This cohort study assesses whether cancer drug trials that show improvement in overall survival or progression-free survival also improve global quality of life in patients with cancer and how unchanged or detrimental quality-of-life outcomes are reported in trial publications.
Key Points
Questions
Are global quality-of-life (QOL) outcomes in phase 3 randomized clinical trials (RCTs) of cancer drugs in the advanced setting associated with other efficacy outcomes, and how do publications of RCTs report unimproved QOL outcomes?
Findings
This cohort study of 45 RCTs found that a minority (24%) of new medicines were associated with improved QOL. Only 22% of trials showing improved progression-free survival also showed improved QOL, and approximately one-half of trials that failed to show improvement in QOL reported these outcomes positively.
Meaning
Most cancer drug trials that show improved progression-free survival do not show improved patient global QOL; however, this outcome is reported favorably.
Abstract
Importance
Although quality of life (QOL) is an important clinical end point, cancer drugs are often approved based on overall survival (OS) or putative surrogate end points such as progression-free survival (PFS) without QOL data.
Objective
To ascertain whether cancer drug trials that show improvement in OS or PFS also improve global QOL of patients with cancer compared with the control treatment, as well as to assess how unchanged or detrimental QOL outcomes are reported in trial publications.
Design, Setting, and Participants
This retrospective cohort study included all patients with cancer in the advanced setting who were enrolled into phase 3 randomized clinical trials (RCTs) of cancer drugs reporting QOL data and published in English language in a PubMed-indexed journal in the calendar year 2019. The systematic search of PubMed was conducted in July 2020.
Main Outcomes and Measures
Association of QOL outcomes with OS and PFS, framing of unchanged QOL outcomes in trial publications, and the association of favorable framing with industry funding of the trials.
Results
A total of 45 phase 3 RCTs enrolling 24 806 participants (13 368 in the experimental arm and 11 438 in the control arm) met the inclusion criteria and were included in the study analyses. Improvement in global QOL with the experimental agent was reported in 11 (24%) RCTs. The RCTs with improved QOL were more likely to also show improved OS vs trials with unimproved QOL (7 of 11 [64%] trials vs 10 of 34 [29%] trials; χ2 = 4.13; P = .04); there was no such association observed for PFS (6 of 11 [55%] trials vs 17 of 34 [50%] trials, χ2 = 0.03; P = .87). Six trials reported worsening QOL, of which 3 (50%) were trials of targeted drugs, and 11 trials reported improvement in QOL, of which 6 (55%) were trials of immunotherapy drugs. Of the 34 trials in which QOL was not improved compared with controls, 16 (47%) reported these results in a positive frame, an observation statistically significantly associated with industry funding (χ2 = 6.35; P = .01).
Conclusions and Relevance
In this cohort study, a small proportion of RCTs of cancer drugs showed benefit in global QOL with the experimental agent. These results showed an association between QOL benefit and OS benefit but no such association with PFS benefit. Trials that failed to show improved QOL often reported their QOL outcomes more favorably. Non–immunotherapy-targeted drugs led to worse QOL more often than did cytotoxic agents.
Introduction
Patients with advanced cancer expect treatments to help them live longer and/or better lives. Thus, overall survival (OS) and quality of life (QOL) are the most important markers of therapeutic benefit of cancer medicines in clinical trials. However, several studies have shown that many cancer drugs receive regulatory approval without any evidence of improvement in either of these end points, based solely on improvement of putative surrogate measures of efficacy.1,2,3 In particular, QOL is less frequently evaluated in randomized clinical trials (RCTs) of cancer drugs and, even when tested, is underreported.4,5
A cancer drug’s effect on QOL cannot be predicted based on toxicity profile or the effect of the drug in delaying progression alone. Studies have shown that progression-free survival (PFS), a commonly used intermediate primary end point, is poorly correlated with QOL and OS and, thus, its claimed surrogacy is usually flawed.4,6 Some physicians and patients also believe that targeted drugs are associated with better QOL than cytotoxic chemotherapy, thus promoting chemotherapy-free regimens in oncology. In addition to data on specific adverse events, the effect of cancer drugs on patients’ overall QOL is important and relevant information for the physician and patient to make shared decisions about cancer treatments.7,8
Although worsening of QOL is clearly an adverse outcome, failure to improve QOL demands special consideration in making risk-benefit trade-off decisions. However, trial publications often report an observed lack of improvement in QOL in a positive frame, such as QOL being maintained, rather than not improved, which may be misinterpreted by the readers to mean beneficial effects on QOL.
