Abstract
Background
The aim of this systematic review was to assess the effectiveness of fibrin sealant compared to sutures in periodontal surgery.
Methods
Five electronic databases (PubMed, Scopus, EBSCO, Cochrane and Web of Science) were screened from initiation to January 2021 for randomized controlled trials (RCTs) comparing fibrin sealant to sutures in periodontal surgery using this search equation: (Periodont* OR Periodontitis) AND (“fibrin tissue adhesive” OR “fibrin glue” OR “fibrin sealant” OR “fibrin sealant system” OR “fibrin adhesive system” OR “fibrin fibronectin sealant system”). Quality assessment of the included studies was performed using the revised tool to assess risk of bias in randomized trials (RoB 2). The level of evidence was evaluated using the GRADE tool.
Results
A total of 240 publications were found as search results in the screened databases. Four RCTs were included in this systematic review based on predetermined inclusion criteria. The trials were published between 1987 and 2014. All the RCTs compared fibrin sealant to sutures in periodontal surgery. The sample size included 101 patients. The overall risk of bias in this systematic review was at high risk in 75% of the studies, while 25% of the studies raised some concerns. The level of evidence evaluated using GRADE tool was very low.
Discussion
The current systematic review indicates a low level of evidence of the use of fibrin sealant as an alternative to sutures in periodontal practice. More interventional and multicentric studies should be conducted to support and confirm the results of the included studies.
Keywords: Fibrin sealant, Sutures, Periodontal surgery, Periodontal wound healing, Hemostasis, Tissue adhesion
Highlights
-
•
First systematic review aiming to compare the effectiveness of fibrin sealants (FS) to sutures (S) in periodontal surgery (PS).
-
•
Only randomized controlled trials (RCTs) were assessed.
-
•
The numerous biases and low level of evidence in the reviewed reports limited the strength of evidence on the effectiveness of FS in PS, compared to S.
1. Introduction
Proper closure of wound margins in their desired position are critical events that influence the success of periodontal surgery. Numerous methods and materials have been used such as sutures, and tissue adhesives [1,2].
Fibrin sealants (FS) are natural adhesives derived from plasma coagulation proteins, that mimic the final stages of blood coagulation [[3], [4], [5]]. The first reports of FS use in periodontal surgery were in the 1980s. Bösch P et al. [6] applied it to retain heterogeneous bone graft in periodontal defects, while Bartolucci et al. [7] used it to fix periodontal flaps and grafts.
Although sutures have been conventionally used, they can present some shortcomings, like acute inflammation and postoperative infection resulting in compromised wound healing. Furthermore, suturing is time consuming, requires skill and an additional visit for suture removal [8,9].
Several studies have reported the usefulness of FS in various surgical fields [4,[10], [11], [12], [13]], given their hemostatic, adhesive, and healing properties, which may reduce operating time, prevent complications, and enhance the overall outcome of many surgical interventions [14].
However, there is still, to this date, no clear evidence suggesting the superiority of FS to sutures in periodontal surgery.
The aim of this systematic review was to assess the effectiveness of FS compared to sutures in periodontal surgery.
2. Methods
The present systematic review was structured following the PRISMA recommendations for transparent reporting of systematic reviews and meta-analysis [15].
The global review protocol was preliminarily registered in the PROSPERO database under the registration number: CRD42021253913.
2.1. Eligibility criteria
2.1.1. Inclusion criteria
Studies were assessed for eligibility based on the following criteria:
-
-
Participants: Patients undergoing periodontal surgery were eligible for inclusion.
-
-
Intervention: Clinical studies comparing two groups, one test (using commercial FS in periodontal surgery) and one control (using sutures in periodontal surgery) were eligible for inclusion.
-
-
Outcome variables were classified into primary outcomes such as inflammation, healing hemostasis and post-operative comfort, and secondary outcomes such as surgical chair time.
-
-
Study design: Randomized controlled trials (RCT) were eligible for inclusion. No restrictions on language or year of publication were placed.
2.1.2. Exclusion criteria
Studies using autologous or animal derived fibrin sealants and studies using fibrin sealants as an adjunct to other materials were excluded. Case reports, case series, editorials, reviews were also excluded.
2.2. Information sources
Medline (PubMed), Scopus, EBSCO, The Cochrane Central Register of Controlled Trials and Web of science were screened up to January 2021 for eligible studies related to the focused question.
A complementary hand search was done in the following journal databases: Journal of Periodontology, Journal of Clinical Periodontology, Journal of Periodontal Research, and The International Journal of Periodontics and Restorative Dentistry.
