptake value; TRUS, Transrectal ultrasonography; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging
1. Introduction
Prostate cancer (PCa) is a common malignant tumor of the genitourinary system in older men.1 With the aging of our society, its incidence and mortality rate are increasing and require our attention.2 Since there are no obvious clinical symptoms of PCa, the best time window for treatment may be missed after patients are diagnosed.3 Imaging diagnosis is of necessity for patients to win treatment time and guide the formulation of the best treatment plan.
Multiparametric magnetic resonance imaging (mpMRI), which involves T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) MRI, has been introduced as a standard for the diagnosis of primary prostate lesions and is useful for guiding prostate biopsy.4,5 However, the usefulness of DCE MRI in diagnosing PCa is controversial.6,7 In prostate imaging reporting and data system (PI-RADS), T2WI and DWI are proposed as the dominant sequence for transition zone and peripheral zone lesions. DCE MRI plays a supplementary role when DWI is not sufficient for diagnosis, and DCE positivity only upgrades DWI score three lesions in the peripheral zone to score 4.8 Considering that DCE MRI does not significantly improve the diagnostic accuracy for PCa but has obvious disadvantages such as being time-consuming and necessitating additional costs, and the potential risk of nephrogenic systemic fibrosis brought by gadolinium contrast reagent, its abandonment has been suggested, and bpMRI has been recommended for more clinical applications.9, 10, 11
Prostate-specific membrane antigen (PSMA) targeted Positron emission tomography/Magnetic resonance imaging (PET/MRI) is considered to be a promising technology for PCa imaging.12 The clinical breakthrough of PSMA ligands was achieved with Gallium 68 (68Ga)-labeled PSMA-11, which has been the most commonly used PSMA ligand in clinical trials.13 However, some limitations for 68Ga-PSMA-11 were gradually exposed in clinical applications, such as requiring an on-site generator to produce with a short half-life, compromised ability to detect smaller lesions with high energy characteristics, and increased PET artifacts surrounding the bladder, due to urethral system excretion.14,15 With this background, fluorine 18 (18F)-labeled PSMA-1007 has recently received wide attention and has been introduced in clinical practice.15 Besides its advantages, such as longer half-life, improved resolution of PET image, and higher labeling yields, 18F-PSMA-1007 facilitates nonurinary background clearance superior to that of 68Ga-PSMA-11 through the early hepatobiliary route leading to a reduced bladder background.16,17 Nevertheless, the clinical reports on 18F-PSMA-1007 PET/MRI are limited. In this study, we evaluate the diagnostic value of 18F-PSMA-1007 PET/MRI and compare it with that of bpMRI for the detection of primary prostate cancer. This study will help promote the clinical applications of 18F-PSMA-1007 PET/MRI and contribute to the literature.
2. Methods
2.1. Study population
Our institution was authorized by the government for the clinical trial research of 18F-PSMA-1007 with financial support. This retrospective study has been approved by the Institutional Ethics Committee (Approved No.2019-01), and written informed consent was obtained from all patients. We initially collected the records of 34 consecutive male patients with suspected PCa preoperatively who underwent 18F-PSMA-1007 PET/MRI and subsequent targeted biopsy for lesions suggestive of PCa between June 2019 and June 2021. Of these patients, 5 were excluded for the following reasons: pathological results were not available for review (n = 2); the patient had other tumors besides PCa (n = 1); the patients had received relevant therapy previously (n = 2). Thus, 29 patients with suspected PCa lesions (n = 48) for targeted biopsy were finally enrolled in our study. Of these lesions, the presence or absence of PCa lesions, tumor Gleason score, and the International Society of Urological Pathology (ISUP) grade for each biopsy specimen were recorded independently according to the 2014 ISUP Modified Grading System.18,19 Tumor with Gleason score = 3 + 3 and tumor size≥0.5 mL (tumor diameter ≥8 mm), or with Gleason score of ≥7 and tumor diameter≥5 mm was defined as clinically significant PCa.8,20
2.2. 18F-PSMA-1007 PET/MRI examination
18F-PSMA-1007 was produced as described by Cardinale et al.21 with radiochemical purity >95%. The patients were injected with 18F-PSMA-1007 at an injection dosage of 3.7 MBq/kg. After sixty minutes of tracer uptake, a whole-body scan (from the top of the skull to the middle and upper femur, five beds, about 40 min) and subsequent prostate bed fine scan (1 bed, about 15–20 min) were performed after using an integrated PET/MRI system 3T Biograph mMRI (Siemens, Erlangen, Germany). During the prostate bed fine scan, the 3D PET imaging combined with MRI imaging, including transversal T1-weighted imaging, transversal T2-weighted imaging, transversal DWI, sagittal T2-weighted imaging, and coronal T2-weighted imaging, was performed to cover the prostate bed. Apparent diffusion coefficient (ADC) maps of the prostate area were automatically reconstructed from the DWI images acquired using different b-values of 0. 800, and 1400 sec/mm2. The data acquired from PET and MRI were reconstructed (3D-iterative) using the ordered subset-expectation maximization algorithm (three iterations, 21 subsets, Gaussian postfiltering full width at half maximum 2). The detailed MRI sequence parameters are listed in Supplemental material Table S1.