To best inform discussions with patients about proposed treatments, it is important to clearly understand and communicate the effects of cancer drugs on patient QOL alongside their effects on OS and intermediate end points such as PFS. Therefore, we conducted this study to (1) describe the proportion of phase 3 RCTs of cancer drugs that found beneficial, null, or detrimental effect on QOL vs the control treatment; (2) assess the association between improved QOL and improved efficacy parameters (OS or PFS); (3) assess the proportion of RCTs that show only improved PFS without showing improved OS or QOL; (4) categorize the association with QOL by cancer drug type (immunotherapy, targeted, or cytotoxic); (5) assess how unimproved QOL findings are framed in publications; and (6) assess if favorable framing of QOL results is associated with industry sponsorship.
Methods
Literature Search
We conducted a systematic search of the PubMed database in July 2020 for all phase 3 RCTs of cancer drugs published in 2019 using the search terms cancer AND [quality of life OR quality-of-life OR patient reported outcomes OR patient-reported-outcomes OR QOL OR PRO], limiting the search to trials in English language and trials in a noncurative (advanced) setting. We limited this analysis to trials in the noncurative setting because treatment goals, preferences for QOL benchmarks, and surrogate end points are different between curative and advanced-treatment settings. We used the year 2019 because it was the most recent full calendar year available at the start of this study.
Titles and abstracts were screened individually, and full-text articles were subsequently downloaded. Full-text articles were individually reviewed for the following exclusion criteria: (1) not a phase 3 randomized trial; (2) study design or study protocol only; (3) not a drug trial (ie, trials evaluating the role of surgery or radiotherapy); (4) trials of decision support, diagnostic modalities, or supportive care; (5) trials assessing behavioral interventions, genetic counseling, or screening; (6) trials only assessing pharmacokinetics; (7) trials with no QOL information; and (8) trials that did not include an efficacy end point (ie, PFS or OS). Only RCTs of cancer drugs reporting both efficacy (PFS and/or OS) and QOL data were included. We also excluded de-escalation trials or trials of different doses or schedules of the same drug because the comparator would be the same agent in these cases.
This study involves data from published literature and does not involve individual patient data; thus, it is exempt from institutional review board approval. This meta-epidemiological study was conducted in accordance with the adaptation of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for meta-epidemiological studies.9
Data Extraction
Data were extracted by 2 authors (J.N.S. and B.G.) individually, and any discrepancies were resolved by consensus. We extracted data on cancer type, drug type, primary end point(s), effect on OS and PFS, tool(s) used to measure QOL, QOL outcomes, sample size, blinding (ie, double blind, open label), and funding (ie, public, industry, or mixed public and industry funding).
The QOL outcomes were separated into 3 categories: superior (statistically significant improvement in the global QOL domain), no statistically significant difference, and inferior (statistically significant decline in global QOL). The emphasis on global QOL for declaring improvement in QOL is in accordance with the principles of the European Society of Medical Oncology–Magnitude of Clinical Benefit Scale (ESMO-MCBS) upgrade criterion for QOL outcomes.10 These comparisons were made for the experimental arm vs the control arm, not before and after comparisons in each arm because the purpose of an RCT is to evaluate whether the new drug provides clinical benefit vs the already existing standard of care, and thereby assist in regulatory and clinical decisions. Thus, in this article, unchanged QOL means no statistically significant difference in global QOL outcomes between the 2 arms, and unimproved QOL means unchanged plus inferior global QOL outcomes with the experimental agent vs the control treatment.
Finally, we extracted QOL statements as reported in the abstract or conclusions of each article to assess how QOL outcomes were framed when reported (favorably vs neutral or negatively). For unchanged QOL outcomes, if it was reported that QOL did not worsen or QOL was maintained rather than QOL did not improve, or if there was downplaying of worse QOL outcomes, this was recorded as favorable interpretation because the expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL. These categorizations were based on consensus.
Statistical Analysis
Potential associations of QOL outcomes with efficacy outcomes (OS and PFS), sponsorship, and apparent favorable interpretation in the QOL report were assessed using Fisher exact test or χ2 test as appropriate. All statistical tests were conducted using STATA, version 15 (StataCorp).