Reference lists of any potential articles and OpenGrey (www.opengrey.eu) database were screened for relevant unpublished studies or papers not identified by electronic searching.
Furthermore, the ISRCTTN registry (www.isrctn.com) and the EU Clinical Trials Register (www.clinicaltrialsregister.eu) were screened for appropriate ongoing on unpublished studies.
2.3. Search strategy
The structured search strategy/equation used in all the databases was as follows: (Periodont* OR Periodontitis) AND ("fibrin tissue adhesive" OR "fibrin glue" OR "fibrin sealant" OR "fibrin sealant system" OR "fibrin adhesive system" OR "fibrin fibronectin sealant system").
No restrictions on language or year of publication were placed.
2.4. Selection process
Titles and abstracts of retrieved papers from our search strategy were screened in duplicate and independently by two reviewers (M.M and F.S). Full text versions of potentially pertinent studies were collected based on the initial screening. These reports were classified as absolutely eligible, absolutely not eligible or controversial.
Disagreement between examiners was resolved through discussion until consensus was reached. If needed, arbitration by a third investigator (A.B.) was planned to determine the final decision. The reports that satisfied all of the inclusion criteria were used for data extraction.
2.5. Data collection and items
All the studies meeting the inclusion criteria underwent data extraction performed by two review authors independently. Both reviewers used a standardized data extraction sheet with the following items: first author/year of publication (country), study design, surgical techniques, sample, follow-up, age range, gender, study groups, measured outcomes and results. The references were added through Mendeley.
2.6. Quality assessment
Quality assessment of the included studies was conducted using the revised tool to assess risk of bias in randomized trials (RoB 2) [16]. Assessment was based on five domains (Randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result). Within each domain, one or more signaling questions were answered. These answers lead to judgments of “low risk of bias”, “some concerns”, or “high risk of bias”. The judgments within each domain lead to an overall risk-of-bias judgment for the result being assessed [16].
Risk of bias assessment was performed by two reviewers (M.M and F.S). Each disagreement was resolved by discussion and consensus. The judgement of a third reviewer (A.B) was planned if a disagreement was not resolved.
The present systematic review has been self-evaluated through the AMSTAR 2 checklist (available in supplementary file) [17]. As no meta-analysis was conducted given the overall limited sample and heterogeneity of outcomes, the level of compliance with AMSTAR 2 came out to be “moderate”.
2.7. Level of evidence assessment
The quality of evidence of all studies was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) [18]. In this review, a narrative GRADE approach was performed [19,20].
Generally, RCTs start as high quality evidence. From the initial rating, the quality of evidence can be rated down by one or two levels when there are serious or very serious concerns, respectively, in any of the following five domains: risk of bias, inconsistency, indirectness, imprecision, or publication bias [19,20] As a result of the assessment, the quality of evidence for each outcome falls into one of four categories: high (the review authors are very confident that the true effect lies close to that of the estimate of the effect), moderate (the review authors are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different), low (the review author's confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect) and very low (the review authors have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect) [21].
3. Results
3.1. Study selection
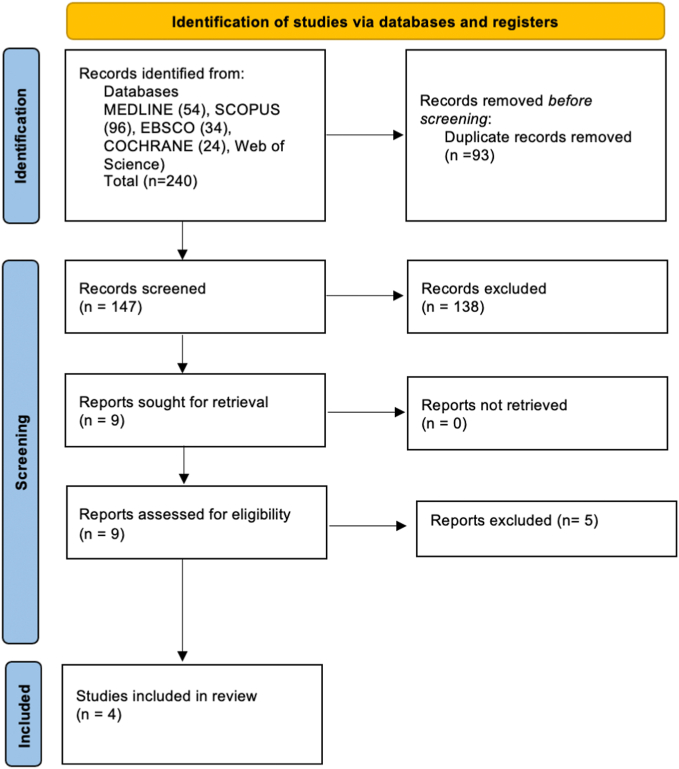
The search strategy generated 240 publications, by screening titles and abstracts and removing duplicates, 9 papers were retained, for which full text versions were obtained for detailed reading. Five of those articles [[22], [23], [24], [25], [26]] were excluded for being incomplete clinical trials, or using other materials in adjunction to FS.