2.3. Image interpretation
A study coordinator prepared a PowerPoint file showing the 18F-PSMA-1007 PET/MRI images of the lesions targeted for biopsy of each patient prior to the assessments. Two readers board-certified in radiology and nuclear medicine with more than 5 years of experience interpreted the images of each lesion using dedicated software Syngovia version 3.0 (Siemens, Erlangen, Germany) and viewed with reference to the PowerPoint file. Firstly, all bpMRI images of each lesion were interpreted by the two readers blinded to the PET images. Lesions were assigned bpMRI assessment categories of 1–5 according to the simplified PI-RADS v2.1 described by Tamada et al.20 using only T2WI images and DWI images with ADC maps. The scoring system for T2WI, DWI, and the algorithm for determining bpMRI assessment categories were listed in Supplemental material Tables S2-S3, and a bpMRI assessment category of 3 or higher was characterized as positive. Subsequently, the PET images were evaluated by the two readers with the MRI images as anatomical background and assigned scores of 1–5 for PET according to PSMA-RADS Version 1.0 proposed by Rowe et al.22 The scoring system was listed in Supplemental material Tables S4. Finally, all lesions were reviewed a second time with access to the individual evaluations of PET and bpMRI images, and a combined PET/MRI interpretation was obtained to determine the final assessment categories by matching the bpMRI assessment categories and PET scores according to the algorithm listed in Supplemental material Tables S5. The PSMA-PET uptake was semi-quantitatively analyzed using the optimal standardized uptake value (SUVmax), which was acquired from the most intense uptake area by drawing a region of interest (ROI). For the ADC values, an ROI was drawn around the lesion on DWI according to the hypointense signal on T2WI. The mean SUVmax and ADC values of a single ROI calculated by the two interpreters were used as observations for analysis and labeled as the SUVmax and ADC of the lesion.
2.4. Biopsy method
All biopsy procedures were performed using the BioJet Fusion Software System (DK Technologies GmbH, Barum, Germany) combined with a transperineal grid transrectal ultrasonography (TRUS) platform (BK Medical, Herlev, Denmark) by an operator who had passed the professional biopsy training. Prior to biopsy, an experienced radiologist was in attendance with targeted lesions marked on MRI and fused to the real-time TRUS images for targeted biopsy. The biopsy core of the target lesion displayed on the fusion image was obtained by biopsy needle (using 18G needles with a sampling length of 17 mm) under TRUS guidance with at least two cores per lesion. During the biopsy, the real-time TRUS images were presented to guarantee the accuracy of needle deployment within the target lesion. The cores were processed for histopathologic analysis by an experienced uropathologist blinded to the imaging results according to the ISUP protocols.
2.5. Statistical Analysis
All statistical analyses were performed using SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA), and MedCalc software version 11.4.2.0 (MedCalc Software Ltd, Ostend, Belgium). The patient clinical characteristics were summarized using descriptive statistics. The continuous variables (ADC) conforming to the normal distribution were represented by the mean ± standard deviation, and the paired sample t-test was used to compare those associated with PCa and non-PCa cases. The continuous variables not conforming to the normal distribution (age, PSA, and SUVmax) were represented by the median with interquartile ranges, and the Mann–Whitney U test was used to compare those associated with PCa and non-PCa cases. The diagnostic accuracy for 18F-PSMA-1007 PET/MRI and bpMRI were assessed by comparing the imaging findings with the histopathological results as the reference standard using a 2 × 2 contingency table. Their sensitivities, specificities, NPVs, and PPVs were calculated with a 95% Confidence interval (CI), and their AUCs were determined using ROC curves. The ROC curves were used to evaluate the diagnostic accuracies of ADC and SUVmax for detecting PCa and determining their optimal values. The correlations between SUVmax, ADC, and the ISUP grade were analyzed using Spearman’s correlation test. P < 0.05 was considered significant.
3. Results
3.1. Clinical characteristics of included patients
The clinical characteristics of the included patients are shown in Table 1. According to the histopathological biopsy results, the patients were divided into the PCa group with 22 patients and the non-PCa group with seven patients. The median age and PSA level of the PCa patients were 71 years (range, 63–75 years) and 11.25 ng/mL (range, 8.5–31.6 ng/mL), while those of the non-PCa patients were 65 years (range, 55–67 years) and 7.6 ng/mL (range, 5.2–10.6 ng/mL), respectively. The differences in age and PSA level between the PCa and non-PCa groups showed no statistical significance (p = 0.199 and p = 0.055, respectively). In 29 patients, 48 suspected PCa lesions were analyzed, which included one lesion in 17 patients, two lesions in eight patients, three lesions in two patients, four lesions in one patient, and five lesions in one patient. Of these 48 lesions, 38 were pathologically confirmed to be clinically significant PCa (6 of ISUP grade 1 with Gleason score 3 + 3, 3 of ISUP grade 2 with Gleason score 3 + 4, 8 of ISUP grade 3 with Gleason score 4 + 3, 8 of ISUP grade 4 with Gleason score 4 + 4, and 13 of ISUP grade 5 with Gleason score 4 + 5/5 + 4/5 + 5, and 10 were non-PCa lesions. Based on the bpMRI, the distribution of the scores for the 38 biopsy-proven PCa lesions were as follows: 3 of PI-RADS score 2, 12 of PI-RADS score 3, 18 of PI-RADS score 4, and 5 of PI-RADS score 5. The distribution of the 10 biopsy-proven non-PCa lesions was as follows: 5 of PI-RADS score 2 and 5 of PI-RADS score 3. Of the 38 biopsy-proven PCa lesions based on the 18F-PSMA PET/MRI, 36 were evaluated as positive and two as negative. All the 10 biopsy-proven non-PCa lesions were labeled as negative. The mean ADC values for the PCa and non-PCa lesions were (0.7966 ± 0.15079) × 10−3 mm2/s and (0.9870 ± 0.2071) × 10−3 mm2/s, respectively. The median SUVmax values of the PCa and non-PCa lesions were 15.95 (range, 9.7–25.6) and 6.15 (range, 5.2–6.3). The differences between the ADC and SUVmax for the PCa and non-PCa lesions were statistically significant (p = 0.007 and p = 0, respectively).
Table 1.