Results
Study Characteristics
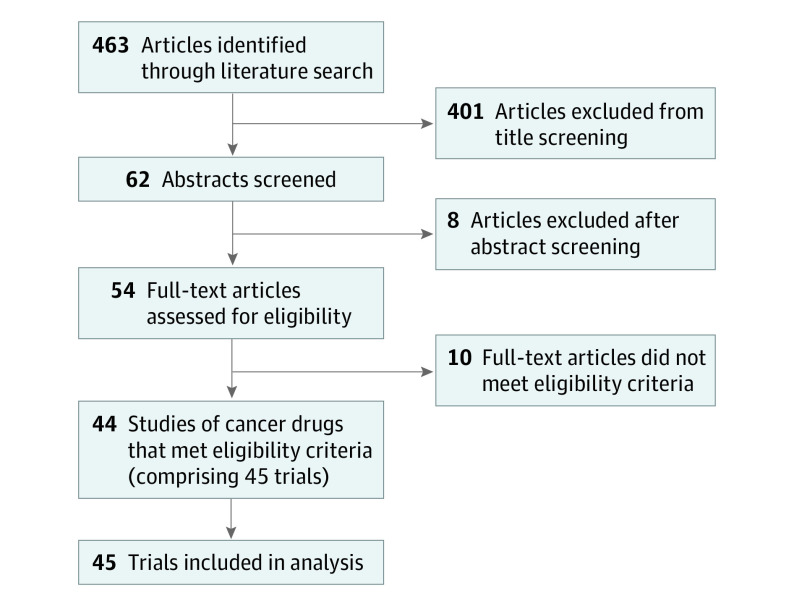
The literature search resulted in 463 articles, of which 45 trials enrolling 24 806 participants (13 368 in the experimental arm and 11 438 in the control arm) met the inclusion criteria and were included in the analyses (Figure).11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 Among the 45 trials, the most commonly reported tumor types were lung (n = 7 [16%]), ovarian (n = 6 [13%]), and breast (n = 5 [11%]), and the most commonly reported cancer drugs were targeted therapies (n = 21 [47%]), cytotoxic drugs (n = 12 [27%]), and immunotherapies (n = 7 [16%]). The majority of trials were double blind, placebo controlled (n = 23 [51%]); received industry funding (n = 34 [76%]); and reported OS as the primary or coprimary end point (n = 16 [36%]). Quality of life was most commonly assessed using various versions of the European Organisation for Research and Treatment of Cancer tool (Table 1). There were 11 (24%), 28 (62%), and 6 (13%) trials that demonstrated superior, unchanged, and inferior QOL for the experimental arm vs the control treatment, respectively.
Figure. PRISMA Diagram for the Selection of Studies.
Table 1. Characteristics of Included Trials (n = 45).
| Characteristic | No. (%) |
|---|---|
| Year of study | 2019 |
| Tumor type | |
| Lung | 7 (16) |
| Ovarian | 6 (13) |
| Breast | 5 (11) |
| Gastric | 4 (9) |
| Prostate | 4 (9) |
| Melanoma | 3 (7) |
| Hematological | 3 (7) |
| Pancreatic | 2 (4) |
| Brain | 2 (4) |
| Other | 9 (20) |
| Type of drug | |
| Cytotoxic | 12 (27) |
| Targeted | 21 (47) |
| Immunotherapy | 7 (16) |
| Other (repurposed drugs) | 5 (11) |
| Type of RCT | |
| Open label | 23 (51) |
| Double blind | 22 (49) |
| Funding | |
| Industry | 33 (73) |
| Public | 9 (20) |
| Mixed | 3 (7) |
| Primary end points | |
| Overall survival | |
| Primary | 12 (27) |
| Coprimary | 5 (11) |
| Progression-free survival | 28 (62) |
| QOL Tool used, No.a | |
| EORTC, various versions | 21 |
| FACT, various versions | 12 |
| EuroQOL, various versions | 12 |
| Lung Cancer Symptom Scale | 3 |
| FOSI | 3 |
| FKSI | 1 |
| No tool mentioned | 1 |
| QOL Assessment time after baseline assessment, No. | |
| Once during treatment period | 2 |
| During treatment period only | 18 |
| During and once after the end of study | 7 |
| During and after until the end of study or disease progression | 16 |
| During with unclear assessment protocol for after therapy | 2 |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; FACT, Functional Assessment of Cancer Therapy; FKSI, Functional Assessment of Cancer Therapy–Kidney Symptom Index; FOSI, Functional Assessment of Cancer Therapy–Ovarian Symptom Index; QOL, quality of life; RCT, randomized clinical trial.
Trials may have used multiple tools.
Association of QOL Benefit With Other Efficacy Measures
Of the 45 included trials, 40 (89%) had data reported for OS and 42 (93%) had data for PFS. Of the 17 trials reporting improved OS, QOL was also improved in 7 (41%), and of the 27 trials reporting improved PFS, QOL was also improved in 6 (22%). Improvements in OS were not accompanied with worsening of QOL in any case, but improvement in PFS was (4 of 27 [15%] trials) (Table 2). Of the 6 trials that showed inferior QOL outcomes for the experimental arm, none demonstrated improved OS, but 4 (67%) demonstrated improved PFS.