In brief, 4 eligible publications [2,[27], [28], [29]] were included in the current systematic review. More details of the data search are described in the flow chart (Fig. 1).
Fig. 1.
Flow chart of the systematic review.
3.2. Study characteristics
Four clinical trials, comparing FS to sutures in periodontal surgery and conducted in India and Italy met the criteria and were included in this systematic review. The trials were published between 1987 and 2014. All the RCTs compared FS to sutures in periodontal surgery. The sample size included 101 patients aged from 9 to 63 years. The characteristics of the studies were presented in Table 1.
Table 1.
Overview of the characteristics of included randomized clinical trials.
| First author/year (country) | Study design | Surgical technique | Sample Follow-up | Age range Gender |
Study groups | Measured outcomes | Results |
|---|---|---|---|---|---|---|---|
| G.P.P. Prato 1986 (Italy) [27] | Split mouth randomized controlled clinical trial |
|
51 patients 7th day, 14th day, 21st day |
9–63 years F: 22 M: 29 |
Control side: 4-0 silk sutures Ethicon® | The differences in the chair time | Difference in overall chair time between FS and S: FGG: 8′30″, Pedicle: 8′, MWF: 4′45″, APF: 6′ |
| No differences in statistical significance reported | |||||||
| Test side: Fibrin glue Tissucol ® kit | Pain and discomfort (no objective measuring tools reported) | 3 patients reported greater pain on the control side, with one reporting pain on the experimental side. Discomfort was often noticed during the removal of sutures. | |||||
| Amount of Tissucol used | Total amount of Tissucol used: FGG: 0.2 ml, Pedicle: 0.2 ml, MWF: 0.3 ml, APF: 0.4 ml | ||||||
| A.G. Manimegalai 2010 (India) [2] | Randomized controlled clinical trial |
Group I: Modified Widman flap Group II: Pedicle Graft Group III: Free Gingival Autograft |
25 patients 1st, 2nd, 3rd, 7th, and 10th days |
20–45 years F: 10 M: 15 |
Control group: 4-0 black braided silk sutures (S) | Chair side time | Total chair side time |
| Group I: FS: 23′43″, S: 35′09″ | |||||||
| Group II: FS: 23′53″, S: 36′40″ | |||||||
| Group III: FS: 24′13″, S: 38′58″ | |||||||
| No differences in statistical significance reported | |||||||
| Test group: Fibrin sealant (FS) (Tisseel®) | Time saved | An average of 5–10 min was saved by using FS. | |||||
| Plaque index | Plaque index | ||||||
| Group I: FS:1st = 0.32, 7th = 0; S: 1st = 1.58, 7th = 0.70 | |||||||
| Group II: FS: 1st = 0.61, 10th = 0; S: 1st = 1.36, 10th = 0.87 | |||||||
| Group III: FS: 1st = 0.36, 10th = 0; S: 1st = 1.05, 10th = 0.46 | |||||||
| No differences in statistical significance reported | |||||||
| Stability of tissues | Stability of tissues (at 5 min) | ||||||
| Group I: FS: 0; S: 1 | |||||||
| Group II: FS: 0; S: 1 | |||||||
| Group III: FS: 0; S: 1 | |||||||
| No differences in statistical significance reported | |||||||
| Postoperative bleeding | Postoperative bleeding (at 1 and 5 min) | ||||||
| Group I: FS: 0; S: 1st = 0, 2nd = 0, 3rd = 1, 7th = 1, 10th = 0 | |||||||
| Group II: FS: 0; S: 1 | |||||||
| Group III: FS: 0; S: 1st = 1, 2nd = 1, 3rd = 5, 7th = 1, 10th = 1 | |||||||
| No differences in statistical significance reported | |||||||
| SJ. Pulikkotil 2013 (India) [28] | Split mouth randomized controlled clinical trial | Periodontal flap surgery | 10 patients 7th day, 14th day, 21st day |
