Key Points
Question
Are there any differences in survival, quality of life, and toxic effects between a transoral surgical approach (TOS) vs radiation as a treatment deescalation approach in human papillomavirus–related oropharyngeal squamous cell carcinoma?
Findings
In this randomized clinical trial of 61 patients with T1-T2N0-2 p16–positive oropharyngeal squamous cell carcinoma, TOS was associated with an excessive risk of treatment-related mortality, but swallowing-related quality of life was high in both treatment arms. The primary radiotherapy approach achieved good preliminary oncologic and toxic effect outcomes.
Meaning
The findings of this study suggest that based on the treatment-related deaths, TOS should be used cautiously, and once survival data from this trial mature, prespecified statistical comparisons with historical controls and between treatment arms are planned.
Abstract
Importance
The optimal approach for treatment deescalation in human papillomavirus (HPV)–related oropharyngeal squamous cell carcinomas (OPSCCs) is unknown.
Objective
To assess a primary radiotherapy (RT) approach vs a primary transoral surgical (TOS) approach in treatment deescalation for HPV-related OPSCC.
Design, Setting, and Participants
This international, multicenter, open-label parallel-group phase 2 randomized clinical trial was conducted at 9 tertiary academic cancer centers in Canada and Australia and enrolled patients with T1-T2N0-2 p16–positive OPSCC between February 13, 2018, and November 17, 2020. Patients had up to 3 years of follow-up.
Interventions
Primary RT (consisting of 60 Gy of RT with concurrent weekly cisplatin in node-positive patients) vs TOS and neck dissection (ND) (with adjuvant reduced-dose RT depending on pathologic findings).
Main Outcomes and Measures
The primary end point was overall survival (OS) compared with a historical control. Secondary end points included progression-free survival (PFS), quality of life, and toxic effects.
Results
Overall, 61 patients were randomized (30 [49.2%] in the RT arm and 31 [50.8%] in the TOS and ND arm; median [IQR] age, 61.9 [57.2-67.9] years; 8 women [13.6%] and 51 men [86.4%]; 31 [50.8%] never smoked). The trial began in February 2018, and accrual was halted in November 2020 because of excessive toxic effects in the TOS and ND arm. Median follow-up was 17 months (IQR, 15-20 months). For the OS end point, there were 3 death events, all in the TOS and ND arm, including the 2 treatment-related deaths (0.7 and 4.3 months after randomization, respectively) and 1 of myocardial infarction at 8.5 months. There were 4 events for the PFS end point, also all in the TOS and ND arm, which included the 3 mortality events and 1 local recurrence. Thus, the OS and PFS data remained immature. Grade 2 to 5 toxic effects occurred in 20 patients (67%) in the RT arm and 22 (71%) in the TOS and ND arm. Mean (SD) MD Anderson Dysphagia Inventory scores at 1 year were similar between arms (85.7 [15.6] and 84.7 [14.5], respectively).
Conclusions and Relevance
In this randomized clinical trial, TOS was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year. Long-term follow-up is required to assess OS and PFS outcomes.
Trial Registration
Clinicaltrials.gov Identifier: NCT03210103
This randomized clinical trial examines a primary radiotherapy approach vs a primary transoral surgical approach in treatment deescalation for HPV-related oropharyngeal squamous cell carcinoma.
Introduction
The incidence of human papillomavirus (HPV)–related oropharyngeal cancer has increased rapidly during the past 2 decades, fueled by endemic oral HPV infections.1,2 These cancers are commonly treated with radiotherapy (RT) and concurrent platinum-based chemotherapy, which are associated with short-term and long-term adverse sequelae, including xerostomia, swallowing dysfunction, dysgeusia, and osteoradionecrosis.3,4 With the better prognosis of HPV-related cancers leading to better long-term survival, strategies to mitigate toxic effects while maintaining excellent outcomes are crucial.
Transoral surgery (TOS) techniques, including transoral laser microsurgery (TLM) and transoral robotic surgery (TORS), have been suggested in retrospective comparisons as better approaches than primary RT to preserve swallowing function.5,6 Contrasting these retrospective studies, the ORATOR randomized clinical trial compared swallowing quality of life (QOL) after treatment with RT vs TORS and demonstrated statistically superior swallowing scores in the RT arm, but the difference did not qualify as a clinically meaningful change.7,8 However, ORATOR was not limited to HPV-related tumors and did not test any treatment deescalation approaches.