Table 2. Overall Survival and Progression-Free Survival in Trials Also Reporting Quality-of-Life Outcomes (n = 45).
| Outcome | Quality-of-life outcome, No. of trials | ||
|---|---|---|---|
| Improved | No difference | Worsened | |
| Overall survival | |||
| Improved | 7 | 10 | 0 |
| No difference | 3 | 16 | 4 |
| No data | 1 | 2 | 2 |
| Progression-free survival | |||
| Improved | 6 | 17 | 4 |
| No difference | 3 | 9 | 2 |
| Worse | 0 | 1 | 0 |
| No data | 2 | 1 | 0 |
Randomized clinical trials with improved QOL were more likely to also show improved OS compared with trials with unimproved QOL (7 of 11 [64%] trials vs 10 of 34 [29%] trials; χ2 = 4.13; P = .04). No such association was observed for PFS (6 of 11 [55%] trials vs 17 of 34 [50%] trials; χ2 = 0.03; P = .87).
Association of QOL With Drug Class
Three of the 6 (50%) trials with inferior QOL were of targeted drugs, 2 (33%) were trials of repurposed drugs for cancer, and 1 (17%) was a cytotoxic agent. The association with QOL differed by the type of cancer drug. Although limited by small sample size, compared with the QOL outcomes of the control treatment, superior QOL was most commonly observed in RCTs of immunotherapy drugs (6 of 11 [54%]) while inferior QOL was most commonly observed among targeted drugs (3 of 6 [50%]) (Fisher exact P = .002; Table 3).
Table 3. Distribution of Quality-of-Life Outcomes by Drug Class Among Included Trials (n = 45).
| Drug class | Quality-of-life outcome, No. of trials | ||
|---|---|---|---|
| Improved | No difference | Worsened | |
| Cytotoxic | 1 | 10 | 1 |
| Targeted | 3 | 15 | 3 |
| Immunotherapy | 6 | 1 | 0 |
| Other (repurposed drugs) | 1 | 2 | 2 |
Favorable Framing of QOL Results
In 3 of 6 (50%) RCTs with inferior QOL outcomes, the article seemed to downplay those outcomes by describing them as not clinically significant despite being statistically significant and without a priori description of what constituted clinically significant difference (2 of 3 [67%] trials) or as small differences, although statistically significant (1 of 3 [33%] trials). Among the 28 trials with unchanged QOL outcomes, 15 (54%) reported the results in a neutral frame. In the remaining 13 (46%) cases, no improvement in QOL was reported as follows: 3 reports highlighted subgroup findings from specific subscales of QOL tools that favored the experimental agent, 3 reports stated that QOL was maintained, and 1 report each stated that there was no deterioration in QOL, there was no sustained deterioration in QOL, QOL was preserved, QOL was not impaired, QOL was not adversely affected, QOL was not worse than placebo, and QOL was not compromised. Thus, favorable reporting of QOL results was present in 16 of 34 (47%) trials with unimproved QOL outcomes. Industry-funded trials were associated with favorable reporting of QOL outcomes (15 of 25 [60%] trials vs 1 of 9 [11%] trials; χ2 = 6.35; P = .01) but class of drug trials were not (χ2 = 1.12; P = .57) (Table 4).
Table 4. Distribution of Favorable Reporting of Quality-of-Life Outcomes by Industry Sponsorship and Drug Classa.
| Characteristic | Quality-of-life outcomes, No. of trials | |
|---|---|---|
| Favorable | Accurate | |
| Drug class | ||
| Cytotoxic | 4 | 7 |
| Targeted | 10 | 8 |
| Immunotherapy | 1 | 0 |
| Other (repurposed drugs) | 1 | 3 |
| Industry sponsorship | ||
| Yes | 15 | 10 |
| No | 1 | 8 |
Of the 45 included trials, 34 had unimproved quality-of-life outcomes.
Discussion
In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ QOL and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved QOL. Improved QOL outcomes were associated with improved OS but not with improved PFS. Importantly, almost half of the cancer drugs drug trials that showed improved PFS showed no improved OS or QOL (ie, PFS-only benefit). Some reports included conclusions regarding QOL findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications.
First, PFS is often considered a surrogate end point for oncology trials. However, in the present study, 44% of trials showed PFS-only benefit (ie, the drugs improved PFS but did not improve either OS or QOL). This is not surprising because the definition of disease progression based on Response Evaluation Criteria in Solid Tumours criteria, and thereby PFS, is arbitrary. In the absence of improvement in either OS or QOL, improving PFS alone does not confer any clinical benefit. When the physical and financial toxicities of cancer drugs are considered, the drugs with PFS-only benefit should be considered a net harm to patients and society. In addition, the fact that 15% of trials with PFS improvement showed inferior QOL outcomes should caution us against extrapolating PFS benefit as QOL benefit. Harms in QOL may be masked by apparent PFS gains. Reassuringly, in no case was a drug that showed OS gain associated with inferior QOL. Indeed, there was no association between improvement in PFS and improvement in QOL in this study. This is consistent with previous studies in this space documenting lack of validation for PFS as a surrogate for QOL outcomes.4,6 Therefore, clinical trials should measure QOL specifically because it cannot be predicted by PFS results.
