Although angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers remain the cornerstones to slow kidney disease progression and reduce cardiovascular events in patients with chronic kidney disease (CKD), sodium-glucose cotransporter2 (SGLT2) inhibitors demonstrate great additional promise to reduce morbidity and mortality in this population.1, 2, 3, 4, 5, 6 The large cardiovascular outcome and subsequent trials have demonstrated significant cardiovascular and kidney benefits of SGLT2 inhibitors in CKD beyond glucose lowering (Item S1), leading to varying indications for reduction of major cardiovascular events, reduction of hospitalization for heart failure, and slowing CKD progression in patients with and without type 2 diabetes mellitus (DM).2, 3, 4, 5, 6 Although primary care and endocrinology providers historically prescribed SGLT2 inhibitors for glucose lowering, the evolution of the evidence surrounding this medication class now centrally positions nephrology providers to initiate SGLT2 inhibitor therapy.
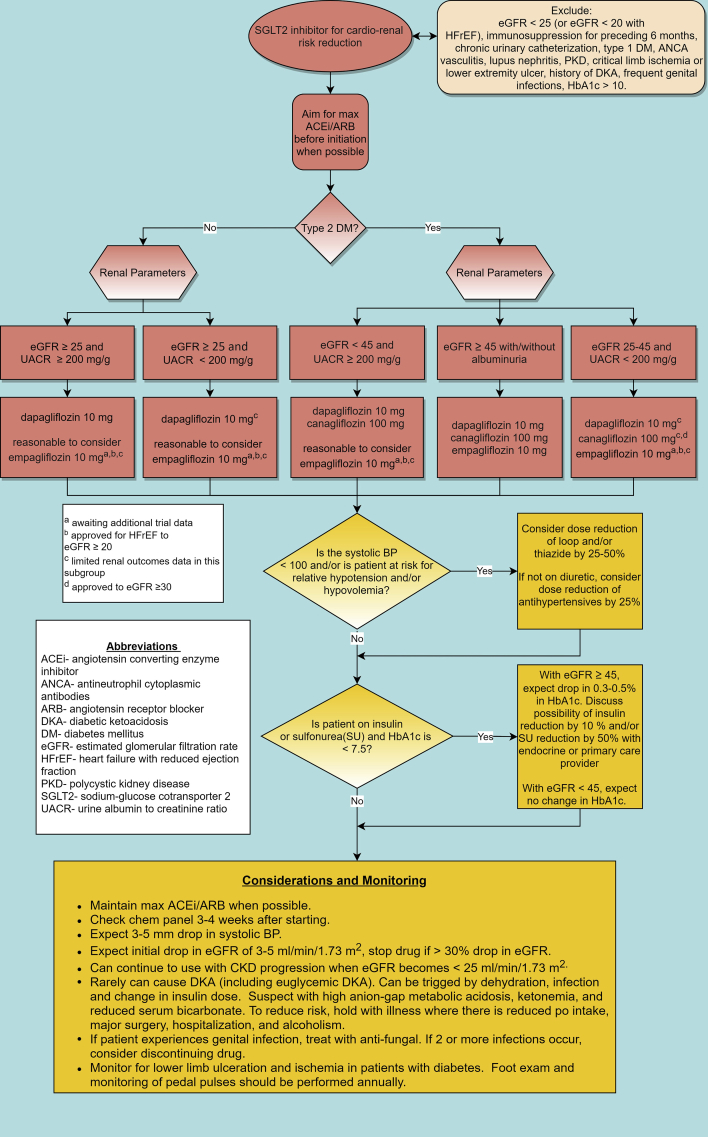
Despite the abundance of trial evidence demonstrating significant cardiovascular and kidney protective benefits and guideline recommendations, SGLT2 inhibitors remain underutilized in patient populations that are most likely to benefit and historically have not been commonly prescribed by nephrologists or cardiologists.7, 8, 9 This is not unexpected, as novel therapies traditionally take a sluggish 17 years from research to implementation in clinical practice due to the many barriers to adopting new practice habits.10 Some relevant barriers pertinent to prescribing SGLT2 inhibitors include: lack of knowledge, concern over medication side effects, lack of decision support, cost, and change resistance.11 A change in clinical practice and overcoming “clinical inertia” is multifactorial, involving a complex interplay of patient, clinician, and systems factors and requires a targeted multi-prong approach.11,12 To overcome prescribing barriers and facilitate evidence uptake with the promotion of SGLT2 inhibitor use, evidence-based research translation strategies are desperately needed. In response to this need, we developed an SGLT2 inhibitor clinical pathway (Fig 1) to aid in medical decision making.
Figure 1.
SGLT2 inhibitor clinical pathway.
Although many patients with CKD are eligible for SGLT2 inhibitor initiation, patients with type 1 DM, immunosuppression in the last 6 months, systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, and polycystic kidney disease were excluded from the major trials2, 3, 4, 5, 6 and are excluded in the clinical pathway. The majority of trial protocols included maximally-tolerated renin-angiotensin-aldosterone system blockade,2, 3, 4, 5, 6 and therefore, the pathway advises to maximize angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker therapy before SGLT2 inhibitor initiation.