To our knowledge, no prior randomized clinical trials have compared surgery and RT as the primary treatment modality in a deescalation approach. The goal of the ORATOR2 trial was to assess long-term survival, oncologic outcomes, and toxic effect outcomes with 2 deescalation approaches: a primary reduced-dose RT approach with weekly treatment with chemotherapy vs a TOS approach followed by treatment with reduced-dose adjuvant RT when necessary.
Methods
Study Design
The ORATOR2 clinical trial was an investigator-initiated, international open-label parallel-group phase 2 randomized study. Patients with T1 to T2, N0 to N2 (staged according to the American Joint Committee on Cancer 8th edition) HPV-related oropharyngeal squamous cell carcinoma (OPSCC) were randomly assigned in a 1:1 ratio to either the primary RT arm, comprising treatment with 60 Gy of RT and concurrent weekly cisplatin chemotherapy (40 mg/m2) in node-positive patients, or the TOS and neck dissection (ND) arm, comprising surgery and ND, with treatment with adjuvant reduced-dose RT depending on pathologic findings. The trial was registered (NCT03210103), and the full protocol (previously published9) is available in Supplement 1. Ethics approval was obtained in all jurisdictions, and all patients provided written informed consent.
Participants
Patients were required to be 18 years or older and have a good performance status (Eastern Cooperative Oncology Group score, 0-2), and stage T1 to T2 N0 to N2 M0 histologically proven OPSCC (American Joint Committee on Cancer 8th edition). The tumor was required to be HPV-related, as determined by positive p16 status, real-time polymerase chain reaction, or in situ hybridization. All patients were assessed at a head-and-neck multidisciplinary clinic (with examination by a surgeon and radiation oncologist) and described to a multidisciplinary tumor board before enrollment. The main exclusion criteria included unambiguous evidence of extranodal extension and history of head-and-neck cancer within 5 years (or prior head-and-neck radiation treatment at any time), with other exclusions listed in the protocol (Supplement 1).
Randomization and Masking
Patients were assigned to groups using a computer-generated randomization list using permuted blocks stratified by smoking status (<10 pack-years vs ≥10 pack-years). Neither patients nor enrolling physicians were masked to treatment allocation.
Procedures
Credentialing for surgery and radiotherapy were required, as described in detail in the study protocol (Supplement 1). In the RT arm, radiation was delivered using intensity-modulated RT. The prescribed dose was 60 Gy in 30 fractions over 6 weeks to areas of gross disease, with lower doses to high-risk and low-risk nodal areas (Supplement 1). Patients with multiple positive nodes or any node larger than 3 cm received treatment with weekly cisplatin (40 mg/m2). For patients not receiving treatment with chemotherapy, the radiation could be accelerated.
In the TOS and ND arm, the primary tumor was excised by credentialed surgeons (see Supplement 1 for credentialing process) using TLM or TORS, with selective ND(s) and mandatory ipsilateral carotid artery ligation. Full surgical details are described in Supplement 1. A tracheostomy at the time of surgery was strongly recommended. Adjuvant RT, at a total dose of 50 Gy in 25 fractions (or 60 Gy in 30 fractions if there were positive margins or extranodal extension), was recommended for patients based on pathologic features. To avoid trimodality treatment, adjuvant chemoradiotherapy was not administered unless there was residual gross disease that could not be resected, which was expected to be rare. Follow-up occurred every 3 months for the first 2 years and every 6 months thereafter until 5 years.
Outcomes
The primary end point was overall survival (OS), compared separately for each arm against a historical control, which was similar to the design of the NRG-HN002 trial.10 Secondary end points included progression-free survival (PFS), QOL including MD Anderson Dysphagia Inventory (MDADI) scores, European Organisation for Research and Treatment of Cancer Quality of Life general and head-and-neck (H&N35) scales, Voice Handicap Index (VHI-10), Neck Dissection Impairment Index, Patient Neurotoxicity Questionnaire, and EuroQol 5-Dimension 5-Level. Toxic effect secondary end points were assessed by the Common Terminology Criteria for Adverse Events scale, version 4. Swallowing end points were measured by the percutaneous feeding tube rate at 1 year and Functional Oral Intake Scale, in addition to the MDADI.