Second, the downplaying of inferior QOL outcomes or portraying of lack of improvement in QOL as “not worse” is concerning. A previous report highlighted the downplaying of toxic effects in trial publications using terms such as “acceptable” or “tolerable” while describing toxic effects.55 Similarly, the oncology community should be cautious in interpreting how the effects of cancer drugs on QOL are framed in trial publications. Any detrimental effects on QOL should be clearly described as such, given how critical these results are for the informed decision-making process. Using phrases such as “no detrimental effects on QOL” or “without adversely affecting QOL” to describe unimproved QOL should be avoided, especially in the advanced or metastatic setting because the only reason for patients to undergo cancer treatment in the advanced setting (in the absence of OS gains) would be to have a better QOL or relief from cancer symptoms. Such downplaying of QOL outcomes was positively associated with industry funding of trials. This association with industry funding is consistent with other studies where industry funding has been observed with reporting bias,56 positive framing of conclusions,57 and using noninferiority design without justification.58 Readers of clinical trials should be aware of such possible bias during interpretation of QOL results. Editors and reviewers should demand clarity in reporting, and readers need to be aware of these potential misrepresentations. We strongly recommend that trial publications report no difference in QOL in a neutral frame, such as “unchanged QOL” rather than “not worse.”
A caveat in interpretation of these QOL outcomes is that they represent QOL outcome comparisons against the control treatment. That is, there could be detrimental effects on QOL from the experimental agent, which would be characterized as improved QOL outcomes in the present analysis if the detriment was not as bad as the detriment from the control treatment. Similarly, improved QOL maybe regarded as unchanged if the control arm also showed similar improvements in QOL. For evaluation of treatment benefits, before and after comparisons are fraught with several confounders, including placebo effect. Therefore, a new drug must be evaluated for QOL effects against the available standard of care, similar to survival outcomes. Indeed, the upgrade or downgrade of ESMO-MCBS grade based on QOL outcomes also requires between-arm comparisons of QOL and not simply before and after comparisons.59 We strongly recommend that trial publications clearly distinguish before and after comparisons of QOL from the between-arm comparisons of QOL. This is also consistent with the recommendations from the Consolidated Standards of Reporting Trials patient-reported outcomes extension,60 as well as the need for a clear taxonomy of objectives as recommended by the Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium.61 Furthermore, application of this benchmark—to improve QOL outcomes vs the control treatment—has been applied uniformly to all of the trials in the present analysis.
Third, many physicians and patients believe that targeted drugs and immunotherapies are preferable owing to their perceived positive effect on patients’ QOL compared with cytotoxic agents. Although the present results for immunotherapy drugs were consistent with these perceptions, we found that worsening of QOL was more common with targeted drugs than with cytotoxic agents. This study challenges the common notion that cytotoxic drugs are necessarily worse for patients’ QOL than targeted drugs. Therefore, physicians and patients should keep in mind that a chemotherapy-free regimen does not necessarily mean better QOL.
Although there is growing recognition of the importance of QOL data for cancer drugs, several studies, including this one, have found various deficiencies in QOL reporting, including not measuring QOL,4 not publishing QOL data,4,5 publishing QOL data after several years,10 and reporting the results favorably when they are finally published. Although QOL data have not often directly affected regulatory decisions and, therefore, there is no incentive for the industry to publish those data immediately (especially when the results are not favorable), several health-technology assessment bodies consider QOL data for making reimbursement decisions. The ESMO-MCBS tool allows for a 1-point upgrade or downgrade (of a total of 5 points) based on effects on QOL.62 The concerned authorities making policy and clinical decisions must be aware of these limitations in interpretation of QOL results from cancer trials.
Assessment of QOL information in clinical trials is challenging, owing in part to the multitude of measures available and to variation among these measures and how they are scored and scaled. Nonetheless, within a clinical trial, interpretation of scores should be similar among the comparison arms. The QOL domains that are most important to patients and that are clinically important to measure differ among trial contexts; thus, several tumor-specific tools have been developed to supplement general oncology QOL assessment tools. The relevance of global QOL spans across tumor types. Furthermore, the Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium has made important recommendations to standardize the analysis of QOL outcomes in cancer-related RCTs.61 The US Food and Drug Administration has also issued a draft guidance to improve the quality and consistency of QOL data in cancer-related RCTs.63 These are all important advancements to promote quality and consistency in the use of QOL end points.
Limitations
These findings must be interpreted in the context of some limitations. Several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.5 Furthermore, the sample from 1 calendar year may not be reflective of all years. However, there is no reason to believe that things have changed in the absence of interventions. The heterogeneity of the tumor types, QOL instruments used, and time points at which QOL assessments were conducted are other potential limitations. The correlations between PFS and/or OS and QOL may differ by tumor types, but the limited sample size prevented us from making definitive conclusions. This will be an area of future study. Finally, although we focused on global QOL, some patients may find specific subdomains of QOL meaningful in some clinical settings, and the results of global QOL may not align with the results of the subdomains of interest. That we focus on noncurative settings only is a strength rather than a limitation of the study because the risk-benefit thresholds are different in curative settings where patients may be willing to accept worsening QOL to a certain degree in exchange for improved odds of cure.