18–60 years | Control group: 3-0 black silk sutures (Johnson Ethicon®) | Number of fibroblasts, blood vessels, inflammatory cells at 8th day postoperatively | Fibroblast: FS:70.45 ± 7.22, S: 42.95 ± 4.344, p < 0.001* |
| Blood vessels: FS: 5.74 ± 2.41, S:1.89 ± 3.64, p = 0.005* | |||||||
| Inflammatory cells: FS: 20.91 ± 4.46, S:32.58 ± 4.29, p < 0.001* | |||||||
| Test group: Fibrin sealant (Reliseal®, Reliance Life Sciences) | Discomfort | Discomfort was noticed on the sutured site. | |||||
| Amount of Fibrin sealant used | An average of 0.2–0.3 ml of FS used on both buccal and lingual aspects on each tooth. | ||||||
| SJ. Pulikkotil 2014 (India) [29] | Split mouth controlled randomized clinical trial | Periodontal flap surgery | 15 patients 7th, 8th, 14th, 21st day, 3 months |
18–60 years F: 9 M: 6 |
Control group: 3-0 black silk sutures (S) (Johnson, Ethicon, USA) |
IL-1β and IL-8 levels (pg/μl) between S and FS side in GCF at 8th day postop | Difference: |
| IL-1β: S = 97.43 ± 41.23, F = (−)55.61 ± 75.73, p < 0.001* | |||||||
| IL-8: S = 92.74 ± 43.61, F = (−) 36.12 ± 46.44, p < 0.001* | |||||||
| Test group: Fibrin sealant (F)(Tisseel ® Baxter Healthcare, Deerfield, IL, USA) | Plaque, bleeding, color, dehiscence, recession, probing depth, pain and discomfort using 100 mm VAS scale | Plaque | |||||
| FS: Baseline:0.0 ± 0.0, 7th day: 0.13 ± 0.35, 3 months: 0.0 ± 0.0 | |||||||
| S: Baseline:0.0 ± 0.0, 7th day: 0.73 ± 0.46, 3 months:0.0 ± 0.0 | |||||||
| P value: 7th day: p < 0.001* | |||||||
| Bleeding | |||||||
| FS: Baseline: 0.32 ± 0.10, 7th day:0.0 ± 0.0, 3 months: 0.0 ± 0.0 | |||||||
| S: Baseline: 0.28 ± 0.12, 7th day: 0.67 ± 0.49, 3 months: 0.0 ± 0.0 | |||||||
| P value: 7th day p < 0.001* | |||||||
| Color | |||||||
| FS: Baseline: 1.52 ± 0.35, 7th day:1.05 ± 0.09, 3 months:1.00 ± 0.0 | |||||||
| FS: Baseline: 1.27 ± 0.24, 7th day: 1.54 ± 0.53, 3 months:1.00 ± 0.0 | |||||||
| P value: Baseline: 0.68, 7th day: 0.003* | |||||||
| Dehiscence | |||||||
| FS: Baseline: 0.0 ± 0.0, 7th day: 0.0 ± 0.0, 3 months: 0.0 ± 0.0 | |||||||
| S: Baseline: 0.0 ± 0.0, 7th day: 0.20 ± 0.41, 3 months: 0.0 ± 0.0 | |||||||
| P value: 7th day: 0.07 | |||||||
| Recession | |||||||
| FS: Baseline: 1.06 ± 0.88, 3 months: 0.93 ± 0.96 | |||||||
| S: Baseline: 0.94 ± 0.8, 3 months: 0.83 ± 0.94 | |||||||
| P value: Baseline: 0.72, 3 months: 0.70 | |||||||
| Probing depth | |||||||
| FS: Baseline: 7.13 ± 1.45, 3 months: 6.07 ± 1.28 | |||||||
| S: Baseline: 6.93 ± 1.27, 3 months: 6.07 ± 1.28 | |||||||
| P value: Baseline: 0.96 | |||||||
| Pain (mm): | |||||||
| FS: Immediately after surgery: 11.6 ± 2.97, at 7 days: 4.93 ± 3.08 | |||||||
| S: Immediately after surgery: 32.0 ± 6.21, at 7 days: 31.26 ± 7.42 | |||||||
| P value: Immediately after surgery: p < 0.001*, at 7 days: p < 0.001* | |||||||
| Discomfort (mm): | |||||||
| FS: Immediately after surgery: 11.8 ± 2.70, at 7 days: 3.46 ± 1.76 | |||||||
| S: Immediately after surgery: 25.60 ± 6.23, at 7 days: 20.53 ± 6.71 | |||||||
| P value: Immediately after surgery: p < 0.001*, at 7 days: p < 0.001* | |||||||
| Amount of fibrin sealant used | An average of 0.2 ml of Tisseel® was used per tooth. |
Sutures (S), Fibrin sealant (FS), Gingival crevicular fluid (GCF), Free gingival graft (FGG), Pedicule sliding flap (Pedicle), Modified Widman flap (MWF), Apically positioned flap (APF), Fibrin (F), F: female, M: male,’ minutes,’’ seconds.