Statistical Methods
The 2-year OS in each arm was estimated to be 94%. To differentiate an OS of 94% vs 84% using a 1-sided 1-sample binomial test with 80% power and an α of .05 with 10% dropout, 70 patients were needed in each arm (140 total). This approach mirrored the design of the HN002 trial,10 although for a different end point (PFS vs OS).
The statistical analysis plan for all end points is provided in Supplement 1. All statistical analyses were performed using SAS, version 9.4 (SAS Institute), with 2-sided statistical testing at the P < .05 significance level.
The data safety monitoring committee met biannually to review toxic effect outcomes. An interim analysis had been planned to occur after the accrual of 70 total patients (35 per arm), with unacceptable toxic effects predefined as a grade 5 toxic effect rate of 5% or more in either arm (ie, 2 grade 5 toxic effects occurring for 35 patients).
In November 2020, after accrual of 61 patients, accrual was stopped because of unacceptable grade 5 toxic effects in the TOS and ND arm. This high up-front risk of mortality was deemed unacceptable in the context of consenting patients to enroll in a deescalation paradigm. In light of the early closure, the results in this article are being reported earlier than the planned analysis because of the need to disseminate these unforeseen toxic effect outcomes. Because of the premature follow-up for survival events, although Kaplan-Meier estimates are provided, statistical comparisons of OS against the historical control and of OS and PFS between arms will be reported once survival data are mature.
Results
Between February 13, 2018, and November 17, 2020, 61 patients were enrolled at 9 institutions in Canada and Australia, with 30 patients (49.2%) randomized to the RT arm and 31 (50.8%) to the TOS and ND arm. The arms were well balanced for baseline characteristics (Table 1). In the entire cohort, the median age was 61.9 years (IQR, 57.2-67.9 years), most patients (31 [51%]) were never smokers, and most (51 [86%]) were male.
Table 1. Baseline Characteristics of All Enrolled Patients.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All patients (n = 61)a | RT arm (n = 30)a | TOS and ND arm (n = 31)a | |
| Age, median (IQR) | 61.9 (57.2-67.9) | 61.8 (55.0-67.3) | 62.2 (57.2-69.8) |
| Smoking status | |||
| Current | 7 (11) | 4 (13) | 3 (10) |
| Previous (>1 y since quitting) | 23 (38) | 10 (33) | 13 (42) |
| Never | 31 (51) | 16 (54) | 15 (48) |
| Smoking pack-years | |||
| <10 (including never smokers) | 45 (74) | 22 (73) | 23 (74) |
| ≥10 | 16 (26) | 8 (27) | 8 (26) |
| Sex | |||
| Male | 51 (86) | 26 (90) | 25 (83) |
| Female | 8 (14) | 3 (10) | 5 (17) |
| Baseline ECOG performance status | |||
| 0 | 40 (73) | 20 (74) | 20 (71) |
| 1 | 15 (27) | 7 (26) | 8 (29) |
| Primary site | |||
| Tonsil or tonsillar fossa | 33 (56) | 17 (59) | 16 (53) |
| Base of tongue | 25 (42) | 11 (38) | 14 (47) |
| Oropharynx | 1 (2) | 1 (3) | 0 |
| Clinical T stage | |||
| T1 | 31 (52) | 16 (55) | 15 (50) |
| T2 | 28 (48) | 13 (45) | 15 (50) |
| Clinical N stage | |||
| N0 | 9 (15) | 5 (17) | 4 (13) |
| N1 | 44 (75) | 21 (73) | 23 (77) |
| N2 | 6 (10) | 3 (10) | 3 (10) |
| Baseline FOIS | |||
| 5: Total oral diet, multiple consistencies, and special preparation | 1 (2) | 0 | 1 (3) |
| 6: Total oral diet, multiple consistencies, and special food limits | 2 (3) | 1 (3) | 1 (3) |
| 7: Total oral diet and no restrictions | 56 (95) | 28 (97) | 28 (94) |
Abbreviations: ECOG, Eastern Cooperative Group; FOIS, Functional Oral Intake Scale; RT, radiotherapy; TOS and ND, transoral surgery and neck dissection.
For some values, totals do not add to the total randomized in that arm because some patients withdrew consent for further data upload/follow-up.