Clinical characteristics of included patients
| Item | PCa∗ | non-PCa | P value |
|---|---|---|---|
| Number of patients | 22 | 7 | |
| Age of patients (year) | 71(63,75) | 65(55,67) | 0.199 |
| PSA level of patients (ng/mL) | 11.25 (8.5,31.6) | 7.6 (5.2,10.6) | 0.055 |
| Number of lesions | 38 | 10 | |
| ISUP grade (Gleason score) | |||
| 1 (3 + 3) | 6 (6) | 0 | |
| 2 (3 + 4) | 3 (3) | 0 | |
| 3 (4 + 3) | 8 (8) | 0 | |
| 4 (4 + 4) | 8 (8) | 0 | |
| 5 (4 + 5/5 + 4/5 + 5) | 13 (9/3/1) | 0 | |
| bpMRI PI-RADS score | |||
| 1 | 0 | 0 | |
| 2 | 3 | 5 | |
| 3 | 12 | 5 | |
| 4 | 18 | 0 | |
| 5 | 5 | 0 | |
| 18F-PSMA PET/MRI assessment category | |||
| Positive (+) | 36 | 0 | |
| Negative (−) | 2 | 10 | |
| ADC of lesions (10−3 mm2/s) | 0.7966 ± 0.15079 | 0.9870 ± 0.2071 | 0.007 |
| SUVmax of lesions | 15.95 (9.7,25.6) | 6.15 (5.2,6.3) | 0 |
All the 38 PCa lesions were defined as clinically significant PCa.
3.2. Diagnostic accuracies of bpMRI and 18F-PSMA-1007 PET/MRI for detecting PCa
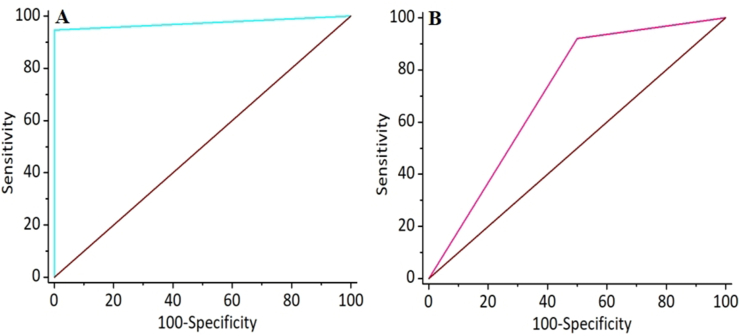
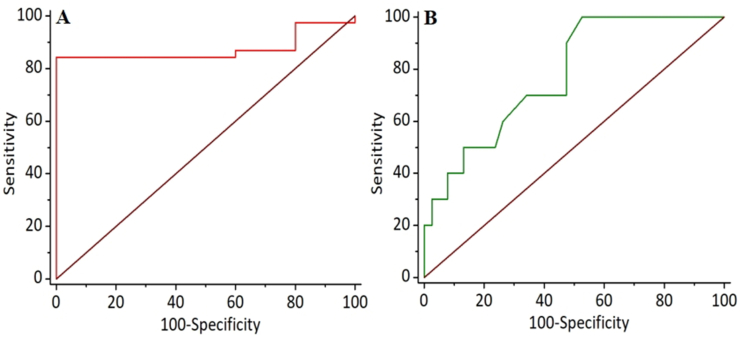
The results of bpMRI and 18F-PSMA-1007 PET/MRI are summarized in Table 2. Of the 38 lesions with biopsy-proven PCa, 36 were positive, and two were negative based on 18F-PSMA-1007 PET/MRI, while 35 were positive and three were negative based on bpMRI. All of the 10 biopsy-proven non-PCa lesions were negative based on 18F-PSMA-1007 PET/MRI, but only 5 were negative based on bpMRI. The lesion-based sensitivities, specificities, PPV, and NPV of 18F-PSMA-1007 PET/MRI and bpMRI were calculated and are shown in Table 3. Both 18F-PSMA-1007 PET/MRI and bpMRI had relatively high and similar sensitivities (a representative case was shown in Fig. 1) with values of 94.74% (95% CI, 82.25%–99.36%) and 92.11% (95% CI, 78.62%–98.34%), but the specificity, PPV, and NPV of 18F-PSMA-1007 PET/MRI were higher than those of bpMRI as follows: 100% (95% CI, 69.15%–100%) versus 50% (95% CI, 18.71%–81.29%), 100% (95% CI, 90.26%–100%) versus 87.50% (95% CI, 73.20%–95.81%), and 83.33% (95% CI, 51.59%–97.91%) versus 62.5% (95% CI, 24.49%–91.48%), respectively. This may be attributed to the false-positive (a representative case was shown in Fig. 2) and false-negative (a representative case was shown in Fig. 3) on bpMRI compared to 18F-PSMA-1007 PET/MRI. Subsequently, the ROC curves for the two imaging examinations for PCa detection were derived, as shown in Fig. 4. The AUC of 18F-PSMA-1007 PET/MRI with the value of 0.974 (95% CI, 0.881–-0.999) was higher than that of bpMRI with the value of 0.711 (95% CI, 0.562–0.832), indicating that 18F-PSMA-1007 PET/MRI have a better diagnostic performance than bpMRI with significance (p = 0.0029).
Table 2.
Diagnostic test evaluation results of bpMRI and18F-PSMA-1007 PET/MRI
| Pathologic results |
||||
|---|---|---|---|---|
| PCa | non-PCa | Total | ||
| Imaging results | 18F-PSMA-1007 PET/MRI | |||
| Positive (+) | 36 | 0 | 36 | |
| Negative (−) | 2 | 10 | 12 | |
| Total | 38 | 10 | 48 | |
| bpMRI | ||||
| Positive (+) | 35 | 5 | 40 | |
| Negative (−) | 3 | 5 | 8 | |
| Total | 38 | 10 | 48 | |
Table 3.
Diagnostic accuracy of bpMRI and18F-PSMA-1007 PET/MRI for detecting PCa
| 18F-PSMA-1007 PET/MRI | bpMRI | |
|---|---|---|
| Sensitivity (95% CI) | 94.74% (82.25%–99.36%) | 92.11% (78.62%–98.34%) |
| Specificity (95% CI) | 100% (69.15%–100%) | 50% (18.71%–81.29%) |
| PPV (95% CI) | 100% (90.26%–100%) | 87.50% (73.20%–95.81%) |
| NPV (95% CI) | 83.33% (51.59%–97.91%) | 62.50% (24.49%–91.48%) |
| AUC (95% CI) | 0.974 (0.881–0.999) | 0.711 (0.562–0.832) |
| p value | 0.0029 | |
Fig. 1.