Conclusions
In this cohort study, we show that improvement in QOL cannot be presumed based on improved PFS alone, and QOL is not necessarily worse with use of cytotoxic drugs. Clinical trial publications frequently frame unimproved QOL outcomes in a positive way. Stakeholders of QOL research, such as physicians, patients, regulators, and payers, must be aware of these issues to interpret QOL data from cancer drug trials accurately and meaningfully.
References
- 1.Gyawali B, Sharma S, Booth CM. Is the number of cancer drug approvals a surrogate for regulatory success? J Cancer Policy. 2019;22:100202. doi: 10.1016/j.jcpo.2019.100202 [DOI] [Google Scholar]
- 2.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers DE, Jenei K, Chisamore TM, Gyawali B. Evaluation of the clinical benefit of cancer drugs submitted for reimbursement recommendation decisions in Canada. JAMA Intern Med. 2021;181(4):499-508. doi: 10.1001/jamainternmed.2020.8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144(7):1746-1751. doi: 10.1002/ijc.31957 [DOI] [PubMed] [Google Scholar]
- 5.Marandino L, La Salvia A, Sonetto C, et al. Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann Oncol. 2018;29(12):2288-2295. doi: 10.1093/annonc/mdy449 [DOI] [PubMed] [Google Scholar]
- 6.Kovic B, Jin X, Kennedy SA, et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: a systematic review and quantitative analysis. JAMA Intern Med. 2018;178(12):1586-1596. doi: 10.1001/jamainternmed.2018.4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haraldstad K, Wahl A, Andenæs R, et al. ; LIVSFORSK network . A systematic review of quality of life research in medicine and health sciences. Qual Life Res. 2019;28(10):2641-2650. doi: 10.1007/s11136-019-02214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haslam A, Herrera-Perez D, Gill J, Prasad V. Patient experience captured by quality-of-life measurement in oncology clinical trials. JAMA Netw Open. 2020;3(3):e200363. doi: 10.1001/jamanetworkopen.2020.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22(4):139-142. doi: 10.1136/ebmed-2017-110713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyawali B, de Vries EGE, Dafni U, et al. Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring. ESMO Open. 2021;6(3):100117. doi: 10.1016/j.esmoop.2021.100117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Lin P, Higano CS, et al. Prognostic association of prostate-specific antigen decline with clinical outcomes in men with metastatic castration-resistant prostate cancer treated with enzalutamide in a randomized clinical trial. Eur Urol Oncol. 2019;2(6):677-684. doi: 10.1016/j.euo.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 12.Brandes AA, Gil-Gil M, Saran F, et al. A randomized phase II trial (TAMIGA) evaluating the efficacy and safety of continuous bevacizumab through multiple lines of treatment for recurrent glioblastoma. Oncologist. 2019;24(4):521-528. doi: 10.1634/theoncologist.2018-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella D, Grünwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20(2):297-310. doi: 10.1016/S1470-2045(18)30778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau I, Fuchs CS, Ohtsu A, et al. Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: exploratory analysis of RAINBOW and REGARD phase III trials. Eur J Cancer. 2019;107:115-123. doi: 10.1016/j.ejca.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 15.Chi KN, Agarwal N, Bjartell A, et al. ; TITAN Investigators . Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. doi: 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 16.Cocks K, Contente M, Simpson S, DeRosa M, Taylor FC, Shaw JW. A Q-TWiST analysis comparing nivolumab and therapy of investigator’s choice in patients with recurrent/metastatic platinum-refractory squamous cell carcinoma of the head and neck. Pharmacoeconomics. 2019;37(8):1041-1047. doi: 10.1007/s40273-019-00798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403-2415. doi: 10.1056/NEJMoa1909707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. ; BFORE Study Investigators . Patient-reported outcomes in the phase 3 BFORE trial of bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia. J Cancer Res Clin Oncol. 2019;145(6):1589-1599. doi: 10.1007/s00432-019-02894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennell DA, Baas P, Taylor P, et al. Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND—a double-blind, randomized, phase II study. J Clin Oncol. 2019;37(10):790-798. doi: 10.1200/JCO.2018.79.0543 [DOI] [PubMed] [Google Scholar]
- 20.Fizazi K, Shore N, Tammela TL, et al. ; ARAMIS Investigators . Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi: 10.1056/NEJMoa1815671 [DOI] [PubMed] [Google Scholar]