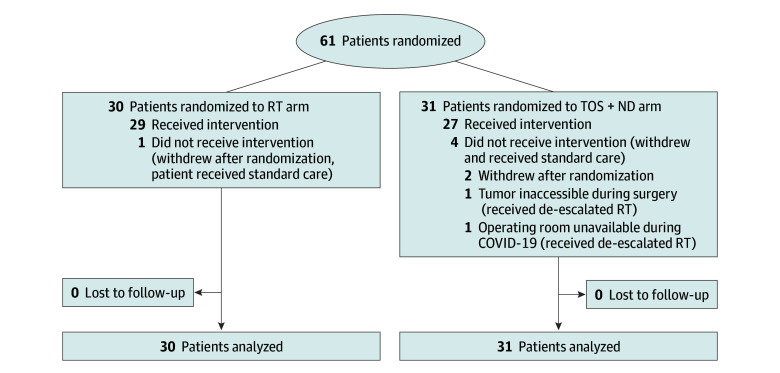
Of the 61 patients randomized (Figure 1), 5 (8%) did not receive protocol-specified treatment (Supplement 1). Of the 27 patients in the TOS and ND arm who underwent surgery, 25 (93%) received TORS and 2 (7%) received TLM. Fifteen patients (56%) underwent an ipsilateral ND, and 12 (44%) underwent bilateral dissection. Compliance with external carotid artery ligation was 100%. Twenty of 31 patients in the surgical arm (65%) received temporary tracheostomies solely for airway protection postoperatively. Margin status was negative in 18 (67%), close (<3 mm) in 7 (26%), and positive in 2 (7%). Twenty-one patients (78%) required treatment with adjuvant RT, with a median (IQR) adjuvant radiation dose of 52 (50-60) Gy; none required treatment with concurrent chemotherapy. In the RT arm, the median (IQR) dose was 60 (60.0-61.8) Gy, and 21 patients (72%) received concurrent chemotherapy.
Figure 1. CONSORT Diagram.
RT indicates radiotherapy; TOS + ND, transoral surgery and neck dissection.
Survival and Oncologic Outcomes
Median follow-up was 17 months (IQR, 15-20 months). For the OS end point, there were 3 death events, all in the TOS and ND arm, including the 2 treatment-related deaths (0.7 and 4.3 months after randomization, respectively) and 1 of myocardial infarction at 8.5 months. There were 4 events for the PFS end point, also all in the TOS and ND arm, which included the 3 mortality events and 1 local recurrence. Early OS and PFS time-to-event analyses are shown in Figure 2, but remain immature. There were no salvage NDs needed in the RT arm.
Figure 2. Preliminary Analyses of Time-to-Event Outcomes for Overall Survival and Progression-Free Survival Stratified by Treatment Arm.

RT indicates radiotherapy; TOS + ND, transoral surgery and neck dissection.
Toxic Effects
In the TOS and ND arm, 2 treatment-related deaths occurred among 31 patients randomized to that arm (7% grade 5 toxic effect rate), both of which occurred after the patients underwent TORS. One patient died of an oropharyngeal hemorrhage in hospital on day 4 after undergoing resection of a well-lateralized base of tongue cancer despite having a tracheostomy in place and having the ipsilateral external carotid artery ligated intraoperatively. A second patient died of cervical vertebral osteomyelitis that occurred on the ipsilateral side of the spine to the resection approximately 1 month after completing treatment with adjuvant RT after undergoing TORS for a tonsil cancer (110 days postsurgery), a complication previously reported after TORS (associated with a 43% mortality rate),11 also known as spondylodiscitis.12 Overall rates of related grade 2 or greater toxic effects were similar between arms (occurring in 20 patients [67%] in the RT arm and 22 [71%] in the TOS and ND arm). Specific grade 2 to 5 adverse events are shown in Table 2, with the RT arm showing worse anorexia (n = 8 vs n = 1; P = .01) and dysgeusia (n = 11 vs n = 4; P = .03).