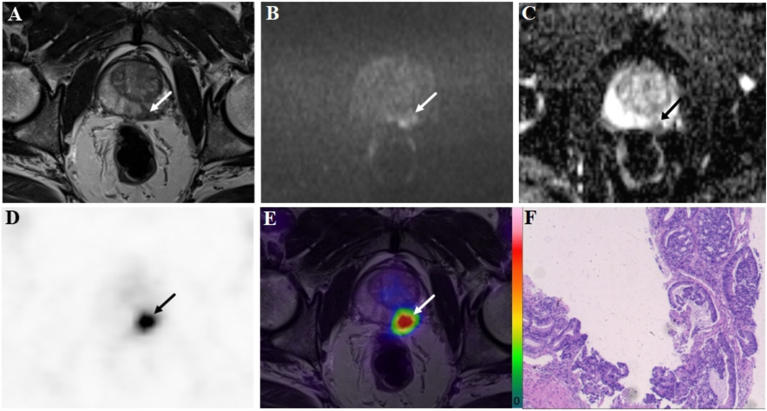
A 72-year-old patient with a PSA level of 50.8 ng/ml. The lesion with tumor a diameter of 11.7 mm (arrow) was evaluated as positive (PI-RADS score 4) on bpMRI with T2WI image (A), DWI image (B), and ADC map (C), indicating PCa lesion. On the 18F-PSMA-1007 PET image (D), the lesion showed an intense uptake with a SUVmax of 79.6 (PSMA-RADS score 5). Combined with bpMRI and PET score, fused 18F-PSMA-1007 PET/MRI image (E) further suggested PCa. Subsequent biopsy results (F) proved PCa lesion of ISUP grade 5 with Gleason score 5 + 4.
Fig. 2.
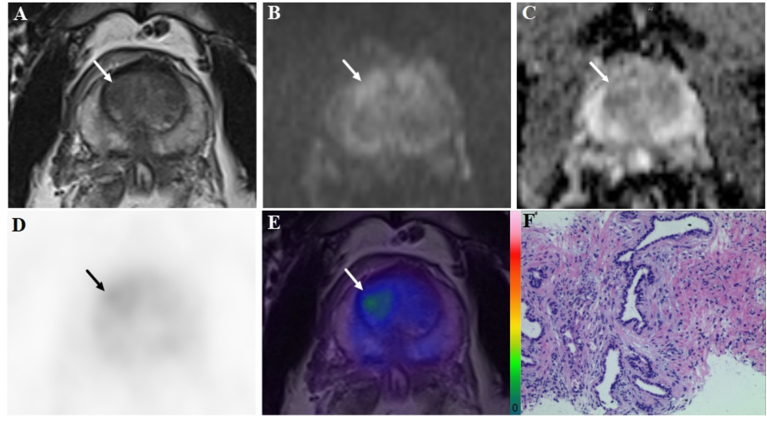
A 51-year-old patient with a PSA level of 7.1 ng/ml. The lesion (arrow) with a tumor diameter of 13.6 mm was evaluated as positive (PI-RADS score 3) on bpMRI with T2WI image (A), DWI image (B), and ADC map (C), indicating PCa lesion. However, on the 18F-PSMA-1007 PET image (D) the lesion showed only a slight uptake with a SUVmax of 3.1 (PSMA-RADS score 2). Combined with bpMRI and PET score, fused 18F-PSMA-1007 PET/MRI image (E) suggested benign prostatic lesion, and it was consistent with subsequent biopsy results (F).
Fig. 3.
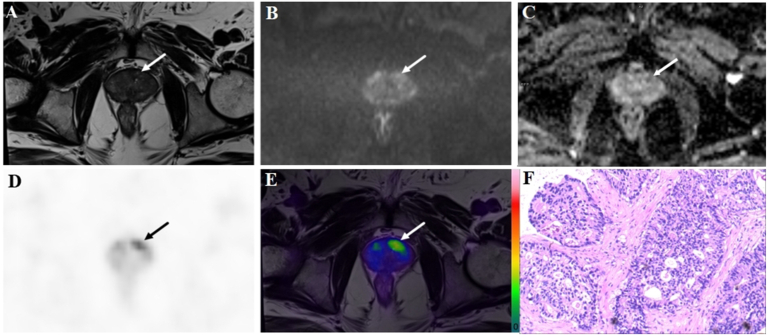
A 69-year-old patient with a PSA level of 13.5 ng/ml. The lesion (arrow) with a tumor diameter of 10.1 mm was evaluated as negative (PI-RADS score 2) on bpMRI with T2WI image (A), DWI image (B), and ADC map (C), indicating benign lesion. However, on 18F-PSMA-1007 PET image (D), the lesion showed an obvious uptake with a SUVmax of 16.2 (PSMA-RADS score 4). Combined with bpMRI and PET score, fused 18F-PSMA-1007 PET/MRI image (E) suggested PCa. Subsequent biopsy proved PCa lesion of ISUP grade 4 with Gleason score 4 + 4(F).
Fig. 4.
ROC curves of 18F-PSMA-1007 PET/MRI (A) and bpMRI (B) for detection of PCa.
3.3. ROC curve analyses of SUVmax and ADC for detecting PCa
Since the differences between the ADC and SUVmax for the PCa and non-PCa lesions were statistically significant, their semi-quantitative analyses and optimal cutoffs for detecting PCa were based on the ROC curves shown in Fig. 5. The ROC curve analyses (Table 4) showed that SUVmax had a better diagnostic AUC than ADC (AUC: 0.874 (95% CI, 0.746–0.952) versus 0.776 (95% CI, 0.633–0.884)), but their differences showed no significant meaning (P = 0.2902). Specifically, SUVmax demonstrated an 84.21% sensitivity and 100% specificity for the optimal cutoff of 6.7, while ADC showed a 100% sensitivity and 47.37% specificity at 0.79.