Stability of tissues (score): 0 – Stable at 5 min; 1 – Unstable at 5 min; Postoperative bleeding: 0 – No bleeding at 1 min; 1 – Bleeding at 1 min; 5 – Bleeding at 5 min; *Statistically significant.
3.3. Assessment of risk of bias
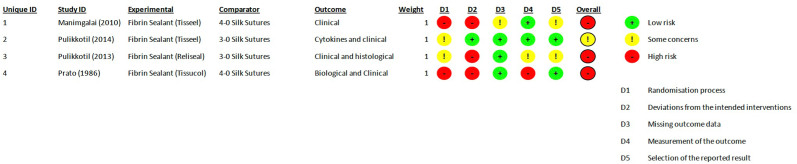
Randomization process was not well described and was at high risk of bias in 50% of the studies, while the other 50% raised some concerns. Deviations from intended interventions was at low risk of bias in 25% of the studies and was at high risk of bias in 75% of the studies. Missing outcome data was at low risk of bias in 75% of the studies and raised some concerns in 25% of the studies. Measurement of the outcome in the included studies was at low risk of bias in 50% of the studies, raised some concerns in 25% of the studies and was at high risk of bias in 25% of the studies. Selection of the reported result was at low risk of bias in 50% of the studies and raised some concerns in 50% of the studies. The overall risk of bias in this systematic review was at high risk in 75% of the studies [2,27,28], while 25% [29] of the studies raised some concerns (Fig. 2).
Fig. 2.
Assessment of risk of bias.
3.4. Level of evidence
A narrative GRADE approach was conducted to assess the quality of evidence across the four studies. The outcome of the overall analysis resulted in a very low quality of evidence. The flaws presented in the risk of bias, inconsistency and imprecision of analysis due to bias, heterogeneity, and number of studies, as demonstrated in Table 2.
Table 2.
Quality of the level of evidence by GRADE narrative assessment.
| Certainty assessment |
Impact | Quality | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | |||
| Inflammation and healing 3 [2,28,29] |
RCT | serious | serious | no | serious | undetected | In three studies, different parameters of inflammation and healing were evaluated. Two studies reported statistically significant results suggesting lower inflammation and better healing in the test groups. However, problems related to bias, heterogeneity, and reduced sample sizes impair risk of bias, inconsistency and imprecision of the found results. | ⊕οοο very low |
Important |
| Hemostasis 4 [2,[27], [28], [29]] |
RCT | serious | serious | no | serious | undetected | In the four studies, postoperative bleeding was evaluated, no statistical measure was done to evaluate the difference between control and test groups. Therefore, risk of bias, inconsistency and impression of this outcome were impaired. | ⊕οοο very low |
Important |
| Postoperative comfort 3 [[27], [28], [29]] |
RCT | serious | serious | no | serious | undetected | In the three studies, postoperative comfort was evaluated, no statistically significant evidence was provided. Therefore, risk of bias, inconsistency and impression of this outcome were impaired. | ⊕οοο very low |
Important |
⊕οοο Very low; ⊕⊕οο Low; ⊕⊕⊕ο Moderate; ⊕⊕⊕⊕ High.
4. Discussion
4.1. Summary of the main results
The results of this systematic review demonstrated that up until today, only 4 RCTs, in the literature compared the effectiveness of FS to sutures in periodontal surgery.
Among these studies, 2 studies concluded that FS enhance early wound healing, while sutures cause inflammation around themselves and cumulate plaque [27,28]. One study demonstrated that FS promote early wound healing by reducing IL-1β and IL-8 levels, two inflammatory mediators present in gingival crevicular fluid [29].