Table 2. Summary of Adverse Events.
| Adverse event | RT arm (n = 30) | TOS and ND arm (n = 31) | P valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G1 | G2 | G3 | G4 | G5 | ||
| Anemia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Anorexia | 2 | 6 | 2 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | .01 |
| Bone infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | >.99 |
| Constipation | 6 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Dehydration | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Dermatitis (radiation) | 12 | 5 | 1 | 0 | 0 | 11 | 5 | 0 | 0 | 0 | .69 |
| Dry mouth | 19 | 5 | 0 | 0 | 0 | 15 | 4 | 0 | 0 | 0 | .73 |
| Dysarthria | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Dysgeusia | 14 | 11 | 0 | 0 | 0 | 10 | 4 | 0 | 0 | 0 | .03 |
| Dyspepsia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Dysphagia | 7 | 7 | 0 | 0 | 0 | 9 | 5 | 2 | 0 | 0 | .94 |
| Fatigue | 9 | 7 | 0 | 0 | 0 | 9 | 3 | 1 | 0 | 0 | .29 |
| Febrile neutropenia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Fever | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .24 |
| Gastrointestinal disorder | 2 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | >.99 |
| Hearing loss (based on audiogram) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Hearing impairment (patient reported) | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .24 |
| Hypokalemia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Hypomagnesemia | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Infection (other) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Joint range of motion decreased | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | >.99 |
| Lung infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | .24 |
| Lymph leakage | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Mucositis (oral) | 9 | 5 | 1 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | .51 |
| Musculoskeletal and connective tissue disorder | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | .49 |
| Nausea | 11 | 1 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | >.99 |
| Neck pain | 1 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | >.99 |
| Neutrophil count decreased | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | .11 |
| Oral hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | .05 |
| Oral/oropharyngeal pain | 9 | 2 | 0 | 0 | 0 | 5 | 4 | 0 | 0 | 0 | .67 |
| Osteonecrosis of jaw | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Pain (not otherwise specified) | 5 | 5 | 1 | 0 | 0 | 5 | 7 | 0 | 0 | 0 | .81 |
| Peripheral sensory neuropathy | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | .49 |
| Rash (maculopapular) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Skin ulceration | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Thromboembolic event | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Thrush | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | .49 |
| Tinnitus | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
| Trismus | 0 | 1 | 0 | 0 | 0 | 5 | 3 | 2 | 0 | 0 | .20 |
| Vomiting | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | >.99 |
| Weight loss | 8 | 2 | 0 | 0 | 0 | 11 | 3 | 0 | 0 | 0 | >.99 |
| White blood cell count decreased | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | .49 |
Abbreviations: G, grade; RT, radiotherapy; TOS and ND, transoral surgery and neck dissection.
P values calculated based on rates of grade 2 or greater adverse events using the χ2 or Fisher exact test as appropriate.
QOL and Functional Outcomes
The mean (SD) MDADI total scores at 1 year were 85.7 (15.6) in the RT arm and 84.7 (14.5) in the TOS and ND arm (P = .85). Comparisons of MDADI, European Organisation for Research and Treatment of Cancer H&N35, Voice Handicap Index, Patient Neurotoxicity Questionnaire, EuroQol 5-Dimension 5-Level, and Neck Dissection Impairment Index scores at 1 year are shown in eTable 1 in Supplement 2, with no differences between arms, except for the H&N35 subdomains of coughing and weight loss, which were worse in the TOS and ND arm (mean [SD] of 10.5 [15.9] vs 27.1 [21.8]; P = .02 and mean [SD] of 0 [0] vs 25.0 [44.7]; P = .04, respectively). One patient in each arm required a percutaneous feeding tube; no patients had feeding tubes in place at one year. Functional Oral Intake Scale scores at 1 year were assessed as total oral diet with no restrictions (highest score) in 13 of 16 patients (81%) in the RT arm vs 5 of 13 patients (38%) in the TOS and ND arm (P = .05). The second highest score, total oral diet with multiple consistencies and special food limits, was assessed in 3 patients (19%) in the RT arm vs 6 patients (46%) in the TOS and ND arm, and 2 patients (15%) in the TOS and ND arm were assessed as needing special preparation. In comparing hematological and biochemical outcomes (eTable 2 in Supplement 2), patients in the RT arm had higher creatinine values at last follow-up (median, 91 [IQR, 86-96] μmol/L vs 74 [IQR, 66-89] μmol/L; P = .04). There were no differences in leukocyte, neutrophil, platelet, and hemoglobin levels or liver function at last follow-up.
Discussion
In this randomized clinical trial comparing primary RT vs primary TOS as a deescalation approach for HPV-related OPSCC, TOS was associated with unacceptable risks of treatment-related mortality. Radiotherapy was associated with a different toxic effect profile, including anorexia, dysgeusia, and higher long-term creatinine values.