The pathway adheres to the current US Food and Drug Administration indications with some noteworthy considerations. The most recent indication for the use of dapagliflozin is in CKD with all levels of albuminuria; however, individuals with CKD with urine albumin-creatinine ratio of <200 (with estimated glomerular filtration rate [eGFR] of >25 mL/min/1.73 m2) were not included in the DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) study, and despite benefits that were seen in a secondary analysis of the DECLARE-TIMI (Dapagliflozin Effect on Cardiovascular Events) study, overall trial data in this subgroup are limited.6,13 Although empagliflozin has no current kidney-specific indication, the most recent Food and Drug Administration indication (October 2021) allows for use in type 2 DM with established cardiovascular disease to an eGFR of >30 mL/min/1.73 m2 (with/without albuminuria) and in heart failure with reduced ejection fraction to an eGFR of >20 mL/min/1.73 m2.14 To address kidney-specific use, the ongoing EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) trial will provide answers regarding kidney outcomes with and without DM and albuminuria, and it will include patients down to the lowest eGFR of all the trials (20 mL/min/1.73 m2).15 The trial results may lead to additional indications for empagliflozin to reduce adverse kidney outcomes. Finally, there is some consensus that the overarching cardiovascular and kidney benefits of SGLT2 inhibitors are likely a class effect with no evidence thus far of substantial interclass variances16; given this, it may be reasonable in certain situations to substitute one SGLT2 inhibitor for another off-label as a result of coverage/cost.
SGLT2 inhibitors cause a reduction of 3-5 mm Hg in the systolic blood pressure, likely because of the resulting natriuresis.17,18 In patients who are relatively hypotensive or at risk of hypotension or hypovolemia, diuretic dose should be reduced by 25%-50%. If such at-risk patients are not on a diuretic, it is reasonable to reduce other antihypertensive medications by approximately 25%.
SGLT2 inhibitors modestly lower the blood glucose levels, an effect that is attenuated by reduced eGFR.19 Although this was of initial concern, increased risk of hypoglycemia was not seen in most study populations with CKD, including in the nondiabetic study population in DAPA-CKD.2, 3, 4, 5, 6,20, 21, 22, 23 SGLT2 inhibitor use with an eGFR of ≥45 mL/min/1.73 m2 results in a 0.3%-0.5% decrease in hemoglobin A1c10; therefore, in patients with hemoglobin A1c < 7.5% and eGFR ≥ 45 mL/min/1.73 m2 who are on insulin and/or sulfonylurea, the pathway guides to reduce the insulin dose by 10%-20% and sulfonylurea dose by 50%. For those patients with eGFR < 45 mL/min/1.73 m2, no adjustment in insulin or sulfonylurea is generally needed.
Regarding side effects, mycotic genital infections were 2-3 times higher with SGLT2 inhibitor use than with placebo use in multiple trials (but with no increased risk seen in the DAPA-CKD study)2, 3, 4, 5, 6,22, 23, 24, 25; therefore, it is reasonable to avoid SGLT2 inhibitors in patients with a history of multiple mycotic genital infections. For patients who develop a genital infection while on SGLT2 inhibitors, antifungal treatment should be initiated, and SGLT2 inhibitors should be discontinued if genital infections become recurrent. Overall, the data show that SGLT2 inhibitors as a class did not increase the risk of urinary tract infections6,22,24,25; therefore, this is not denoted in the pathway.
The use of SGLT2 inhibitors results in an initial eGFR decline of 3-5 mL/min/1.73 m2, an effect which is likely hemodynamically mediated via afferent arteriolar vasoconstriction.18 This initial decline levels off and tends to return to baseline with the stabilization of kidney function over time.1,6,16,22,26,27 Despite this reduction in eGFR, several analyses now show that SGLT2 inhibitors actually reduce the risk of acute kidney injury.16,28 In light of these findings, the pathway indicates to only consider the discontinuation of SGLT2 inhibitors if the eGFR decreases by >30%.
A rare but important side effect of SGLT2 inhibitors is diabetic ketoacidosis (DKA), including euglycemic DKA. In a large population cohort study, the incidence of DKA with SGLT2i use was three times than that with dipeptidyl peptidase-4 inhibitor use.29 Although the clinical trials show a 2 times higher risk of DKA with SGLT2 inhibitor use compared with placebo use in patients with type 2 DM, the overall absolute incidence of DKA was low (0.18%).30 Reassuringly, the DAPA-CKD study found no increased risk of DKA with SGLT2 inhibitor use, regardless of DM status.6 Patients often present with classic symptoms of DKA including nausea, vomiting, abdominal pain, fatigue, and shortness of breath.31 Changes in oral intake, alcohol use, insulin adjustment, history of DKA, and major surgery have been identified as precipitating events in some, but not all, cases.31, 32, 33 The pathway advises of appropriate situations to hold SGLT2 inhibitors and signs of DKA.