Pulikkotil et al. reported on the seventh day of follow-up, a statistically significant difference between test side (FS) and control side (sutures), in the following clinical parameters: plaque index, bleeding, color and soft tissue dehiscence. The control group showed more plaque accumulation, gingival redness and soft tissue dehiscence [29]. It is worth mentioning that the statistical differences observed between FS and sutures, were compared according to each day of follow up, instead of comparing the mean differences during the follow-ups. Manimegalai et al. reported that the plaque and gingival index scores, and pocket depth were reduced postoperatively in test groups, compared to control groups. Although, no statistically significant test was reported [2].
Two studies reported that FS provide better and early hemostasis than sutures [2,27], and that they can form a better alternative in terms of tissue stability [2,27].
When comparing clinical manipulation, three studies concluded that FS is easier and more comfortable to use than sutures and reduces the surgical time considerably [2,27,29].
Three studies reported pain and discomfort on the control side [2,[27], [28], [29]]. The Visual Analogue Scale (VAS) was used only in one study [29], no measuring tools were mentioned in the other studies.
4.2. Agreements and disagreements with other studies or reviews
The use of FS in periodontal surgery has been addressed in the literature, which highlighted positive findings regarding its use in local hemostatic measures, correction of periodontal bony defects, and as a tissue adhesive. Nevertheless, the present systematic review compares the outcomes of periodontal parameters among included studies. The findings from the present work tend to agree with the conclusions of the previous publications, with some reservations based on bias detected in the included trials.
There are some drawbacks to using FS, mainly the high cost, which limited the cost/benefit ratio. Another theoretical drawback is the risk of transmission of infectious agents, such as parvovirus B19 [30,31] and prions, as FS is a blood derived product. However, careful selection of donors and improved viral inactivation techniques in the manufacturing process has largely minimized these concerns [5,11,32].
4.3. Overall completeness and applicability of evidence
The present systematic review is the fruit of screening five databases, using complementary searches in specific journals and screening reference lists of any potential articles and grey literature database for relevant unpublished studies. Nevertheless, the number of included studies is limited compared to published reports in medical sciences.
The adopted search strategy resulted in 4 RCTs. The overall risk of bias in this systematic review was at high risk in 75% of the studies while 25% of the studies presented some concerns. This high risk mainly concerned randomization process, deviations from intended interventions and measurement of the outcome domains.
Moreover, the quality of the level of evidence of all the outcomes evaluated was classified very low using the GRADE tool.
Based on the analysis of extracted data, it seems that the consistency of the evidence is rather complex. Differences in protocols, variability across studies and measured outcomes make the comparison more complicated.
4.4. Advantages and potential limitations in the review process
To our knowledge, this is the first systematic review aiming to compare the effectiveness of FS to sutures in periodontal surgery. All of the included articles were RCTs. The present systematic review was structured following the PRISMA recommendations for transparent reporting of systematic reviews and meta-analysis [15]. The research design was carried out to be reproducible based on a specific search strategy. Overall, the level of bias of the whole review process would be estimated as low.
4.5. Implications for clinical practice and for further research
The present systematic review, conducted on patients undergoing periodontal surgery, shows that FS has potential uses in periodontal practice. Surgery performed with FS is technically less demanding than sutures. However, the numerous biases in the reviewed reports limited the strength of the evidence on this issue. The overall convenience of the use of FS should be evaluated on the basis of its cost and benefits.
The current clinical use of FS in periodontology might be limited by the challenges above.
Thus, further trials with larger samples are required to determine clearly whether fibrin sealant can completely replace sutures in periodontal surgery.
The design of the upcoming trials must be carefully performed, should not neglect defining the FS's pharmaceutical form and assess more periodontal parameters following its use. It should also be more specific to the site and type of intervention. The ideal would be to have homogeneous outcomes measured and a standardized protocol. All possible side effects during clinical trials have to be clearly notified without any selective reporting.
5. Conclusion
Within the limitations of the extracted data from the RCTs, it seems that FS would have some clinical benefits such as saving more surgical time compared to sutures. However, the numerous biases in the reviewed reports limited the strength of the evidence on this issue.
Given various detected biases in the included trials, and the very low level of evidence, we do not suggest the use of FS as an alternative to sutures in periodontal surgery.
Our results should guide researchers in carrying out more RCTs with rigorous protocols in order to provide a clear response to our focused question.
FS and other sealing products should have as much attention in periodontal research as conventional sutures in order to face the increasing esthetic and comfort demands of patients.
Registration of research studies
Name of registry: PROSPERO.
Unique Identifying number of registration ID: CRD42021253913.