The rate of treatment-related mortality after TORS varies in the literature but has been reported to be 1.7% at 90 days in the US National Cancer Database.13 The 90-day window would not have captured the case of spondylodiscitis in this trial, which occurred 110 days postoperatively. Oropharyngeal bleeding after TORS may be more common than after other head-and-neck oncologic surgeries because flaps are generally not used to protect the surgical site. Severe bleeding can occur despite external carotid artery ligation (as occurred in both ORATOR trials), and these bleeds present risks of asphyxiation and exsanguination. A tracheostomy may allow for better control of the airway and packing of the oropharynx, but is not a failsafe. While the overall rate of grade 3 to 5 bleeding in the E3311 study (6.1%) was similar to the present trial, fatal bleeding was rare; however, the control of such bleeds can be tenuous, and the difference between a grade 3 to 4 and a grade 5 bleed may be a matter of chance.
Limitations
This trial has limitations that must be considered. The early closure diminishes the power of the trial to be able to ascertain small differences between arms in toxic effects, QOL, or survival outcomes. The early closure because of toxic effects may bias survival estimates towards the RT arm at early follow-up; therefore, longer follow-up for survival outcomes is paramount. However, the diminished sample size did not prevent the trial from reaching its primary goal of determining which of the 2 arms was most suitable for deescalation in phase 3 studies and supports the use of deescalated chemoradiotherapy as an experimental arm in the ongoing HN005 phase 3 noninferiority study.
Conclusions
The results of this randomized clinical trial suggest that a primary TOS approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach. Long-term follow-up data are forthcoming to assess oncologic and survival outcomes.
Trial protocol
eTable 1. Quality of life scores
eTable 2. Follow-up hematologic findings
eAppendix. Patients who did not receive protocol treatment
Data sharing statement
References
- 1.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus–attributable cancers—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724-728. doi: 10.15585/mmwr.mm6833a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550-4559. doi: 10.1200/JCO.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chia SH, Gross ND, Richmon JD. Surgeon experience and complications with transoral robotic surgery (TORS). Otolaryngol Head Neck Surg. 2013;149(6):885-892. doi: 10.1177/0194599813503446 [DOI] [PubMed] [Google Scholar]
- 4.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582-3589. doi: 10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh DH, Tam S, Fung K, et al. Transoral robotic surgery vs. radiotherapy for management of oropharyngeal squamous cell carcinoma—a systematic review of the literature. Eur J Surg Oncol. 2015;41(12):1603-1614. doi: 10.1016/j.ejso.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Dowthwaite SA, Franklin JH, Palma DA, Fung K, Yoo J, Nichols AC. The role of transoral robotic surgery in the management of oropharyngeal cancer: a review of the literature. ISRN Oncol. 2012;2012:945162. doi: 10.5402/2012/945162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. 2019;20(10):1349-1359. doi: 10.1016/S1470-2045(19)30410-3 [DOI] [PubMed] [Google Scholar]
- 8.Nichols AC, Theurer J, Prisman E, et al. Randomized trial of radiotherapy versus transoral robotic surgery for oropharyngeal squamous cell carcinoma: long-term results of the ORATOR trial. J Clin Oncol. 2022;40(8):866-875. doi: 10.1200/JCO.21.01961 [DOI] [PubMed] [Google Scholar]
- 9.Nichols AC, Lang P, Prisman E, et al. Treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma with radiotherapy vs. trans-oral surgery (ORATOR2): study protocol for a randomized phase II trial. BMC Cancer. 2020;20(1):125. doi: 10.1186/s12885-020-6607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002). J Clin Oncol. 2021;39(9):956-965. doi: 10.1200/JCO.20.03128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier C, Bobillier C, Blanchard D, et al. Spondylodiscitis after transoral robotic surgery: Retrospective 7-case series from the GETTEC group. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(3):179-183. doi: 10.1016/j.anorl.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121-138. doi: 10.1093/bmb/ldw003 [DOI] [PubMed] [Google Scholar]
- 13.Oliver JR, Persky MJ, Wang B, et al. Transoral robotic surgery adoption and safety in treatment of oropharyngeal cancers. Cancer. 2022;128(4):685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Quality of life scores
eTable 2. Follow-up hematologic findings
eAppendix. Patients who did not receive protocol treatment
Data sharing statement