In summary, research translation of SGLT2 inhibitors into clinical practice remains imperative, and nephrology providers have the opportunity to lead at the helm of this initiative. The traditional barriers to the uptake of new knowledge impede practice transformation and can be overcome with evidence-based reinforcement systems. The SGLT2 inhibitor clinical pathway presented here may be an impactful tool to increase the initiation of SGLT2 inhibitors and ultimately improve the outcomes of patients with CKD.
Article Information
Authors’ Full Names and Academic Degrees
Laura Nishi, DScPAS, Cybele Ghossein, MD, and Anand Srivastava, MD, MPH.
Support
This work was supported by the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857. Dr Srivastava is supported by NIH grants K23DK120811 and U01AI163081, Kidney Precision Medicine Project Opportunity Pool grant under award U2CDK114886, and core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857.
Financial Disclosure
Dr Srivastava reports personal fees from Horizon Therapeutics PLC, CVS Caremark, AstraZeneca, Bayer, and Tate & Latham (medicolegal consulting). Dr Ghossein reports to be local PI on Chemocentryx and Travere studies and has been on a scientific advisory board for Horizon Therapeutics. Dr Nishi declares that she has no relevant financial interests.
Peer Review
Received November 3, 2021. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form January 15, 2022.
Footnotes
Item S1: SGLT2 Inhibitors Background and Evidence Review.
Supplementary Material
Item S1.
References
- 1.Perkovic V., de Zeeuw D., Mahaffey K.W., et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 6.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 7.Hamid A, Vaduganathan M, Oshunbade AA, et al. Antihyperglycemic therapies with expansions of US Food and Drug Administration indications to reduce cardiovascular events: prescribing patterns within an academic medical center. J Cardiovasc Pharmacol. 2020;76:313–320. doi: 10.1097/FJC.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol. 2018;72(25):3370–3372. doi: 10.1016/j.jacc.2018.08.2202. [DOI] [PubMed] [Google Scholar]
- 9.McCoy RG, Dykhoff HJ, Sangaralingham L. et. al. Adoption of new glucose-lowering medications in the U.S. The case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21:702–712. doi: 10.1089/dia.2019.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Crossing the Quality Chasm : A New Health System for the 21st Century. National Academy Press; 2001. Accessed October 24, 2021. http://www.ihi.org/resources/Pages/Publications/CrossingtheQualityChasmANewHealthSystemforthe21stCentury.aspx
- 11.Andreozzi F, Candido R, Corrao S, et al. Clinical inertia is the enemy of therapeutic success in the management of diabetes and its complications: a narrative literature review. Diabetol Metab Syndr. 2020;12(52):1–11. doi: 10.1186/s13098-020-00559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh T. How to Implement Evidenced Based Health Care. Wiley-Blackwell; 2017. [Google Scholar]
- 13.Zelniker T.A., Raz I., Mosenzon O., et al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2021;6(7):801–810. doi: 10.1001/jamacardio.2021.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drug approvals and database. US Food and Drug Administration. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases Updated September 23, 2021.
- 15.The study of heart and kidney protection with empagliflozin. ClinicalTrials.gov identifier: NCT03594110. Updated October 5, 2021. Accessed October 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03594110
- 16.Neuen B.L., Young T., Heerspink H.J.L., et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 17.Scheen A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 18.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 19.Heerspink H.J., Perkins B.A., Fitchett D.H., Husain M., Cherney D.Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 20.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 21.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 22.Toyama T., Neuen B.L., Jun M., et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(5):1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Zhang M., Lv Q., Tong N. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes and moderate renal function impairment: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;140:295–303. doi: 10.1016/j.diabres.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 24.Li D., Wang T., Shen S., Fang Z., Dong Y., Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(3):348–355. doi: 10.1111/dom.12825. [DOI] [PubMed] [Google Scholar]
- 25.Puckrin R., Saltiel M.P., Reynier P., Azoulay L., Yu O.H.Y., Filion K.B. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–514. doi: 10.1007/s00592-018-1116-0. [DOI] [PubMed] [Google Scholar]
- 26.Seidu S., Kunutsor S.K., Cos X., Gillani S., Khunti K. for and on behalf of Primary Care Diabetes Europe. SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: a systematic review and meta-analysis. Prim Care Diabetes. 2018;12(3):265–283. doi: 10.1016/j.pcd.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 28.Heerspink H.J.L., Cherney D., Postmus D., et al. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022;101(1):174–184. doi: 10.1016/j.kint.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Douros A, Lix LM, Fralick M, et al. Sodium–glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis. Ann Intern Med. 2020;173(6):417–425. doi: 10.7326/M20-0289. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Li L., Li S., et al. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22(9):1619–1627. doi: 10.1111/dom.14075. [DOI] [PubMed] [Google Scholar]
- 31.Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187–194. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Voss EA, Weaver J, et al. Diabetic ketoacidosis in patients with type 2 diabetes treated with sodium glucose co-transporter 2 inhibitors versus other antihyperglycemic agents: an observational study of four US administrative claims databases. Pharmacoepidemiol Drug Saf. 2019;28(12):1620–1628. doi: 10.1002/pds.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1.