- 21.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Martín A, Pothuri B, Vergote I, et al. ; PRIMA/ENGOT-OV26/GOG-3012 Investigators . Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi: 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 23.Gore M, Hackshaw A, Brady WE, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol. 2019;153(3):541-548. doi: 10.1016/j.ygyno.2019.03.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Q, Li Q, Wang J, et al. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: a preliminary, three-center, clinical trial study. Medicine (Baltimore). 2019;98(27):e16234. doi: 10.1097/MD.0000000000016234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafizi M, Kalanaky S, Moaiery H, et al. A randomized, double-blind, placebo-controlled investigation of BCc1 nanomedicine effect on survival and quality of life in metastatic and non-metastatic gastric cancer patients. J Nanobiotechnology. 2019;17(1):52. doi: 10.1186/s12951-019-0484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubner RA, Cubillo A, Blanc JF, et al. Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5-fluorouracil and leucovorin. Eur J Cancer. 2019;106:24-33. doi: 10.1016/j.ejca.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 27.Kelly JD, Tan WS, Porta N, et al. ; BOXIT Investigators . BOXIT-A randomised phase III placebo-controlled trial evaluating the addition of celecoxib to standard treatment of transitional cell carcinoma of the bladder (CRUK/07/004). Eur Urol. 2019;75(4):593-601. doi: 10.1016/j.eururo.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Park S, Im SA, et al. ; Korean Cancer Study Group . Quality of life outcomes including neuropathy-associated scale from a phase II, multicenter, randomized trial of eribulin plus gemcitabine versus paclitaxel plus gemcitabine as first-line chemotherapy for HER2-negative metastatic breast cancer: Korean Cancer Study Group trial (KCSG BR13-11). Cancer Commun (Lond). 2019;39(1):29. doi: 10.1186/s40880-019-0375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 30.Ludwig H, Moreau P, Dimopoulos MA, et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9(3):23. doi: 10.1038/s41408-019-0181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott DF, Shah R, Gupte-Singh K, et al. Quality-adjusted survival of nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone among treatment-naive patients with advanced melanoma: a quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis. Qual Life Res. 2019;28(1):109-119. doi: 10.1007/s11136-018-1984-3 [DOI] [PubMed] [Google Scholar]
- 32.Montillo M, Illés Á, Robak T, et al. Idelalisib addition has neutral to beneficial effects on quality of life in bendamustine/rituximab-treated patients: results of a phase 3, randomized, controlled trial. Health Qual Life Outcomes. 2019;17(1):173. doi: 10.1186/s12955-019-1232-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morabito A, Piccirillo MC, Maione P, et al. Effect on quality of life of cisplatin added to single-agent chemotherapy as first-line treatment for elderly patients with advanced non-small cell lung cancer: joint analysis of MILES-3 and MILES-4 randomised phase 3 trials. Lung Cancer. 2019;133:62-68. doi: 10.1016/j.lungcan.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Nielsen LK, Klausen TW, Jarden M, et al. Clarithromycin added to bortezomib-cyclophosphamide-dexamethasone impairs health-related quality of life in multiple myeloma patients. Eur J Haematol. 2019;102(1):70-78. doi: 10.1111/ejh.13175 [DOI] [PubMed] [Google Scholar]
- 35.Oudard S, Latorzeff I, Caty A, et al. Effect of adding docetaxel to androgen-deprivation therapy in patients with high-risk prostate cancer with rising prostate-specific antigen levels after primary local therapy: a randomized clinical trial. JAMA Oncol. 2019;5(5):623-632. doi: 10.1001/jamaoncol.2018.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park IH, Im SA, Jung KH, et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED Trial (KCSG BR 11-01). Cancer Res Treat. 2019;51(1):43-52. doi: 10.4143/crt.2017.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérol M, Winfree KB, Cuyun Carter G, Lin Cui Z, Bowman L, Garon EB. Association of baseline symptom burden with efficacy outcomes: exploratory analysis from the randomized phase III REVEL study in advanced non-small-cell lung cancer. Lung Cancer. 2019;131:6-13. doi: 10.1016/j.lungcan.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 38.Pimentel I, Lohmann AE, Ennis M, et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast. 2019;48:17-23. doi: 10.1016/j.breast.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 39.Platania M, Pasini F, Porcu L, et al. Oral maintenance metronomic vinorelbine versus best supportive care in advanced non-small-cell lung cancer after platinum-based chemotherapy: the MA.NI.LA. multicenter, randomized, controlled, phase II trial. Lung Cancer. 2019;132:17-23. doi: 10.1016/j.lungcan.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 40.Ray-Coquard I, Pautier P, Pignata S, et al. ; PAOLA-1 Investigators . Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 41.Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137-147. doi: 10.1016/j.ejca.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 42.Rinke A, Neary MP, Eriksson J, et al. Health-related quality of life for long-acting octreotide versus placebo in patients with metastatic midgut neuroendocrine tumors in the phase 3 PROMID trial. Neuroendocrinology. 2019;109(2):141-151. doi: 10.1159/000499469 [DOI] [PubMed] [Google Scholar]