Hyperlink to registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021253913.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
Research studies involving patients require ethical approval. Please state whether approval has been given, name the relevant ethics committee and the state the reference number for their judgement.
No ethical approval was required as this research project is a systematic review of previous studies.
Please state any sources of funding for your research
All sources of funding should be declared as an acknowledgement at the end of the text. Authors should declare the role of study sponsors, if any, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. If the study sponsors had no such involvement, the authors should so state.
This work was not funded by any institution.
Author contribution
Mariam Mounsif: Writing and Editing the first draft, Article's screening, Data extraction, Data analysis, Fatima ezzahraa Smouni: Conceptualization, Article's screening, Data extraction, Data analysis, Writing initial draft. Amal Bouziane: Conceptualization, Data analysis, Supervision, Review and editing. All: Approval of the final version of the manuscript.
Please state any conflicts of interest
All authors must disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
No potential conflict of interest relevant to this article was reported.
Annals of medicine and surgery
The following information is required for submission. Please note that failure to respond to these questions/statements will mean your submission will be returned. If you have nothing to declare in any of these categories then this should be stated.
Consent
Studies on patients or volunteers require ethics committee approval and fully informed written consent which should be documented in the paper.
Authors must obtain written and signed consent to publish a case report from the patient (or, where applicable, the patient's guardian or next of kin) prior to submission. We ask Authors to confirm as part of the submission process that such consent has been obtained, and the manuscript must include a statement to this effect in a consent section at the end of the manuscript, as follows: "Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Patients have a right to privacy. Patients’ and volunteers' names, initials, or hospital numbers should not be used. Images of patients or volunteers should not be used unless the information is essential for scientific purposes and explicit permission has been given as part of the consent. If such consent is made subject to any conditions, the Editor in Chief must be made aware of all such conditions.
Even where consent has been given, identifying details should be omitted if they are not essential. If identifying characteristics are altered to protect anonymity, such as in genetic pedigrees, authors should provide assurance that alterations do not distort scientific meaning and editors should so note.
No consent was required as this research project is a systematic review of previous studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103539.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Burkhardt R., Lang N.P. Influence of suturing on wound healing. Periodontol. 2000;68(1):270–281. doi: 10.1111/prd.12078. 2015. [DOI] [PubMed] [Google Scholar]
- 2.Manimegalai A. A comparative study on the efficacy of a commercial fibrin adhesive (Tisseel ®) vis-à-vis silk suture on wound closure following periodontal surgical procedures. J. Indian Soc. Periodontol. 2010;14(4):231. doi: 10.4103/0972-124X.76925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fattahi T., Mohan M., Caldwell G.T. Clinical applications of fibrin sealants. J. Oral Maxillofac. Surg. 2004;62(2):218–224. doi: 10.1016/j.joms.2003.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Spotnitz W.D. Commercial fibrin sealants in surgical care. Am. J. Surg. 2001;182(2 SUPPL. 1):8–14. doi: 10.1016/s0002-9610(01)00771-1. [DOI] [PubMed] [Google Scholar]
- 5.Sierra D.H. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J. Biomater. Appl. 1993;7(4):309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 6.Bösch P., Lintner F., Arbes H., Brand G. Experimental investigations of the effect of the fibrin adhesive on the Kiel heterologous bone graft. Arch. Orthop. Trauma. Surg. 1980;96(3):177–185. doi: 10.1007/BF00457781. [DOI] [PubMed] [Google Scholar]
- 7.Bartolucci E.G., Prato G.P. Preliminary observations on the use of a biologic sealing system (Tissucol®) in periodontal surgery. J. Periodontol. 1982;53(12):731–735. doi: 10.1902/jop.1982.53.12.731. [DOI] [PubMed] [Google Scholar]