Fig. 5.
ROC curve analyses of SUVmax (A) and ADC (B) for the detection of PCa.
Table 4.
Results of the ROC curve analyses
| AUC | optimal cut-off points | Sensitivity | Specificity | 95% CI | P value | |
|---|---|---|---|---|---|---|
| SUVmax | 0.874 | 6.7 | 84.21% | 100.0% | 0.746–0.952 | 0.2902 |
| ADC | 0.776 | 0.79 | 100.0% | 47.37% | 0.633–0.884 |
3.4. Correlations between SUVmax, ADC, and ISUP grade
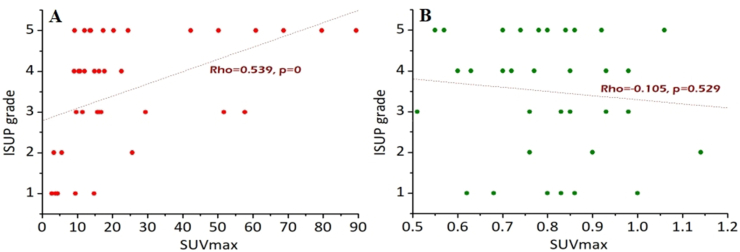
The correlations between the SUVmax, ADC, and the ISUP grade are shown in Fig. 6. The ISUP grade was moderately associated with SUVmax (Rho = 0.539, P = 0), but not ADC (Rho = −0.105, P = 0.529).
Fig. 6.
Correlations between SUVmax (A), ADC (B), and ISUP grade.
4. Discussion
PCa is the second commonly diagnosed cancer in men, and it is predominant in older adults,1,2 as in our study with a median age of 71 years (range, 63–75 years). For primary PCa, the therapy plan is completely different with different assessments. Radical prostatectomy is the main treatment for early-stage PCa, and is feasible to achieve good therapeutic effects and even cure, while only conservative treatment can be administered to advanced metastatic patients using androgen deprivation therapy or chemotherapy.23, 24, 25 Thus, early detection of PCa is directly related to the patient’s treatment plan and prognosis. Since PCa has nonspecific clinical symptoms, early laboratory screening and imaging examinations are important for its detection and diagnosis. Prostate-specific antigen (PSA), a glycoprotein produced by prostate cells, is the commonly used biomarker for screening, and elevated PSA values detected by PSA screening aids in the early diagnosis of PCa.26,27 However, elevated PSA may also be associated with non-PCa diseases such as benign prostatic hyperplasia and prostatitis.28 In agreement with previous reports, our study showed an elevated serum PSA level for both PCa and non-PCa patients, and their differences showed no statistical significance. Thus, for patients with an elevated serum PSA concentration, further imaging is urgently needed to confirm the diagnosis.
The clinical application of mpMRI has become the standard for prostate imaging according to PI-RADS.6,10 However, bpMRI has gradually been recommended for more clinical applications, given the increase in recent reports,22,29,30 indicating that it has almost the same diagnostic value as mpMRI in detecting PCa and avoids the disadvantages of mpMRI, including being time-consuming with additional costs and the potential risk of nephrogenic systemic fibrosis brought by gadolinium contrast reagent.31 Nevertheless, although mpMRI and bpMRI have been introduced as novel imaging approaches for diagnosis and the localization and characterization of primary prostate lesions and shown to be useful for detecting PCa and guiding prostate biopsy, the problem of relatively low specificity and poor detection of low-grade disease and lower sensitivity within the transitional zone tumors still exists, resulting in missed diagnoses.32, 33, 34 As shown in this study, it also showed that the sensitivity of bpMRI for the detection of PCa reached above 90%, but the specificity was only 50%, resulting in a low AUC of 0.711.
With the development of molecular imaging techniques, PSMA PET was introduced into clinical practice for PCa detection. Different from the anatomical signs obtained from MRI, PSMA PET depicts PCa lesions by detecting the PSMA expression of PCa cells at a molecular level. As PSMA tracer is a transmembrane type II glycoprotein specifically high-expressed in PCa cells with several hundred or thousand more than normal cells and non-PCa cells,35 PSMA PET exhibits much higher sensitivity and specificity for the detection of PCa than MRI, especially for the detection of occult and small lesions. Among the different PSMA ligands, 68Ga-PSMA-11 is the most used and represents a significant technological breakthrough for clinical applications. However, some disadvantages, such as shorter half-life, higher energy characteristics, and increased PET artifacts surrounding the bladder, limited its widespread use.14,15,36 Recent studies have shown that 18F-PSMA-1007, with the same target molecule, made up for these disadvantages. Especially, it was beneficial in overcoming the high background caused by the metabolism of the urethral system during the 68Ga-PSMA-11 process due to the variation in early hepatobiliary metabolism.15, 16, 17 Actually, 18F-PSMA-1007 has been recently introduced to clinical practice after successful preclinical studies.37, 38, 39 To date, most of the recent studies on PCa detection and local staging with 18F-PSMA-1007 were conducted using PET/CT, and the results40, 41, 42, 43, 44, 45, 46, 47 showed that 18F-PSMA-1007 PET/CT were performed with sensitivities ranging from 71% to 100%, specificities ranging from 76% to 100%, and accuracies ranging from 80% to 100%. Although the overall diagnostic value was high, limited by the low resolution of soft tissue on CT, the false-negative and false-positive findings should still be taken into account in some studies, resulting in a relatively low diagnostic accuracy. Promising results in reducing this frequency have been achieved by dual imaging with 18F-PSMA-1007 PET/CT and MRI,45,47 suggesting that a combination of these two methods will improve the detection of PCa. In recent years, the development of integrated PET/MRI equipment for imaging diagnosis has realized the simultaneous fusion of PET and MRI information, as well as anatomy, function, and metabolism, which may maximize the combined effects of these two technologies.48, 49, 50 In this regard, integrated 18F-PSMA-1007 PET/MRI is expected to show great diagnostic value for PCa imaging. However, the studies on the availability of this method for clinical trials are still limited.15 Thus, our clinical study was conducted on this premise. Our results confirmed a high diagnostic value of 18F-PSMA-1007 PET/MRI for the detection of PCa lesions with AUC of 0.974, a sensitivity of 94.74%, and a specificity of 100%. Some false-negative and false-positive findings were validly reduced with 18F-PSMA-1007 PET/MRI compared to bpMRI.