- 43.Robson M, Ruddy KJ, Im SA, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer. 2019;120:20-30. doi: 10.1016/j.ejca.2019.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719-729. doi: 10.1007/s10549-018-05125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shitara K, Yamanaka T, Denda T, et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol. 2019;30(2):259-265. doi: 10.1093/annonc/mdy526 [DOI] [PubMed] [Google Scholar]
- 46.Tomita Y, Fukasawa S, Shinohara N, et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup 3-year follow-up analysis from the phase III CheckMate 025 study. Jpn J Clin Oncol. 2019;49(6):506-514. doi: 10.1093/jjco/hyz026 [DOI] [PubMed] [Google Scholar]
- 47.Vergote I, Bergfeldt K, Franquet A, et al. A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecol Oncol. 2020;156(2):293-300. doi: 10.1016/j.ygyno.2019.11.034 [DOI] [PubMed] [Google Scholar]
- 48.Vergote I, du Bois A, Floquet A, et al. Overall survival results of AGO-OVAR16: a phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2019;155(2):186-191. doi: 10.1016/j.ygyno.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Huang C, Yang JJ, et al. A randomised phase II clinical trial of nab-paclitaxel and carboplatin compared with gemcitabine and carboplatin as first-line therapy in advanced squamous cell lung carcinoma (C-TONG1002). Eur J Cancer. 2019;109:183-191. doi: 10.1016/j.ejca.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 50.Watanabe S, Yoshioka H, Sakai H, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous non-small cell lung cancer: a phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan. Lung Cancer. 2019;129:55-62. doi: 10.1016/j.lungcan.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 51.Wick W, Krendyukov A, Junge K, Höger T, Fricke H. Longitudinal analysis of quality of life following treatment with Asunercept plus reirradiation versus reirradiation in progressive glioblastoma patients. J Neurooncol. 2019;145(3):531-540. doi: 10.1007/s11060-019-03320-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo KH, Lee SJ, Cho J, et al. A randomized, open-label, phase II study comparing pemetrexed plus cisplatin followed by maintenance pemetrexed versus pemetrexed alone in patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer after failure of first-line EGFR tyrosine kinase inhibitor: KCSG-LU12-13. Cancer Res Treat. 2019;51(2):718-726. doi: 10.4143/crt.2018.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37(26):2317-2328. doi: 10.1200/JCO.19.01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jouve J-L, Lecomte T, Bouché O, et al. ; PRODIGE-11 investigators/collaborators . Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71(3):516-522. doi: 10.1016/j.jhep.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 55.Gyawali B, Shimokata T, Honda K, Ando Y. Reporting harms more transparently in trials of cancer drugs. BMJ. 2018;363:k4383. doi: 10.1136/bmj.k4383 [DOI] [PubMed] [Google Scholar]
- 56.Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med. 2010;153(3):158-166. doi: 10.7326/0003-4819-153-3-201008030-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang F, Zhu J, Mo M, et al. Role of industry funders in oncology RCTs published in high-impact journals and its association with trial conclusions and time to publication. Ann Oncol. 2018;29(10):2129-2134. doi: 10.1093/annonc/mdy305 [DOI] [PubMed] [Google Scholar]
- 58.Gyawali B, Tessema FA, Jung EH, Kesselheim AS. Assessing the justification, funding, success, and survival outcomes of randomized noninferiority trials of cancer drugs: a systematic review and pooled analysis. JAMA Netw Open. 2019;2(8):e199570. doi: 10.1001/jamanetworkopen.2019.9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherny NI, Sullivan R, Dafni U, et al. ESMO-Magnitude of Clinical Benefit Scale V.1.0 questions and answers. ESMO Open. 2016;1(5):e000100. doi: 10.1136/esmoopen-2016-000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814-822. doi: 10.1001/jama.2013.879 [DOI] [PubMed] [Google Scholar]
- 61.Coens C, Pe M, Dueck AC, et al. ; Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium . International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21(2):e83-e96. doi: 10.1016/S1470-2045(19)30790-9 [DOI] [PubMed] [Google Scholar]
- 62.Wilke H, Muro K, Van Cutsem E, et al. ; RAINBOW Study Group . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. doi: 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 63.FDA in brief: FDA provides guidance on measuring patient-reported outcomes in cancer clinical trials. News release. US Food and Drug Administration. June 9, 2021. Accessed March 27, 2022. https://www.fda.gov/news-events/press-announcements/fda-brief-fda-provides-guidance-measuring-patient-reported-outcomes-cancer-clinical-trials