- 8.Leknes K.N., Selvig K.A., Bøe O.E., Wikesjö U.M.E. Tissue reactions to sutures in the presence and absence of anti-infective therapy. J. Clin. Periodontol. 2005;32(2):130–138. doi: 10.1111/j.1600-051X.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 9.Leknes K.N., Røynstrand I.T., Selvig K.A. Human gingival tissue reactions to silk and expanded polytetrafluoroethylene sutures. J. Periodontol. 2005;76(1):34–42. doi: 10.1902/jop.2005.76.1.34. [DOI] [PubMed] [Google Scholar]
- 10.Matras H. Fibrin seal: the state of the art. J. Oral Maxillofac. Surg. 1985;43(8):605–611. doi: 10.1016/0278-2391(85)90129-6. [DOI] [PubMed] [Google Scholar]
- 11.Clark R.A.F. Fibrin sealant in wound repair: a systematic survey of the literature. Expet Opin. Invest. Drugs. 2000;9(10):2371–2392. doi: 10.1517/13543784.9.10.2371. [DOI] [PubMed] [Google Scholar]
- 12.Radosevich M., Goubran H.A., Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72(3):133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 13.Albala D.M. Fibrin sealants in clinical practice. Cardiovasc. Surg. 2003;11(SUPPL. 1):5–11. doi: 10.1016/S0967-2109(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas B.V.V., Rupa N., Kumari K.V.H., Rajender A., Reddy M. Treatment of gingival recession using free gingival graft with fibrin fibronectin sealing system: a novel approach. J. Pharm. BioAllied Sci. 2015;7(6):S734–S739. doi: 10.4103/0975-7406.163524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J., Savović J., Page M.J., Sterne J.A.C. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Br. Med. J. 2019;(July):1–24. [Google Scholar]
- 17.Shea B., Reeves B., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;21(358):j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Granholm A., Alhazzani W., Møller M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019;123(5):554–559. doi: 10.1016/j.bja.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Dijkers M. Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development. e-newsletter Cent. Knowl. Transl. Disabil. Rehabil. Res. 2013;1(5):1–9. [Google Scholar]
- 21.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Shah C., Parmar M., Desai K., Budhiraja S., Kumar S. Evaluation and comparison of healing of periodontal flaps when closed with silk sutures and N-butyl cyanoacrylate. A Clinico – Histol. Stud. 2013;(November):7–14. [Google Scholar]
- 23.Kızıltoprak M., Uslu M.Ö. Comparison of the effects of injectable platelet-rich fibrin and autologous fibrin glue applications on palatal wound healing: a randomized controlled clinical trial. Clin. Oral Invest. 2020;24(12):4549–4561. doi: 10.1007/s00784-020-03320-6. [DOI] [PubMed] [Google Scholar]
- 24.Trombelli L., Scabbia A., Scapoli C., Calura G. Clinical effect of tetracycline demineralization and fibrin-fibronectin sealing system Application on healing response following flap debridement surgery. J. Periodontol. 1996;67(7):688–693. doi: 10.1902/jop.1996.67.7.688. [DOI] [PubMed] [Google Scholar]
- 25.Jung U.W., Chang S.J., Choi S.H., Kim C.S., Chai J.K. Effects of mixture of fibrin-fibronectin sealant system and calcium carbonate in periodontal intrabony defects. Key Eng. Mater. 2006;309–311:1397–1400. [Google Scholar]
- 26.Yen C.A., Griffin T.J., Cheung W.S., Chen J. Effects of platelet concentrate on palatal wound healing after connective tissue graft harvesting. J. Periodontol. 2007;78(4):601–610. doi: 10.1902/jop.2007.060275. [DOI] [PubMed] [Google Scholar]
- 27.Pini Prato G.P., Coltellini P., Agudio G., Clauser C. Human fibrin glue versus sutures in periodontal surgery. J. Periodontol. 1987;58(6):426–431. doi: 10.1902/jop.1987.58.6.426. [DOI] [PubMed] [Google Scholar]
- 28.Pulikkotil S.J., Nath S. Fibrin sealant as an alternative for sutures in periodontal surgery. J. Coll. Physicians Surg. Pakistan. 2013;23(2):164–165. [PubMed] [Google Scholar]
- 29.Pulikkotil S.J., Nath S. Effect on interleukin-1β and interleukin-8 levels following use of fibrin sealant for periodontal surgery. Aust. Dent. J. 2014;59(2):156–164. doi: 10.1111/adj.12178. [DOI] [PubMed] [Google Scholar]
- 30.Morita Y., Nishii O., Kido M., Tsutsumi O. Parvovirus infection after laparoscopic hysterectomy using fibrin glue hemostasis. Obstet. Gynecol. 2000;95(Supplement):1026. doi: 10.1016/s0029-7844(00)00873-5. [DOI] [PubMed] [Google Scholar]
- 31.Hino M., Ishiko O., Honda K.I., Yamane T., Ohta K., Takubo T., et al. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br. J. Haematol. 2000;108(1):194–195. doi: 10.1046/j.1365-2141.2000.01818.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacob S., Nath S. Fibrin sealant: a review of its applications in periodontal surgery. Int. J. Exp. Dent. Sci. 2015;4(1):40–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.