The SUVmax value obtained from PET and the ADC value obtained from the DWI component are important semi-quantitative parameters of the lesions, which may reflect lesion characteristics and aggressiveness.51,52 Their association based on integrated 18F-PSMA-1007 PET/MRI findings is rarely reported. Our study found that the SUVmax and ADC for the PCa and non-PCa lesions showed statistical differences, and they can be used for differential diagnosis. For this reason, we separately studied their ROC curves for diagnosing PCa, and the results showed that SUVmax had a higher diagnostic value over ADC, but there are no significant differences. At their optimal cut-off points, SUVmax and ADC presents a specificity of 100% and sensitivity of 100%, respectively, indicating that a combing of SUVmax and ADC would be beneficial to improving the diagnosis accuracy. Meanwhile, SUVmax value of 18F-PSMA-1007 showed a positive correlation with the ISUP score of the lesion, indicating that 18F-PSMA-1007 overexpression in the PCa cells was associated with a higher ISUP grade, probably resulting in a higher incidence of metastasis and castration resistance.53 Thus, 18F-PSMA-1007 may be useful for prognostic evaluation and staging of PCa.
Our study had limitations that should be acknowledged. First, 18F-PSMA-1007 PET/MRI is still in the pre-clinical promotion stage, our study was limited by the retrospective, single-center design with a relatively small number of patients. Second, no standardized scoring system is yet available for detecting PCa lesion for the application of bpMRI or 18F-PSMA-1007 PET/MRI in clinical practice. We have formulated a unified algorithms with reference to the literature and strictly implement it to minimize that concern, but further clinical studies are still needed to standardized the scoring system of bpMRI or 18F-PSMA-1007 PET/MRI and confirmed their value. Nevertheless, this study had indicated the high diagnostic value of 18F-PSMA-1007 PET/MRI for the diagnosis of PCa lesions and revealed the associations between the semi-quantitative values of SUVmax and ADC and the ISUP grades for PCa lesions. Our study may provide a reference for the clinical applications of 18F-PSMA-1007 PET/MRI and enrich the literature.
5. Conclusion
18F-PSMA-1007 PET/MRI has a greater diagnostic value for detecting PCa than bpMRI, and SUVmax shows a good correlation with the ISUP grade. For clinical applications, a high SUVmax may indicate a lesion with a high ISUP grade.
Credit Author Statement
Yuping Zeng: Data curation, Methodology, Writing–original draft, Writing–review &editing. Xiaoming Leng: Validation, Investigation. Hengbin Liao: Resources, Investigation. Guihua Jiang: Supervision, Conceptualization, Writing–review and editing. Ping Chen: Supervision, Conceptualization, Writing–review and editing.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was supported by the Municipal Health Science and Technology Project of Guangzhou (No.20201A011111 and No.20211A011110) and the Scientific Research Project of Universal Medical Imaging Science and Technology Co., Ltd (UV-2020M06).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2022.03.003.
Contributor Information
Guihua Jiang, Email: jianggh@gd2h.org.cn.
Ping Chen, Email: chenping@uvclinic.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mehdi A., Cheishvili D., Arakelian A., Bismar T.A., Szyf M., Rabbani S.A. DNA methylation signatures of Prostate Cancer in peripheral T-cells. BMC Cancer. 2020;20(1):588. doi: 10.1186/s12885-020-07078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng R.M., Zong Y.N., Cao S.M., Xu R.H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarty D., Huang L., Kahn M., Tewari A.K. Immunotherapy for Metastatic Prostate Cancer: Current and Emerging Treatment Options. Urol Clin. 2020;47(4):487–510. doi: 10.1016/j.ucl.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Stabile A., Giganti F., Rosenkrantz A.B., Taneja S.S., Villeirs G., Gill I.S., et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17(1):41–61. doi: 10.1038/s41585-019-0212-4. [DOI] [PubMed] [Google Scholar]
- 5.Drost F.H., Osses D., Nieboer D., Bangma C.H., Steyerberg E.W., Roobol M.J., et al. Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur Urol. 2020;77(1):78–94. doi: 10.1016/j.eururo.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Brancato V., Di Costanzo G., Basso L., Tramontano L., Puglia M., Ragozzino A., et al. Assessment of DCE Utility for PCa Diagnosis Using PI-RADS v2.1: Effects on Diagnostic Accuracy and Reproducibility. Diagnostics. 2020;10(3):164. doi: 10.3390/diagnostics10030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mussi T.C., Martins T., Dantas G.C., Garcia R.G., Filippi R.Z., Lemos G.C., et al. Comparison between multiparametric MRI with and without post-contrast sequences for clinically significant prostate cancer detection. Int Braz J Urol. 2018;44(6):1129–1138. doi: 10.1590/S1677-5538.IBJU.2018.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J., et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho J., Ahn H., Hwang S.I., Lee H.J., Choe G., Byun S.S., et al. Biparametric versus multiparametric magnetic resonance imaging of the prostate: detection of clinically significant cancer in a perfect match group. Prostate Int. 2020;8(4):146–151. doi: 10.1016/j.prnil.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scialpi M., D’Andrea A., Martorana E., Malaspina C., Aisa M.C., Napoletano M., et al. Biparametric MRI of the prostate. Turk J Urol. 2017;43(4):401–409. doi: 10.5152/tud.2017.06978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai G.H., Yang Q.H., Chen W.B., Liu Q.Y., Zeng Y.R., Zeng Y.J. Diagnostic Performance of PI-RADS v2, Proposed Adjusted PI-RADS v2 and Biparametric Magnetic Resonance Imaging for Prostate Cancer Detection: A Preliminary Study. Curr Oncol. 2021;28(3):1823–1834. doi: 10.3390/curroncol28030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubmüller B., Baltzer P., Hartenbach S., D’Andrea D., Helbich T.H., Haug A.R., et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin Cancer Res. 2018;24(24):6300–6307. doi: 10.1158/1078-0432.CCR-18-0768. [DOI] [PubMed] [Google Scholar]
- 13.Kratochwil C., Afshar-Oromieh A., Kopka K., Haberkorn U., Giesel F.L. Current Status of Prostate-Specific Membrane Antigen Targeting in Nuclear Medicine: Clinical Translation of Chelator Containing Prostate-Specific Membrane Antigen Ligands into Diagnostics and Therapy for Prostate Cancer. Semin Nucl Med. 2016;46(5):405–418. doi: 10.1053/j.semnuclmed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Afshar-Oromieh A., Haberkorn U., Schlemmer H.P., Fenchel M., Eder M., Eisenhut M., et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imag. 2014;41(5):887–897. doi: 10.1007/s00259-013-2660-z. [DOI] [PubMed] [Google Scholar]
- 15.Awenat S., Piccardo A., Carvoeiras P., Signore G., Giovanella L., Prior J.O., et al. Diagnostic Role of 18F-PSMA-1007 PET/CT in Prostate Cancer Staging: A Systematic Review. Diagnostics. 2021;11(3):552. doi: 10.3390/diagnostics11030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zippel C., Ronski S.C., Bohnet-Joschko S., Giesel F.L., Kopka K. Current Status of PSMA-Radiotracers for Prostate Cancer: Data Analysis of Prospective Trials Listed on Clinical Trials.gov. Pharmaceuticals. 2020;13(1):12. doi: 10.3390/ph13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta S., Krishnan M.A., Chattopadhyay S., Chelvam V. Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Rep. 2019;2(4):e1169. doi: 10.1002/cnr2.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40(2):244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 19.Egevad L., Delahunt B., Srigley J.R., Samaratunga H. International Society of Urological Pathology (ISUP) grading of prostate cancer-An ISUP consensus on contemporary grading. APMIS. 2016;124(6):433–435. doi: 10.1111/apm.12533. [DOI] [PubMed] [Google Scholar]
- 20.Tamada T., Kido A., Yamamoto A., Takeuchi M., Miyaji Y., Moriya T., et al. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1. J Magn Reson Imag. 2021;53(1):283–291. doi: 10.1002/jmri.27283. [DOI] [PubMed] [Google Scholar]
- 21.Giesel F.L., Hadaschik B., Cardinale J., Radtke J., Vinsensia M., Lehnert W., et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imag. 2017;44(4):678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe S.P., Pienta K.J., Pomper M.G., Gorin M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. J Nucl Med. 2018;59(3):479–485. doi: 10.2967/jnumed.117.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo M.Y., Rathkopf D.E., Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komura K., Sweeney C.J., Inamoto T., Ibuki N., Azuma H., Kantoff P.W. Current treatment strategies for advanced prostate cancer. Int J Urol. 2018;25(3):220–231. doi: 10.1111/iju.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swami U., McFarland T.R., Nussenzveig R., Agarwal N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer. 2020;6(8):702–715. doi: 10.1016/j.trecan.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Dunn M.W. Prostate Cancer Screening. Semin Oncol Nurs. 2017;33(2):156–164. doi: 10.1016/j.soncn.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Chung J.S., Morgan T.M., Hong S.K. Clinical implications of genomic evaluations for prostate cancer risk stratification, screening, and treatment: a narrative review. Prostate Int. 2020;8(3):99–106. doi: 10.1016/j.prnil.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filella X., Foj L. Novel Biomarkers for Prostate Cancer Detection and Prognosis. Adv Exp Med Biol. 2018;1095:15–39. doi: 10.1007/978-3-319-95693-0_2. [DOI] [PubMed] [Google Scholar]
- 29.Christophe C., Montagne S., Bourrelier S., Roupret M., Barret E., Rozet F., et al. Prostate cancer local staging using biparametric MRI: assessment and comparison with multiparametric MRI. Eur J Radiol. 2020;132:109350. doi: 10.1016/j.ejrad.2020.109350. [DOI] [PubMed] [Google Scholar]
- 30.Woo S., Suh C.H., Kim S.Y., Cho J.Y., Kim S.H., Moon M.H. Head-to-Head Comparison Between Biparametric and Multiparametric MRI for the Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2018;211(5):W226–W241. doi: 10.2214/AJR.18.19880. [DOI] [PubMed] [Google Scholar]
- 31.Junker D., Steinkohl F., Fritz V., Bektic J., Tokas T., Aigner F., et al. Comparison of multiparametric and biparametric MRI of the prostate: are gadolinium-based contrast agents needed for routine examinations? World J Urol. 2019;37:691–699. doi: 10.1007/s00345-018-2428-y. [DOI] [PubMed] [Google Scholar]
- 32.Arafa M.A., Rabah D.M., Khan F.K., Farhat K.H., Al-Atawi M.A. Effectiveness of magnetic resonance imaging-targeted biopsy for detection of prostate cancer in comparison with systematic biopsy in our countries with low prevalence of prostate cancer: our first experience after 3 years. Prostate Int. 2021;9(3):140–144. doi: 10.1016/j.prnil.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer M.D., Choyke P.L., Turkbey B. PI-RADS v2: How we do it. J Magn Reson Imag. 2017;46:11–23. doi: 10.1002/jmri.25645. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Bao J., Ping X., Hu C., Hou J., Dong F., et al. The diagnostic value of PI-RADS V1 and V2 using multiparametric MRI in transition zone prostate clinical cancer. Oncol Lett. 2018;16:3201–3206. doi: 10.3892/ol.2018.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perner S., Hofer M.D., Kim R., Shah R.B., Li H., Möller P., et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Matushita C.S., da Silva A.M.M., Schuck P.N., Bardisserotto M., Piant D.B., Pereira J.L., et al. 68Ga-Prostate-specific membrane antigen (psma) positron emission tomography (pet) in prostate cancer: a systematic review and meta-analysis. Int Braz J Urol. 2021;47(4):705–729. doi: 10.1590/S1677-5538.IBJU.2019.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robu S., Schmidt A., Eiber M., Schottelius M., Günther T., Yousefi B.H., et al. Synthesis and preclinical evaluation of novel 18F-labeled Glu-urea-Glu-based PSMA inhibitors for prostate cancer imaging: a comparison with 18F-DCFPyl and 18F-PSMA-1007. EJNMMI Res. 2018;8(1):30. doi: 10.1186/s13550-018-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardinale J., Schäfer M., Benešová M., Bauder-Wüst U., Leotta K., Eder M., et al. Preclinical Evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J Nucl Med. 2017;58(3):425–431. doi: 10.2967/jnumed.116.181768. [DOI] [PubMed] [Google Scholar]
- 39.Foley R.W., Redman S.L., Graham R.N., Loughborough W.W., Little D. Fluorine-18 labelled prostate-specific membrane antigen (PSMA)-1007 positron-emission tomography-computed tomography: normal patterns, pearls, and pitfalls. Clin Radiol. 2020;75(12):903–913. doi: 10.1016/j.crad.2020.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Anttinen M., Ettala O., Malaspina S., Jambor I., Sandell M., Kajander S., et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE) Eur Urol. Oncol. 2021;4(4):635–644. doi: 10.1016/j.euo.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Giesel F.L., Hadaschik B., Cardinale J., Radtke J., Vinsensia M., Lehnert W., et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imag. 2017;44:678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietlein F., Kobe C., Hohberg M., Zlatopolskiy B.D., Krapf P., Endepols H., et al. Intraindividual Comparison of 18F-PSMA-1007 with Renally Excreted PSMA Ligands for PSMA PET Imaging in Patients with Relapsed Prostate Cancer. J Nucl Med. 2020;61(5):729–734. doi: 10.2967/jnumed.119.234898. [DOI] [PubMed] [Google Scholar]
- 43.Kuten J., Fahoum I., Savin Z., Shamni O., Gitstein G., Hershkovitz D., et al. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J Nucl Med. 2020;61(4):527–532. doi: 10.2967/jnumed.119.234187. [DOI] [PubMed] [Google Scholar]
- 44.Sprute K., Kramer V., Koerber S.A., Meneses M., Fernandez R., Soza-Ried C., et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J Nucl Med. 2021;62(2):208–213. doi: 10.2967/jnumed.120.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong J.J., Liu B.L., Wang Z.Q., Tang K., Ji X.W., Yin W.W., et al. The value of 18F-PSMA-1007 PET/CT in identifying non-metastatic high-risk prostate cancer. EJNMMI Res. 2020;10:138. doi: 10.1186/s13550-020-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesch C., Vinsensia M., Radtke J.P., Schlemmer H.P., Heller M., Ellert E., et al. Intraindividual Comparison of 18F-PSMA-1007 PET/CT, Multiparametric MRI, and Radical Prostatectomy Specimens in Patients with Primary Prostate Cancer: A Retrospective, Proof-of-Concept Study. J Nucl Med. 2017;58(11):1805–1810. doi: 10.2967/jnumed.116.189233. [DOI] [PubMed] [Google Scholar]
- 47.Privé B.M., Israël B., Schilham M.G.M., Muselaers C.H.J., Zámecnik P., Mulders P.F.A., et al. Evaluating F-18-PSMA-1007-PET in primary prostate cancer and comparing it to multi-parametric MRI and histopathology. Prostate Cancer Prostatic Dis. 2021;24:423–430. doi: 10.1038/s41391-020-00292-2. [DOI] [PubMed] [Google Scholar]
- 48.Freitag M.T., Radtke J.P., Hadaschik B.A., Kopp-Schneider A., Eder M., Kopka K., et al. Comparison of hybrid (68)Ga-PSMA PET/MRI and (68)Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imag. 2016;43:70–83. doi: 10.1007/s00259-015-3206-3. [DOI] [PubMed] [Google Scholar]
- 49.Eiber M., Weirich G., Holzapfel K., Souvatzoglou M., Haller B., Rauscher I., et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol. 2016;70(5):829–836. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 50.Freitag M.T., Radtke J.P., Afshar-Oromieh A., Roethke M.C., Hadaschik B.A., Gleave M., et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imag. 2017;44:776–787. doi: 10.1007/s00259-016-3594-z. [DOI] [PubMed] [Google Scholar]
- 51.Uslu-Beşli L., Bakır B., Asa S., Güner E., Demirdağ Ç., Şahin O.E., et al. Correlation of SUVmax and Apparent Diffusion Coefficient Values Detected by Ga-68 PSMA PET/MRI in Primary Prostate Lesions and Their Significance in Lymph Node Metastasis: Preliminary Results of an On-going Study. Mol Imaging Radionucl Ther. 2019;28:104–111. doi: 10.4274/mirt.galenos.2019.63825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J., Mangarova D.B., Brangsch J., Kader A., Hamm B., Brenner W., et al. Correlation between Intraprostatic PSMA Uptake and MRI PI-RADS of [68Ga]Ga-PSMA-11 PET/MRI in Patients with Prostate Cancer: Comparison of PI-RADS Version 2.0 and PI-RADS Version 2.1. Cancers. 2020;12(12):3523. doi: 10.3390/cancers12123523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bravaccini S., Puccetti M., Bocchini M., Ravaioli S., Celli M., Scarpi E., et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci Rep. 2018;8:4254. doi: 10.1038/s41598-018-22594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.